Abstract
We demonstrate that a Rho kinase inhibitor (Y-27632), in combination with fibroblast feeder cells, induces normal and tumor epithelial cells from many tissues to proliferate indefinitely in vitro, without transduction of exogenous viral or cellular genes. Primary prostate and mammary cells, for example, are reprogrammed toward a basaloid, stem-like phenotype and form well-organized prostaspheres and mammospheres in Matrigel. However, in contrast to the selection of rare stem-like cells, the described growth conditions can generate 2 × 106 cells in 5 to 6 days from needle biopsies, and can generate cultures from cryopreserved tissue and from fewer than four viable cells. Continued cell proliferation is dependent on both feeder cells and Y-27632, and the conditionally reprogrammed cells (CRCs) retain a normal karyotype and remain nontumorigenic. This technique also efficiently establishes cell cultures from human and rodent tumors. For example, CRCs established from human prostate adenocarcinoma displayed instability of chromosome 13, proliferated abnormally in Matrigel, and formed tumors in mice with severe combined immunodeficiency. The ability to rapidly generate many tumor cells from small biopsy specimens and frozen tissue provides significant opportunities for cell-based diagnostics and therapeutics (including chemosensitivity testing) and greatly expands the value of biobanking. In addition, the CRC method allows for the genetic manipulation of epithelial cells ex vivo and their subsequent evaluation in vivo in the same host.
See related Commentary on page 443
The unlimited propagation of adult mammalian, nonkeratinocyte epithelial cells offers exciting opportunities for gene and cell-based therapies, as well as regenerative and personalized medicine. However, it has not been possible to passage and rapidly expand cells derived from adult glandular tissues that retain lineage commitment and normal growth and differentiation potential. This has been particularly problematic, for example, with cells derived from the prostate, lung, and liver (either normal or neoplastic) and has severely limited their in vitro characterization.1,2 The propagation of adult epithelial cells requires specialized medium and is limited by the early onset of senescence. Although it is possible to bypass this senescence block using viral oncogenes, such as SV40 large T antigen3 or the E6/E7 proteins of the oncogenic human papillomaviruses,4–8 the resultant cell lines have aberrant p53 and Rb regulatory pathways. It is also possible to immortalize primary human adult cells with exogenous human Telomerase reverse transcriptase (hTERT) and additional cellular genes, such as cdk4,9 but as we show in this study, breast cells immortalized with hTERT alone have disrupted differentiation, as assayed by mammosphere formation in Matrigel. In addition, some somatic cells can be reprogrammed and expanded into induced pluripotent stem (iPS) cells.10–15 Although iPS techniques extend cell life span, this method is relatively inefficient and uses the transduction of exogenous genes that can induce alterations in the cellular genome16–18 and antigenicity.19,20 Also, precise control of the fate of iPS cells is still undergoing experimental refinement. To date, it has not been possible to continuously propagate adult epithelial cells in culture without permanently compromising their normal phenotype.
Recently, we established a method to indefinitely extend the life span of primary human keratinocytes using both fibroblast feeder cells and a Rho-associated kinase (ROCK) inhibitor, Y-27632.21 However, the culture of nonkeratinocytes has proved to be difficult over the years, and feeder cells are not generally used to culture these cells. There are synthetic media (without feeders) that can generate limited populations of some nonkeratinocyte cells, but none that can induce unrestricted proliferation. Indeed, some human epithelial cells, such as prostate, lung, and liver, have extremely short in vitro life spans and can only be passaged for a few times before they cease proliferation.1,2,9,22–25 This limited proliferation is also characteristic of primary human cancers, such as those derived from the prostate.2 Interestingly, the principal prostate cancer cells available for research have been derived from aggressive metastatic tumors. Later, we describe a widely applicable tissue culture method that rapidly and conditionally reprograms normal and tumor epithelial cells to a highly proliferative state during which they maintain their original karyotypes. As shown previously with keratinocytes, removal of these conditions restores the capacity for cell differentiation. We speculate on a potential mechanism that is operative in the generation of these conditionally reprogrammed cells (CRCs).
Materials and Methods
Harvesting of Tissues
Normal or tumor human mammary/prostate specimens were collected with the informed consent of the patients, according to Georgetown University Institutional Review Board (Washington, DC) protocols. Mammary tissues were minced and digested with a mixture of dispase and collagenase 1A (StemCell Technologies Inc, Vancouver, BC, Canada), and fat was removed with a cell strainer (70 μm; BD Biosciences, Bedford, MA). Prostate tissues were chopped into 1-mm fragments and digested with trypsin. In addition to cells derived from tissue, we also obtained primary normal epithelial cells (human mammary epithelial cell, herein called mammary, and human prostate epithelial cell, herein called prostate) from Lonza (Walkersville, MD) and tracheal/bronchial lung cells from Lifeline (Lifeline Cell Technology, Walkersville, MD). Hepatocytes were harvested using a two-step collagenase perfusion technique. Briefly, liver tissues were first perfused with calcium and magnesium-free Hanks' buffer at 80 to 100 mL/minute for 10 to 15 minutes; the second perfusion was performed with 0.5 g/L collagenase solution at 50 to 70 mL/minute for 10 minutes. The two perfusion steps were performed at 37°C to 38°C. After perfusion, the liver capsule was incised. The thick fibrous connective tissue was discarded, and cell suspensions were harvested. The cell suspensions were further digested at 37°C for 10 to 15 minutes. RPMI 1640 medium was used for cessation of digestion, and the released cells were filtered through three-layer sterilized gauze and washed by three centrifugations (50 × g).
Cryopreservation of Human Tissue
Fresh human breast tissue from a patient who underwent reduction mammoplasty was minced into small pieces or thin slices with a maximal size of 1 to 3 mm. The tissue pieces were then frozen in cryopreservation medium [90% fetal calf serum (Invitrogen, Gaithersburg, MD)/10% dimethyl sulfoxide (v/v; Sigma-Aldrich, St. Louis, MO) 5 μmol/L Y-27632 (Enzo Life Sciences, Lausen, Switzerland)] at −80°C or in liquid nitrogen. At later times, the frozen tissue was thawed at 37°C, pelleted, and suspended in F medium for a brief digestion with dispase-collagenase (StemCell). The cell suspensions were subjected to the culture system, as described later.
