Abstract
Intestinal fibrostenosis is a hallmark of severe Crohn's disease and can lead to multiple surgeries. Patients with certain TNFSF15 variants overexpress TL1A. The aim of this study was to determine the effect of TL1A overexpression on intestinal inflammation and the development of fibrostenosis. We assessed the in vivo consequences of constitutive TL1A expression on gut mucosal inflammation and fibrostenosis using two murine models of chronic colitis. In the dextran sodium sulfate (DSS) and adoptive T-cell transfer models, there was proximal migration of colonic inflammation, worsened patchy intestinal inflammation, and long gross intestinal strictures in Tl1a transgenic compared to wild-type littermates. In the DSS model, myeloid- and T-cell–expressing Tl1a transgenic mice had increased T-cell activation markers and interleukin-17 expression compared to wild-type mice. In the T-cell transfer model, Rag1−/− mice receiving Tl1a transgenic T cells had increased interferon-γ expression but reduced T-helper 17 cells and IL-17 production. Narrowed ureters with hydronephrosis were found only in the Tl1a transgenic mice in all chronic colitis models. In human translational studies, Crohn's disease patients with higher peripheral TL1A expression also exhibited intestinal fibrostenosis and worsened ileocecal inflammation with relative sparing of rectosigmoid inflammation. These data show that TL1A is an important cytokine that not only modulates the location and severity of mucosal inflammation, but also induces fibrostenosis.
Crohn's disease (CD) is a chronic inflammatory condition with pathological features such as patchy transmural gut inflammation and fibrostenosis with relative sparing of the rectum.1,2 Fibrostenosis is a characteristic of severe, often treatment-resistant, disease, a source of significant morbidity, and in nearly every case, requires surgical intervention. Variants in the TNFSF15 gene have been found to be associated with inflammatory bowel disease (IBD); and its protein product, TL1A, is elevated in the intestinal mucosa of IBD patients. Certain TNFSF15 haplotypes are associated with susceptibility in non-Jewish Caucasian CD and UC patients. In addition, TNFSF15 haplotype-B is not only associated with risk, but also with severity in Jewish CD patients.3–5 Moreover, monocytes from Jewish patients carrying the risk haplotype-B express higher peripheral monocyte TL1A expression in response to FcγR stimulation.3 These results show that CD-associated TNFSF15 genetic variations contribute to enhanced induction of TL1A, resulting in severe, chronic mucosal inflammation- and that modulation of TL1A may be a potential target for therapeutic development.
TL1A signals via death domain receptor 3 (DR3) and modulates the adaptive immune response. In the T-helper (Th)-1 effector arm, TL1A enhances interferon (IFN)-γ production from peripheral and mucosal T cells.6,7 In addition to mediating the Th-1 response, TL1A also plays a role in augmenting Th-2 and Th-17 effector cell function.8–11 The differential effect of TL1A on Th-17 cells was demonstrated by a recent report showing that TL1A inhibited Th-17 development in naive T cells but maintained the effector characteristics of committed Th-17 cells.12
In addition to human reports, several studies in mice also implicate the TL1A/DR3 signaling pathway in mucosal inflammation.13,14 Neutralizing TL1A antibody ameliorates inflammation in DSS and Gαi2−/− T-cell transfer chronic colitis models.10 Additionally, constitutive TL1A expression in mice leads to mild spontaneous ileitis and increased collagen deposition.15–17
To determine the in vivo consequences of constitutive TL1A expression under colitogenic settings, chronic dextran sodium sulfate (DSS)-induced [myeloid and T-cell Tl1a transgenic (Tg) mice] and adoptive-transfer (T-cell Tl1a transgenic mice) models were used. In all models, there was proximal migration of colonic inflammation, worsened patchy intestinal inflammation, and strictures in the intestine and colon with constitutive TL1A expression. Similarly, CD patients with TNFSF15 haplotype-B and elevated TL1A expression in primary monocytes showed worsened ileocecal inflammation with relative rectosigmoid sparing and intestinal fibrostenosis. These novel murine Tl1a-Tg models resemble a complicated form of severe human CD. Results from these Tl1a-Tg experimental models coupled with findings in CD patients suggest that TNFSF15 is a severity gene and that the cytokine, TL1A, when overexpressed, plays a central role in generating one phenotypic form of complicated CD.
Materials and Methods
Transgenic Mice and Induction of Chronic Colitis
LCK-CD2-Tl1a-GFP (L-Tg) and FMS-Tl1a-GFP Tg (M-Tg) mice were generated and genotyped as described.17 Chronic DSS colitis was induced as described,10 except 2% (w/v) DSS (MP Biomedicals, Irvine, CA) drinking water was used due to lethality in Tl1a-Tg mice when DSS concentration ≥2.5% was used. In the adoptive-transfer model, colitis was induced by intraperitoneal injection of 500,000 CD4+CD45RBhi naive T cells isolated from either L-Tg or wild-type (WT) mice to Rag1−/− mice.18 All mice were maintained under specific pathogen-free conditions in the Animal Facility at Cedars-Sinai Medical Center (CSMC). Littermate control mice were used. This study was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal studies were approved by the CSMC Animal Care and Use Committee (protocol 2269).
Quantitation of DSS Water Consumption
WT, L-Tg, and M-Tg mice (n = 5 in each group) were housed individually in a cage and administered 2% DSS water on days 1 to 5, 8 to 12, 15 to 19, and 22 to 26. The weight of DSS water was measured at the beginning of each cycle (days 1, 8, 15, and 22) and at the end of the cycle (days 5, 12, 19, and 26). DSS water consumption was measured by subtracting DSS water weight at the beginning of the treatment cycle from the end of the treatment cycle.
