Abstract
Dendritic cells (DCs) use all-trans retinoic acid (ATRA) to promote characteristic intestinal responses, including Foxp3+ Treg conversion, lymphocyte gut homing molecule expression, and IgA production. How this ability to generate ATRA is conferred to DCs in vivo remains largely unstudied. Here, we observed that among DCs, retinaldehyde dehydrogenase (ALDH1), which catalyzes the conversion of retinal to ATRA, was preferentially expressed by small intestine CD103+ lamina propria (LP) DCs. Retinoids induced LP CD103+ DCs to generate ATRA via ALDH1 activity. Either biliary or dietary retinoids were required to confer ALDH activity to LP DCs in vivo. Cellular retinol-binding protein II (CRBPII), a cytosolic retinoid chaperone that directs enterocyte retinol and retinal metabolism but is redundant to maintain serum retinol, was required to confer ALDH activity to CD103+ LP DCs. CRBPII expression was restricted to small intestine epithelial cells, and ALDH activity in CRBPII−/− DCs was restored by transfer to a wild-type recipient. CD103+ LP DCs from CRBPII−/− mice had a decreased capacity to promote IgA production. Moreover, CD103+ DCs preferentially associated with the small intestine epithelium and LP CD103+ DC ALDH activity, and the ability to promote IgA production was reduced in mice with impaired DC–epithelia associations. These findings demonstrate in vivo roles for the expression of epithelial CRBPII and lumenal retinoids to imprint local gut DCs with an intestinal phenotype.
The intestinal immune system is positioned at the interface of the host with the environment, resulting in continuous exposure to an array of substances derived from food and the lumenal microbiota. In this precarious environment, the immune system must both protect from infection and avoid the untoward outcomes of overly exuberant or inappropriate responses. Dendritic cells (DCs) are critical in initiating and shaping adaptive immunity, and accordingly play a key role in properly guiding mucosal immune responses.
Through the release of the biologically active vitamin A metabolite all-trans retinoic acid (ATRA), DCs induce the expression of gut homing molecules on responding lymphocytes, promote the conversion of naive T cells into Foxp3-expressing Tregs, and promote the development of IgA-producing plasma cells.1–8 These outcomes are characteristic of homeostatic intestinal immune responses, and among DCs, CD103+ DCs from the intestine and associated lymphoid tissues have the ability to provide ATRA and are the most efficient at promoting these homeostatic outcomes.1,9,10 Understanding how this subset of DCs acquires this ATRA-releasing property has been the focus of much attention.
ATRA is unstable, and most studies indicate that DCs supplying ATRA have the capacity to generate ATRA from the stable parent compound, retinol. The conversion of retinol to ATRA occurs by a two-step process in which retinol is reversibly converted to retinal via alcohol dehydrogenases (ADHs) and subsequently irreversibly converted to ATRA via cytosolic retinaldehyde dehydrogenase family members (RALDHs, also referred to as ALDH1). Although ADHs are widely expressed by multiple cell types, ALDH1 expression is more restricted and therefore is viewed as a key component defining the ability of cell types, including DCs, to generate ATRA. In vitro studies revealed granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, and ATRA as factors inducing ALDH1 expression in DCs.11–13 In addition, ALDH1 expression is reduced in DCs from vitamin A–deficient (VAD) mice,12 further supporting the role of ATRA in this process. Observations that ALDH1 expression in these DCs can be rescued by supplying a vitamin A–sufficient diet,12,14 and in vitro studies demonstrating that co-culture of bone marrow–derived DCs (BMDCs) with epithelial cell lines induces DC ALDH1 expression,12,14,15 support an elegant model wherein lumenal vitamin A acts as an environmental cue shaping the phenotype of DCs and their attendant responses. In this model, locally elevated ATRA levels derived from epithelial cell conversion of lumenal retinoids supply a signal, inducing ALDH1 expression in DCs near the epithelium. These DCs subsequently imprint ensuing immune responses with a phenotype characteristic of intestinal homeostasis, thus responding to this intestine-specific environmental cue. Despite these supporting findings, in vivo confirmation and dissection of this model, which implies a role for lumenal retinoids, epithelial-derived ATRA, and DC–epithelial cell associations, has been difficult, in part due to the complexities of vitamin A biology limiting interpretations of the relevant retinoid source.
The essential nutrient vitamin A, or retinoids, are a group of compounds found in food of animal origin as an ester, primarily retinyl palmitate, or in foods of plant origin as a provitamin, including β-carotene.16,17 Within the intestinal lumen, dietary retinyl esters are hydrolyzed into retinol and absorbed by epithelial cells, whereas β-carotene is absorbed directly by enterocytes and subsequently converted to retinol. Within enterocytes, retinol is esterified with long chain fatty acids, packaged into chylomicrons, and transported to the liver for storage within hepatic stellate cells, which contain a several-month supply of vitamin A. In addition epithelial cells have ALDH1 activity, and can generate ATRA from retinol via retinal.18 Due to the instability of the hormonally active retinoids ATRA and 9-cis retinoic acid, retinol serves as both a parent compound and as a means to distribute highly unstable active retinoids. Thus, homeostasis is regulated at the level of serum retinol levels maintained by the release of hepatic stores, which are converted to biologically active retinoids within target tissues. In addition to the serum, hepatic retinoids are also released in the bile19,20 and in vitro studies have demonstrated that bile can induce ALDH1 expression in BMDCs and endow them with the ability to induce expression of the gut homing molecule CCR9 on responding lymphocytes.21 Therefore, at least three retinoid sources could serve to generate ATRA and induce ALDH1 expression in DCs: serum, which is present systemically, and bile and diet, which are present within the intestinal lumen. Interpreting the effects of vitamin A status manipulation on DC ALDH1 expression to gain insight into how DCs acquire this property is confounded by these multiple sources of retinoids and their coincident regulation. Thus, decreased ALDH1 expression by DCs from VAD mice does not identify the relevant retinoid source for the induction of ALDH1, because these mice have depleted hepatic stores and lack both systemic (serum) and lumenal (bile and dietary) retinoids. Likewise, observations of the rescue of DC ALDH1 expression in VAD mice with a vitamin A–sufficient diet has ambiguous interpretations, because lumenal dietary retinoids can be rapidly transferred to the systemic pool.22
We investigated the in vivo role of lumenal retinoid sources, epithelial-derived retinoids, and DC–epithelial proximity in imprinting intestinal DCs. Here, we report that lumenal retinoids from the diet or bile, as opposed to systemic retinoids, were required to imprint intestinal DCs with ALDH activity in vivo. This process was dependent on intestinal epithelial cell expression of the cytosolic retinol and retinal chaperone protein cellular retinol binding protein II (CRBPII) and was disrupted in mice in which epithelial–DC interactions were impaired. Together, these findings provide in vivo evidence for the role of epithelial-derived retinoids from lumenal sources in imprinting local gut DCs to promote homeostatic intestinal immune responses.
Materials and Methods
Mice
C57BL/6 mice and B6SJL mice, a congenic strain carrying the CD45.1 allele, were purchased from The Jackson Laboratory (Bar Harbor, ME). CRBPII−/− mice23 and LTβR−/− mice24 were bred for seven or more generations onto the C57BL/6 background before use in experiments. Animals were housed in a specific pathogen–free facility and fed a diet containing 28 IU/g vitamin A unless otherwise specified. Animals were 8 to 20 weeks of age at the time of analysis. Bone marrow transplants were performed as previously described.25 Animal procedures and protocols were performed in accordance with the institutional review board at Washington University School of Medicine.
Isolation of Cellular Populations and Flow Cytometry
Small intestines and colons were harvested, rinsed with PBS, and Peyer's patches (PP) were removed. Epithelial cells and the associated hematopoietic cellular populations were released by three consecutive 15-minute incubations in Hanks' balanced salt solution medium (BioWhittaker, Lonza, Walkersville, MD) containing 5 mmol/L EDTA at 37°C in a rotating incubator. Isolation of splenic and LP cellular populations was performed as previously described.26 Cellular suspensions were then passed through a 70-μm nylon filter, and the cells were prepared for flow cytometric analysis or sorting. Staining with 7-amino-actinomycin D (7-AAD) (eBioscience, San Diego, CA) was used to distinguish live cellular populations. Antibodies used for flow cytometry include: anti-mouse CD103 (BD Biosciences, clone M290), anti-mouse CD4 (clone GK1.5), anti-mouse CD11c (clone N418), anti-mouse CD45 (clone 30.F11), and anti-mouse MHCII (clone M5/114.15.2; all from eBioscience, except as noted). The Aldefluor assay (Stem Cell Technologies, Vancouver, BC, Canada) to detect ALDH activity was performed per the manufacturer's recommendations. The percentage of ALDH+ cells was determined by comparison with a control population treated with the ALDH inhibitor diethylaminobenzaldehyde (DEAB).