Cell Culture
Two different culture conditions were used to propagate both commercially available and harvested epithelial cells in standard tissue culture flasks.1 The primary method of epithelial cell propagation used a fibroblast feeder cell system.26 Epithelial cells were cocultivated with irradiated (3000 rad) Swiss 3T3 fibroblasts (J2 strain) in F medium [3:1 (v/v) F-12 Nutrient Mixture (Ham)–Dulbecco's modified Eagle's medium (Invitrogen), 5% fetal bovine serum, 0.4 μg/mL hydrocortisone (Sigma-Aldrich), 5 μg/mL insulin (Sigma-Aldrich), 8.4 ng/mL cholera toxin (Sigma-Aldrich), 10 ng/mL epidermal growth factor (Invitrogen), and 24 μg/mL adenine (Sigma-Aldrich)] with addition of 5 to 10 μmol/L Y-27632 (Enzo Life Sciences). All cells were maintained at 37°C in a humidified incubator, with 5% CO2, and passaged at a 1:4 (cells without feeder fibroblasts) or a 1:8 to 1:32 (cells with feeder fibroblasts) split ratio when 80% to 90% confluent. The growth curves were plotted as population doublings versus time (days).21 Mammary, prostate, and lung cells were also grown in defined media [mammary epithelial growth medium and prostate epithelial growth medium (PrEGM) from Lonza and keratincytes growth medium from Invitrogen] without serum.
Separation of Feeder and Epithelial Cells
Differential trypsinization was used to separate feeder and epithelial cells during passaging. Briefly, feeder/epithelial co-cultures were rinsed with PBS and incubated with 0.05% trypsin solution at room temperature for 30 seconds to 1 minute, with close monitoring by phase microscopy. When the feeder cells rounded up and began to detach from the substrate, the cultures were gently tapped and the detached cells were removed by aspiration. The epithelial colonies remained tightly adherent. The epithelial cells were rinsed again with PBS and then retrypsinized at 37°C for 3 to 5 minutes. After gentle pipetting to disperse the epithelial cells into a single-cell suspension, the cells were pelleted through PBS containing 10% serum (to neutralize trypsin). After centrifugation at 500 × g, the cell pellets were resuspended in F medium for passaging or in cryopreservative medium (see previous description) for freezing.
Transduction of Mammary Epithelial Cells with Myc and hTERT
Human primary mammary epithelial cells were transduced with the Myc T58A or hTERT genes, and cell lines were established by continued passaging, as described in a previous publication.22
Three-Dimensional Culture
Single-cell suspensions of epithelial cells were dispersed into serum-free keratinocyte growth medium (Invitrogen) containing 2% Matrigel (BD Biosciences). Morphogenesis assays (and the harvesting of acini, immunostaining, and confocal microscopy) were performed as previously described.27,28
Real-Time RT-PCR
Total RNA was extracted using an RNeasy kit (Qiagen, Valencia, CA). Total RNA, 1 μg, was reverse transcribed in 20 μL of reaction mixture using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCRs containing 100 ng of total cDNA were performed using TaqMan Gene Expression Assays (Applied Biosystems) on the Applied Biosystems 7900HT Fast Real-Time PCR System using fast mode. The assay identification numbers of the validated genes were as follows: androgen receptor, Hs00171172_m1; NK3 homeobox 1, Hs00171834_m1; and prostate stem cell antigen, Hs04177224_g1. Amplification of human β-actin mRNA (4310881E) was used as an endogenous control to standardize the amount of sample added to the reaction. To obtain relative values, the following arithmetic formula was used: 2ΔCT, where ΔCT = difference between the threshold cycles of the target and an endogenous reference (β-actin mRNA). The real-time PCR conditions were as follows: 1 cycle at 90°C for 20 minutes, then 40 cycles at 95°C for 1 second, and 60°C for 20 seconds. Assays consisted of three technical replicates. hTERT mRNA was measured using the methods and primers that have been previously published.29,30
Immunofluorescence Staining
Cells were grown on sterile glass coverslips and fixed in 4% (w/v) paraformaldehyde, permeabilized with 0.1% saponin in PBS containing 0.2% gelatin, and labeled with the primary (mouse anti-p63; Santa Cruz Biotechnologies, Santa Cruz, CA, sc-863) and secondary (Alexa Fluor 488 donkey anti-mouse IgG; Invitrogen) antibody, according to the manufacturer's protocol. DNA in the cells was stained for 3 minutes at room temperature with 0.5 μg/mL Hoescht (number 33342) in PBS and washed three times with PBS. Coverslips were mounted on glass slides using ProLong anti-fade mounting medium (Invitrogen) for 1 hour at room temperature and were stored at 4°C. A Zeiss Axioskop microscope (Carl Zeiss, Inc., Thornwood, NY), equipped with a ×63 objective lens and a Hammamutsu charge-coupled device camera, was used to image the cells. Images were processed using Openlab software version 3.0.7 (Improvision, Coventry, UK).
Conventional Cytogenetic Analysis
Prostate cells at passage 33 were cultured, and chromosomes were prepared and G-banded using standard protocols.31 Chromosomes were identified and classified according to standard cytogenetic nomenclature.32
STR Analysis
Short tandem repeat (STR) analysis (ie, DNA fingerprinting) was performed using a commercially available kit (Cell ID System; Promega Corporation, Madison, WI). This system allows the co-amplification and three-color detection of 10 loci (nine STR loci and the Y-chromosome–specific Amelogenin). This approach provides a powerful level of discrimination of approximately 2.92 × 109. The following STR markers were tested in addition to the Amilogenin locus: CSF1PO, TPOX, TH01, vWA, D21S11, D16S539, D7S820, D13S317, and D5S818. The PCR amplification was performed according to the manufacturer's recommended protocol and as previously described.33 Detection of the amplified fragments was achieved with the ABI 3100 genetic analyzer (Applied Biosystems). Data analysis and allele size determination were performed using GeneMapper Software (Applied Biosystems).
Xenograft Assays
Exponentially growing normal or tumor prostate cells (1 × 106) were trypsinized, dispersed into single cells, and suspended in 200 μL of Matrigel HC (BD Biosciences). The Matrigel-suspended cells were injected s.c. into the left and right flanks of 6-week-old male imprinting control region mice with severe combined immunodeficiency (Taconic, Germantown, NY). The growth of xenografts was measured weekly with calipers. Animals were housed at the Georgetown University animal care facility, according to institutional guidelines.
Study Approval
Samples of normal or tumor tissue were collected with the informed consent of the patients, according to Georgetown University Institutional Review Board protocols. All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committees of Georgetown University Medical Center.