Disease Activity Index, Myeloperoxidase, and Macroscopic and Histopathological Analyses
The disease activity index (DAI) was calculated by scoring from 0 to 4 abnormalities regarding changes in body weight (0, no weight loss; 1, 1% to 5% weight loss; 2, 5% to 10% weight loss; 3, 10% to 15% weight loss; 4, more than 15% weight loss), stool consistency (0, firm dry stool; 1, moist stool; 2, soft adherent stool; 3, large soft pliable stool; 4, liquid stool), stool blood performed on Hemoccult Sensa (Beckman Coulter, Brea, CA; 0, no color; 1, flecks of blue; 2, up to 50% blue; 3, more than 50% blue; 4, gross red blood), and summing the results.19 DAI was determined every other day for the DSS model and twice a week for the adoptive-transfer model. Myeloperoxidase activity was assessed using the EnzChek Myeloperoxidase Activity Assay Kit according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). Macroscopic evidence of inflammation was scored blinded to the mice genotype using the established classification.10,18 Briefly, normal gut morphology was assigned a score of 0; mild bowel wall thickening without hyperemia was assigned a score of 1; moderate bowel wall thickening with hyperemia was assigned a score of 2; severe bowel wall thickening with rigidity and hyperemia was assigned a score of 3; and severe bowel wall thickening with rigidity, hyperemia, and adhesions was assigned a score of 4. Samples were processed and stained with hematoxylin and eosin (H&E) by the CSMC Histology Core Laboratory. Masson-Trichrome and vimentin staining were performed as described.20 Histopathological scores of colons and intestine were assigned in a blinded manner by two trained animal pathologists (D.Q.S. and H.W.K.) using established methods.10,18 For the DSS model, histology was scored as follows: inflammation (I): 0, none; 1, mild; 2, moderate; 3, severe; extent (E): 0, none; 1, mucosa; 2, mucosa and submucosa; 3, transmural; regeneration (R): 0, complete regeneration; 1, almost complete regeneration; 2, regeneration with crypt depletion; 3, surface epithelium not intact; 4, no tissue repair; crypt damage (C): 0, none; 1, basal one-third damaged; 2, basal two-thirds damaged; 3, only surface epithelium intact; 4, entire crypt and epithelium lost; percentage involvement (P): 1, 1% to 25%; 2, 26% to 50%, 3, 51% to 75%, 4, 76% to 100%. Total histology score is given as I + E + R + C + P. For the adoptive-transfer model, histopathological assessments of colons were scored as follows: inflammatory infiltrate in lamina propria (I): 1, 1% to 25%; 2, 26% to 66%; 3, 66% to 100%; mucin depletion (M): 0, normal; 1, up to 70% depleted; 2, over 70% depleted; epithelial atypia (E): 0, normal; 1, epithelial hyperplasia; 2, epithelial atypia; 3, epithelial atypia with nuclear changes; crypt architecture (C): 0, normal; 1, distortion; 2, branching or atrophy; 3, complete loss; crypt abscess (A): 0, none; 1, few cells; 2, abscess; ulceration (U): 0, normal; 1, erosion; 2, ulceration; depth of involvement (D): 1, to submucosa; 2, transmural. Total colonic histology score for adoptive-transfer model is given as I + M + E + C + A + U + D. Histopathological scoring of small intestine in the adoptive transfer model was performed as follows: inflammation (I): 0, normal; 1, mild; 2, moderate; 3, severe; crypt damage (C): 0, none; 1, basal one-third damaged; 2, basal two-third damaged; 3, more than two-third damages; percent involvement (P): 1, mucosa; 2, submucosa; 3, transmural; villus change (V): 0, normal; 1, distortion; 2, branching; 3, atrophy and blunting. The total intestinal score for the adoptive- transfer model is given as I + C + P + V. The histological score, vimentin-positive cells, and collagen thickness were calculated from observation of ≥10 different fields per gut region at ×200 magnification from ≥10 mice in each group.
Expression Analysis
Total RNA was isolated and reverse-transcription polymerase chain reaction (RT-PCR) was performed as described.21 β-Actin, IGF-1, and TGF-β mRNA were amplified as described.10,20 Amount of transcript was analyzed and expressed as percentage of β-actin. Cytokine concentration was assayed by enzyme-linked immunosorbent assay (ELISA) using kits for IFN-γ, IL-17, IL-13, IL-6, and TNFα (eBioscience, San Diego, CA) per the manufacturer's protocol.
Cell Isolation, Culture, Intracellular Cytokine Expression, and Flow Cytometry
Isolation and culture of lamina propria mononuclear cells (LPMC), mesenteric lymph node (MLN), and splenic cells and their subsequent stimulation by anti-CD28 and anti-CD3 were performed as reported.10 We used the whole colon and the distal 10 cm of the ilea for LPMC isolation. Intracellular staining and antibodies used were described previously.17 Cells were acquired on a Cyan flow cytometer (Dako-Cytomation, Carpinteria, CA) and analyzed using FlowJo analysis software (Tree Star, Ashland, OR).
Human Subjects
Patients enrolled in this study were seen at the CSMC Inflammatory Bowel Disease Center and had been diagnosed with CD according to standard clinical, endoscopic, radiological, and histological findings. Written informed consent was obtained and approved by the CSMC Institutional Review Board (IRB nos. 3358 and 2673). Clinical course, laboratory values, and endoscopic findings were collected, and histology was scored by two pathologists blinded to TL1A status (D.Q.S. and M.V.). The biopsy specimens from the first available colonoscopy at CSMC after IBD diagnosis were used. Ten or more histological sections per gut region from each patient were scored at ×200 magnification.22 Assessment of TL1A levels, genotyping, and haplotype assignment had been performed.3 Patients defined as high TL1A producers were Jewish subjects with homozygous risk haplotype-B whose immune complex–stimulated TL1A at 6 hours was >0.15 ng/mL/1 × 106 peripheral monocytes. Patients defined as low TL1A producers were Jewish subjects with homozygous protective haplotype-A whose immune complex–stimulated TL1A at 6 hours was <0.15 ng/mL/1 × 106 peripheral monocytes.
Statistical Analysis
Data are presented as the mean ± SD. Comparison between two groups was performed by a two-tailed Fisher's exact test for categorical variables and Student's t-test for continuous variables. Parametric and nonparametric tests were used depending on the fulfillment of the test assumptions. Comparison between three groups was done using analysis of variance test, followed by pairwise post hoc analysis with Tukey's HSD test and Behrens-Fisher test correction for the multiple comparisons. P < 0.05 was considered significant.
Results
Constitutive TL1A Expression Leads to Reduced Weight in the Adoptive T-Cell Transfer Model
DSS-induced and adoptive T-cell transfer models were used to determine the in vivo consequences of TL1A overexpression. Adaptive immunity plays an important role in chronic colitis induced by multiple cycles of DSS10,23 and by adoptively transferring naive CD4+CD45RBhi T cells into immune-deficient Rag1−/− mice.18 No significant differences in weight were observed in the DSS model (Figure 1A). In general, there was no difference in disease activity index between WT and Tg mice (data not shown). To determine that changes in the disease activity index were not due to differences in consumed DSS water between the different mice groups, DSS water consumption was assessed. We found that the quantity of consumed DSS water was similar for each of the four DSS treatment cycles among the different groups of mice (see Supplemental Figure S1 at http://ajp.amjpathol.org).
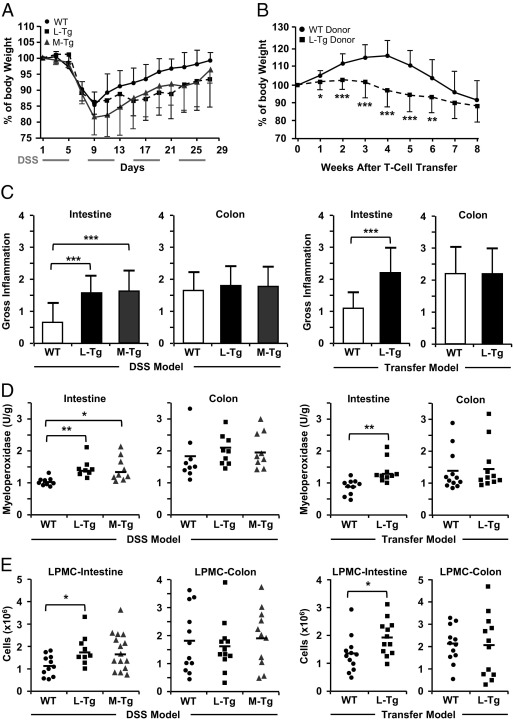
Figure 1.