ATRA Reporter Assay
The Sil-15 cell line, an F9 teratocarcinoma cell line transfected with a β-galactosidase reporter construct driven by tandem repeats of the retinoic acid response element,27 was subcloned, and clone E5B6, which displayed optimal retinoid sensitivity was selected for use in these studies (see Supplemental Figure S1 at http://ajp.amjpathol.org). Cellular populations to be tested were isolated and cultured overnight in RPMI medium containing 10% fetal calf serum, 5 × 10−5 mol/L 2-ME, 2 mmol/L l-glutamine, 10 mmol/L HEPES, 50 U/mL penicillin, 50 μg/mL streptomycin, and 1 mmol/L sodium pyruvate at 37°C 5% CO2 in the presence or absence of 1 μmol/L retinol or 200 nmol/L ATRA (Sigma-Aldrich, St. Louis, MO). Cellular populations were washed three times in PBS, and transferred to 96-well plates containing the 1 × 105 Sil-15 subclone E5B6 cells in the presence or absence of 1 μmol/L citral (MP Biomedicals, Solon, OH), an ALDH inhibitor, and cultured at 37°C 5% CO2. Twenty-four hours later, supernatants were removed, and the presence of β-galactosidase activity was assessed using a β-Galactosidase Assay (Pierce, Rockford, IL) per the manufacturer's recommendations. Concentrations of experimental samples in equivalents of ATRA were interpolated from a standard curve constructed from known concentrations of ATRA (Sigma-Aldrich). Culture with citral did not affect the ability of Sil-15 E5B6 cells to respond to exogenous ATRA (data not shown).
In Vitro ALDH Induction
Cellular populations were isolated as above and cultured overnight at 37°C 5% CO2 in retinoid-free X-VIVO 10 medium (Lonza) containing 5 × 10−5 mol/L 2-ME, 2 mmol/L l-glutamine, 10 mmol/L HEPES, 50 U/mL penicillin, 50 μg/mL streptomycin, and 1 mmol/L sodium pyruvate at 37°C 5% CO2 in the presence of the indicated concentration of ATRA. RNA was isolated from cellular populations and used for real-time quantitative PCR as described below.
Immunohistochemistry
Immunohistochemistry on frozen sections was performed as previously described.28 Antibodies used for immunohistochemistry include: anti-CD11c (clone HL3, BD Bioscience), anti-CD103 (clone M290, BD Bioscience), anti-CD3ε (clone 145-2C.11, eBioscience), and anti-CD49f (clone GoH3, eBioscience). Pseudo-colored black and white images were obtained with an Axioskop 2 microscope using Axiovision software (Carl Zeiss MicroImaging, Thornwood, NY). To enumerate the DCs associating with the epithelium, or epithelial-associated DCs, mid jejunal sections of intestine from two or more LTβR−/− or wild-type mice were stained for CD11c, CD3ε, and CD49f. Ten or more random photographs of villus cross sections were obtained, and the number of DCs (CD11c+ CD3ε− cells) lying above or within the basement membrane, as determined by CD49f staining, per villus were counted.
In Vitro IgA Induction Assay
Flow cytometrically sorted LP DC subtypes were co-cultured at a 1:1 ratio with flow cytometrically sorted splenic CD19+ IgM+ B cells in RPMI medium containing 10% fetal calf serum, 5 × 10−5 mol/L 2-ME, 2 mmol/L l-glutamine, 10 mmol/L HEPES, 50 U/mL penicillin, 50 μg/mL streptomycin, and 1 mmol/L sodium pyruvate at 37°C 5% CO2 in the presence of 5 μg/mL anti-CD40 (Axxora, San Diego, CA) and 100 nmol/L ATRA and 10 ng/mL IL-6 where indicated. After 6 days of culture, the number of IgA-producing cells and the concentration of IgA were measured by enzyme-linked immunosorbent spot and enzyme-linked immunosorbent assay (ELISA) as previously described.29
IL-6 ELISA
Flow cytometrically sorted CD103+ LP DCs from C57BL/6 and LTβR−/− mice were cultured in the above medium and stimulated with 5 μg/mL anti-CD40 antibody for 72 hours. Following stimulation, IL-6 was measured in culture supernatants using an IL-6 ELISA (R and D Systems, Minneapolis, MN) per the manufacturer's recommendations.
Quantitative Real-Time PCR Assay
RNA was extracted from cellular populations isolated as above, treated with DNase, and transcribed into cDNA using Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Primers used for Real-time-PCR include: 18S forward 5′-CGGCTACCACATCCAAGGAA-3′ and reverse 5′-GCTGGAATTACCGCGGCT-3′, ALDH1A1 forward 5′-GGGAAAGAGCCCTTGCATTGTGTT-3′ and reverse 5′-GCGACACAACATTGGCCTTGATGA-3′, ALDH1A2 forward 5′-ACCGTGTTCTCCAACGTCACTGAT-3′ and reverse 5′-TGCATTGCGGAGGATACCATG AGA-3′, ALDH1A3 forward 5′-TCAACAAGATAGCCTTCACCGGCT-3′ and reverse 5′-TTGAAGAACACTCCCTGGTGAGCA-3′, and CRBPII forward 5′-GGGTGGAGTTTGACGAACACACAA-3′ and reverse 5′-TTTGAACACTTGTCGGCACACCTG-3′. The absolute copy number of the target was calculated from standards that were constructed as previously described.30
Bile Duct Ligation
Mice were transferred to cages containing vitamin A–free bedding and placed on diet AIN-93M (Purina Test Diet, Greenfield, IN) containing 0.0 IU/g (VAD diet) or 28 IU/g (control diet) vitamin A. Seven days later, mice received either a sham surgery or ligation of the common bile duct proximal to the union with the pancreatic duct. Forty-eight hours after surgery, LP DC populations were analyzed by flow cytometry as described above.
Statistical Analysis
Data analysis using Student's t-test or a one-way analysis of variance was performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
ALDH Activity Is Enriched in the Small Intestine LP and Is Largely Restricted to CD103+ DCs
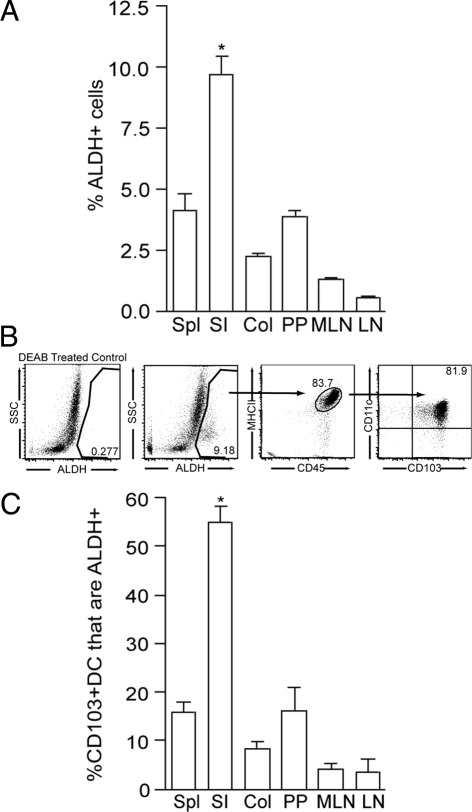
Intestinal DCs, including those from the PP, mesenteric lymph node (MLN), and LP express ALDH1.1,4,12 To gain insight into the environment within the intestine where ALDH1 expression is the greatest and therefore the location in which ALDH may be induced, we evaluated ALDH activity using the Aldefluor assay11,12,21,31 (Stem Cell Technologies, Vancouver, BC, Canada) in total cell populations and DCs from small intestine immune compartments, colon, spleen, and peripheral lymph node. We found that the small intestine LP had the largest population of cells with ALDH activity and that ALDH activity was progressively less in the PP, MLN, and peripheral lymph node, which correlates with the distance of these cell populations from the small intestine epithelium (Figure 1A). Furthermore, we observed that the majority of the cell population with ALDH activity in the small intestine LP was CD45+, CD11c+, MHCII+, and CD103+, corresponding to the CD103+ DC population (Figure 1B). In addition, we observed that the percentage of CD103+ DCs with ALDH activity was largest in the small intestine LP (Figure 1C), indicating that the differences in the ALDH+ populations between the examined compartments was not solely due to differences in the size of the CD103+ LP DC populations within each compartment.
Figure 1.
ALDH activity is enriched in the small intestine LP and is largely restricted to CD103+ DCs. To assess which compartment contained the largest population of cells with the ability to generate ATRA, and by extension, where this ATRA generating capacity may be imprinted, cellular populations were isolated from intestinal and systemic compartments and evaluated for the presence of ALDH activity using the flow cytometric based Aldefluor assay. A: The small intestine LP contained a substantially larger population of ALDH+ cells when compared with all other intestinal and non-intestinal compartments. B: Analysis of the LP ALDH+ cells revealed that the majority of this population was CD45+ MHCII+ CD11c+ CD103+, consistent with CD103+ DCs. LP cellular populations treated with DEAB, an ALDH inhibitor served as a control to set the gate for positive staining. C: Among CD103+ DCs, those from the small intestine LP were significantly more likely to be ALDH+, indicating the differences in the ALDH+ populations from each tissue are not solely due to differences in the size of the CD103+ DC populations. Col, colonic LP; LN, peripheral lymph node; MLN, mesenteric lymph node; PP, Peyer's patch; SI, small intestine LP; Spl, spleen. n = 2 in A and C. Data in B are representative of 1 of 8 experiments. *P < 0.05.