Results
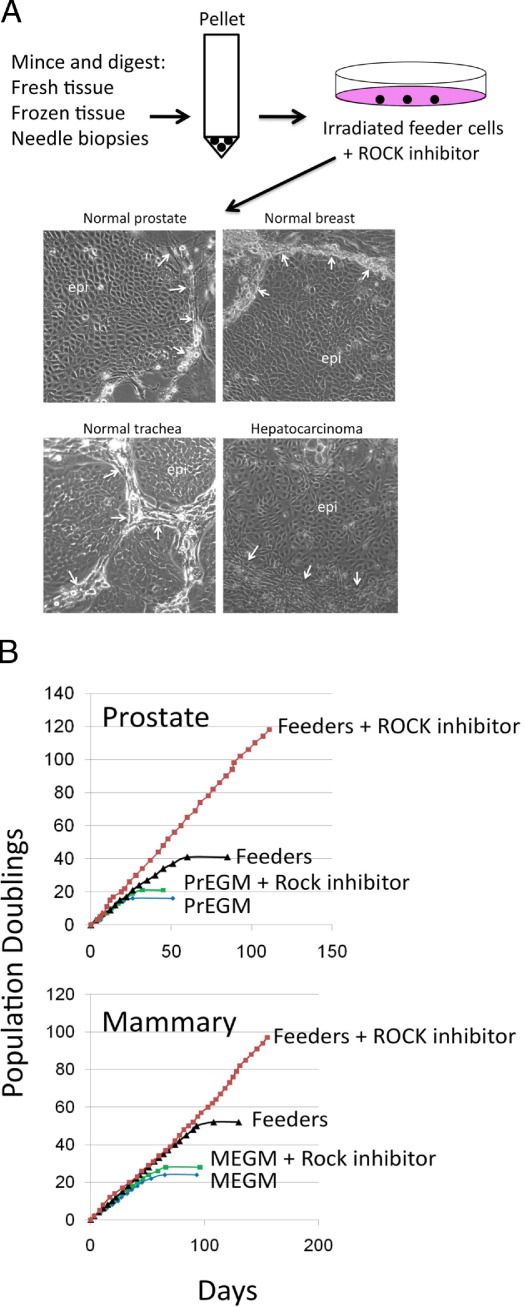
Figure 1A describes the technique for establishing CRCs from normal and neoplastic human epithelium. An Institutional Review Board–approved protocol was used to obtain biopsy specimens or samples of fresh surgical specimens in the Department of Pathology, Georgetown University Hospital. The samples were dispersed into single cells by digestion with collagenase/trypsin (see Materials and Methods), and the cells were plated in medium containing irradiated Swiss 3T3 J2 cells26 and 5 to 10 μmol/L Y-27632. Epithelial colonies were readily observed at 2 days and rapidly proliferated to reach confluence in approximately 5 days. Examples of human prostate, breast, lung, and liver cell (hepatocellular carcinoma) colonies are shown. Mouse and rat CRC cultures have also been established successfully from primary breast, prostate, and liver tissue using an identical protocol (see Supplemental Figures S1 and S2 at http://ajp.amjpathol.org).34,35 The average success rate of establishing CRCs is approximately 88% from a variety of animal tissues, although some tissues exhibit 100% success rates (Table 1).
Figure 1.
Propagation and immortalization of human adult epithelial cells. A: Prostate, breast, tracheal, and liver (hepatocellular carcinoma) tissues were harvested and digested with trypsin-collagenase, as described in Materials and Methods. Cells isolated from the tissues were plated on a feeder layer of irradiated (3000 rad) Swiss 3T3 cells (J2 subclone) and grown in F medium containing 10 μmol/L ROCK inhibitor (Y-27632). Small colonies could be observed after 1 day. At day 5 (shown), there were large islands of epithelial (epi) cells that compressed the surrounding feeder cells (white arrows). B: The prostate and breast epithelial cells were passaged repetitively using trypsinization techniques described for keratinocytes.21 The cell number was recorded at each passage, and a plot of population doublings versus time (days) was constructed. The cells were grown under four different conditions: with both feeder cells and Y-27632 in F medium, with feeders only in F medium, with Y-27632 in mammary epithelial growth medium or PrEGM or mammary epithelial growth medium or PrEGM alone. Only cells grown in F medium containing feeders and Y-27632 continued to proliferate with a constant growth rate.
Table 1.
Tissues Used for Cell Isolation and Culture
| Species | Tissue type | Cases | Total samples | Cell lines | Normal/diseased |
|---|---|---|---|---|---|
| Human | Breast | 13 | 25 | 25 | 18/4B/3T |
| Prostate | 9 | 16 | 16 | 9/7T | |
| Lung | 6 | 8 | 8 | 5/3T | |
| Liver | 5 | 6 | 3 | 1/2T | |
| Colon | 4 | 8 | 4 | 1/4T | |
| Pancreas | 5 | 8 | 4 | 2/2T | |
| Endothelium | 1 | 1 | 1 | 1/0 | |
| Ovary | 1 | 1 | 1 | 1/0 | |
| Pancreas (met) | 1 | 1 | 1 | 0/1T | |
| Oral (H&N) | 2 | 3 | 3 | 1/2T | |
| Mouse | Breast | 7 | 7 | 7 | 6/1T |
| Prostate | 3 | 3 | 3 | 3/0 | |
| Rat | Breast | 5 | 5 | 5 | 1/4T |
| Dog | Skin epithelium | 1 | 1 | 1 | 1/0 |
| Oral epithelium | 1 | 1 | 1 | 1/0 | |
| Total | NA | 64 | 94 | 83 | NA |
The tissues for which biopsy specimens were obtained for culture are indicated for each of the mammalian species. The number of cases (patient or animal) is indicated, as is the total number of samples taken from those cases. The cell lines that were generated from these samples are also indicated, and the success rate was determined by dividing the number of generated cell lines by the total number of samples placed into tissue culture. The success rate is defined as the ability of the cultured cells to reach 50 population doublings or, in some cases (colon and pancreas), as three times the population doublings observed with current, optimal culture conditions.
B, BRCA1/2 mutation carrier; H&N, head and neck cancer; met, metastatic; NA, not applicable; T, tumor.
After the first plating, the breast CRCs in Figure 1A were passaged every 3 days at 1:4 to 1:8 dilutions and the prostate cells were passaged at 1:16 to 1:32 dilutions, verifying the robust growth of these epithelial cultures. When plated at single-cell densities, colony-forming efficiency varied from 40% to 70%. However, when cells were passaged at a normal density (eg, 1:16 splits), there was no morphological evidence of cell death. The rapid reprogramming of normal adult epithelial cells to a proliferative state is consistent with their expression of p63, a marker of basal cell phenotype (see Supplemental Figure S3 at http://ajp.amjpathol.org).