Intestinal and colonic disease features in WT, L-Tg, and M-Tg mice. Percentage of body weight change is shown for DSS-induced colitis (n = 15 for WT, M-Tg, and L-Tg) (A) and adoptive T-cell transfer model (n = 12 for WT donor and L-Tg donor) (B). Data are expressed as mean ± SD. C: Gross appearance of intestine and colon were measured using a standard scoring system.18D: Myeloperoxidase activity was measured, and data are expressed as arbitrary unit (U) per gram (g) of protein. E: Total numbers of mononuclear cells were isolated from the distal 10 cm of intestine or colon. For D, each symbol represents independent mice. Each symbol in E represents the mean of triplicates. *P < 0.05, **P < 0.01, and ***P < 0.001.
In the adoptive-transfer model, Rag1−/− mice that received naive L-Tg (but not WT) T cells had significantly lower weight by week 1 that persisted up to week 6 after T-cell transfer (Figure 1B). No differences in the amount of stool blood or consistency were found (data not shown). We observed that constitutive TL1A expression led to reduced weight in the adoptive T-cell transfer model.
Increased Intestinal Inflammation in Tl1a-Tg Mice
Inspection of the gut revealed increased gross inflammation in the intestine of Tl1a-Tg compared to WT mice in both chronic colitis models (Figure 1C). Another measure of gut inflammation was to determine myeloperoxidase activity.17 Compared to WT mice, increased myeloperoxidase activity was found in the ileum of Tl1a-Tg mice in both models of chronic colitis (Figure 1D). Additionally, greater numbers of LPMC were isolated from the intestine of both Tl1a-Tg models than from WT mice (Figure 1E).
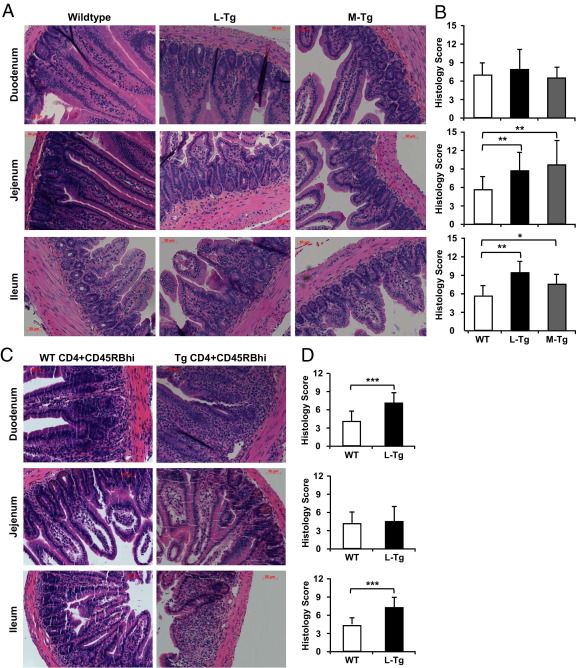
Histological examination of the intestine revealed blunting of the villi, crypt damage, worsened inflammatory changes, and increased mononuclear cell infiltration of the lamina propria (LP), particularly in the ileum from Tl1a-Tg compared to WT mice in both the DSS (Figure 2A) and adoptive-transfer models (Figure 2C). Depending on the murine colitis model used, different intestinal areas with increased inflammatory changes were observed. For example, histological changes were reflected by a significant increase in the inflammatory score of Tl1a-Tg mice in the jejunum and ileum of the DSS model (Figure 2B) and in the duodenum and ileum of the T-cell transfer model (Figure 2D). Taken together, these results indicate that constitutive TL1A expression in either T cells or myeloid lineages resulted in patchy intestinal inflammation.
Figure 2.
Tl1a-Tg mice have patchy intestinal inflammation. Representative H&E stained DSS (A) and adoptive-transfer (C) intestinal sections are shown. Quantitative histology scores for DSS (B) and adoptive transfer (D) were determined. Data are expressed as mean ± SD. Fields were scored at ×200 magnification. *P < 0.05, **P < 0.01, ***P < 0.001.
Proximal Migration of Colonic Inflammation in Tl1a-Tg Mice
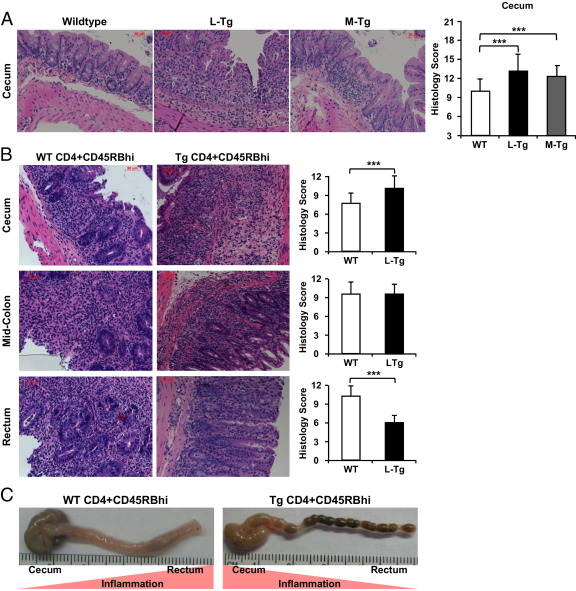
No differences were found in the overall gross inflammatory score (Figure 1C), myeloperoxidase activity (Figure 1D), or LPMC recovery (Figure 1E) in the colon between WT and Tl1a-Tg mice in both chronic colitis models. In the DSS-induced colitis model, enhanced inflammation was observed in the Tl1a-Tg compared to WT cecum but no other colonic regions (Figure 3A and data not shown).
Figure 3.
Tl1a-Tg mice have proximal migration of colonic inflammation. Representative H&E-stained DSS (A) and adoptive-transfer (B) colonic sections are shown with corresponding quantitative histology scores. C: Representative colonic specimen showing proximal migration of gross inflammation in the mice receiving L-Tg T cells compared to WT T cells. Data are expressed as mean ± SD. Fields were scored at ×200 magnification. ***P < 0.001.
In the adoptive T-cell transfer model, proximal migration of colonic inflammation was observed in T cells from Rag1−/− mice transferred with Tl1a-Tg compared to WT T cells (Figure 3B). Compared to WT mice, there was exacerbation of cecal inflammation but relative sparing of rectal inflammation in Tl1a-Tg mice (Figure 3B). Closer inspection of colonic specimens revealed increased hyperemia, wall thickening, edema, and adhesions in the cecum with relative sparing in the rectum in the Rag1−/− mice transferred with Tl1a-Tg compared to WT T cells (Figure 3C). These data showed constitutive TL1A expression led to proximal migration of colonic inflammation with relative rectal sparing in the adoptive T-cell transfer model.
