Abstract
Avascular, hypoxic retina has been postulated to be a source of angiogenic factors that cause aberrant angiogenesis and intravitreal neovascularization (IVNV) in retinopathy of prematurity. Vascular endothelial growth factor (VEGF) is an important factor involved. However, VEGF is also required for normal retinal vascular development, which raises concerns about inhibiting its activity to treat IVNV in retinopathy of prematurity. Therefore, understanding the effects that VEGF has on other factors in the development of avascular retina is important to prevent aberrant angiogenesis and IVNV. Here, we show that STAT3 was activated by increased retinal VEGF in the rat 50/10 oxygen-induced retinopathy model. Phospho-STAT3 colocalized with glutamine synthetase-labeled Müller cells. Inhibition of STAT3 reduced avascular retina and increased retinal erythropoietin (Epo) expression. Epo administered exogenously also reduced avascular retina in the model. In an in vitro study, hypoxia-induced VEGF inhibited Epo gene expression by STAT3 activation in rat Müller cells. The mechanism by which activated STAT3 regulated Epo was by inhibition of Epo promoter activity. Together, these findings show that increased retinal VEGF contributes to avascular retina by regulating retinal Epo expression through Janus kinase/STAT signaling. Our results suggest that rescuing Epo expression in the retina before the development of IVNV may promote normal developmental angiogenesis and, therefore, reduce the stimulus for later pathologic IVNV.
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness.1 It is characterized first by a delay in developmental retinal angiogenesis and potentially some capillary constriction, resulting in the clinical appearance of avascular retina and then later by dilated and tortuous retinal vessels and intravitreous neovascularization (IVNV), that is, blood vessels that proliferate into the vitreous. Several clinical studies have found an association between large areas of peripheral avascular retina and worse outcomes in ROP.2,3 Besides ROP, proliferative, uncontrolled angiogenesis occurs as a result of avascular retina in other conditions, including proliferative diabetic retinopathy and retinal vein occlusions.4 Currently, efforts have been focused on inhibiting aberrant angiogenesis in these conditions5,6 and less on reducing avascular retina,7 the causes of which are incompletely understood.8–13 In this study, we seek to understand the causes and mechanisms for avascular retina in retinal diseases with IVNV. We report our findings with the use of a model developed by Penn et al,14 in which newborn rat pups are exposed to fluctuations in oxygen levels between 50% and 10%, yielding arterial oxygen concentrations similar to transcutaneous oxygen levels reported in human severe ROP.14,15 The model also produces a characteristic appearance of severe ROP with peripheral avascular retina similar to zone II ROP,14,16 followed by retinal tortuosity,17 and then IVNV at the junctions of vascular and avascular retina, similar to stage 3 ROP.16,18 With the use of this model, we found that vascular endothelial growth factor (VEGF), one of the most notable angiogenic factors that causes aberrant retinal angiogenesis,19,20 was significantly increased in retinas from pups with avascular retina, compared with retinas from age-matched pups raised in room air that had full vascularization of the inner retinal plexus.21 Therefore, a question was raised whether the increase in retinal VEGF protein played a role in the persistence of avascular retina.
Janus kinase (JAK)/STAT signaling pathway is a main signaling mechanism for cytokines and growth factor receptors. Activation of JAK/STAT signaling involves ligand binding and dimerization of cell membrane receptors, which result in the activation of receptor-associated JAKs and phosphorylation of receptors at cytoplasmic tyrosine residues. STATs dock on these phosphotyrosine motifs by their Src homology 2 domains. Receptor-bound STATs then are phosphorylated on conserved carboxy-terminal tyrosines and then dimerize. Dimerized STATs translocate into the nucleus to regulate gene transcription,22 resulting in cell proliferation, differentiation, migration, and apoptosis. The regulatory mechanism of STATs on target genes requires DNA binding and coactivator or corepressor recruitment. The transcriptional activity of STATs is potentiated by phosphorylation of a conserved carboxy-terminal serine residue, which increases interactions with coactivators or corepressors. Therefore, JAK/STAT signaling can lead to increased or reduced transcription of a gene. Besides being activated by a cytokine or interferon, JAK/STAT signaling can be triggered by hypoxia and reactive oxygen species. Downstream effects can include regulation of angiogenic genes such as VEGF and hypoxia inducible factor-1 α.23 We previously reported that activation of JAK2 and STAT3 signaling exacerbated the severity of retinopathy in a model of oxygen-induced retinopathy (OIR) rescued in supplemental oxygen.24
In this study, we hypothesized that activation of STATs would contribute to the persistence of avascular retina after repeated oxygen fluctuations by promoting apoptosis or inhibiting angiogenic factor expression. We found that the angiogenic cytokine, erythropoietin (Epo), was down-regulated by VEGF-mediated STAT3 activation and was involved in the persistence of avascular retina. We tested our hypothesis with both in vitro cell cultures and the relevant 50/10 OIR model.
Materials and Methods
Animals
All animal procedures were conducted in accordance with the University of Utah and University of North Carolina (Guide for the Care and Use of Laboratory Animals) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Rat 50/10 OIR Model
A bioactive gas controller (Oxycycler; BioSpherix, New York, NY) that regulates the atmosphere inside an incubator by injecting either nitrogen or oxygen was used to induce OIR in newborn Sprague-Dawley rats (Charles River, Wilmington, MA), as previously reported.14 Within 4 hours of birth, pups and their mothers were placed into the incubator, which cycled oxygen between 50% and 10% every 24 hours for 14 days. Carbon dioxide in chambers was monitored and flushed from the system by maintaining sufficient gas flow and using soda lime. Litter numbers were maintained between 12 and 14 pups for each experiment to assure consistency in outcomes, all animals were weighed, and mean body weight of litters was found to be within ±2 g of each other at the time of treatment.
Animals Treated with AG490 or Erythropoietin or Neutralizing VEGF Antibody
AG490 (LC Laboratories, Woburn, MA) is a synthetic protein tyrosine kinase inhibitor that inhibits JAK225 and was used to inhibit the JAK/STAT pathway in rats.26 To test whether JAK2/STAT3 inhibition affects angiogenesis and ultimately leads to avascular retina in the 50/10 OIR model rat, pups were treated with 10 mg/kg AG490 in 5% dimethyl sulfoxide (DMSO; MP Biomedicals, Solon, OH) in PBS (Sigma-Aldrich, St. Louis, MO) by i.p. injection. This dose was based on earlier studies that showed an inhibitory effect on IVNV in the 50/10 OIR model rescued in room air.24 Intraperiotneal injections were administered daily from postnatal day (p) 3 to p13. Control animals received i.p. injections of sterile 5% DMSO in PBS. Separate litters received i.p. injections of Epo (human Procrit; 41.6 μg/kg; R&D Systems, Minneapolis, MN) or PBS control at multiple time points (p2, p4, and p6). Before the decision to use human Procrit, rat Epo was compared with Procrit, and no difference was found in the resulting measured hematologic parameters (data not shown). Fifty nanograms of neutralizing VEGF antibody (recognizes only VEGF164 splice variant) or control IgG (R&D Systems) was delivered to one eye of pups raised in the 50/10 OIR model by intravitreous injection at p12. The fellow eye was used as a noninjection control. All of the injections were done during the 50% O2 cycle. Percentage of avascular retina, platelet counts in whole blood, and interested proteins in whole retinas were measured at p14. Pups were removed from cycling for <20 minutes for treatments. Pup weights were obtained before each treatment and the time of assay and were not found to be significantly different between experimental and control 50/10 OIR groups.