CD103+ LP DCs Express ALDH1A2 and Generate ATRA in an ALDH-Dependent Manner in Response to Retinoids
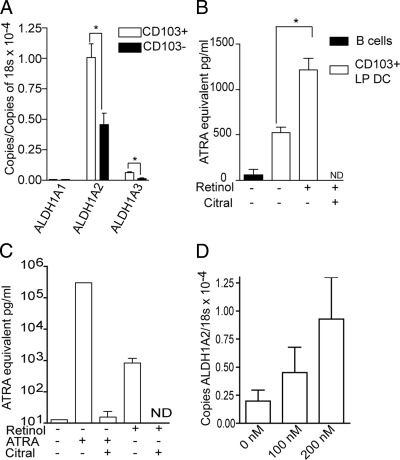
Of the multiple mammalian ALDH family members, three murine members, ALDH1A1, ALDH1A2, and ALDH1A3, can efficiently use retinal as a substrate, and are therefore indicators of the ability to generate ATRA.32–34 Antigen-presenting cells from the intestine have been shown to variously express these ALDH1 isoforms, with PP DCs expressing ALDH1A1,2 MLN DCs and LP CD11chi CD11bhi LP DCs expressing ALDH1A2,2,7,12 and LP macrophages expressing both ALDH1A1 and ALDH1A2.35 To evaluate which of the retinal-converting ALDH isoforms CD103+ LP DCs express, we performed real-time quantitative PCR on RNA from sorted small intestine LP DCs. Small intestine LP DCs predominantly expressed the ALDH1A2 family member, and consistent with the assays of ALDH activity, this expression was greatest in CD103+ DCs (Figure 2A). Bulk populations of MLN DCs have an augmented ability to convert retinol into ATRA when compared with splenic DCs.2 To assess the ability of small intestine LP CD103+ DCs to generate ATRA, we evaluated the presence of ATRA in cultures of sorted LP CD103+ DC populations using a biological reporter assay.27 Consistent with the presence of ALDH activity, we observed that CD103+ LP DCs generate ATRA, and that ATRA generation was enhanced when the CD103+ DCs were cultured with retinol, the upstream parent compound of ATRA, and inhibited by the ALDH inhibitor citral (Figure 2B). These findings both directly demonstrate the ability of LP CD103+ DCs to generate ATRA and indicate that this capacity may be augmented by retinol or, more likely, ATRA itself, because CD103+ DCs can convert retinol to ATRA via ALDH1. To evaluate these possibilities, LP cellular populations were pretreated with retinol or ATRA, washed extensively, and evaluated for ATRA generation in the presence or absence of the ALDH inhibitor citral. Treatment with retinol or ATRA significantly increased the generation of ATRA, and this was inhibited in both conditions by citral (Figure 2C). However, treatment with ATRA resulted in the generation of much more ATRA than treatment with retinol. The difference between the ATRA-treated and ATRA- and citral-treated cultures was >100 ng/mL, whereas in comparison, the difference between retinol-treated and retinol- and citral-treated cultures was ∼1 ng/mL, indicating that ATRA treatment greatly augmented the ability to convert retinol to ATRA via ALDH activity. In vitro culture with ATRA augmented ALDH1A2 mRNA expression (Figure 2D), indicating that inducing ALDH1A2 at the transcriptional level is one way in which ATRA augments its own production. Therefore, in LP DCs, ATRA is both an end product and a stimulus for its own generation.
Figure 2.
LP CD103+ DCs express ALDH1A2 and generate ATRA in response to exogenous retinoids. The murine ALDH family members ALDH1A1, ALDH1A2, and ALDH1A3 have the capacity to convert retinal to ATRA. To evaluate whether LP DCs express ALDH1 and which ALDH1 family members they express, RNA was isolated from CD103+ and CD103− sorted LP DCs, and the expression of ALDH1 family members was measured by quantitative real-time PCR. A: CD103+ LP DCs expressed ALDH1A2 and to a lesser extent ALDH1A3, whereas CD103− LP DCs expressed ALDH1A2 and ALDH1A3 at lower levels than CD103+ LP DCs. Expression of ALDH1A1 was low in both CD103+ and CD103− LP DCs. B: To confirm the ability of CD103+ DCs to release ATRA, 2 × 105 sorted CD103+ LP DCs were assayed for ATRA release using a biological reporter assay as described in Materials and Methods. Splenic B cells, which are not known to produce ATRA, were used as a negative control. CD103+ LP DCs generated biologically active ATRA, and the ATRA-generating capacity was enhanced by pretreatment with 1 μmol/L retinol, and inhibited by the ALDH inhibitor citral, indicating that retinoids induced the generation of ATRA via ALDH activity. C: To evaluate which retinoid, retinol or its downstream metabolite ATRA, effectively induced ATRA release, 5 × 105 LP cells were pretreated with retinoids and evaluated for ATRA generation in the presence or absence of citral using a biological reporter assay. Pretreatment with ATRA resulted in a dramatic increase in ATRA release that was >99.9% suppressed by ALDH inhibition, whereas pretreatment with retinol resulted in a significant but less dramatic increase in ATRA release that was suppressed by ALDH inhibition. D: Overnight culture of LP cells in increasing doses of ATRA demonstrated that ATRA induced ALDH1A2 mRNA expression, thus indicating that ATRA induces its own generation via the induction of ALDH1A2 expression. *P < 0.05. Data are representative of one of two experiments.
Lumenal Retinoids from Bile or Diet Imprint LP DCs with ALDH Activity
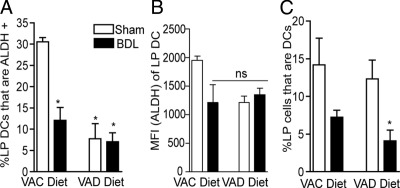
Multiple cellular populations throughout the body have the ability to convert retinol to ATRA, including intestinal epithelial cells and stromal cells in the peripheral lymph nodes.12,18,36 Systemic vitamin A homeostasis is regulated at the level of serum retinol, which is released from hepatic stores. In the adult mouse, hepatic vitamin A stores are sufficient to maintain serum retinol levels for months in the absence of ingestion of vitamin A from the diet. Although compartments throughout the body are bathed in retinol derived from serum, only cellular populations located in the mucosa would be exposed to the potentially elevated levels of retinoids from lumenal sources, which include the diet and bile. To evaluate whether diet and/or bile are the relevant source of retinoids for the induction of ALDH activity in LP DCs in vivo, we placed adult mice on a vitamin A–deficient diet or a control diet and performed a sham operation or ligation of the common bile duct to interrupt the flow of bile. Mice were evaluated 48 hours after surgery for the presence of LP DCs with ALDH activity. In the absence of bile and/or dietary vitamin A, there was a significant decrease in the ALDH+ LP DC population (Figure 3A). There was a trend toward a decrease in LP DC ALDH activity in all of the treatment groups, as measured by the mean fluorescence intensity of ALDH staining (Figure 3B). BDL resulted in a trend toward a decrease in the LP DC population, and this decrease became significant when both bile and dietary vitamin A were removed (Figure 3C). Although dietary retinoids were slightly more effective at inducing ALDH activity in LP DCs, these findings indicate that lumenal retinoids from bile or diet play a role in imprinting gut DCs in vivo, and suggest a role for bile and dietary vitamin A in maintaining the LP DC population.
Figure 3.
Lumenal retinoids from bile or diet imprint LP DCs with ALDH activity. To evaluate the relevant source of retinoids imprinting LP DCs with ALDH activity, we evaluated LP DC ALDH activity in mice receiving a sham surgery or common bile duct ligation (BDL) on vitamin A deficient (VAD) diets or control diets. A: Mice on the control diet receiving BDL, mice on a VAD diet receiving a sham surgery, and mice on a VAD diet receiving BDL had a significant decrease in the population of ALDH + LP DCs. B: Removal of either biliary and/or dietary retinoids resulted in a trend toward decreased LP DC ALDH activity, as determined by the mean fluorescence intensity. C: Removal of lumenal bile resulted in trend toward a decrease in the LP DC population, which became significant when both bile and dietary vitamin A were removed. *P < 0.05. ns = not significant. n = 3 or more mice in each group for A and C, n = 2 mice in each group for B. Data are representative of one of two experiments. VAC, Vitamin A control.