To determine the contributions of feeder cells and Y-27632 to the exuberant growth of nonkeratinocyte CRCs, we assayed the growth kinetics of human mammary and prostate cells under different culture conditions (Figure 1B). Both cell types proliferated continuously in F medium containing feeder cells and Y-27632. The growth of the prostate cells was particularly rapid, with the culture reaching 120 population doublings in <120 days. As shown, continuous cultures could not be initiated with either breast or prostate cells using F medium and feeder cells without Y-27632. In addition, breast and prostate cells grown in commercial medium designed for these cells (mammary epithelial growth medium and PrEGM, respectively; Lonza) became senescent at early times, either in the presence or absence of the ROCK inhibitor. Finally, cells cultured in F medium alone (containing neither feeders nor Y-27632) immediately ceased proliferation and underwent rapid terminal differentiation, due in part to the serum and higher calcium levels present in the F medium. Thus, both feeder cells and Y-27632 were necessary for the highly efficient, long-term establishment of cell cultures from primary tissue.
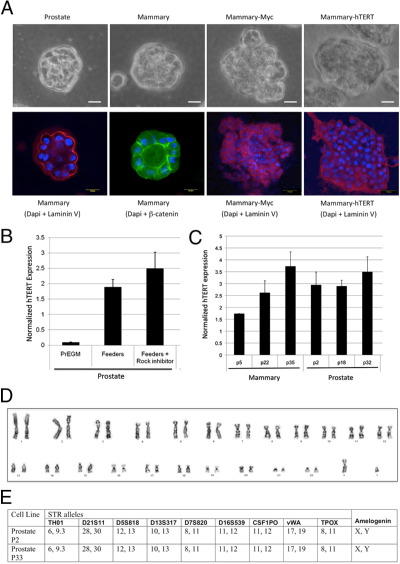
Despite the initial requirement for feeders and Y-27632, late-passage mammary and prostate CRCs continued to proliferate (at least for limited doublings) when transferred to Matrigel (in keratincytes growth medium) in the absence of these two components. In Matrigel, they formed well-defined prostaspheres and mammospheres (Figure 2A). The mammospheres were characterized by a well-defined cell/Matrigel interface and showed polarized synthesis of laminin that surrounded each colony (Figure 2A). In addition, the mammospheres expressed β-catenin in the cytoplasm and adjacent to plasma membranes (Figure 2A), typical for normal epithelial cells. Lumen formation was observed in both prostaspheres and mammospheres and is best illustrated in cells stained with β-catenin. In contrast, mammary cells that were transformed by a Myc mutant (T58A)22 formed solid masses of disorganized colonies with extensions into the Matrigel, a characteristic of transformed or tumorigenic cells (Figure 2A). Corresponding to this morphological alteration, laminin deposition by the cells was not polarized to the cell/Matrigel interface, but was dispersed in the sphere (Figure 2A). Similarly, mammary cells that were immortalized by hTERT (but not transformed as defined by growth in agarose) formed aberrant Matrigel colonies consisting of solid masses (Figure 2A) with generalized, nonpolarized accumulation of laminin (Figure 2A).
Figure 2.
Characterization of late-passage breast and prostate cells. A: The differentiation potential of prostate and breast cells was evaluated by culture in Matrigel, without feeders or Y-27632, as described in Materials and Methods. Both prostate and breast cells formed normal prostaspheres (A, top row, left panel) and mammospheres (A, top row, second panel from left) with glandular lumens. The mammospheres showed normal polarized laminin deposition around the periphery of the sphere (A, bottom row, left panel). In addition, the mammospheres also showed the characteristic cytoplasmic/plasma membrane localization of β-catenin observed in normal epithelial cells (A, bottom row, second panel from left, green). In contrast, mammary cells immortalized with the Myc T58A mutant showed abnormal differentiation and formed abnormally shaped solid spheres with extensions into the Matrigel (A, top row, second panel from right). Scale bars: 25 μm (white); 20 μm (yellow). These Myc-immortalized cells also exhibited abnormal deposition of laminin within the sphere and without polarized localization to the colony periphery (A, bottom row, second panel from right). Mammary cells immortalized by hTERT also formed abnormal solid spheres (A, top row, right panel) with aberrant, dispersed distribution of laminin (A, bottom row, right panel). B: The cause of the high hTERT expression in the epithelial cells is the result of the in vitro culture conditions. When prostate cells are grown in commercial medium (PrEGM), the level of hTERT mRNA is low. However, when those cells are transferred to F medium with feeders (with or without Y-27632), there is an approximate 20-fold increase in hTERT expression. The greatest impact is the presence of feeder cells. C: Induction of hTERT mRNA in breast and prostate cells is similar and occurs early during passaging. Quantitative real-time PCR for hTERT was performed at the indicated passage numbers. Both cell types show an early induction of telomerase expression. In prostate, for example, the induction is maximal already at passage 2, indicating that selection during in vitro culture is not the cause of this induction. D: Chromosomal analysis of late-passage prostate cells revealed a normal 46,XY karyotype. E: DNA fingerprinting of early- and late-passage prostate cells demonstrated that they have nine identical STR loci and the Y-specific Amelogenin locus, thereby verifying their genetic identity. Data are presented as mean ± SEM.
Telomerase plays a critical role in primary cell immortalization, and our initial studies indicated that feeder cells induced pronounced telomerase activity and hTERT expression in keratinocytes.21,29 To determine whether hTERT induction was also observed in nonkeratinocytes, we quantified hTERT mRNA in prostate cells in medium with and without feeders and Y-27632 (Figure 2, B and C). Prostate cells grown in F medium with feeders or feeders plus Y-27632 exhibited a 20-fold increase in hTERT expression compared with cells grown in synthetic PrEGM medium (Figure 2B). Mammary cells also exhibited a similar induction of hTERT expression levels when propagated on feeder cells (Figure 2C). We next examined whether the late-passage CRCs from normal tissues retained a diploid karyotype. The results for prostate cells (at population doubling 93) are shown in Figure 2D and verify that their chromosomes are structurally and numerically normal, with a 46,XY karyotype. The breast cells were also normal by karyotype analysis (46,XX; X.L., B.R.H, R.S., unpublished data). To verify that our immortalized CRC cultures were not contaminated with another cell line during prolonged passaging, we performed DNA fingerprinting analysis at nine STR loci and at the Y-specific Amelogenin locus (Cell ID System; Promega). The data in Figure 2E demonstrate that the prostate cell cultures, when analyzed at early and late passages, are identical at all 10 loci, thus confirming their identity.