Dissecting Retinal Tissue for Flatmounts and Cryosections
Pups were anesthetized by i.p. injection of ketamine (20 mg/kg) and xylazine (6 mg/kg). Both eyes were enucleated. For flatmounts, eyes were fixed in 4% paraformaldehyde (PFA) for 2 hours. Within the first 10 minutes, anterior segments were removed, and retinas with intact ora serratas were carefully dissected and placed into PBS with care taken to remove the hyaloidal vessels and any remaining vitreous. Then, each retina was placed onto a microscope slide and flattened by making four incisions, each 90° apart, beginning at the ora serrata and extending centrally from the equator, stopping short of the optic nerve opening.
For cryosections, eyes were fixed in 4% PFA containing 10 mmol/L sodium orthovanadate for 10 minutes. Anterior segments were removed, and retinas were placed into 4% PFA that contained 10 mmol/L sodium orthovanadate for another 15 minutes. Retinas were then placed into 30% sucrose/PBS overnight. Each retina was dried with filter paper, soaked in optimal cutting temperature compound (Tissue Tek; product no. 4583; EMS, Hatfield, PA), and kept at −80°C for future analysis. In most analyses, one eye from each pup was used for flatmount and the other for fresh tissue analysis.
Tissue Staining for Flatmounts
Flattened retinas were incubated in 4% PFA overnight at 4°C, then washed with 1× Tris buffer (50 mmol/L Trizma Maleate, 50 mmol/L sodium hydroxide, pH 7.2; Sigma-Aldrich) for three times. After the washes, retinas were incubated with 1× Tris buffer/1% Triton X-100 for 15 minutes at room temperature, followed by three washes with 1× Tris buffer. Retinas then were incubated with ADPase staining solution (200 mmol/L Trizma Maleate, pH 7.2, 3 mmol/L lead nitrate, 6 mmol/L magnesium chloride, 2 mmol/L ADPase; Sigma-Aldrich) for 15 to 25 minutes at 37°C. After rinsing in 1× Tris buffer three times, retinas were then placed in ammonium sulfide (1:30 diluted in water; Fisher Scientific, Fair Lawn, NJ) for 1 to 2 minutes at room temperature, mounted in Fluoromount-G (Southern Biotech, Birmingham, AL), and placed on slides with coverslips. Images of the superficial blood vessel layers were captured with an inverted microscope (Olympus 1 × 81; Olympus, Tokyo, Japan) and digitally stored for analysis. Image sections were stitched with the commercial image management software (Photoshop version 7.0; Adobe Systems, Mountain View, CA) and assembled with methods that maintain the original image dimensions and do not induce image distortion (PhotoFit Premium version 1.44; TekMate Inc., Tokyo, Japan; or Photoshop; Adobe Systems).
Tissue Staining for Cryosections
Retinas cut into 10-μM sections were incubated in 20 μg/mL proteinase K in TE buffer (pH 8.0) for 1.5 minutes, washed with cold PBS, incubated with 5% goat serum/1× PBS for 1 minute, and then washed with cold PBS followed by PBS/1% Triton X-100 three times. Sections were incubated in 10% normal goat serum for 1 hour to block nonspecific binding of the primary antibody. Mouse anti-glutamine synthetase (GS; 1:2000; BD Transduction Laboratories, Franklin Lakes, NJ) and rabbit anti-phosphorylated STAT3 (p-STAT3; Y705; 1:50; Cell Signaling Technology Inc., Danvers, MA) were used, and the sections were incubated overnight at 4°C. After three washes in PBS, sections were incubated for 1 hour with a 1:500 dilution of Alexa 594-conjugated goat anti-mouse secondary antibody (Invitrogen, Carlsbad, CA) for GS or Alexa 488-conjugated goat anti-rabbit secondary antibody (Invitrogen) for p-STAT3. The sections were rinsed in PBS and mounted in Fluoromount-G (Southern Biotech). Images were captured with an inverted microscope (Olympus 1 × 81) and digitally stored for analysis.
Measurement of Avascular Areas
To measure avascular retinal areas, digitized retinal image sections were assembled with methods that maintain original image dimensions (PhotoFit Premium version1.44; TekMate Inc.). Total retinal and summed peripheral avascular retinal27 areas were computed in pixels with Image Tool version 3 (University of Texas, San Antonio, TX) and converted to mm2 (using a calibration bar on each image). Avascular retinal areas were expressed as a percentage of total retinal area for each eye. Measurements were performed by two independent masked reviewers. A third reviewer was used to rectify discrepancies in measurements, and a final consensus was determined.
RNA Isolation and Analysis
Samples were removed from RNAlater, and total RNA was extracted with the RNeasy Mini kit (Qiagen, Valencia, CA). RNA was quantified by NanoDrop. cDNA was generated with the use of High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). RT-PCR was performed on an ABI Prism 7500 sequence detector (Applied Biosystems) with the use of TaqMan probes (Applied Biosystems). Expression levels for all genes were normalized to the mean value of internal control GAPDH.
Protein Extraction and Western Blot Analysis
Retinal samples frozen in modified RIPA buffer (20 mmol/L Tris base, 120 mmol/L NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol) with protease cocktail inhibitor (1:100; Sigma-Aldrich) and orthovanadate (1 mmol/L; Sigma-Aldrich) were homogenized and centrifuged at 13,000 rpm for 10 minutes at 4°C. Total protein in the supernatant fluid was quantified by BCA Protein Assay (Pierce, Rockford, IL). Total protein (50 μg) for each sample was separated by NuPAGE 4% to 12% Bis-Tris Gels (Invitrogen), transferred to a PVDF membrane, and incubated with primary antibodies overnight at 4°C: p-STAT3, STAT3 (1:1000; Cell Signaling Technology Inc.), active caspase 3 (1:1000; Chemicon International, Temecula, CA), VEGF (all splice variants), and erythropoietin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed three times in Tris-buffered saline/0.1% Tween-20, probed with horseradish peroxidase-linked secondary antibody, and visualized by enhanced chemiluminescence (Millipore Corp, Billerica, MA). All membranes were reprobed with β-actin (1:2000; Abcam, Cambridge, MA) to ensure equal protein loading. Densitometry analysis was done on exposed films with the use of the software UN-SCAN-IT version 6.1 (Silk Scientific, Orem, UT).