Constitutive TL1A Expression Leads to Gut Fibrostenosis
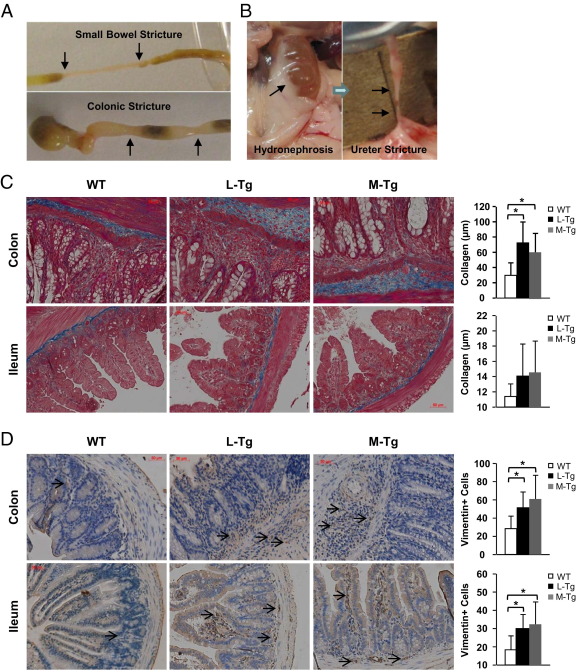
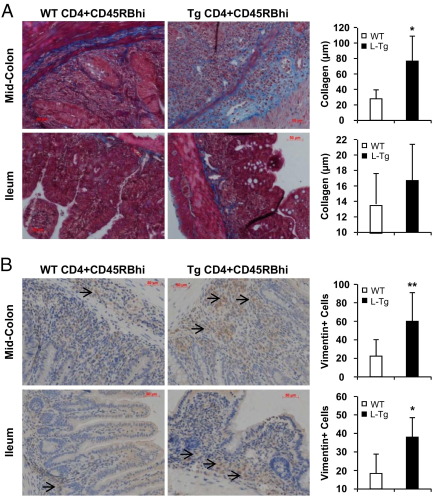
Mice with constitutive TL1A expression developed both intestinal and colonic gross stricturing disease in both chronic colitis models. In the DSS model, gross strictures were present only in the Tl1a-Tg mice: intestine up to 3 cm in length (WT, 0 of 15 mice; L-Tg, 3 of 14 mice; M-Tg, 3 of 15 mice), colon <1 cm in length (WT, 0 of 15 mice; L-Tg, 3 of 14 mice; M-Tg, 3 of 15 mice). In the adoptive-transfer model, gross strictures were only present in Rag1−/− mice transferred with Tl1a-Tg T cells: intestine up to 3 cm in length (WT, 0 of 12 mice; L-Tg, 4 of 12 mice), colon (WT, 0 of 12 mice; L-Tg, 3 of 12 mice). An example of fibrostenotic disease in the colon and intestine is shown in Figure 4A. At a low frequency, hydronephrosis with ureteral stricture was observed in the DSS model from the Tl1a-Tg mice (WT, 0 of 15; L-Tg, 1 of 14; M-Tg, 1 of 15) and in the adoptive T-cell transfer model (WT, 0 of 12; L-Tg, 1 of 14). An example of hydronephrosis with ureteral stricture is shown in Figure 4B.
Figure 4.
Tl1a-Tg mice have fibrostenotic disease. A: Representative pictures of intestinal and colonic stricture (arrows) are shown. B: Representative pictures of hydronephrosis due to ureteral stricture (arrows) are shown. C: Masson-Trichrome staining of collagen deposition in nonstrictured tissue sections of mouse at mid-colon and distal 3 cm of ilea is shown for the DSS model. Thickness of collagen deposition (stained blue) was quantitated and represented as mean ± SD. D: Vimentin stain of fibroblasts in nonstrictured tissue sections were performed for the DSS model. Fibroblasts were stained brown (arrows). Vimentin-positive cells were quantitated and represented as mean ± SD. Fields were scored at ×200 magnification. *P < 0.05.
To determine whether there was increased collagen deposition, Masson-Trichrome stain was performed on colonic and intestinal regions without gross stricture. Increased Masson-Trichrome stain in the colon, but not in the ilea, of Tl1a-Tg mice undergoing chronic DSS treatment was observed (Figure 4C). The increased Masson-Trichrome stain was correlated with increased thickness of colonic collagen deposition in both L-Tg and M-Tg mice when compared to WT mice (Figure 4C). Collagen is secreted by fibroblasts and can be identified by the marker vimentin.20 Compared with WT controls, an increased number of vimentin-positive cells was observed in the nonstrictured colon and ilea of DSS-treated Tl1a-Tg mice (Figures 4D). Similar to the DSS model, increased collagen deposition was found in the nonstrictured colon in Rag1−/− mice transferred with Tl1a Tg T cells (Figure 5A). Collagen deposition was not different in the nonstrictured ilea (Figure 5A). Increased numbers of vimentin-positive cells were observed in both the nonstrictured ileum and colon of Rag1−/− mice transferred with Tl1a-Tg than with WT T cells (Figure 5B).
Figure 5.
Adoptive transfer of L-Tg mice can lead to fibrostenotic disease. A: Masson-Trichrome staining of collagen deposition in nonstrictured tissue sections of mouse at mid-colon and distal 3 cm of ilea is shown for the adoptive-transfer model. Thickness of collagen deposition was quantitated and represented as mean ± SD. B: Vimentin stain of fibroblasts in nonstrictured tissue sections was performed for the adoptive-transfer model. Fibroblasts were stained brown (arrows). Vimentin-positive cells were quantitated and represented as mean ± SD. Fields were scored at ×200 magnification for A and B. *P < 0.05, **P < 0.01.
As increased colonic mRNA expression of pro-fibrogenic factors TGF-β1 and IGF-1 in CD patients had been reported,24,25 the expression of these genes was assessed. Compared to WT mice (1.1% ± 0.34% actin), increased expression of colonic TGF-β1 in the L-Tg (2.0% ± 0.94%, P = 0.02 versus WT) and M-Tg mice (2.29% ± 1.4% actin, P = 0.02 versus WT) undergoing chronic DSS treatment was found in the nonfibrotic colonic sections. The expression of TGF-β1 in the intestine was not different between WT and Tl1a-Tg mice (data not shown). No difference in the expression of IGF-1 was detected in both chronic colitis models (data not shown). Together, these results demonstrated that constitutive TL1A expression in T cells and myeloid cells led to severe fibrostenotic disease in the murine gut, which may in part be due to elevated expression of the pro-fibrogenic factor TGF-β1.