Epithelial Cellular Retinol Binding Protein II Plays a Critical Role in Imprinting CD103+ LP DCs with ALDH Activity
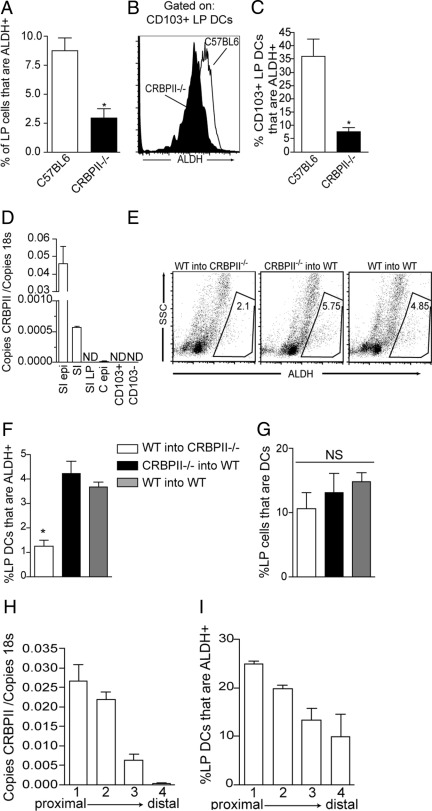
Due to its hydrophobicity, intracellular retinol is bound to carrier proteins, or cellular retinol binding proteins, within the aqueous environment of the cytosol. In the adult small intestine, CRBPII is expressed by absorptive villous enterocytes, but not by gut lymphoid tissue epithelium,37 and acts as an intracellular chaperone directing the metabolism of its ligands, retinol and retinal. In vitro studies of CRBPII overexpression in epithelial cell lines demonstrate that CRBPII facilitates retinol uptake.38 However, in vivo studies of CRBPII−/− mice on a vitamin A–enriched diet have shown that this role is redundant for the maintenance of normal serum retinol levels.23 Therefore, although CRBPII is not necessary to maintain serum retinol concentrations when vitamin A is plentiful in the diet, its chaperone function to direct the metabolism of retinol and retinal within the cytosol of villous epithelial cells could increase local retinoid concentrations and play a role imprinting local gut DCs. To evaluate the role of CRBPII in imprinting LP DCs, we examined the ALDH activity of LP cells and LP DCs isolated from CRBPII−/− mice. We observed that ALDH activity was decreased in LP cells from CRBPII−/− mice (Figure 4A) and that this correlated with decreased ALDH activity in the CD103+ LP DCs and a decrease in the population of ALDH+ CD103+ LP DCs (Figure 4, B and C). In addition to the small intestine epithelial cells, CRBPII expression has been found in placental tissues and fetal liver23,39; however, its expression in DCs has not been evaluated. Consistent with prior reports, we observed that CRBPII was highly expressed by small intestine epithelial cells and was undetectable in the small intestine LP and CD103+ and CD103− LP DCs (Figure 4D). We observed low levels of CRBPII expression in colonic epithelial cells (Figure 4D), consistent with earlier reports of inducible CRBPII expression in a colonic epithelial cell line.40 To further confirm the role of nonhematopoietic sources of CRBPII in imprinting LP DCs, we performed bone marrow transfers of wild-type or CRBPII−/− bone marrow into wild-type or CRBPII−/− recipients using congenic CD45.1 or CD45.2 markers to identify donor-derived populations. Consistent with a role for nonhematopoietic-, or epithelial-, derived CRPBII in imprinting ALDH activity, we observed that ALDH activity was significantly decreased in CRBPII−/− recipients of wild-type bone marrow, but not in wild-type recipients of CRBPII−/− bone marrow (Figure 4, E and F). We did not observe a difference in the LP DC populations between any of the groups (Figure 4G). In the rat intestine, CRBPII was found to be expressed in a proximal-to-distal gradient.37 We hypothesize that epithelial CRBPII plays a critical role in imprinting ALDH activity in intestinal DCs by providing a local elevation in ATRA, and accordingly, we investigated the correlation between the gradient of CRBPII expression and ALDH activity by LP DCs. Consistent with earlier reports of a proximal-to-distal gradient of CRBPII expression in the rat intestine, we observed that CRBPII was expressed in a proximal-to-distal gradient in the murine intestine and that this expression correlated with the ALDH activity in LP DCs (Figure 4, H and I).
Figure 4.
Epithelial CRBPII plays a critical role in imprinting CD103+ LP DCs with ALDH activity. A–C: To evaluate a role for CRBPII in imprinting LP DCs, LP cellular populations were isolated from wild-type and CRBPII−/− mice and evaluated for ALDH activity. CRBPII−/− mice had a decrease in the population of ALDH+ LP cells (A), which correlated with a decreased level of ALDH expression in CD103+ LP DCs from CRBPII−/− mice and with a decrease in the population of CD103+ LP DCs that are ALDH + (B and C). D: Quantitative real-time polymerase chain reaction was used to define the cellular population expressing CRBPII. CRBPII was highly expressed by small intestine epithelium, was expressed at low levels in the colonic epithelium, and was undetectable in the small intestine LP and CD103+ and CD103− LP DCs. E andF: Bone marrow transfers confirmed the requirement for CRBPII on nonhematopoietic cells for the induction of ALDH activity on LP DCs, as transfer of wild-type bone marrow into CRBPII−/− mice resulted in a significant reduction in the population of ALDH+ LP DCs, whereas conversely, transfer of CRBPII−/− bone marrow into wild-type recipients resulted in an ALDH+ LP DC population that was equivalent to the transfer of wild-type bone marrow into wild-type recipients. G: There was no significant difference in the size of the LP DC population between the groups of bone marrow recipients. H and I: Evaluation of the distribution of epithelial CRBPII expression and LP DC ALDH activity in four equivalent sections of small intestine revealed a correlating proximal to distal gradient of CRBPII expression and ALDH activity in LP DCs. C epi, colon epithelial cells; NS, not significant; SI, full-thickness small intestine; SI epi, small intestine epithelial cells; SI LP, small intestine LP. *P < 0.05. n = 4 for data in A–C, H, and I, n = 3 for data in E–G; data are representative of one of two experiments in D.
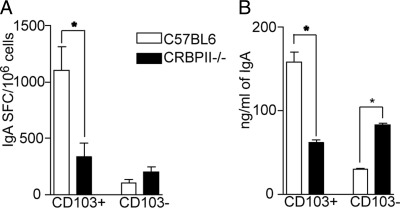
CD103+ LP DCs from CRBPII−/− Mice Have an Impaired Ability to Facilitate IgA Production
Intestinal DCs facilitate the generation of IgA-producing plasma cells, and this process is mediated in part by ATRA.4,5,7 Using an in vitro co-culture model, we evaluated the ability of LP DCs from CRBPII−/− mice to facilitate the generation of IgA-producing plasma cells from naive B cells. Consistent with their elevated ALDH activity, we found that wild-type CD103+ LP DCs were more efficient at inducing IgA production when compared with their CD103− LP DC counterparts (Figure 5, A and B). Furthermore, consistent with the decreased ALDH activity seen in CD103+ LP DCs from CRBPII−/− mice, we observed that these DCs had an impaired ability to facilitate IgA production from naive B cells (Figure 5, A and B). Interestingly, CD103− LP DCs from CRBPII−/− mice had an increased capacity to support IgA production when compared with their wild-type counterparts; however, this remained well below the capacity of wild-type CD103+ LP DCs to support IgA production (Figure 5, A and B).
Figure 5.
CD103+ LP DCs from CRBPII−/− mice have an impaired ability to facilitate IgA production. To assess for a defect in CD103+ LP DCs from CRBPII−/− mice related to their reduced ALDH activity, CD103+ and CD103− LP DCs from CRBPII−/− mice and wild-type mice were isolated and cultured with naive B cells and evaluated for the development of IgA-producing plasma cells by enzyme-linked immunosorbent spot (A) and IgA production by ELISA (B) as described in Materials and Methods. CD103+ LP DCs from wild-type mice had an augmented capacity to facilitate IgA production, whereas CD103+ LP DCs from CRBPII−/− mice were less effective at facilitating IgA production and were comparable to the CD103− population. *P < 0.05. Data are representative of one of two experiments.