Construction of Pluc-MCS-rEpo-Promoter
The luciferase reporter vector Pluc-MCS was obtained from Promega (Madison, WI). Plasmid Pluc-MCS-rEpo-promoter was generated by ligating the sequences from the rat Epo promoter (−300 to +48) into the pluc-MCS vector at the XhoI and HindIII sites. The integrity of the plasmid construct was verified by the DNA sequencing (HSC Core Facility, The University of Utah, Salt Lake City, UT).
Cell Culture and Transfections
Rat Müller cells (rMC-1s; kindly provided by V.P. Sarthy, Northwestern University, Evanston, IL) were maintained in DMEM/high glucose (1000 mg/L; Sigma-Aldrich) with 10% FBS. Cells were placed in an incubator (BioSpherix) in hypoxia (1% oxygen) or in room air (21% oxygen) with either Tryphostin AG490 (final concentration, 10 μg/mL in DMSO; LC Laboratories), human anti-VEGF165 antibody (final concentration, 600 ng/mL in PBS; R&D Systems), DMSO (control for AG490) or IgG (control for anti-VEGF165). Six hours after treatment, cells were collected for either Western blot analyses or real-time PCR for detection of expression levels of p-STAT3, total STAT3, Epo, and VEGF.
Transfections of Cos-7 cells were performed in 12-well plates with a total amount of plasmid DNA of 0.7 μg/well with 0.75 μL of JetPRIME (Polyplus-transfection Inc., New York, NY). These DNA mixtures included Pluc-MCS (0.5 μg) or Pluc-MCS-rEpo-Promoter (0.4 μg), rat STAT3 expressing construct pExpress-1-rSTAT3 (0.2 μg; Open Biosystems; Lafayette, CO), and β-galactosidase (0.1 μg). Cells were treated with recombinant VEGF164 (20 ng/mL; R&D Systems), AG490 (10 μg/mL), and VEGF and AG490 together for 6 hours. Cells were collected for luciferase and β-galactosidase assay within 24 hours of transfection.
ChIP Assay
Chromatin immunoprecipitation (ChIP) assays were performed with a commercial reagent (Millipore Corp) according to the manufacturer's protocol. Briefly, rMCs grown in 10-cm2 dishes were treated with control or with AG490 (10 μg/mL), VEGF (20 ng/mL), or both together and placed into the hypoxia incubator at 1% O2 for 6 hours. Cells were grown in 21% O2 incubator as a control. For the cross-linking step, an aliquot of formaldehyde [37% (v/v)] was added directly to the culture medium to achieve a final concentration of 1%, and the incubation was done for 10 minutes. Cells were then rinsed twice with cold PBS, harvested with cell scrapers, and lysed in 10 mL of an SDS buffer (1% SDS, 10 mmol/L EDTA, 50 mmol/L Tris, pH 8.1). The lysates were sonicated to shear genomic DNAs to achieve lengths of between 200 and 1000 bp. The sheared lysates were centrifuged at 16,000 × g for 10 minutes; supernatant fluids were collected and diluted 10-fold with ChIP dilution buffer (24 mL of 0.01% SDS, 1.1% Triton X-100, 1.2 mmol/L EDTA, 16.7 mmol/L Tris-HCl, pH 8.1, 167 mmol/L NaCl) in the presence of protease inhibitors (Roche Diagnostics, Indianapolis, IN). An aliquot (75 μL) of a 50% slurry of salmon sperm DNA-protein A-agarose was added and incubated for 30 minutes. Agarose beads were precipitated by brief centrifugation, and the precleared supernatant fluid was collected. Antibodies against STAT3 and control IgG antibody (Cell Signaling Technology Inc.) were added to this 2-mL supernatant fraction and incubated overnight at 4°C with gentle mixing. Sixty microliters of the salmon sperm DNA-protein A-agarose slurry was added and incubated for an additional 1 hour at 4°C. The antibody/histone/DNA complex was collected by gentle centrifuge. The presence of target DNA fragments was detected by PCR after the DNA was de-cross-linked with proteins. The primers used for rat Epo promoter were 5′-TGGCGACCCCTCACGCACAC-3′ and 5′-TCCCTAGCCTGTGAGTACTCAC-3′.
Statistical Analyses
Significant differences between groups were determined by ANOVA with post hoc protected t-tests. A minimum P value of <0.05 was considered statistically significant.
Results
STAT3 Is Activated in Retinas from Rat Pups Raised in 50/10 OIR
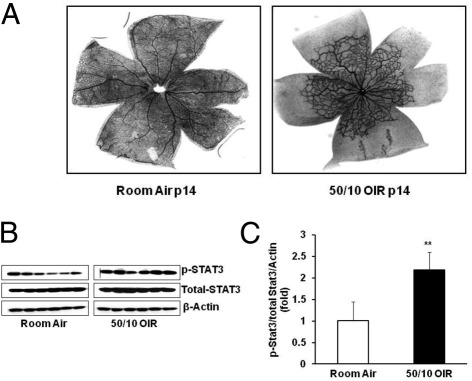
We first determined whether the JAK/STAT signaling pathway was activated in the retina after repeated fluctuations in oxygen. At p14, pups raised in the 50/10 OIR model show approximately 60% to 70% retinal vascular coverage of the inner plexus and, therefore, have 30% to 40% avascular retinal area. However, pups raised in room air have full vascularization of the inner plexus and, therefore, no avascular retina (Figure 1A). Compared with age-matched p14 pups raised in room air, retinal p-STAT3 was significantly increased in retinas from pups raised in the 50/10 OIR model (Figure 1, B and C), whereas p-STAT1 and p-STAT5 were not changed (data not shown). These results show that STAT3 activation is associated with persistent avascular retina after repeated fluctuations in oxygen in the 50/10 OIR model.
Figure 1.
Pups raised in 50/10 OIR model have increased avascular retina and activation of retinal STAT3 at p14. A: Retinal vessels by flatmount with ADPase staining in pups raised in room air and 50/10 OIR model (n = 18). B: Western blot analysis of STAT3 phosphorylation in retina from pups raised in room air and 50/10 OIR. C: Quantification of Western gels shown in panel B. **P < 0.01 versus room air (ANOVA). Data shown in panels B and C are representative of 12 independent samples. Results are means ± SEMs.