Accelerated T-Cell Activation and Enhanced IL-17 Production in the Chronic DSS Colitis Model
To assess the effect of TL1A expression on immune-cell composition, we performed flow cytometric analysis. Flow cytometry did not reveal differences in the frequencies of CD3+, CD4+, CD8+, CD11c+, or F4/80+ cells in the spleen and MLN between L-Tg, M-Tg, or WT littermate mice (data not shown). To determine the effect of constitutive TL1A expression on the activation state of antigen-presenting cells, the expression of the activation marker CD86 was compared. Flow cytometry did not reveal differences in the CD86 expression between dendritic cells (CD11c+) and macrophages (F4/80+) between Tl1a-Tg and WT mice (data not shown). Similar frequencies of T cells expressing the gut-homing markers chemokine receptor-9 and chemokine receptor-10 were found (data not shown).
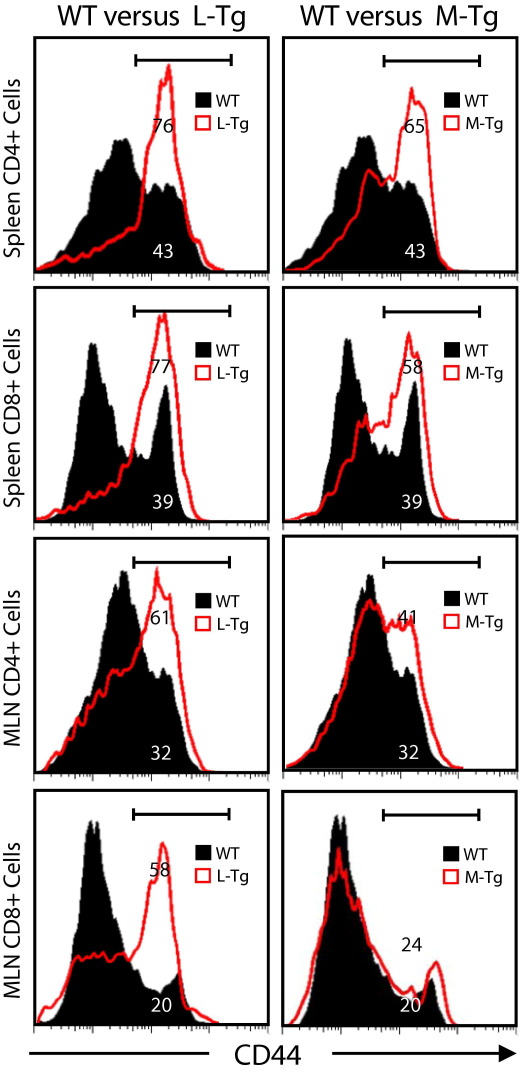
To assess whether constitutive TL1A expression can enhance the co-stimulation of T cells in the DSS model, we compared the expression of CD44 on CD4+ and CD8+ cells between Tg and WT littermate controls. CD4+CD45RBlowCD25+ Treg cells were gated out to examine the expression of activation markers on conventional T cells. Compared to WT mice, a higher percentage of Tl1a-Tg CD4+ and CD8+ cells expressed the activation marker CD44, particularly in the L-Tg mice (Figure 6).
Figure 6.
Sustained TL1A expression leads to increased percentage of activated T cells in DSS-induced chronic colitis. Flow cytometry plot of splenocytes and MLN cells showing expression of activation markers CD44. CD4+ and CD8+ cells were gated as indicated. Data shown are representative of at least five independent experiments.
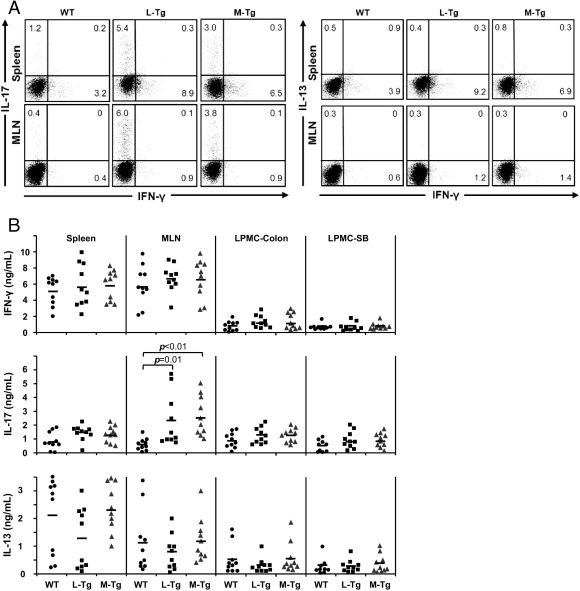
To assess the molecular consequences of increased T-cell activation in the Tl1a-Tg mice, the expression of IFN-γ, interleukin (IL)-13, and IL-17 was measured. Figure 7A shows increased frequency of CD4+IL-17+ T cells in Tl1a-Tg mice compared to WT mice that reached significance in the frequency of CD4+IL-17+ in the MLN (WT 0.6 ± 0.2 versus L-Tg 6.0 ± 2.8, P = 0.03; WT 0.6 ± 0.2 versus M-Tg 4.0 ± 1.9, P = 0.04). No difference in the proportion of CD4+IL-13+ and CD4+FN-γ+ cells was found. Similar to what was shown with intracellular staining; higher IL-17 production in the MLN for both Tl1a-Tg mice was found (Figure 7B). No differences in the production of IFN-γ, IL-13, TNFα, or IL-6 were observed (Figure 7B and data not shown).
Figure 7.
Sustained TL1A expression leads to increased IL-17 expression in DSS-induced chronic colitis. A: Flow cytometry plots of gated CD4+ cells from spleen and MLN, stained for intracellular IFN-γ, IL-17, and IL13 expression, are shown. Data shown are representative of ≥5 mice per group. B: IFN-γ, IL-17, and IL13 secretion after stimulation with anti-CD3 and anti-CD28 were assessed by enzyme-linked immunosorbent assay. Each data point represents cytokine expression for splenocytes, MLN cells, or LPMC from an individual mouse. P values are indicated where significant.
Constitutive TL1A Expression Leads to Enhanced Th-1 but Reduced Th-17 Immune-Responses in the Adoptive T-Cell Transfer Model
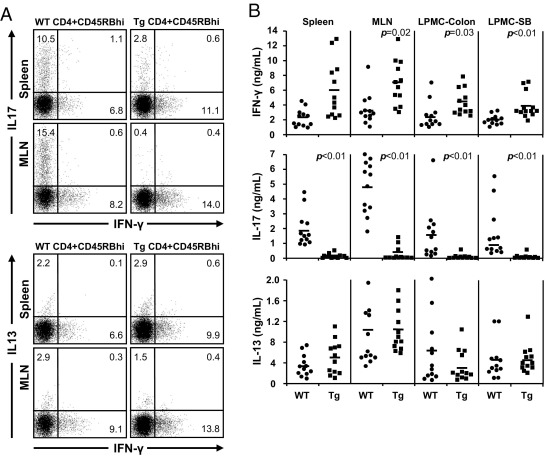
In the adoptive T-cell transfer model, no differences in the frequencies of CD3+, CD4+, chemokine receptor-9 and -10, activation markers (CD44 and CD86), CD11c+, or F4/80+ cells in the spleen, and MLN between Rag1−/− mice transferred with WT or Tl1a-Tg T cells were observed (data not shown). Intracellular cytokine staining showed that the frequency of CD4+IFN-γ+ was significantly elevated in the spleen (WT 7.7 ± 0.9 versus L-Tg 11.1 ± 1.5, P = 0.03) and MLN (WT 9.5 ± 0.8 versus L-Tg 13.1 ± 2.0, P = 0.04) of Rag1−/− mice transferred with T cells expressing TL1A compared to WT T cells (Figure 8A). There were very low numbers of CD4+IL-17+ T cells in Rag1−/− mice transferred with Tl1a-Tg T cells in the spleen (WT 11.5 ± 3.4 versus L-Tg 4.4 ± 2.1, P = 0.04) and MLN (WT 18.3 ± 2.2 versus L-Tg 1.7 ± 1.2, P < 0.01) (Figure 8A). No differences in the frequency of CD4+IL13+ cells were found (Figure 8A).