LP DCs from Mice with Impaired Epithelial Cell–DC Associations Have Reduced ALDH Activity
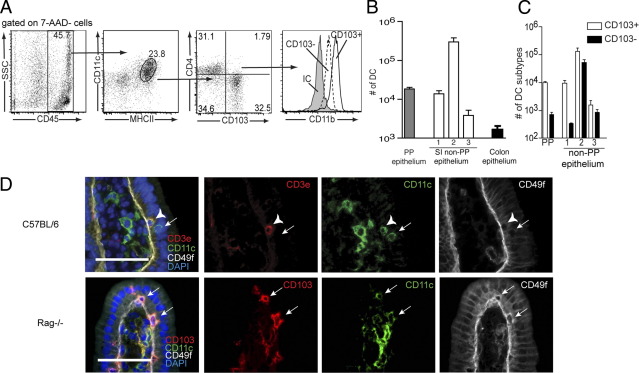
The above findings implicate an important role for local or mucosal/epithelial-derived retinoids in imprinting ALDH activity in LP DCs. The basement membrane of the villous epithelium is invested with pores through which phagocytic cells protrude.41,42 Furthermore, immunohistochemical studies have demonstrated the presence of dendritic cells closely associated with intestinal epithelial cells in the small intestine,43,44 and that DCs are released with the isolation of small intestine epithelial cells.45,46 To better assess the characteristics of the DCs released with the small intestine epithelial cells, and therefore the DCs that might be imprinted by epithelial cells, we compared the populations of cells released with the non–PP-bearing small intestine. Consistent with prior observations, we found that CD45+ CD11c+ MHCII+ cells, or DCs, were present in the released epithelial cell populations (Figure 6A).45,46 This DC population could be divided into a CD103+ and CD103− population, which had low expression of CD4 (Figure 6A). Both the CD103+ and CD103− population expressed CD11b, consistent with the phenotype of the majority of LP DCs (Figure 6A).45,47 Intraepithelial T lymphocytes can also express CD11c.48 By immunohistochemistry, we observed that DCs (CD11c+ CD3ε−) associated with the epithelium above or within the basement membrane border (Figure 6B). Furthermore, evaluating intestines from Rag−/− mice, which lack T lymphocytes and simplify the immunohistochemical staining strategy, we observed that some of these DCs were CD103+, consistent with the ALDH-expressing population (Figure 6B). The number of DCs released with the epithelium was greatest with the second fraction of isolated epithelium and outnumbered all of the DCs released with the PP follicle-associated epithelium by 10-fold (Figure 6C). We observed that the colonic epithelium contained very few DCs (Figure 6C). Consistent with prior observations, the EDTA treatments did not disrupt the villous architecture49,50 (see Supplemental Figure S2 at http://ajp.amjpathol.org). The CD103+ DCs were released more easily by the EDTA treatments as evidenced by their prominence in the first EDTA treatment, suggesting they may be more closely associated with the small intestine epithelium (Figure 6D). These observations demonstrate that DCs associating with the small intestine epithelium can have the same phenotype as the DCs imprinted with ALDH activity.
Figure 6.
CD103+ DCs associate with the small intestine epithelium. Flow cytometry and immunohistochemistry were used to evaluate the phenotype of the DCs associating with the epithelial compartment. A: Gating on the live (7AAD−) cellular population released with epithelial cells by treatment with EDTA revealed the presence of CD45+ CD11c+ MHCII+ cells that were predominantly CD103+ or CD103− CD4lo. Both the CD103+ and CD103− DCs isolated with the epithelium were CD11b+, consistent with the cell surface phenotype of the majority of LP DCs. B: Enumeration of the DCs isolated from the Peyer's patch–free intestine by successive EDTA incubations revealed the majority of DCs were isolated in the second EDTA incubation, and that the number of DCs isolated with the third incubation was dramatically decreased (∼100-fold). By comparison, the number of DCs released from the Peyer's patch follicle-associated epithelium in all three incubations combined was 10-fold less, and the number of DCs released from the colonic epithelium was >100-fold less, than that released from the non-Peyer's patch–bearing small intestine epithelium. C: CD103+ DCs were significantly enriched in the population isolated with the first incubation. D: Immunohistochemistry on frozen sections from intestines of wild-type mice confirmed the presence of DCs (CD11c+ CD3ε−) cells lying within or above the basement membrane as identified by CD49f staining, an integrin expressed on the basolateral surface of the small intestine epithelial cells. To simplify the immunohistochemical staining strategy, staining was performed on intestines from Rag−/− mice, lacking T cells. Consistent with the flow cytometry findings, immunohistochemistry revealed that some of the DCs observed to be lying above the small intestine basement membrane were CD103+. IC, isotype control. Arrows denote DCs as determined by the presence of CD11c staining and lack of CD3ε in wild-type mice or the CD11c staining in Rag−/− mice. Arrowheads denote a CD11c+ intraepithelial (CD3ε+) T lymphocyte in wild-type mice. 1 = first EDTA incubation, 2 = second EDTA incubation, 3 = third EDTA incubation, *P < 0.05. Data in A are representative of one of six experiments. n = 3 for data in B and C. Scale bars in D: 50 μm.
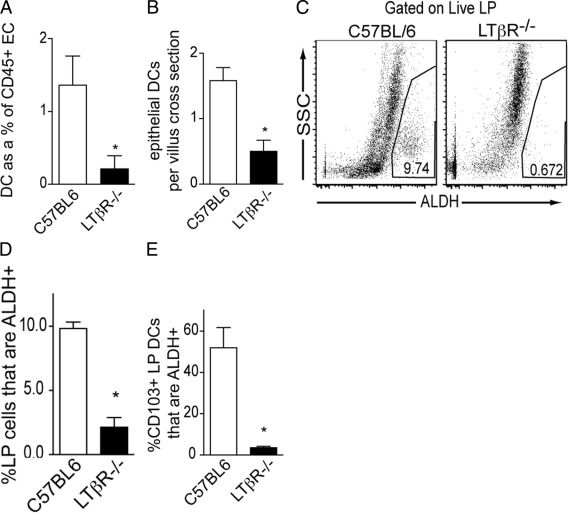
The lymphotoxin β receptor (LTβR) is a tumor necrosis receptor superfamily member whose ligation on nonhematopoietic cells results in the production of multiple chemokines.51 The LTβR is expressed on intestinal epithelial cells,52 and epithelial expression of the LTβR plays an important role in recruiting leukocytes to the intestine.53 We evaluated a role for the LTβR in recruiting DCs to associate with the epithelium by assessing the population of DCs released with the isolation of intestinal epithelial cells from LTβR−/− mice. We found that LTβR−/− mice had a diminished population of DCs released with the epithelium (Figure 7A). Using immunohistochemistry to assess the population of DCs (CD11c+ CD3ε−) lying above the basement membrane, demarcated by CD49f staining, we observed a similar decrease in the DC population in the intestines of LTβR−/− mice (Figure 7B). We also observed a corresponding decrease in the population of LP cells with ALDH activity and accordingly a decrease in the percentage of CD103+ LP DCs with ALDH activity in the intestines of LTβR−/− mice (Figure 7, C–E).
Figure 7.
Epithelial–DC associations are disrupted in the absence of the LTβR, and ALDH activity is diminished in LP CD103+ DCs from LTβR−/− mice. The epithelial compartment from wild-type and LTβR−/− mice was isolated as described in Materials and Methods and evaluated by flow cytometry for the presence of DCs. A: Flow cytometry revealed that LTβR−/− mice had a significantly decreased population of DCs released with the epithelium. B: Immunohistochemical staining revealed a similar decrease in the epithelial DC population defined as CD11c+CD3ε− cells lying above the CD49f+ small intestine basement membrane.C–E: The decreased population of DCs associated with the epithelium correlated with a decreased population of ALDH+ LP cells (C and D) and a decrease in the percentage of CD103+ LP DCs that were ALDH+ (E). EC, epithelial compartment. *P < 0.05. n = 4 mice in each group for data in A, and C–E. Data in B are representative of one of two replicates performed as described in Materials and Methods.
CD103+ LP DCs from LTβR−/− Mice Have an Impaired Ability to Facilitate IgA Production
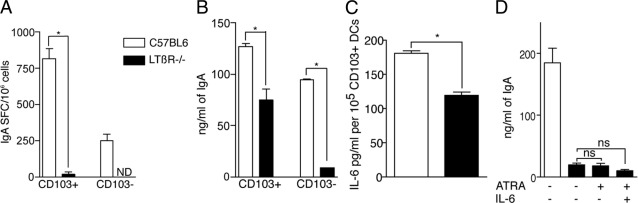
ATRA produced by dendritic cells plays a role promoting the development of IgA-producing plasma cells.5 To evaluate the ability of LP DCs from LTβR−/− mice, which have decreased ALDH activity, to facilitate the production of IgA, we cultured sorted CD103+ LP DCs with naive B cells and evaluated the development of IgA-producing plasma cells and IgA production as described above. We observed that, consistent with their decreased ALDH activity, CD103+ LP DCs from LTβR−/− mice had a diminished capacity to promote the development of IgA-producing plasma cells, and IgA production (Figure 8, A and B). IL-6 production by intestinal DCs has been shown to promote IgA production, and the addition of exogenous IL-6 and ATRA to co-cultures of nonintestinal DCs and B cells has been shown to facilitate IgA production equivalent to that seen in co-cultures with intestinal DCs.5,54,55 We observed that LTβR−/− LP DCs were deficient in IL-6 production (Figure 8C) and that the addition of exogenous ATRA and IL-6 could not restore their ability to promote IgA production (Figure 8D). These observations indicate that LP DCs from LTβR−/− mice have defects related to an intestinal phenotype, ALDH activity, as well as other defects that cannot be rescued by exogenous IL-6.
Figure 8.
CD103+ DCs from the LP of LTβR−/− mice have an impaired ability to facilitate IgA production that cannot be rescued by exogenous ATRA and IL-6. To evaluate the ability of CD103+ DCs from LTβR−/− mice to facilitate IgA production, CD103+ and CD103− LP DCs from wild-type and LTβR−/− mice were isolated and cultured with naïve B cells as described in Materials and Methods. CD103+ DCs from LTβR−/− mice displayed a diminished ability to facilitate the development of IgA-producing plasma cells (A) and IgA production (B). C: CD103+ LP DCs from LTβR−/− mice had diminished production of IL-6. D: The addition of 100 nmol/L ATRA or 100 nmol/L ATRA and 10 ng/mL IL-6 did not rescue the ability of CD103+ LP DCs from LTβR−/− mice to promote IgA production, suggesting additional defects in this DC population. *P < 0.05. Data are representative of one of two experiments.