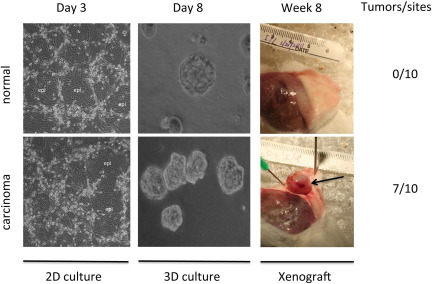
The ability to generate matched normal and cancer cells from patients provides a renewable source for comparative genetic and biochemical analysis, as well as drug screening. As an example, we obtained matched biopsy specimens of normal and malignant prostate tissue from the same patient and processed them as described. By day 3 after plating, epithelial colonies had already formed, expanded, and compressed adjacent feeder cells (Figure 3). The growth rate and morphological characteristics of the normal and tumor cells were similar. However, the normal and tumor cells differed in the expression of several markers. In tumors cells, the androgen receptor and prostate stem cell antigen were increased, whereas the NK3 homeobox 1 prostate tumor suppressor protein was decreased (see Supplemental Figure S4 at http://ajp.amjpathol.org). The normal and tumor cells also displayed biological differences in three-dimensional Matrigel cultures (Figure 3). The normal prostate cells formed prostaspheres, as previously shown in Figure 2A, whereas the tumor cells formed large undifferentiated aggregates, similar to those observed for breast cells transformed by Myc. The normal and tumor cells could also be distinguished by xenograft assays. When the normal and tumor prostate cells were suspended in Matrigel and injected s.c. into mice with severe combined immunodeficiency, tumors formed rapidly (in 8 weeks) only in mice receiving the cells derived from prostate cancer (Figure 3). Finally, there were also karyotype differences between these two cell cultures. The karyotype of the prostate tumor cells exhibited instability of chromosome 13 (X.L., R.S., unpublished data), in contrast to the normal karyotype of the matched normal prostate cells. STR fingerprinting analysis verified that these two prostate cell cultures were, indeed, derived from the same patient (X.L., R.S., unpublished data).
Figure 3.
Establishment of normal prostate and prostate adenocarcinoma cell cultures from the same patient. Biopsy specimens of normal and malignant prostate tissue from the same patient were processed and propagated in vitro, as described in Materials and Methods. For two-dimensional cultures, cells were photographed at days 2 and 3, demonstrating the rapid and equivalent outgrowth of cells from the biopsy specimens. For in vitro three-dimensional cultures, matched cells were also grown in the three-dimensional Matrigel system, as described in Materials and Methods. The normal prostate cells formed prostaspheres, whereas the tumor cells formed large undifferentiated aggregates. For an in vivo xenograft, 1 million normal and tumor cells were injected s.c. into the flanks of five mice with severe combined immunodeficiency at two different sites (a total of 10 sites for each cell type). The prostate cancer cells induced tumors at 7 of 10 sites within 8 weeks; a representative tumor is shown (arrow). The normal prostate cells did not induce tumors (0 of 10 sites). epi, epithelial cells.
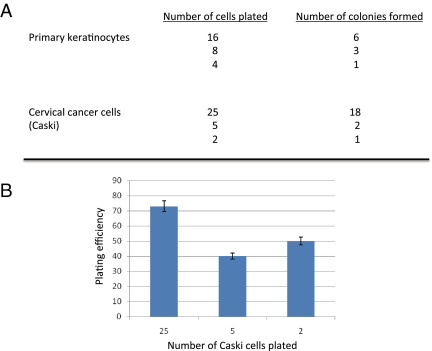
We have also established CRC cultures from as few as two to four viable cells (Figure 4), suggesting potential applications to the study of circulating tumor cells. Perhaps more important, we generated cell cultures from human tissues frozen in cryopreservative medium, a finding that will affect the practice of pathology and biobanking (see Supplemental Figure S5 at http://ajp.amjpathol.org).
Figure 4.
Colony-forming efficiency of primary human cells. Human keratinocytes and cervical cancer cells (Caski) were plated at the indicated cell numbers onto the feeder cell cultures. After 7 to 10 days, the feeder cells were removed and the epithelial colonies were fixed for 5 minutes with 3.7% paraformaldehyde, and then stained for 30 minutes with 0.05% crystal violet. The plates were photographed and the colonies were counted with ChemiDoc and QuantityOne software (Bio-Rad Laboratories, Inc, Hercules, CA). A: The number of cells plated was plotted versus the number of colonies observed after 7 to 10 days. B: The efficiency of colony formation for the cervical cancer cells is depicted for the indicated number of Caski cells plated. The plating efficiency for low-density seeding ranged from 40% to 70%. Data are presented as mean ± SEM.
Discussion
In contrast to current methods for culturing primary epithelial cells or for inducing pluripotent stem cells (iPS cells), establishing CRC cultures with feeders and Y-27632 is rapid and efficient. For example, we have generated 1 × 106 to 2 × 106 normal prostate cells from a single mouse prostate in 6 days (see Supplemental Figure S1 at http://ajp.amjpathol.org) and 2 × 106 breast carcinoma cells from a core biopsy specimen of a rat breast cancer in 5 days (see Supplemental Figure S2 at http://ajp.amjpathol.org). We have also demonstrated that cultured cells derived from breast tumors of transgenic human epidermal growth factor receptor 2/neu mice (Friend's leukemia virus B strain) induced tumors when injected into mammary fat pads of the same strain (data not shown). The ability to generate matched normal and cancer cells from patients is a fundamental breakthrough in cell culture technology. This system allows, for the first time to our knowledge, the direct comparison of the molecular and genetic profile of the normal and transformed epithelium, enabling patient-specific ex vivo pathobiological analysis. In addition, the ability to culture metastatic lesions will enable identification of the cellular changes that occur and potentially contribute to invasion and metastasis.
We first observed the dramatic reprogramming of adult epithelial cells to a proliferative state with keratinocytes.21 Even epithelial cells that were entering a senescence phase were immediately converted to a proliferative state when transferred to F medium containing feeder cells and Y-27632. These findings demonstrate that the combination of feeder cells and Y-27632 is directly altering cell growth, rather than selecting for a small subpopulation of stem-like cells.24,25,36–40 The mechanism for this reprogramming is unclear, although there are insightful parallels with the process of cell immortalization induced by the human papillomaviruses (Figure 5). The high-risk human papillomaviruses encode two oncoproteins, E6 and E7, that are required for the efficient immortalization of primary cells. One of the most critical roles for the E6 protein in cell immortalization is the induction of hTERT.4,41,42 We have demonstrated that the predominant factor for hTERT induction in CRCs is growth in F medium with feeder cells and that Y-27632 has a minimal contribution to this induction. The mechanism by which telomerase is induced by the F medium plus feeder cells is under investigation.
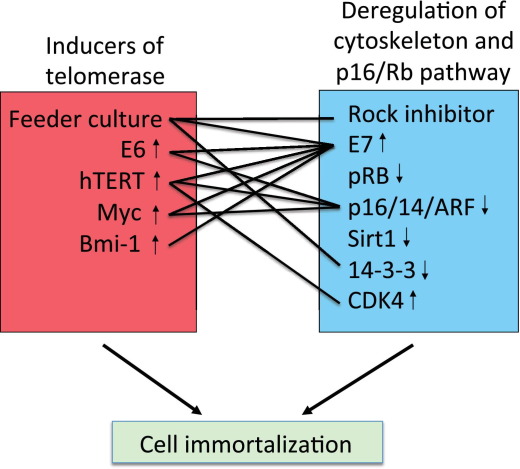
Figure 5.