Inhibition of STAT3 Reduces Avascular Retina Independent of Retinal VEGF and Active Caspase 3
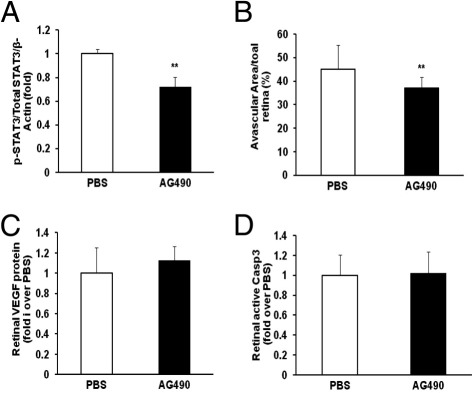
To investigate whether STAT3 activation led to the persistence of avascular retina, pups raised in the 50/10 OIR model were administered a chemical inhibitor of JAK/STAT signaling, AG490 (5 to 20 mg/kg), or control PBS. On the basis of the inhibitory effects on STAT3 activation, we chose the 10-mg/kg dose of AG490 delivered as an i.p. injection (5 μL/g of body weight) daily from p3 to p13. Rat pups were sacrificed at p14, and one eye was analyzed for retinal p-STAT3 and the other for percentage of avascular retina. Retinal p-STAT3 was reduced in pups that received AG490 compared with control (Figure 2A). Treatment with systemic AG490 significantly reduced avascular retina compared with control (Figure 2B), providing evidence that JAK/STAT signaling contributed to avascular retina after repeated fluctuations in oxygen in the 50/10 OIR model. There was no difference in body weight gain between AG490 and control groups.
Figure 2.
Decreased avascular retina with sustained retinal VEGF expression and caspase 3 activity in AG490-treated rat pups raised in the 50/10 OIR model. A: Western blot analysis of p-STAT3 and total STAT3 in retina. **P < 0.01 versus PBS (ANOVA; n = 8). B: Avascular retina analysis by measuring the ratio of avascular area to total retina area with the use of an image tool. *P < 0.01 versus PBS (ANOVA; n = 18). C and D: Western blot analyses of retinal VEGF protein (C) and active caspase 3 (D). Data shown in panels C and D are representative of 8 independent samples. Results are means ± SEMs.
Next, we sought to determine the potential mechanisms by which retinal STAT3 activation contributed to avascular retina in the 50/10 OIR model. We initially tested the effect of STAT3 activation on VEGF production, because inhibition of VEGF could lead to reduced retinal vascularization by inhibiting developmental angiogenesis.28,29 However, no difference was observed in retinal VEGF protein between AG490-treated and control pups (Figure 2C). In a previous study,11 we found that pharmacologic inhibition of NADPH oxidase activation by apocynin reduced avascular retina and retinal cleaved caspase 3, a downstream effector of both the intrinsic and extrinsic pathways of apoptosis. In addition, cleaved caspase 3 colabeled with NG2-positive pericytes and glial fibrillary acidic protein-positive Müller cells in the inner nuclear layer. We postulated that loss of these cells might interfere with normal retinal vascular development and increase avascular retina by reducing endothelial cell support from pericytes or from glial growth factor expression. We also found evidence for direct endothelial cell apoptosis with colabeling of CD31 and cleaved caspase 3. We, therefore, asked whether JAK/STAT signaling contributed to avascular retina in the 50/10 OIR model by triggering apoptosis.22 We measured retinal cleaved caspase 3 and found no difference between AG490-treated and control rat pups (Figure 2D).
Inhibition of STAT3 Increases Retinal Epo Expression
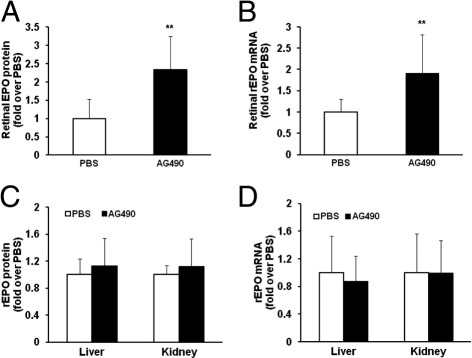
The data in Figure 2 do not support apoptosis or VEGF expression as downstream events of activated STAT3-mediated avascular retina. We, therefore, considered other factors. Epo has been shown to be important in stabilizing newly developed retinal vessels7 and has been correlated with improved cognitive function later in childhood.30 However, Epo has also been shown to cause pathologic IVNV in OIR models,31 and, when used to treat anemia of prematurity in human preterm infants, Epo has been associated with more severe ROP.32 To determine whether STAT3 activation mediated the expression of Epo in the retina, we measured retinal Epo protein in pups raised in the 50/10 OIR model that had been administered AG490 or control PBS. We found AG490-treated pups had significantly increased retinal Epo protein (Figure 3A) compared with control. Epo has been reported as up-regulated in the liver and kidney in other models of OIR33; therefore, we postulated that increased Epo in other organs could enter the retinal or choroidal circulation and be detected as increased in the retina. To determine whether the increased Epo protein was from retina rather than from systemic sources, we also measured retinal Epo mRNA in pups raised in the 50/10 OIR model treated with AG490 or control. As shown in Figure 3B, inhibition of STAT3 phosphorylation led to greater retinal Epo mRNA expression compared with control, similar to protein expression. We also tested the effect of AG490 given as an i.p. injection on Epo expression in liver and kidney. We found no difference in liver and kidney Epo mRNA and protein in pups treated with AG490 compared with control (Figure 3, C and D). These results further support a local effect of STAT3 activation on retinal Epo expression.
Figure 3.
Increased retinal Epo expression in AG490-treated rat pups raised in the 50/10 OIR model. A: Western blot analysis of Epo protein in retina. **P < 0.01 versus PBS (ANOVA; n = 7). B: Retinal Epo mRNA analysis by real-time PCR. **P < 0.01 versus PBS (ANOVA; n = 8). C: Western blot analysis of Epo protein in liver and kidney (n = 7). D: Real-time PCR of Epo mRNA in liver and kidney (n = 8). Results are means ± SEMs.
Pups Administered Exogenous Epo Have Reduced Avascular Retina
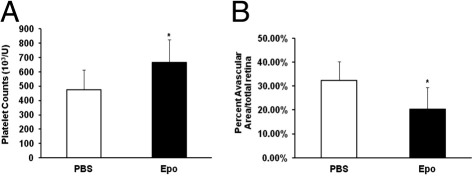
To determine whether the down-regulation of Epo mediated by STAT3 activation in the retinas of pups raised in the 50/10 OIR model contributed to increased avascular retina, we administered pups exogenous Epo and measured the percentage of avascular retina. Pups raised in the 50/10 OIR model were given i.p. Epo (41.6 μg/kg or 5000 IU/kg) on days 2, 4, and 6. This dose of Epo was sufficient to significantly increase blood platelet counts compared with PBS control (Figure 4A). This dose was also reported to cause plasma Epo levels similar to a dose being tested in a clinical trial for neuroprotection34 in human preterm infants. At day 14, avascular retina was significantly reduced in Epo-treated pups compared with control (Figure 4B).
Figure 4.
Decreased avascular retina by exogenous Epo given at early time points in the 50/10 OIR model. A: Platelet count of pups treated with Epo and PBS injection exposed to the 50/10 OIR model. *P < 0.05 versus PBS (ANOVA; n = 8). B: Analysis of avascular retina area. *P < 0.05 versus PBS (ANOVA; n = 8). Results are means ± SEMs.