Figure 8.
Sustained TL1A expression leads to increased IFN-γ and reduced IL-17 expression in the adoptive-transfer model. A: Flow cytometry plots of gated CD4+ cells from spleen and MLN, stained for intracellular IFN-γ, IL-17, and IL13 expression, are shown. Data shown are representative of ≥5 mice per group. B: IFN-γ, IL-17, and IL13 secretion after stimulation with anti-CD3 and anti-CD28 were assessed by enzyme-linked immunosorbent assay. Each data point represents cytokine expression for splenocytes, MLN cells, or LPMC of an individual mouse. P values are indicated where significant.
Consistent with the results from intracellular staining, higher IFN-γ production was found in the spleen, MLN, and LPMC in Rag1−/− mice transferred with Tl1a-Tg T cells compared to WT T cells (Figure 8B). Significantly reduced IL-17 production was observed in all of the tissues tested (Figure 8B). No differences in the production of IL-13, TNFα, and IL-6 were observed (Figure 8B and data not shown). These data indicated that constitutive expression of TL1A in naive CD4+CD45RBhi T cells may affect the proliferation, differentiation, or maintenance of Th-1 and -17 cells in the peripheral immune compartments and inflamed tissues.
Proximal Migration of Colonic Inflammation with Relative Rectal Sparing and Intestinal Fibrostenosis in High TL1A Expressing CD Patients
Given the findings in mice, we examined whether humans with elevated TL1A expression had similar proximal migration of colonic inflammation with relative rectal sparing. A blinded retrospective analysis of CD patients with known TL1A expression in peripheral monocytes was performed.3 In our previous study, TL1A level of 0.15 ng/mL/1 × 106 peripheral monocytes at 6 hours post immune complex stimulation distinguished over 90% of Jewish CD patients with risk TL1A haplotype-B and protective haplotype-A.3 Therefore, high TL1A producers were defined as subjects with homozygous risk haplotype-B whose immune complex–stimulated TL1A at 6 hours was ≥0.15 ng/mL/1 × 106 peripheral monocytes, and low TL1A producers had homozygous protective haplotype-A whose immune complex–stimulated TL1A at 6 hours was ≤0.15 ng/mL/1 × 106 peripheral monocytes. The patients' demographics, clinical features, and medications within 2 months of colonoscopy are provided in Table 1. Significant increases in intestinal strictures and in scores from the Harvey-Bradshaw Index were found in the high-TL1A compared to the low-TL1A group (Table 1).
Table 1.
Summary of Clinical and Laboratory Data
| High TL1A | Low TL1A | P value | |
|---|---|---|---|
| Sex (M/F) | 4/4 | 6/4 | NS |
| SB stricture | 62.5% (5/8) | 10% (1/10) | 0.04 |
| Colon stricture | 12.5% (1/8) | 20% (2/10) | NS |
| Biologic use | 25% (2/8) | 70% (7/10) | NS |
| Failed 1 biologic⁎ | 62.8% (5/8) | 5/10 (50%) | NS |
| Failed ≥2 biologics⁎ | 25% (2/8) | 0% | NS |
| Immunomodulator† use | 27.5% (3/8) | 60% (6/10) | NS |
| Immunomodulator† failure | 75% (6/8) | 50% (5/10) | NS |
| 5-ASA‡ failure | 87.5% (7/8) | 80% (8/10) | NS |
| Enema use | 0% | 0% | NS |
| Ureteral stricture | 25% (2/8) | 0% | NS |
| Previous surgery | 87.5% (7/8) | 70% (7/10) | NS |
| TL1A (ng/mL) | 0.29 ± 0.12 | 0.08 ± 0.04 | 0.01 |
| Harvey-Bradshaw Index | 9 ± 3.45 | 2.61 ± 3.43 | <0.01 |
| Time to surgery (years) | 11 ± 10.05 | 8.75 ± 6.8 | NS |
| WBC (×100/μL) | 8.40 ± 4.34 | 8.10 ± 3.31 | NS |
| ESR (mm/hour) | 20.64 ± 15.31 | 14.36 ± 7.20 | NS |
| CRP (mg/dL) | 1.17 ± 1.59 | 1.76 ± 3.31 | NS |
Data are expressed as percentages with n/total or as the mean ± SD as indicated.
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; F, female; M, male; NS, not significant; SB, small bowel; WBC, white blood cell count.
Biologics include infliximab, adalimumab, certolizumab, thalidomide, and natalizumab.
Immunomodulators include azathioprine, 6-mercaptopurine, methotrexate, and tacrolimus.
5-ASAs include mesalamine and sulfasalazine.
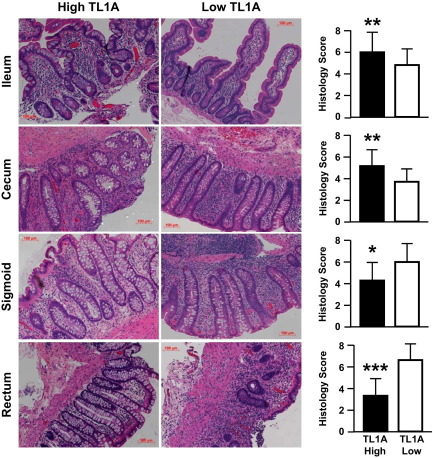
To assess whether TL1A expression level is associated with a specific mucosal pattern of inflammation, H&E-stained histological sections from ileum, cecum, sigmoid, and rectum were examined. Compared to the low-TL1A group, the high-TL1A group had more severe ileocecal inflammation with relative rectosigmoid sparing of inflammation (Figure 9). Together, these data suggest that CD patients who express elevated TL1A levels develop worsened inflammation in the ileocolonic region and proximal migration of colonic inflammation with relative sparing of inflammation in the sigmoid and rectum.
Figure 9.