Potential model for the cooperative effects of F medium plus feeders and the ROCK inhibitor in cell immortalization. Class 1 genes induce telomerase activity, and class 2 genes alter both the p16/Rb pathway and the cell cytoskeleton. The connecting lines indicate documented interactions between class 1 and class 2 genes that result in cell immortalization.
The second component of human papillomavirus–mediated cell immortalization is the function of the E7 protein. In addition to its role in inactivating the Rb pathway, E7 has recently remodeled the actin cytoskeleton43 and inactivated Rho.44 Alterations of actin/myosin activity are the main consequence of ROCK inhibition,45,46 and this cytoskeletal network has a primary role in regulating cell proliferation.45–48 It is also possible that Y-27632 is perturbing the p16 pathway, although our previous studies21 indicate that p16 expression is maintained under our culture conditions. Overall, we believe that the combination of F medium containing feeder cells and Y-27632 supplies two distinct functions that promote unrestricted cell proliferation: (1) induction of telomerase and (2) cytoskeletal remodeling and/or interference with the p16/Rb pathway. In Figure 5, we have listed several cellular genes that fall into these two designated pathways. The genes and proteins in the first class (red box) have induced telomerase activity, whereas the genes and the ROCK inhibitor that compose the second class (blue box) modulate both p16/Rb and/or perturb the cellular cytoskeleton. The interconnecting lines indicate functional pairs of cellular and/or viral genes and inhibitors known to collaborate in cellular immortalization.4,6,7,9,21,22,41,43,49–54
In conclusion, a critical finding in this study is that the combination of feeder cells and ROCK inhibitor induces continued cell proliferation without the use of exogenous viral or cellular gene transduction. It is significant that this reprogramming of cell growth and differentiation is conditional, thereby allowing facile in vitro manipulation of cell behavior. In addition, we have also observed that CRCs immortalized by the feeder/ROCK system display a more normal cellular phenotype than cells immortalized by hTERT. Mammary CRCs, in contrast to hTERT-immortalized cells, form mammospheres in Matrigel that exhibit a well-defined cell-stroma interface and polarized localization of laminin. The ability to rapidly generate CRCs from both normal and tumor (or disease) tissue offers an exciting opportunity to integrate live cell biology into the practice of pathology. In addition, the efficiency and robust nature of establishing CRCs should greatly expand the value of biobanking and provide an inexhaustible supply of patient-specific cells for comparative genetic and molecular analysis.
Acknowledgments
We thank Robert Clarke, Leena Clarke, Anni Warri, Mary Beth Martin, Blair Marshall, and Ruth He for helpful discussions and for providing cell and tissue samples and Janice D. Rone for technical assistance.
Footnotes
Supported by NIH grants R01 CA129003 (C.A.), R01 CA113477 (A.R.), and R01 CA106400 (R.S.); a Department of Defense fellowship (W81XWH-10-1-0747 to V.O.); and two internal development grants (NCI CCSG 5P30CA051008 to X.L. and A.R., respectively). This work was funded, in part, by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases and the NIH.
Disclosures: X.L., A.M., and R.S. have submitted a patent application on this technology. S.C. and A.M. have submitted a patent application on “Use of a ROCK Inhibitor to Sustain Primary Human Keratinocytes in a Proliferative State” (PCT/US2009/066844).
A guest editor acted as editor-in-chief for the final disposition of this article.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.10.036.
Supplementary data
Propagation of mouse glandular epithelial cells (epi). Mouse mammary, prostate, and liver cells were isolated and successfully propagated, as described in Materials and Methods. In addition, mammary cell cultures were also successfully established from several transgenic mouse strains. mMEC FVB, normal mouse mammary epithelial cells were generated from mammary glands of 8-week-old FVB mice; mMEC rtTA amplified in breast cancer I (AIB1) Δ4, normal mouse mammary epithelial cells overexpressing Δ4, a truncated form of AIB1 previously described34; mMEC rtTA AIB1 high, normal mouse mammary epithelial cells overexpressing AIB1, previously described; mMEC MMTV/Neu, normal mouse mammary epithelial cells overexpressing HER2 in the mammary glands. The MMTV-HER2 transgenic mice were previously described.35
Propagation of rat mammary cells from normal or tumor tissues. A: A photomicrograph of breast cell colonies derived from excised normal rat breast tissue. B: A photomicrograph of a colony of rat breast adenocarcinoma cells (7,12-dimethylbenz-alpha-anthracene (DMBA)-induced tumor). The tumor cultures were initiated from a single-needle biopsy specimen of a primary rat breast tumor. Cells (2 × 106) were generated in 5 days from the needle biopsy specimen.
Mammary and prostate cells propagated on feeder cells with Y-27632 express p63, a marker of basal cell differentiation. The indicated cells were plated onto glass coverslips using the described cell culture conditions. Two days after plating, the cells were fixed with 3.7% paraformaldehyde, permeabilized with saponin, stained with DAPI dye, and then reacted with antibody against p63, followed by rhodamine-labeled secondary antibody. The cultured cells reacted with anti-p63 antibodies are shown in red; mouse fibroblast feeder cells are shown as arrows. The nuclei of the feeder cells display punctate DAPI staining because of their previous irradiation.
Gene expression profiles in prostate epithelial cells. Semiquantitative RT-PCR of the following: androgen receptor (AR) (A), NK3 homeobox 1 (Nkx3.1) (B), and prostate stem cell antigen (PSCA) normalized to β-actin mRNA expression (C). Relative mRNA gene expression values were obtained using the following arithmetic formula: 2ΔCT. The Δ(CT) = CT (target gene)-CT (β-actin mRNA). Assays consisted of three technical replicates. Data are presented as mean ± SEM.
Cell cultures established from frozen human tissue. Fresh human breast tissue from a patients who underwent reduction mammoplasty was minced into small pieces, with a maximal size of 1 to 3 mm and frozen in 90% fetal calf serum/10% dimethyl sulfoxide (v/v)/5 μmol/L Y-27632, as described in Materials and Methods. Cell suspensions from the frozen tissue were thawed at 37°C, pelleted, and suspended in F medium for brief digestion with dispase-collagenase. The cell suspensions were cultured and photographed 5 days (top panel) and 8 days (bottom panel) after plating.