Retinal STAT3 Activation in Pups Raised in 50/10 OIR Is Inhibited by Intravitreous VEGF Antibody
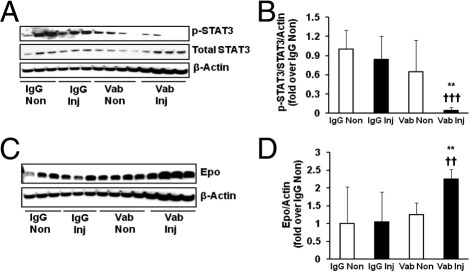
We previously found that repeated fluctuations in oxygen significantly increased the expression of the pathologic splice variant of VEGF, VEGF164, compared with a single hypoxic episode.35 We, therefore, postulated that VEGF, which was up-regulated in the 50/10 OIR model, might trigger JAK/STAT signaling to locally regulate Epo expression. To test this, we administered a neutralizing antibody to VEGF164 as an intravitreal injection at p12 to one eye of pups raised in the 50/10 OIR model. The dose and timing of the injection were previously found to significantly reduce VEGF signaling and IVNV.13 Compared with control nonimmune IgG injections in pups within the same litters and to fellow noninjected eyes of pups from both experimental and control groups, neutralizing VEGF bioactivity with antibody significantly reduced phosphorylation of STAT3 (Figure 5, A and B), providing support that VEGF mediated STAT3 activation at p14 in the 50/10 OIR model.
Figure 5.
Decreased STAT3 phosphorylation and increased Epo expression in retinas of pups with VEGF-neutralizing antibody injection in the 50/10 OIR model. A and B: Retinal p-STAT3 measured by Western blot analysis with representative gels (A) and quantification of gels (B). Overall ANOVA, P < 0.001; post hoc Newman–Keuls multiple comparison testing: Vab versus Vab non-Inj, **P < 0.01, and Vab versus Vab IgG-Inj †††P < 0.001 (n = 6 to 8). C and D: Retinal Epo protein by Western blot analysis with representative gels (C) and quantification of gels (D). Overall ANOVA, P < 0.01; post hoc Newman–Keuls multiple comparison testing: Vab versus Vab non-Inj, **P < 0.01; Vab versus Vab IgG-Inj, ††P < 0.001 (n = 8). Results are means ± SEMs. IgG Inj, IgG injection; IgG Non, IgG noninjection; Vab Inj, VEGF antibody injection; Vab Non, VEGF antibody noninjection.
Because retinal Epo expression was regulated by STAT3 activity (Figure 3, A and B), we then measured Epo protein in the same retinas of eyes treated with anti-VEGF antibody or control IgG and found that local retinal Epo was significantly increased in eyes treated with the intravitreal-neutralizing antibody to VEGF (Figure 5, C and D). This supported the thinking that VEGF triggered JAK/STAT signaling to reduce local Epo expression in the retina.
p-STAT3 Is Colocalized in Müller Cells in Retinas of Pups Raised in the 50/10 OIR Model
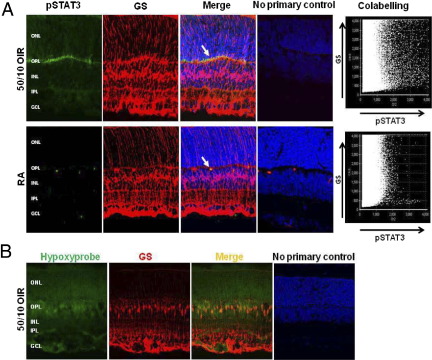
The results above indicate that down-regulation of Epo by VEGF-mediated STAT3 activation in the retina contributes to the persistence of avascular retina. We then labeled retinal cryosections from eyes of pups raised in room air or in the 50/10 OIR model for p-STAT3 at the tyrosine 705 site. p-STAT3 labeling was found mainly in the outer plexiform layer in eyes from pups raised in room air and in the 50/10 OIR model. Colabeling with the Müller cell marker, GS (Figure 6A), was qualitatively greater in sections from the 50/10 OIR model than from pups raised in room air (Figure 6A). Because p-STAT3 was localized to Müller cells and the 50/10 OIR model caused retinal hypoxia,36 we also tested whether Müller cells were hypoxic in the 50/10 OIR model. Pups were given an i.p. injection of pimonidazole (Hypoxyprobe, HPI, Inc., Burlington, MA [60 mg/kg], a marker for hypoxia, 90 minutes before sacrifice. Retinal cryosections from eyes of rats raised in the 50/10 OIR model were stained with GS and Hypoxyprobe. Colabeling of Hypoxyprobe with the Müller cell marker, GS, was seen in sections from the 50/10 OIR model (Figure 6B).
Figure 6.
A: Increased p-STAT3 is localized in retinal Müller cells of pups raised in the 50/10 OIR model compared with pups raised in room air. Immunohistochemical labeling of cryosection that shows p-STAT3 colocalized with Müller cells (arrows) in room air and 50/10 OIR, using antibodies for p-STAT3 and Müller cell marker GS. No primary antibody control sections show mainly DAPI. Density of p-STAT3 colabeling with GS was quantified by confocal colocalization analysis and shows increased pSTAT3 in GS colabeled cells in the 50/10 OIR model compared with room air. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer. Representative of three separate experiments. B: Retinal Müller cells of pups raised in the 50/10 OIR model at p18 are hypoxic as shown by staining with antibody to GS and to Hypoxyprobe.
STAT3 Is Activated by VEGF in Rat Müller Cells
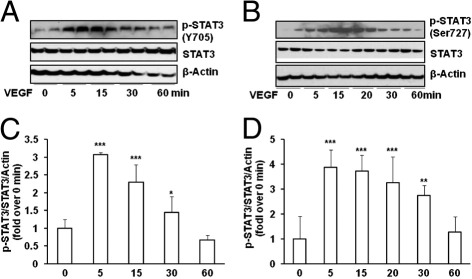
To investigate the mechanisms involved in STAT3 activation in the 50/10 OIR model, we used a rat Müller cell line (rMC-1). We initially tested the hypothesis that VEGF stimulation would increase p-STAT3 and regulate Epo expression. Compared with control, p-STAT3 was significantly increased in rMC-1 cells treated with VEGF (20 ng/mL) at both the tyrosine 705 (Figure 7, A and C) and serine 727 (Figure 7, B and D) sites in a time-dependent manner, peaking 5 minutes after VEGF treatment.
Figure 7.
STAT3 is activated by VEGF in rat Müller cells (rMC-1). Western blot analysis of p-STAT3 at Tyr 705 (A and C) and Ser 727 (B and D), total STAT3 and loading control β-actin at different time points after VEGF (20 ng/mL) treatment (A and B). Representative quantification of gels (C and D). Overall ANOVA. P < 0.0001; post hoc Newman–Keuls multiple comparison testing: ***P < 0.001, **P < 0.01, and *P < 0.05 versus 0 minute of VEGF treatment. Results are means ± SEMs (n = 3). Data are representative of three independent experiments.