CD patients with elevated TL1A expression have increased ileocecal inflammation but reduced rectosigmoid inflammation. Representative H&E-stained intestinal and colonic sections are shown, and corresponding histology scores were determined. Data are expressed as mean ± SD. Sections were scored at ×200 magnification by two pathologists blinded to the TL1A haplotype and its expression level. *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
This study shows that constitutive TL1A expression resulted in severe patchy intestinal inflammation with proximal migration of colonic inflammation under colitogenic conditions. Furthermore, Tl1a-Tg mice spontaneously developed ileitis, but not colitis, by 10 months of age.15–17 The severity of ileitis was more dramatic in these induced chronic colitis models. As colitis is the predominant gut pathology seen in both the DSS and adoptive-transfer models, the ileitis reported in this study was likely driven by constitutive TL1A expression. Depending on the model, TL1A expression led to either additional inflammation in the jejunum (DSS) or duodenum (adoptive-transfer). Constitutive TL1A expression also altered the colitis pattern from the more distal rectosigmoid inflammation to the more proximal cecal inflammation. These inflammatory changes were likely caused by TL1A and not the cell type that expresses TL1A as both L-Tg and M-Tg mice exhibited a similar pattern of inflammation. Relative sparing of rectal inflammation was seen in the adoptive-transfer model using Tl1a-Tg T cells. TNFSF15 is a severity gene, which when overexpressed results in a change in location to the proximal parts of the colon and small intestine, and away from the rectum.
Prior studies reported that TL1A is associated with expansion of T-helper subsets and can synergize with IL-12 and -18 to enhance Th-1 response and IFN-γ production.6,7,26,27 Other investigations found that mice lacking TL1A display reduced experimental encephalitis disease activity that is associated with decreased Th-17 cell differentiation and proliferation.9 Consistently, in DSS-induced models of chronic colitis, TL1A enhances Th-1 and Th-17 effector functions by up-regulating IFN-γ and IL-17 production, respectively, in CD4+ T cells under Th-1/Th-17 polarizing conditions.10 TL1A had also been found to be involved in Th-2–mediated ova-induced lung disease and in spontaneous ileitis.8,15,16 These reports indicated that the TL1A/DR3 signaling pathway might have a universal role in regulating T-helper effector outcomes.
In line with our previous report,10 increased IL-17 production was found in DSS-induced chronic colitis with constitutive TL1A expression. In the adoptive-transfer model, TL1A expression led to a Th-1–dominant immune response with almost a complete absence of IL-17 production. The differences in the T-helper response may be based on the differentiation state of the CD4+ T cells. In the DSS-induced chronic colitis model, TL1A can enhance the effector characteristics of committed Th-17 cells. In the adoptive-transfer model, TL1A/DR3 signaling may inhibit Th-17 development in newly challenged naive T cells. Differential responsiveness of naive and committed T cells to TL1A/DR3 signaling had also been recently proposed.12
Another explanation for the difference in the TL1A-dependent T-helper immune response between the DSS and adoptive T-cell transfer models may be different gut microflora. We bred WT and Tl1a-Tg littermates in our facility for the DSS-induced chronic colitis experiments. For the adoptive-transfer model, we purchased Rag1−/− recipient mice from Jackson Laboratory (Bar Harbor, ME). Previous studies had demonstrated that genetically identical inbred mice raised in different animal facilities had different immune composition due to the different gut microflora.28–30 The potential immunomodulatory roles of gut microbiota–host interaction between mice may have contributed to the different TL1A-mediated T-helper immune response.
Compared to WT mice, increased collagen deposition was observed in older Tl1a-Tg mice.17 Notably, the severity of fibrosis was worse in the induced chronic colitis models with constitutive TL1A expression where gross (not just histological) colonic and intestinal strictures were observed. This may reflect the fact that the induced chronic colitis models led to an accelerated T-helper effector function in which the effector phase of the disease is reached earlier. In addition, TL1A may be expressed by an independent pro-fibrogenic gene to initiate the process of fibrosis since we previously found that TL1A expression resulted in increased colonic collagen deposition in the absence of detectable histological inflammation.17 Furthermore, in the current study, visible colonic strictures were observed in the mid-colon of only the Tl1a-Tg mice despite a similar degree of inflammation as compared to WT mice (Figures 3 and 4). The pro-fibrotic effects of TL1A may be mediated by TGF-β1 as its expression was increased in the nonstrictured colon of Tg compared to WT mice (Figure 5C). However, other factors are likely involved and need to be explored further. To our knowledge, these Tl1a-Tg animal models are the only in vivo model in which overexpression of a single IBD-associated gene lead to gross intestinal and colonic fibrostenotic disease that is characteristic of some forms of CD.
Compared to CD patients with low TL1A expression, higher TL1A-producing CD patients had increased inflammation in the ileum and cecum with reduced inflammation in the sigmoid and rectum (Figure 9). None of the CD patients in our study cohort used rectally administered IBD therapy; therefore, the reduction in rectosigmoid inflammation may be due to elevated TL1A expression. A significantly higher rate of intestinal strictures was found in CD patients with higher TL1A levels (Table 1), suggesting that TL1A may induce intestinal strictures in humans. This is consistent with previous reports showing that TNFSF15 haplotype-B, which is associated with increased TL1A levels, is also characterized by the increased need for surgery due to fibrostenotic CD disease.3,4 One limitation of the retrospective analysis is the small sample size, thus our findings need to be confirmed in an independent prospective study.
Extraintestinal manifestations of CD can include arthropathy, erythema nodosum, and more rarely, hydronephrosis due to strictures along the genitourinary tract.1,31,32 The presence of IBD-like skin lesions and arthropathy in older Tl1a-Tg mice were reported.17 In this study, hydronephrosis due to ureteral strictures was only observed in Tl1a-Tg mice during the development of chronic colitis (7%, 3 of 43). Ureteral involvement has been reported to occur in 3% to 6% of CD cases.32 Interestingly, a higher frequency of ureteral stricturing was found in our small cohort of CD patients expressing higher TL1A levels (25%, 2 of 8, Table 1), suggesting that TL1A may also cause ureteral fibrosis.
In summary, TL1A modulates the severity and location of inflammation in the intestine and colon under colitogenic conditions. Sustained TL1A expression also induces stricturing disease in the gut that is caused by increased collagen deposition and number of fibroblasts. Results from these Tl1a-Tg experimental models coupled with findings in CD patients suggest that TNFSF15 is a severity gene and that the cytokine TL1A, when overexpressed, plays a central role in generating one phenotypic form of complicated CD.
Acknowledgments
We thank Loren Karp for critical reading of the manuscript, Patricia Lin and Gislaine Martins for assistance with flow cytometry, Jeremy Chen for assistance in making histology figures, and Hanlin Wang for assistance with obtaining human histology.
Footnotes
Supported by U.S. Public Health Service grant DK056328.
R.B. and X.Z. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.10.026.
Supplementary data
WT and Tl1a-Tg mice consume similar amounts of DSS water. DSS water consumption was assessed for each cycle and represented as grams (g) of DSS water consumed. Data are expressed as mean ± SD.