Discussion
The intestinal immune system uses a variety of strategies to guard against infection and avoid overly exuberant immune responses, including the production of IgA antibodies to prevent lumenal organisms from breaching the epithelial barrier,56 the development of Foxp3+ regulatory T cells to control immune responses,57 and the selective homing of responding lymphocytes to the mucosal surfaces to localize appropriate immune responses.58,59 Recent work has shown that DCs play a role in generating each of these characteristic, or “homeostatic,” intestinal immune responses, in part through the generation of the biologically active vitamin A metabolite ATRA,1–8 and that this property is enhanced in a subset of DCs from the intestine.5,6,9,59 Consequently, understanding how this ATRA-generating capacity is bestowed on a subset of DCs has been the focal point of multiple studies.
Vitamin A is an essential nutrient supplied through the diet, and following the above discoveries of the effects of DC-derived ATRA in guiding immune responses, it has been intriguing to postulate that sources of vitamin A specific to the intestine act as a cue to “inform” DCs of their surroundings and direct their outcomes. However, investigating this model in vivo has been complicated because the biologically active vitamin A metabolites are unstable, and systemic vitamin A levels are regulated by the release of hepatic stores to maintain serum retinol levels, to which all DCs are exposed.
ATRA is generated from the stable parent compound retinol at target sites by the sequential actions of ADHs, reversibly converting retinol to retinal, and ALDH1 irreversibly converting retinal to ATRA. Because ALDH is expressed more selectively, its expression and activity are viewed as a key phenotypic component of cellular populations having the ability to generate ATRA. In accordance with this, studies have shown that intestinal DCs can express ALDH1 family members,2,7,12,35 and that the above intestinal-type responses mediated by DCs can be inhibited by ALDH inhibitors or retinoic acid receptor antagonists.1,2,4 In contrast to this, it has also been reported that in vitro–derived DCs can act as sources of biologically active ATRA for short periods in the presence of an ALDH inhibitor.60
Studies investigating how DCs acquire ATRA-generating capacity have largely followed two approaches that include using in vitro models to induce ALDH expression and activity in BMDCs or the manipulation of vitamin A status through the generation of VAD mice. In vitro studies identified GM-CSF, IL-4, and ATRA as factors inducing ALDH expression.11–13 Studies also support toll-like receptor (TLR) activation enhancing ALDH expression by DCs11,61,62; however, this may not be a strict requirement because ALDH activity was maintained in MLN DCs in which the TLR signaling pathways were disrupted.12 Co-culture of BMDCs with an intestinal epithelial cell line induced the expression of CD103 on the DCs and endowed the DCs with an increased capacity to generate Foxp3+ T-regulatory cells and control colitis; this BMDC transformation was mediated in part by ATRA.14 Additionally, in vivo studies of VAD mice support the role of retinoids inducing ALDH expression by DCs, because MLN DCs from VAD mice have decreased ALDH activity and ALDH1A2 expression, which could be reversed by resuming a vitamin A–containing diet.12,13,21 However, in the VAD animal, dietary-derived retinoids bypass esterification and storage in the hepatic pool and are rapidly shunted to the systemic pool to maintain vitamin A homeostasis.22 Therefore, although supplementing VAD mice with dietary vitamin A reversed the defect in ALDH expression by intestinal DCs, consistent with a role for vitamin A inducing ALDH expression,12 interpreting this observation to support a role for lumenal retinoids as opposed to systemic sources is equivocal. Interpreting the above findings may be further complicated by the narrow time window and dose effect with which ATRA can induce ALDH activity in DCs,13 such that in vitro models may not reflect events in vivo.
Other observations suggest bile as a source of lumenal, or local, retinoids inducing ALDH activity in intestinal DCs.21 The concentration of retinol in bile is up to three times that of serum.21 Related to this, bile can induce ALDH activity in BMDCs, and intestinal DCs from mice lacking dietary vitamin A for a short-term, 4 weeks, retained ALDH activity.21 Although these observations are consistent with the possibility that biliary retinoids imprint ALDH activity in intestinal DCs, they do not rule out contributions from serum-derived retinoids, which would be unaffected in mice consuming a diet deficient in vitamin A for the short term, nor do they account for the estimated 10-fold dilution of bile by intestinal secretions and diet that could significantly lower the effective concentration of biliary-derived lumenal retinoids.
Here, we have evaluated the in vivo requirements for imprinting intestinal DCs with a “homeostatic” mucosal phenotype. Prior studies have shown that DCs from the PP, MLN, and LP displayed ALDH activity and expressed ALDH1 family members; however, a systematic assessment of which compartment had the largest populations of DCs with ALDH activity within the same animals was relatively uninvestigated. We observed that LP CD103+ DCs displayed the highest ALDH activity and expressed predominantly ALDH1A2. Moreover, we observed that these DCs could generate biologically active ATRA, and consistent with in vitro studies of BMDCs, we observed that this capacity was enhanced by retinol, reduced by ALDH inhibition, and markedly induced by ATRA itself. Given that ATRA is inherently unstable, the above observations indicate that DCs receiving this signal would need to be in close approximation to the ATRA source. To determine the relative contributions of systemic (serum) and lumenal (diet and bile) retinoid sources, we manipulated lumenal retinoid sources for short periods of time and evaluated the effects on LP DC ALDH activity. We observed that both dietary and biliary retinoid sources could imprint LP DCs, with dietary sources being somewhat more effective. Interestingly, there were differences between loss of bile or dietary vitamin A on LP DCs, with loss of bile more dramatically affecting the size of the total LP DC population, and loss of dietary vitamin A having a slightly greater effect on DC ALDH activity. This could be explained in part by differences in the duration of each manipulation, because mice were on a VAD diet for 7 days and had disruption of bile for 2 days. This could also suggest that bile contains biologically active substances not present in the diet that play a role in recruiting or maintaining the LP DC population. In conjunction with our above observations, these findings are indicative of a local intestinal site where DCs become imprinted by lumenal retinoids to confer homeostatic mucosal properties.
CRBPII is highly and selectively expressed by small intestine villous epithelial cells, and serves as a chaperone directing the metabolism of its ligands, retinol and retinal.37,63 In vitro studies demonstrated that CRBPII facilitates retinol uptake and processing by epithelial cells, and the inference was that CRBPII played an important role in total body vitamin A homeostasis by facilitating the uptake and processing of dietary retinoids.40 Supporting this assumption, jejunal segments from CRBPII−/− mice had a 50% reduction in retinol uptake at low retinol concentrations. However, at higher retinol concentrations, there was no difference in retinol uptake between wild-type and CRBPII−/− intestines.23 CRBPII−/− mice had no overt phenotype with respect to growth, development, or the maintenance of normal serum retinol levels while on a vitamin A–enriched diet containing 25 IU retinyl ester/g,23 further suggesting a redundancy for CRBPII's role in systemic retinol homeostasis when dietary vitamin A is plentiful. Although hepatic retinoid stores were reduced by ∼40% in CRBPII−/− mice, the significance of this was unclear because hepatic retinoid stores were far from depleted, and serum retinol levels were maintained.23
Building on the above observations, we evaluated the LP DC population in CRBPII−/− mice ingesting a vitamin A–enriched diet (28 IU/g), which allows us to assess potential effects due to the loss of epithelial retinoid uptake and/or the loss of appropriate chaperoning of retinal and retinol within epithelial cells for downstream metabolism, while maintaining normal serum retinol levels. LP DCs from CRBPII−/− mice had reduced ALDH activity. In wild-type mice, CRBPII expression was restricted to the epithelium of the small intestine, and importantly, LP DCs did not express CRBPII. The defect in ALDH activity in LP DCs from CRBPII−/− mice could be rescued when they were transferred to a CRBPII-sufficient recipient. When wild-type LP DCs were transferred to a CRBPII−/− recipient, they displayed reduced ALDH activity. Moreover, we observed a proximal-to-distal gradient of CRBPII expression and a proximal-to-distal gradient of LP DC ALDH activity, which was also recently reported by others,62 further strengthening the association of epithelial CRBPII in the induction of LP DC ALDH activity. Consistent with prior reports of ATRA generated by DCs facilitating IgA production,5 we found that CD103+ LP DCs from CRBPII−/− mice were less effective at this process. Our current understanding is that CD103+ MLN DCs arise from the migration of CD103+ LP DCs.31 ALDH1A2 expression and ALDH activity increase in MLN DCs from 3 weeks of age to adulthood.12 Of note, this time course correlates with changes in the expression of CRBPII in intestinal epithelial cells, such that small intestinal enterocyte CRBPII expression decreases after birth during the suckling period and increases after weaning into adulthood,64 thus providing further support for a link between epithelial cell CRBPII expression and ADLH expression and activity in intestinal DCs. Somewhat in contradiction to our observations that ATRA was the more efficient retinoid in inducing ALDH dependent ATRA generation by CD103+ LP DCs, and our observation that ALDH activity in CD103+ LP DCs was diminished in CRBPII−/− mice, in vitro studies of mucosal scrapings from CRBPII−/− mice revealed no defect in their ability to generate ATRA when supplied with retinal.65 However, with this assay, neither wild-type nor CRBPII−/− scrapings produced ATRA when supplied with retinol.65 Earlier observations indicate that intestinal epithelial CRBPII expression may play an important role facilitating lumenal retinoid uptake to maintain total body vitamin A levels when vitamin A is limiting in the diet. Our observations demonstrate another important role for epithelial CRBPII by facilitating the production of an environmental cue to imprint LP DCs even when dietary vitamin A is not limiting.