Epo Is Down-Regulated by VEGF Stimulated STAT3 Activation in Müller Cells
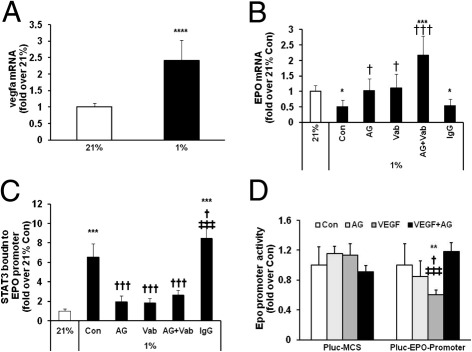
Our study with Hypoxyprobe staining showed that repeated fluctuations of oxygen in the 50/10 OIR model caused retinal36 and Müller cell hypoxia (Figure 6B). Therefore, we postulated that Epo gene expression was regulated by hypoxia-induced VEGF-stimulated STAT3 activation in Müller cells. To test this prediction, rMC-1 cells were exposed to 21% or 1% O2 for 6 hours. As shown in Figure 8A, compared with 21% O2, Vegfa mRNA level was significantly increased in rMC-1 cells incubated in 1% O2, and a parallel reduction in Epo gene expression was also seen (Figure 8B). The down-regulation of Epo in 1% O2 was prevented by AG490 or by treatment with a neutralizing antibody to VEGF. However, in the presence of both AG490 and VEGF antibody, Epo mRNA was significantly elevated, twofold over control in 21% O2 and was significantly increased compared with either AG490 and VEGF antibody treatment alone (Figure 8B). These data support the postulate that hypoxia-inhibited Epo gene expression was mediated by VEGF-induced STAT3 activation in rMC-1 cells and also suggested that parallel signaling pathways could be involved in the regulation of Epo gene expression in hypoxia when VEGF-mediated STAT3 activation was inhibited.
Figure 8.
Activated STAT3 by hypoxia-induced VEGF suppresses Epo gene transcription by a STAT3 binding site within Epo promoter in rMC-1 cells. A and B: Real-time PCR of vegfa mRNA (A) and Epo mRNA (B) in rMC-1 cells exposed to 21% and 1% O2. Overall ANOVA, P < 0.0001; post hoc Newman–Keuls multiple comparison testing: *P < 0.05, ***P < 0.001, and ****P < 0.0001 versus 21% O2; †P < 0.05 and †††P < 0.001 versus 1% O2 Con. C: ChIP assay of STAT3 bound to a STAT3 binding site within a region of rat Epo promoter in rMC-1 cells exposed to 21% and 1% O2. Overall ANOVA, P < 0.0001; post hoc Newman-Keuls multiple comparison testing: ***P < 0.001 versus 21% O2, †P < 0.05, and †††P < 0.001 versus Con of 1% O2; ‡‡‡P < 0.001 versus AG, Vab or Vab+AG. D: Transient transfection of Cos-7 cells with Pluc-MCS reporter gene containing 300-bp promoter region from rat Epo gene with rat STAT3-expressing construct. Overall ANOVA, P < 0.001; post hoc Newman–Keuls multiple comparison testing: **P < 0.01 versus Con of Pluc-Epo-promoter; †P < 0.05 versus AG; ‡‡‡P < 0.001 versus VEGF+AG. AG, AG490; Con, control; Vab, VEGF antibody. Results are means ± SEMs (n = 3). Data are representative of two independent experiments.
STAT3 is a transcriptional factor. After activation, STAT3 can enter the nucleus and regulate gene transcription by directly binding to promoters of target genes. A sequence TTCCCGAA within the 5′ promoter region (−164 to −156) of the rat Epo gene shares similarities to the consensus STAT3 binding motif: TTC(N)2–4GAA or TT(N)4–6AA. To test the prediction that this potential STAT3 binding site was involved in the transcription of the Epo gene, we first determined whether STAT3 was capable of binding to this site within the promoter of the Epo gene with the use of a ChIP assay. PCR quantification of the ChIP assay showed that rMC-1 cells cultured in 1% O2 induced STAT3 recruitment to the promoter of the Epo gene (Figure 8C). However, in the presence of AG490 or VEGF antibody, hypoxia-induced p-STAT3 recruitment to the Epo promoter was significantly reduced compared with control. However, some p-STAT3 remained bound to the Epo promoter, potentially because STAT3 activation was not completely inhibited by AG490 and the neutralizing VEGF antibody. These data suggest that the binding of p-STAT3 to the Epo promoter is mediated by VEGF-induced STAT3 activation.
We next determined whether the STAT3 binding motif was involved in the inhibition of Epo gene transcription. A reporter gene containing the STAT3 binding motif within the 5′ promoter of the rat Epo gene (−300 to +48 from transcriptional start site) was cotransfected with a rat STAT3-expressing construct into Cos-7 cells for 24 hours at 21% O2. In the last 6 hours of transfection, 20 ng/mL VEGF was added to activate STAT3. After the incubation, cells were lysed and assayed with a luciferase assay. Compared with control, VEGF treatment significantly inhibited Epo promoter activity. Treatment with both VEGF and AG490 restored the transcriptional activity of the Epo promoter, whereas AG490 alone had no effect on Epo gene transcription (Figure 8D).
Altogether, these results are consistent with our findings in vivo; we postulate that hypoxia after repeated oxygen fluctuations causes an increase in Müller cell VEGF expression that triggers activation of STAT3, and activated STAT3 inhibits Epo gene expression by directly binding to and inhibiting the activity of the Epo promoter.
Discussion
ROP is a complex disease that involves genetic37 and environmental factors, including oxygen stresses (eg, repeated fluctuations in oxygen and hyperoxia), inflammation, and oxidative stress.38,39 Peripheral avascular retina and IVNV are two main features in ROP. It has long been proposed that retinal hypoxia from avascular retina caused by a delay in retinal vascular development and constriction of newly developed retinal capillaries leads to pathologic angiogenesis in ROP.40–42 Our laboratory has been interested in the causes of avascular retina. We have focused our studies on environmental effects of oxygen stresses and use a relevant model18 that exposes newborn pups to oxygen stresses similar to human preterm infants with severe ROP. Our studies and those from other laboratories have shown that VEGF signaling,43,44 NADPH oxidase activation,13,36,39 and JAK2/STAT3 signaling24,45 are important components in the pathologic angiogenesis in ROP.