References
- 1.Castell J.V., Gomez-Lechon M.J. Liver cell culture techniques. Methods Mol Biol. 2009;481:35–46. doi: 10.1007/978-1-59745-201-4_4. [DOI] [PubMed] [Google Scholar]
- 2.Miki J., Rhim J.S. Prostate cell cultures as in vitro models for the study of normal stem cells and cancer stem cells. Prostate Cancer Prostatic Dis. 2008;11:32–39. doi: 10.1038/sj.pcan.4501018. [DOI] [PubMed] [Google Scholar]
- 3.Van der Haegen B.A., Shay J.W. Immortalization of human mammary epithelial cells by SV40 large T-antigen involves a two step mechanism. In Vitro Cell Dev Biol. 1993;29A:180–182. doi: 10.1007/BF02634177. [DOI] [PubMed] [Google Scholar]
- 4.Liu X., Dakic A., Chen R., Disbrow G.L., Zhang Y., Dai Y., Schlegel R. Cell-restricted immortalization by human papillomavirus correlates with telomerase activation and engagement of the hTERT promoter by Myc. J Virol. 2008;82:11568–11576. doi: 10.1128/JVI.01318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawley-Nelson P., Vousden K.H., Hubbert N.L., Lowy D.R., Schiller J.T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson J.B., Bedell M.A., McCance D.J., Laiminis L.A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990;64:519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munger K., Phelps W.C., Bubb V., Howley P.M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlegel R., Phelps W.C., Zhang Y.L., Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988;7:3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roig A.I., Eskiocak U., Hight S.K., Kim S.B., Delgado O., Souza R.F., Spechler S.J., Wright W.E., Shay J.W. Immortalized epithelial cells derived from human colon biopsies express stem cell markers and differentiate in vitro. Gastroenterology. 2010;138:1012–1021. doi: 10.1053/j.gastro.2009.11.052. [DOI] [PubMed] [Google Scholar]; e1011-e1015
- 10.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Aasen T., Raya A., Barrero M.J., Garreta E., Consiglio A., Gonzalez F., Vassena R., Bilic J., Pekarik V., Tiscornia G., Edel M., Boue S., Izpisua Belmonte J.C. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka S., Blau H.M. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okita K., Yamanaka S. Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci. 2011;366:2198–2207. doi: 10.1098/rstb.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unternaehrer J.J., Daley G.Q. Induced pluripotent stem cells for modelling human diseases. Philos Trans R Soc Lond B Biol Sci. 2011;366:2274–2285. doi: 10.1098/rstb.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gore A., Li Z., Fung H.L., Young J.E., Agarwal S., Antosiewicz-Bourget J., Canto I., Giorgetti A., Israel M.A., Kiskinis E., Lee J.H., Loh Y.H., Manos P.D., Montserrat N., Panopoulos A.D., Ruiz S., Wilbert M.L., Yu J., Kirkness E.F., Izpisua Belmonte J.C., Rossi D.J., Thomson J.A., Eggan K., Daley G.Q., Goldstein L.S., Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussein S.M., Batada N.N., Vuoristo S., Ching R.W., Autio R., Narva E., Ng S., Sourour M., Hamalainen R., Olsson C., Lundin K., Mikkola M., Trokovic R., Peitz M., Brustle O., Bazett-Jones D.P., Alitalo K., Lahesmaa R., Nagy A., Otonkoski T. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 18.Lister R., Pelizzola M., Kida Y.S., Hawkins R.D., Nery J.R., Hon G., Antosiewicz-Bourget J., O'Malley R., Castanon R., Klugman S., Downes M., Yu R., Stewart R., Ren B., Thomson J.A., Evans R.M., Ecker J.R. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor C.J., Bolton E.M., Bradley J.A. Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond B Biol Sci. 2011;366:2312–2322. doi: 10.1098/rstb.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavazava N. Immunity of embryonic stem cell-derived hematopoietic progenitor cells. Semin Immunopathol. 2011;33:613–617. doi: 10.1007/s00281-011-0273-9. [DOI] [PubMed] [Google Scholar]
- 21.Chapman S., Liu X., Meyers C., Schlegel R., McBride A.A. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thibodeaux C.A., Liu X., Disbrow G.L., Zhang Y., Rone J.D., Haddad B.R., Schlegel R. Immortalization and transformation of human mammary epithelial cells by a tumor-derived Myc mutant. Breast Cancer Res Treat. 2009;116:281–294. doi: 10.1007/s10549-008-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garraway I.P., Sun W., Tran C.P., Perner S., Zhang B., Goldstein A.S., Hahm S.A., Haider M., Head C.S., Reiter R.E., Rubin M.A., Witte O.N. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate. 2010;70:491–501. doi: 10.1002/pros.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terunuma A., Limgala R.P., Park C.J., Choudhary I., Vogel J.C. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng Part A. 2010;16:1363–1368. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Valdez J.M., Zhang B., Wei L., Chang J., Xin L. ROCK inhibitor Y-27632 suppresses dissociation-induced apoptosis of murine prostate stem/progenitor cells and increases their cloning efficiency. PLoS One. 2011;6:e18271. doi: 10.1371/journal.pone.0018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rheinwald J.G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 27.Debnath J., Muthuswamy S.K., Brugge J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 28.Lee G.Y., Kenny P.A., Lee E.H., Bissell M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu B., Quintero J., Baker C.C. Keratinocyte growth conditions modulate telomerase expression, senescence, and immortalization by human papillomavirus type 16 E6 and E7 oncogenes. Cancer Res. 2003;63:7815–7824. [PubMed] [Google Scholar]
- 30.Liu X., Roberts J., Dakic A., Zhang Y., Schlegel R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology. 2008;375:611–623. doi: 10.1016/j.virol.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barch M.J., Knutsen T., Spurbeck J.L., editors. The AGT Cytogenetics Laboratory Manual. ed 3. Lippincott-Raven; New York: 1997. [Google Scholar]
- 32.Shaffer LG T.N. S. Karger; Basel: 2005. ISCN: An International System for Human Cytogenetic Nomenclature. [Google Scholar]
- 33.Rae J.M., Creighton C.J., Meck J.M., Haddad B.R., Johnson M.D. MDA-MB-435 cells are derived from M14 melanoma cells: a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 34.Nakles R.E., Shiffert M.T., Díaz-Cruz E.S., Cabrera M.C., Alotaiby M., Miermont A.M., Riegel A.T., Furth P.A. Altered AIB1 or AIB1Δ3 expression impacts ERα effects on mammary gland stromal and epithelial content. Mol Endocrinol. 2011;25:549–563. doi: 10.1210/me.2010-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guy C.T., Webster M.A., Schaller M., Parsons T.J., Cardiff R.D., Muller W.J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Nishikawa S., Muguruma K., Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Meng G., Krawetz R., Liu S., Rancourt D.E. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 2008;17:1079–1085. doi: 10.1089/scd.2007.0247. [DOI] [PubMed] [Google Scholar]