The instability of ATRA dictates that DCs would need to be in close proximity to the source of ATRA for efficient ALDH induction. The above findings and prior in vitro studies support a role for epithelial cells in DC induction of ALDH activity. Multiple observations suggest that a subset of LP DCs are in close association with intestinal epithelial cells and may contact epithelial cells via pores in the basement membrane.41–46 We evaluated the phenotype of the DCs released with epithelial cells during their isolation and similar to an earlier report,45 found that the majority of these DCs were CD103+, consistent with the population that displays ALDH activity. We considered lymphoid tissues as alternative sources for the DCs released with the epithelium. However, we do not favor this explanation because the DCs released with the villous epithelium outnumber those released from the PP follicle–associated epithelium by 10-fold, making this an unlikely source for these DCs even if the Peyer's patches were to remain in place. Furthermore, the DCs released with the epithelium have a cell surface phenotype that is distinct from those of the solitary intestinal lymphoid tissues,30 and the solitary intestinal lymphoid tissues remain intact following the removal of the epithelial cells (see Supplemental Figure S2 at http://ajp.amjpathol.org).
LTβR is expressed by nonhematopoietic cells, including intestinal epithelial cells,52 and directs the production of multiple chemokines.51 Intestinal epithelial cell–specific expression of the LTβR plays an important role in recruiting leukocytes to the intestine,53 and accordingly, we evaluated the population of DCs associating with the epithelium in LTβR−/− mice and found that LTβR−/− mice had a diminished population of DCs released with the epithelium. Our above findings demonstrate that an epithelial-specific retinoid pathway imprints LP DCs via the unstable retinoid ATRA, and consistent with the requirement for close association of epithelial cells and DCs for this process, ALDH activity was diminished in the CD103+ LP DCs from the LTβR−/− mice. Moreover, this defect translated to a diminished ability of the CD103+ LP DCs from the LTβR−/− mice to promote IgA production. Prior studies have shown that IL-6 produced by intestinal DCs promotes IgA production,54,55 and that exogenous IL-6 can allow nonintestinal DCs to facilitate IgA production.5 Accordingly, we observed that LP DCs from LTβR−/− mice had diminished IL-6 production, and that exogenous IL-6 and ATRA were unable to restore their ability to promote IgA production. Thus LTβR−/− LP DCs have deficits related to an intestinal phenotype, ALDH activity, which could be related to their inability to associate with the epithelium, as well as deficits related to other DC properties that cannot be overcome with exogenous IL-6 and ATRA. These additional defects could be related to the well-documented absence of lymphoid tissues in the LTβR−/− mice.24,66–68 Prior studies have shown that LTβR−/− mice have diminished intestinal IgA production, which has been linked to the lack of intestinal lymphoid tissues, and altered lymphocyte homing because of defective chemokine production.24,25,66,69 Our observations suggest additional defects in the intestinal DCs from LTβR−/− mice. Although this could contribute to the decreased IgA production seen in the LTβR−/− mice, this defect is likely overshadowed by the lack of lymphoid tissues, which provide an environment for DC–lymphocyte interactions.
It has long been appreciated that the intestinal immune system has characteristics that are distinct from the systemic immune system including the predominance of IgA, the predominance of Foxp3+ Tregs, and the specific homing properties of lymphocytes. Recent work has shown that these characteristic intestinal-type responses are guided in part by ATRA generated by a subset of DCs. Understanding how DCs are endowed with this property has been the focus of multiple studies, whose results suggest that vitamin A is an intestine-specific environmental cue imprinting these properties on DCs. Here, we provide in vivo data supporting this model, and evidence that CRBPII, an intestinal epithelial cell–specific cytosolic retinol and retinal chaperone, plays an integral role in transforming lumenal retinoids into a local cue to imprint gut DCs with this intestinal phenotype.
Acknowledgments
We thank the Washington University School of Medicine Digestive Diseases Research Core Center (DDRCC) for assistance with morphology services and the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital (St. Louis, MO) for the use of the High Speed Cell Sorter Core, which provided flow cytometric cell sorting services.
Footnotes
This work was supported in part by grants DK064798 (R.D.N.), AI083538 (R.D.N.), DK085941 (L.W.W.), and DK050446 (M.S.L.). The DDRCC is supported by grant P30-DK52574. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant P30-CA91842.
K.G.M. and M.R.L. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.11.009.
Supplementary data
Response of Sil-15 subclone E5B6 to retinoids: The Sil-15 cell line, a teratoma cell line containing a β galactosidase reporter construct driven by tandem repeats of the retinoic acid response element, was subcloned, and lines expressing optimal sensitivity to the appropriate retinoids were selected. Shown is the response profile for subclone E5B6 used in these studies. Abs OD, absorbance optical density.
The intestinal villi and solitary lymphoid tissues (SILT) remain intact after removal of epithelial cells by EDTA treatment: To confirm the villus architecture and SILT were not disrupted by the process of removing the epithelial cells, the epithelium was removed from the small intestine by EDTA treatment and stained with antibodies against CD11c (brown). Shown is a photomicrograph of a full-thickness segment of small intestine containing intact villi and SILT (denoted by the white arrow) after the removal of the epithelium.
References
- 1.Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Kang S.G., Lim H.W., Andrisani O.M., Broxmeyer H.E., Kim C.H. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 4.Massacand J.C., Kaiser P., Ernst B., Tardivel A., Bürki K., Schneider P., Harris N.L. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS ONE. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora J.R., Iwata M., Eksteen B., Song S.Y., Junt T., Senman B., Otipoby K.L., Yokota A., Takeuchi H., Ricciardi-Castagnoli P., Rajewsky K., Adams D.H., von Andrian U.H. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 6.Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uematsu S., Fujimoto K., Jang M.H., Yang B.G., Jung Y.J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K.J., Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 8.Annacker O., Coombes J.L., Malmstrom V., Uhlig H.H., Bourne T., Johansson-Lindbom B., Agace W.W., Parker C.M., Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J.L., Berg P.L., Davidsson T., Powrie F., Johansson-Lindbom B., Agace W.W. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson-Lindbom B. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokota A., Takeuchi H., Maeda N., Ohoka Y., Kato C., Song S.Y., Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molenaar R., Knippenberg M., Goverse G., Olivier B.J., de Vos A.F., O'Toole T., Mebius R.E. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol. 2011;186:1934–1942. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- 13.Feng T., Cong Y., Qin H., Benveniste E.N., Elson C.O. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol. 2010;185:5915–5925. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliev I.D., Mileti E., Matteoli G., Chieppa M., Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 15.Iliev I.D., Spadoni I., Mileti E., Matteoli G., Sonzogni A., Sampietro G.M., Foschi D., Caprioli F., Viale G., Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 16.Napoli J.L. Biochemical pathways of retinoid transport, metabolism, and signal transduction. Clin Immunol Immunopathol. 1996;80:S52–S62. doi: 10.1006/clin.1996.0142. [DOI] [PubMed] [Google Scholar]
- 17.Harrison E.H. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- 18.Thomas S., Prabhu R., Balasubramanian K.A. Retinoid metabolism in the rat small intestine. Br J Nutr. 2005;93:59–63. doi: 10.1079/bjn20041306. [DOI] [PubMed] [Google Scholar]
- 19.Dunagin P.E., Jr., Meadows E.H., Jr., Olson J.A. Retinoyl beta-glucuronic acid: a major metabolite of vitamin a in rat bile. Science. 1965;148:86–87. doi: 10.1126/science.148.3666.86. [DOI] [PubMed] [Google Scholar]
- 20.Bohdal M., Hruba F. [Vitamin A excretion in the bile]: German. Acta Biol Med Ger. 1962;8:60–65. [PubMed] [Google Scholar]
- 21.Jaensson-Gyllenback E., Kotarsky K., Zapata F., Persson E.K., Gundersen T.E., Blomhoff R., Agace W.W. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 2011;4:438–447. doi: 10.1038/mi.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blomhoff R., Helgerud P., Rasmussen M., Berg T., Norum K.R. In vivo uptake of chylomicron [3H]retinyl ester by rat liver: evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc Natl Acad Sci U S A. 1982;79:7326–7330. doi: 10.1073/pnas.79.23.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.E X., Zhang L., Lu J., Tso P., Blaner W.S., Levin M.S., Li E. Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J Biol Chem. 2002;277:36617–36623. doi: 10.1074/jbc.M205519200. [DOI] [PubMed] [Google Scholar]