References
- 1.Peyrin-Biroulet L., Loftus E.V., Jr., Colombel J.F., Sandborn W.J. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 2.Shih D.Q., Targan S.R. Insights into IBD pathogenesis. Curr Gastroenterol Rep. 2009;11:473–480. doi: 10.1007/s11894-009-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michelsen K.S., Thomas L.S., Taylor K.D., Yu Q.T., Mei L., Landers C.J., Derkowski C., McGovern D.P., Rotter J.I., Targan S.R. IBD-associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. PLoS One. 2009;4:e4719. doi: 10.1371/journal.pone.0004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picornell Y., Mei L., Taylor K., Yang H., Targan S.R., Rotter J.I. TNFSF15 is an ethnic-specific IBD gene. Inflamm Bowel Dis. 2007;13:1333–1338. doi: 10.1002/ibd.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prehn J.L., Mehdizadeh S., Landers C.J., Luo X., Cha S.C., Wei P., Targan S.R. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112:66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Papadakis K.A., Prehn J.L., Landers C., Han Q., Luo X., Cha S.C., Wei P., Targan S.R. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172:7002–7007. doi: 10.4049/jimmunol.172.11.7002. [DOI] [PubMed] [Google Scholar]
- 8.Fang L., Adkins B., Deyev V., Podack E.R. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappu B.P., Borodovsky A., Zheng T.S., Yang X., Wu P., Dong X., Weng S., Browning B., Scott M.L., Ma L., Su L., Tian Q., Schneider P., Flavell R.A., Dong C., Burkly L.C. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205:1049–1062. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takedatsu H., Michelsen K.S., Wei B., Landers C.J., Thomas L.S., Dhall D., Braun J., Targan S.R. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meylan F., Davidson T.S., Kahle E., Kinder M., Acharya K., Jankovic D., Bundoc V., Hodges M., Shevach E.M., Keane-Myers A., Wang E.C., Siegel R.M. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29:79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones G.W., Stumhofer J.S., Foster T., Twohig J.P., Hertzog P., Topley N., Williams A.S., Hunter C.A., Jenkins B.J., Wang E.C., Jones S.A. Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation. FASEB J. 2011;25:409–419. doi: 10.1096/fj.10-166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber T.H., Wolf D., Podack E.R. The role of TNFRSF25: TNFSF15 in disease. and health? Adv Exp Med Biol. 2011;691:289–298. doi: 10.1007/978-1-4419-6612-4_30. [DOI] [PubMed] [Google Scholar]
- 14.Shih D.Q., Michelsen K.S., Barrett R.J., Biener-Ramanujan E., Gonsky R., Zhang X., Targan S.R. Insights into TL1A and IBD pathogenesis. Adv Exp Med Biol. 2011;691:279–288. doi: 10.1007/978-1-4419-6612-4_29. [DOI] [PubMed] [Google Scholar]
- 15.Meylan F., Song Y.J., Fuss I., Villarreal S., Kahle E., Malm I.J., Acharya K., Ramos H.L., Lo L., Mentink-Kane M.M., Wynn T.A., Migone T.S., Strober W., Siegel R.M. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4:172–185. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taraban V.Y., Slebioda T.J., Willoughby J.E., Buchan S.L., James S., Sheth B., Smyth N.R., Thomas G.J., Wang E.C., Al-Shamkhani A. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011;4:186–196. doi: 10.1038/mi.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih D.Q., Barrett R., Zhang X., Yeager N., Koon H.W., Phaosawasdi P., Song Y., Ko B., Wong M.H., Michelsen K.S., Martins G., Pothoulakis C., Targan S.R. Constitutive TL1A (TNFSF15) expression on lymphoid or myeloid cells leads to mild intestinal inflammation and fibrosis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016090. e16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostanin D.V., Pavlick K.P., Bharwani S., D'Souza D., Furr K.L., Brown C.M., Grisham M.B. T cell-induced inflammation of the small and large intestine in immunodeficient mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G109–G119. doi: 10.1152/ajpgi.00214.2005. [DOI] [PubMed] [Google Scholar]
- 19.Rachmilewitz D., Karmeli F., Shteingart S., Lee J., Takabayashi K., Raz E. Immunostimulatory oligonucleotides inhibit colonic proinflammatory cytokine production in ulcerative colitis. Inflamm Bowel Dis. 2006;12:339–345. doi: 10.1097/01.MIB.0000217335.30689.77. [DOI] [PubMed] [Google Scholar]
- 20.Koon H.W., Shih D., Karagiannides I., Zhao D., Fazelbhoy Z., Hing T., Xu H., Lu B., Gerard N., Pothoulakis C. Substance P modulates chronic inflammation-induced colonic fibrosis. Am J Pathol. 2010;177:2300–2309. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih D.Q., Kwan L.Y., Chavez V., Cohavy O., Gonsky R., Chang E.Y., Chang C., Elson C.O., Targan S.R. Microbial induction of inflammatory bowel disease associated gene TL1A (TNFSF15) in antigen presenting cells. Eur J Immunol. 2009;39:3239–3250. doi: 10.1002/eji.200839087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Haens G.R., Geboes K., Peeters M., Baert F., Penninckx F., Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 23.Dieleman L.A., Ridwan B.U., Tennyson G.S., Beagley K.W., Bucy R.P., Elson C.O. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 24.Lawrance I.C., Maxwell L., Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16–26. doi: 10.1097/00054725-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann E.M., Li L., Hou Y.T., Mohapatra N.K., Pucilowska J.B. Insulin-like growth factor I and insulin-like growth factor binding protein 5 in Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1022–G1029. doi: 10.1152/ajpgi.2001.280.5.G1022. [DOI] [PubMed] [Google Scholar]
- 26.Bamias G., Martin C., 3rd, Marini M., Hoang S., Mishina M., Ross W.G., Sachedina M.A., Friel C.M., Mize J., Bickston S.J., Pizarro T.T., Wei P., Cominelli F. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171:4868–4874. doi: 10.4049/jimmunol.171.9.4868. [DOI] [PubMed] [Google Scholar]
- 27.Migone T.S., Zhang J., Luo X., Zhuang L., Chen C., Hu B., Hong J.S., Perry J.W., Chen S.F., Zhou J.X., Cho Y.H., Ullrich S., Kanakaraj P., Carrell J., Boyd E., Olsen H.S., Hu G., Pukac L., Liu D., Ni J., Kim S., Gentz R., Feng P., Moore P.A., Ruben S.M., Wei P. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479–492. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov I.I., Frutos Rde L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H.J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruglik G.D., Neiman H.L., Sparberg M., Nudelman E., Mintzer R.A., Rogers L.F. Urological complications of regional enteritis. Gastrointest Radiol. 1977;1:375–378. doi: 10.1007/BF02256400. [DOI] [PubMed] [Google Scholar]
- 32.Ruffolo C., Angriman I., Scarpa M., Polese L., Barollo M., Bertin M., Pagano D., D'Amico D.F. Minimally invasive management of Crohn's disease complicated by ureteral stenosis. Surg Laparosc Endosc Percutan Tech. 2004;14:292–294. doi: 10.1097/00129689-200410000-00014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT and Tl1a-Tg mice consume similar amounts of DSS water. DSS water consumption was assessed for each cycle and represented as grams (g) of DSS water consumed. Data are expressed as mean ± SD.