On the basis of previous studies that used the 50/10 OIR model, we found that repeated fluctuations in oxygen increased retinal VEGF in association with persistent peripheral avascular retina before the development of IVNV.21 This raised the question why increased VEGF was unable to support ongoing physiological retinal vascularization but instead caused IVNV. In this study, a series of experiments that used the 50/10 OIR model was performed to identify whether increased VEGF caused by oxygen stresses affected retinal vascularization and what the underlying molecular mechanisms were. After 14 days of repeated fluctuations in oxygen, pups had an obvious delay in retinal vascularization, producing an increased percentage of avascular retina compared with room air-raised counterparts and a concomitant activation in retinal STAT3 but not other STATs, such as STAT1 and STAT5. We previously found that one mechanism for increased avascular retinal area in the 50/10 OIR model was by apoptosis.11 Therefore, we postulated that STAT-induced apoptosis of vascular or supporting cells might be involved in the persistence of avascular retina. We also proposed that VEGF might be involved, because it is an angiogenic growth factor important in retinal vascular development,28,29 so inhibiting it might reduce vascularization. However, we found the STAT3-mediated avascular retina appeared independent of apoptosis or VEGF expression. However, Epo, an important factor in retinal capillary stabilization,7 was reduced by STAT3 activation. It also appeared that the inhibition of Epo was local to the retina and not because of reduced production in liver or kidney caused by systemic inhibition of JAK/STAT signaling.
The use of recombinant human Epo in the preterm infant has been associated with greater severity of ROP.46 However, it also has beneficial effects as a neuroprotective agent in that children who had received recombinant Epo as preterm infants were found to do better on cognitive testing.34 Epo was reported to stabilize retinal endothelial cells in a model of hyperoxia-induced retinopathy,33 but in the same model, small-interfering RNA to Epo given at a different time point reduced the severity of later retinopathy.47 Therefore, controversy exists as to the role of Epo in prematurity and its potential effects in ROP. To elucidate whether increased Epo, as an angiogenic agent, can improve retinal vascularization, exogenous recombinant Epo was given to pups raised in the 50/10 OIR model at early ages (p2, p4, and p6) by i.p. injection. Our results show that pups with increased Epo had greater retinal vascularization and reduced avascular retinal area, supporting the notion that inhibition of retinal Epo by oxygen fluctuations in the 50/10 OIR model might be a cause of delayed retinal vascularization and, therefore, increased avascular retina.
JAK/STAT is a major signaling pathway for a number of growth factors and cytokines. The finding that VEGF was increased in the 50/10 OIR model encouraged us to determine whether VEGF-mediated STAT3 activation would reduce Epo expression. We found evidence to support this prediction after using a neutralizing antibody delivered into the vitreous of pups in the 50/10 OIR model. Further, p-STAT3 colocalized with Müller cells in sections of retina. In cultured rMC-1 cells, we observed VEGF-induced STAT3 phosphorylation at both the tyrosine705 and serine727 sites, which are critical for STAT3 dimerization, nuclear translocation, and transcriptional activity,48–50 decreased Epo gene expression caused by hypoxia. This last observation differs from reports in which hypoxia-induced factor-2 can up-regulate Epo expression31 and may indicate that hypoxia-regulated Epo gene expression varies according to cell type and the activation status of JAK/STAT signaling. As a transcriptional factor, STAT3 regulates gene expression by directly targeting the promoter of genes. In this case, the inhibitory effect of activated STAT3 by VEGF on Epo gene expression may require the recruitment of STAT3 to the Epo promoter. We predicted a STAT3 binding site located at a region in the 5′ promoter of the Epo gene (−164 to −156 from transcriptional start site). We clearly show by ChIP analysis that, in response to hypoxia, STAT3 appears on the promoter of the Epo gene containing the predicted STAT3 binding site. Binding of STAT3 to the Epo promoter was reduced to that of room air levels in the presence of AG490 or neutralizing VEGF antibody. This suggests that the binding of STAT3 to the Epo promoter is induced by hypoxia, depending on VEGF, and that activated p-STAT3 is capable of binding to the promoter of the Epo gene. We further determined that the Epo promoter activity was suppressed by and depended on the activation of STAT3. The results together indicate that the inhibitory effect of VEGF-mediated p-STAT3 on Epo gene transcription directly targets the Epo promoter. A coregulator may also be recruited to the promoter of the Epo gene. In future studies, we will determine what phosphorylation site(s) of STAT3 regulates its binding to the Epo promoter and the effects on potential recruitment of coregulators.
In summary, we demonstrate that an increase in retinal VEGF, sufficient to lead to pathologic avascular retina and IVNV in the 50/10 OIR model, mediated STAT3 activation and inhibited retinal Epo expression. Exogenous Epo provided at early time points in the model, before the development of IVNV, led to reduced avascular retina. However, the timing for Epo treatment appears critical so as not to increase the risk of pathologic angiogenesis, as suggested from studies that used the mouse OIR model49. Clinical studies have reported a reduction in ROP severity after inhibition of VEGF bioactivity with a neutralizing antibody.51 However, the treatment is given late in the course of ROP, and persistent avascular retina remains a concern. In the 50/10 OIR and mouse OIR models, VEGF-neutralizing antibody decreased retinal IVNV but had no significant effect on avascular retina.13,52,53 Our findings suggest that inhibiting VEGF signaling alone may not be sufficient to promote retinal vascularization and that other angiogenic factors, such as Epo, may be important. However, timing appears critical, and further study is needed to determine whether Epo expression during vasoproliferation might lead to later recurrences of IVNV.
Footnotes
Supported by the NIH (grants R01 R01EY015130 and R01EY017011 to M.E.H.) and March of Dimes (grant MOD 6-FY08-590 to M.E.H.).