- 38.Takehara T., Teramura T., Onodera Y., Kakegawa R., Fukunaga N., Takenoshita M., Sagawa N., Fukuda K., Hosoi Y. Rho-associated kinase inhibitor Y-27632 promotes survival of cynomolgus monkey embryonic stem cells. Mol Hum Reprod. 2008;14:627–634. doi: 10.1093/molehr/gan061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Krawetz R., Liu S., Meng G., Rancourt D.E. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580–589. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- 40.Hanna J.H., Saha K., Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiyono T., Foster S.A., Koop J.I., McDougall J.K., Galloway D.A., Klingelhutz A.J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 42.Liu X., Dakic A., Zhang Y., Dai Y., Chen R., Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Natl Acad Sci U S A. 2009;106:18780–18785. doi: 10.1073/pnas.0906357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yue J., Shukla R., Accardi R., Zanella-Cleon I., Siouda M., Cros M.P., Krutovskikh V., Hussain I., Niu Y., Hu S., Becchi M., Jurdic P., Tommasino M., Sylla B.S. Cutaneous HPV 38 E7 regulates actin cytoskeleton structure for increasing cell proliferation through CK2 and the eukaryotic elongation factor 1A. J Virol. 2011;85:8477–8494. doi: 10.1128/JVI.02561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charette S.T., McCance D.J. The E7 protein from human papillomavirus type 16 enhances keratinocyte migration in an Akt-dependent manner. Oncogene. 2007;26:7386–7390. doi: 10.1038/sj.onc.1210541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong M., Yan B.P., Liao J.K., Lam Y.Y., Yip G.W., Yu C.M. Rho-kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today. 2010;15:622–629. doi: 10.1016/j.drudis.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirth A. Rho kinase and hypertension. Biochim Biophys Acta. 2010;1802:1276–1284. doi: 10.1016/j.bbadis.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Pawlak G., Helfman D.M. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev. 2001;11:41–47. doi: 10.1016/s0959-437x(00)00154-4. [DOI] [PubMed] [Google Scholar]
- 48.Fridman A.L., Tainsky M.A. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975–5987. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dellambra E., Golisano O., Bondanza S., Siviero E., Lacal P., Molinari M., D'Atri S., De Luca M. Downregulation of 14-3-3sigma prevents clonal evolution and leads to immortalization of primary human keratinocytes. J Cell Biol. 2000;149:1117–1130. doi: 10.1083/jcb.149.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denicourt C., Dowdy S.F. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–855. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- 51.Haga K., Ohno S., Yugawa T., Narisawa-Saito M., Fujita M., Sakamoto M., Galloway D.A., Kiyono T. Efficient immortalization of primary human cells by p16INK4a-specific short hairpin RNA or Bmi-1, combined with introduction of hTERT. Cancer Sci. 2007;98:147–154. doi: 10.1111/j.1349-7006.2006.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X., Disbrow G.L., Yuan H., Tomaic V., Schlegel R. Myc and human papillomavirus type 16 E7 genes cooperate to immortalize human keratinocytes. J Virol. 2007;81:12689–12695. doi: 10.1128/JVI.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., Zhang M., Dong H., Yong S., Li X., Olashaw N., Kruk P.A., Cheng J.Q., Bai W., Chen J., Nicosia S.V., Zhang X. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 54.Zhong Z., Yeow W.S., Zou C., Wassell R., Wang C., Pestell R.G., Quong J.N., Quong A.A. Cyclin D1/cyclin-dependent kinase 4 interacts with filamin A and affects the migration and invasion potential of breast cancer cells. Cancer Res. 2010;70:2105–2114. doi: 10.1158/0008-5472.CAN-08-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Propagation of mouse glandular epithelial cells (epi). Mouse mammary, prostate, and liver cells were isolated and successfully propagated, as described in Materials and Methods. In addition, mammary cell cultures were also successfully established from several transgenic mouse strains. mMEC FVB, normal mouse mammary epithelial cells were generated from mammary glands of 8-week-old FVB mice; mMEC rtTA amplified in breast cancer I (AIB1) Δ4, normal mouse mammary epithelial cells overexpressing Δ4, a truncated form of AIB1 previously described34; mMEC rtTA AIB1 high, normal mouse mammary epithelial cells overexpressing AIB1, previously described; mMEC MMTV/Neu, normal mouse mammary epithelial cells overexpressing HER2 in the mammary glands. The MMTV-HER2 transgenic mice were previously described.35
Propagation of rat mammary cells from normal or tumor tissues. A: A photomicrograph of breast cell colonies derived from excised normal rat breast tissue. B: A photomicrograph of a colony of rat breast adenocarcinoma cells (7,12-dimethylbenz-alpha-anthracene (DMBA)-induced tumor). The tumor cultures were initiated from a single-needle biopsy specimen of a primary rat breast tumor. Cells (2 × 106) were generated in 5 days from the needle biopsy specimen.
Mammary and prostate cells propagated on feeder cells with Y-27632 express p63, a marker of basal cell differentiation. The indicated cells were plated onto glass coverslips using the described cell culture conditions. Two days after plating, the cells were fixed with 3.7% paraformaldehyde, permeabilized with saponin, stained with DAPI dye, and then reacted with antibody against p63, followed by rhodamine-labeled secondary antibody. The cultured cells reacted with anti-p63 antibodies are shown in red; mouse fibroblast feeder cells are shown as arrows. The nuclei of the feeder cells display punctate DAPI staining because of their previous irradiation.
Gene expression profiles in prostate epithelial cells. Semiquantitative RT-PCR of the following: androgen receptor (AR) (A), NK3 homeobox 1 (Nkx3.1) (B), and prostate stem cell antigen (PSCA) normalized to β-actin mRNA expression (C). Relative mRNA gene expression values were obtained using the following arithmetic formula: 2ΔCT. The Δ(CT) = CT (target gene)-CT (β-actin mRNA). Assays consisted of three technical replicates. Data are presented as mean ± SEM.
Cell cultures established from frozen human tissue. Fresh human breast tissue from a patients who underwent reduction mammoplasty was minced into small pieces, with a maximal size of 1 to 3 mm and frozen in 90% fetal calf serum/10% dimethyl sulfoxide (v/v)/5 μmol/L Y-27632, as described in Materials and Methods. Cell suspensions from the frozen tissue were thawed at 37°C, pelleted, and suspended in F medium for brief digestion with dispase-collagenase. The cell suspensions were cultured and photographed 5 days (top panel) and 8 days (bottom panel) after plating.