- 24.Futterer A., Mink K., Luz A., Kosco-Vilbois M.H., Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 25.Newberry R.D., McDonough J.S., McDonald K.G., Lorenz R.G. Postgestational lymphotoxin/lymphotoxin beta receptor interactions are essential for the presence of intestinal B lymphocytes. J Immunol. 2002;168:4988–4997. doi: 10.4049/jimmunol.168.10.4988. [DOI] [PubMed] [Google Scholar]
- 26.Newberry R.D., Stenson W.F., Lorenz R.G. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat Med. 1999;5:900–906. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M., Han B., Jessell T.M. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992;116:55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]
- 28.McDonald K.G., McDonough J.S., Wang C., Kucharzik T., Williams I.R., Newberry R.D. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170:1229–1240. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newberry R.D., McDonough J.S., McDonald K.G., Lorenz R.G. Postgestational lymphotoxin/lymphotoxin Beta receptor interactions are essential for the presence of intestinal B lymphocytes. J Immunol. 2002;168:4988–4997. doi: 10.4049/jimmunol.168.10.4988. [DOI] [PubMed] [Google Scholar]
- 30.McDonald K.G., McDonough J.S., Dieckgraefe B.K., Newberry R.D. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am J Pathol. 2010;176:2367–2377. doi: 10.2353/ajpath.2010.090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz O., Jaensson E., Persson E.K., Liu X., Worbs T., Agace W.W., Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu L.C., Chang W.C., Hoffmann I., Duester G. Molecular analysis of two closely related mouse aldehyde dehydrogenase genes: identification of a role for Aldh1, but not Aldh-pb, in the biosynthesis of retinoic acid. Biochem J. 1999;339(Pt 2):387–395. [PMC free article] [PubMed] [Google Scholar]
- 33.Duester G. Genetic dissection of retinoid dehydrogenases. Chem Biol Interact. 2001 doi: 10.1016/s0009-2797(00)00292-1. –132:469–480130. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida A., Rzhetsky A., Hsu L.C., Chang C. Human aldehyde dehydrogenase gene family. Eur J Biochem. 1998;251:549–557. doi: 10.1046/j.1432-1327.1998.2510549.x. [DOI] [PubMed] [Google Scholar]
- 35.Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 36.Molenaar R., Greuter M., van der Marel A.P., Roozendaal R., Martin S.F., Edele F., Huehn J., Forster R., O'Toole T., Jansen W., Eestermans I.L., Kraal G., Mebius R.E. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–6402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 37.Crow J.A., Ong D.E. Cell-specific immunohistochemical localization of a cellular retinol-binding protein (type two) in the small intestine of rat. Proc Natl Acad Sci U S A. 1985;82:4707–4711. doi: 10.1073/pnas.82.14.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin M.S. Cellular retinol-binding proteins are determinants of retinol uptake and metabolism in stably transfected Caco-2 cells. J Biol Chem. 1993;268:8267–8276. [PubMed] [Google Scholar]
- 39.Li E., Demmer L.A., Sweetser D.A., Ong D.E., Gordon J.I. Rat cellular retinol-binding protein II: use of a cloned cDNA to define its primary structure, tissue-specific expression, and developmental regulation. Proc Natl Acad Sci U S A. 1986;83:5779–5783. doi: 10.1073/pnas.83.16.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin M.S., Davis A.E. Retinoic acid increases cellular retinol binding protein II mRNA and retinol uptake in the human intestinal Caco-2 cell line. J Nutr. 1997;127:13–17. doi: 10.1093/jn/127.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi-Iwanaga H., Iwanaga T., Isayama H. Porosity of the epithelial basement membrane as an indicator of macrophage-enterocyte interaction in the intestinal mucosa. Arch Histol Cytol. 1999;62:471–481. doi: 10.1679/aohc.62.471. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi T., Gonda T. Distribution of the pores of epithelial basement membrane in the rat small intestine. J Vet Med Sci. 2004;66:695–700. doi: 10.1292/jvms.66.695. [DOI] [PubMed] [Google Scholar]
- 43.Chabot S., Wagner J.S., Farrant S., Neutra M.R. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol. 2006;176:4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 44.Maric I., Holt P.G., Perdue M.H., Bienenstock J. Class II MHC antigen (Ia)-bearing dendritic cells in the epithelium of the rat intestine. J Immunol. 1996;156:1408–1414. [PubMed] [Google Scholar]
- 45.Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., Stanley E.R., Nussenzweig M., Lira S.A., Randolph G.J., Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wendland M., Czeloth N., Mach N., Malissen B., Kremmer E., Pabst O., Forster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.-D., Shakhar G., Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 48.Huleatt J.W., Lefrancois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J Immunol. 1995;154:5684–5693. [PubMed] [Google Scholar]
- 49.McDonald K.G., Newberry R.D. Whole-mount techniques to evaluate subepithelial cellular populations in the adult mouse intestine. BioTechniques. 2007;43:50–54. doi: 10.2144/000112514. [DOI] [PubMed] [Google Scholar]
- 50.Velázquez P., Wei B., McPherson M., Mendoza L.M.A., Nguyen S.L., Turovskaya O., Kronenberg M., Huang T.T., Schrage M., Lobato L.N., Fujiwara D., Brewer S., Arditi M., Cheng G., Sartor R.B., Newberry R.D., Braun J. Villous B cells of the small intestine are specialized for invariant NK T cell dependence. J Immunol. 2008;180:4629–4638. doi: 10.4049/jimmunol.180.7.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dejardin E., Droin N.M., Delhase M., Haas E., Cao Y., Makris C., Li Z.W., Karin M., Ware C.F., Green D.R. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 52.Browning J.L., French L.E. Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J Immunol. 2002;168:5079–5087. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Koroleva EP, Kruglov AA, Kuprash DV, Nedospasov SA, Fu YX, Tumanov AV: Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 32:403–413 [DOI] [PMC free article] [PubMed]
- 54.Sato A., Hashiguchi M., Toda E., Iwasaki A., Hachimura S., Kaminogawa S. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171:3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 55.Ramsay A.J., Husband A.J., Ramshaw I.A., Bao S., Matthaei K.I., Koehler G., Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 56.Macpherson A.J., McCoy K.D., Johansen F.E., Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 57.Barnes M.J., Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Mora J.R., von Andrian U.H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 59.Mora J.R., Bono M.R., Manjunath N., Weninger W., Cavanagh L.L., Rosemblatt M., von Andrian U.H. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 60.Saurer L., McCullough K.C., Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol. 2007;179:3504–3514. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- 61.Manicassamy S., Ravindran R., Deng J., Oluoch H., Denning T.L., Kasturi S.P., Rosenthal K.M., Evavold B.D., Pulendran B. Toll-like receptor 2–dependent induction of vitamin A–metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villablanca E.J., Wang S., de Calisto J., Gomes D.C., Kane M.A., Napoli J.L., Blaner W.S., Kagechika H., Blomhoff R., Rosemblatt M., Bono M.R., von Andrian U.H., Mora J.R. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141:176–185. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ong D.E. A novel retinol-binding protein from rat. Purification and partial characterization, J Biol Chem. 1984;259:1476–1482. [PubMed] [Google Scholar]
- 64.Levin M.S., Li E., Ong D.E., Gordon J.I. Comparison of the tissue-specific expression and developmental regulation of two closely linked rodent genes encoding cytosolic retinol-binding proteins. J Biol Chem. 1987;262:7118–7124. [PubMed] [Google Scholar]
- 65.Wongsiriroj N., Piantedosi R., Palczewski K., Goldberg I.J., Johnston T.P., Li E., Blaner W.S. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008;283:13510–13519. doi: 10.1074/jbc.M800777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lorenz R.G., Chaplin D.D., McDonald K.G., McDonough J.S., Newberry R.D. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 67.Rennert P.D., James D., Mackay F., Browning J.L., Hochman P.S. Lymph node genesis is induced by signaling through the lymphotoxin beta receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 68.Taylor R.T., Lügering A., Newell K.A., Williams I.R. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173:7183–7189. doi: 10.4049/jimmunol.173.12.7183. [DOI] [PubMed] [Google Scholar]
- 69.Kang H.-S., Chin R.K., Wang Y., Yu P., Wang J., Newell K.A., Fu Y.-X. Signaling via LTβR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol. 2002;3:576–582. doi: 10.1038/ni795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Response of Sil-15 subclone E5B6 to retinoids: The Sil-15 cell line, a teratoma cell line containing a β galactosidase reporter construct driven by tandem repeats of the retinoic acid response element, was subcloned, and lines expressing optimal sensitivity to the appropriate retinoids were selected. Shown is the response profile for subclone E5B6 used in these studies. Abs OD, absorbance optical density.
The intestinal villi and solitary lymphoid tissues (SILT) remain intact after removal of epithelial cells by EDTA treatment: To confirm the villus architecture and SILT were not disrupted by the process of removing the epithelial cells, the epithelium was removed from the small intestine by EDTA treatment and stained with antibodies against CD11c (brown). Shown is a photomicrograph of a full-thickness segment of small intestine containing intact villi and SILT (denoted by the white arrow) after the removal of the epithelium.