References
- 1.Chen J., Smith L.E. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 2.The Early Treatment for Retinopathy of Prematurity Cooperative Group The Early Treatment for Retinopathy Of Prematurity Study: structural findings at age 2 years. Br J Ophthalmol. 2006;90:1378–1382. doi: 10.1136/bjo.2006.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaffer D.B., Palmer E.A., Plotsky D.F., Metz H.S., Flynn J.T., Tung B., Hardy R.J. Prognostic factors in the natural course of retinopathy of prematurity. Ophthalmology. 1993;100:230–237. doi: 10.1016/s0161-6420(93)31665-9. [DOI] [PubMed] [Google Scholar]
- 4.Aiello L.P. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997;8:19–31. doi: 10.1097/00055735-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson B., Schachat A. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:915–930. doi: 10.1007/s00417-010-1315-z. [DOI] [PubMed] [Google Scholar]
- 6.Wolf-Schnurrbusch U.E., Ghanem R., Rothenbuehler S.P., Enzmann V., Framme C., Wolf S. Predictors of short-term visual outcome after anti-VEGF therapy of macular edema due to central retinal vein occlusion. Invest Ophthalmol Vis Sci. 2011;52:3334–3337. doi: 10.1167/iovs.10-6097. [DOI] [PubMed] [Google Scholar]
- 7.Chen J., Connor K.M., Aderman C.M., Smith L.E. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lofqvist C., Chen J., Connor K.M., Smith A.C., Aderman C.M., Liu N., Pintar J.E., Ludwig T., Hellstrom A., Smith L.E. From the Cover: iGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci U S A. 2007;104:10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor A.R., Stephenson T., Johnson A., Tobin M.J., Moseley M.J., Ratib S., Ng Y., Fielder A.R. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–18. doi: 10.1542/peds.109.1.12. [DOI] [PubMed] [Google Scholar]
- 10.Chang K.H., Chan-Ling T., McFarland E.L., Afzal A., Pan H., Baxter L.C., Shaw L.C., Caballero S., Sengupta N., Calzi S.L., Sullivan S.M., Grant M.B. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci U S A. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito Y., Geisen P., Uppal A., Hartnett M.E. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007;13:840–853. [PMC free article] [PubMed] [Google Scholar]
- 12.Niesman M.R., Johnson K.A., Penn J.S. Therapeutic effect of liposomal superoxide dismutase in an animal model of retinopathy of prematurity. Neurochem Res. 1997;22:597–605. doi: 10.1023/a:1022474120512. [DOI] [PubMed] [Google Scholar]
- 13.Geisen P., Peterson L.J., Martiniuk D., Uppal A., Saito Y., Hartnett M.E. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Mol Vis. 2008;14:345–357. [PMC free article] [PubMed] [Google Scholar]
- 14.Penn J.S., Henry M.M., Tolman B.L. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham S., Fleck B.W., Elton R.A., Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 16.Hartnett M.E., Martiniuk D.J., Saito Y., Geisen P., Peterson L.J., McColm J.R. Triamcinolone reduces neovascularization. capillary density and IGF-1 receptor phosphorylation in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2006;47:4975–4982. doi: 10.1167/iovs.06-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K., Akula J.D., Falk C., Hansen R.M., Fulton A.B. The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2006;47:2639–2647. doi: 10.1167/iovs.06-0016. [DOI] [PubMed] [Google Scholar]
- 18.Penn J.S., Tolman B.L., Henry M.M. Oxygen-induced retinopathy in the rat: relationship of retinal nonperfusion to subsequent neovascularization. Invest Ophthalmol Vis Sci. 1994;35:3429–3435. [PubMed] [Google Scholar]
- 19.Robinson G.S., Aiello L.P. Angiogenic factors in diabetic ocular disease: mechanisms of today, therapies for tomorrow. Int Ophthalmol Clin. 1998;38:89–102. [PubMed] [Google Scholar]
- 20.Adamis A.P., Miller J.W., Bernal M.T., DAmico D.J., Folkman J., Yeo T.K., Yeo K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 21.Budd S.J., Thompson H., Hartnett M.E. Association of retinal vascular endothelial growth factor with avascular retina in a rat model of retinopathy of prematurity. Arch Ophthalmol. 2010;128:1014–1021. doi: 10.1001/archophthalmol.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y.H., Huang M.L. Organogenesis and tumorigenesis: insight from the JAK/STAT pathway in the Drosophila eye. Dev Dyn. 2010;239:2522–2533. doi: 10.1002/dvdy.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q., Briggs J., Park S., Niu G., Kortylewski M., Zhang S., Gritsko T., Turkson J., Kay H., Semenza G.L., Cheng J.Q., Jove R., Yu H. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 24.Byfield G.E., Budd S., Hartnett M.E. Supplemental oxygen can cause intravitreous neovascularization through JAK/STAT pathways in a model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2009;50:3360–3365. doi: 10.1167/iovs.08-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meydan N., Grunberger T., Dadi H., Shahar M., Arpaia E., Lapidot Z., Leeder J.S., Freedman M., Cohen A., Gazit A., Levitzki A., Roifman C.M. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 26.Banes A.K., Shaw S., Jenkins J.A., Redd H., Amiri F., Pollock D.M., Marrero M.B. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol. 2004;286:F653–F659. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 27.Penn J.S., McCollum G.W., Barnett J.M., Werdich X.Q., Koepke K.A., Rajaratnam V.S. Angiostatic effect of penetrating ocular injury: role of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2006;47:405–414. doi: 10.1167/iovs.05-0673. [DOI] [PubMed] [Google Scholar]
- 28.Chan-Ling T., Gock B., Stone J. The effect of oxygen on vasoformative cell division: evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- 29.Stone J., Itin A., Alon T., Peer J., Gnessin H., Chan-Ling T., Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart A.L., Rifkin L., Amess P.N., Kirkbride V., Townsend J.P., Miller D.H., Lewis S.W., Kingsley D.P.E., Moseley I.F., Foster O., Murray R.M. Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet. 1999;353:1653–1657. doi: 10.1016/s0140-6736(98)07130-x. [DOI] [PubMed] [Google Scholar]
- 31.Morita M., Ohneda O., Yamashita T., Takahashi S., Suzuki N., Nakajima O., Kawauchi S., Ema M., Shibahara S., Udono T., Tomita K., Tamai M., Yamamoto M., Fujii-Kuriyama Y. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–1146. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suk K.K., Dunbar J.A., Liu A., Daher N.S., Leng C.K., Leng J.K., Lim P., Weller S., Fayard E. Human recombinant erythropoietin and the incidence of retinopathy of prematurity: a multiple regression model. J AAPOS. 2008;12:233–238. doi: 10.1016/j.jaapos.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Sears J.E., Hoppe G., Ebrahem Q., Anand-Apte B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. Proc Natl Acad Sci U S A. 2008;105:19898–19903. doi: 10.1073/pnas.0805817105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown M.S., Eichorst D., LaLa-Black B., Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009;124:e681–e687. doi: 10.1542/peds.2008-2701. [DOI] [PubMed] [Google Scholar]
- 35.McColm J.R., Geisen P., Hartnett M.E. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004;10:512–520. [PMC free article] [PubMed] [Google Scholar]
- 36.Saito Y., Uppal A., Byfield G., Budd S., Hartnett M.E. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49:1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bizzarro M.J., Hussain N., Jonsson B., Feng R., Ment L.R., Gruen J.R., Zhang H., Bhandari V. Genetic susceptibility to retinopathy of prematurity. Pediatrics. 2006;118:1858–1863. doi: 10.1542/peds.2006-1088. [DOI] [PubMed] [Google Scholar]
- 38.Cervantes-Munguía R., Espinosa-López L., Gómez-Contreras P., Hernández-Flores G., Domínguez-Rodríguez J., Bravo-Cuéllar A. Retinopathy of prematurity and oxidative stress ]in Spanish] An Pediatr (Barc) 2006;64:126–131. doi: 10.1157/13084171. [DOI] [PubMed] [Google Scholar]
- 39.Al Shabrawey M., Bartoli M., El Remessy A.B., Platt D.H., Matragoon S., Behzadian M.A., Caldwell R.W., Caldwell R.B. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Path. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patz A., Eastham A., Higginbotham D.H., Kleh T. Oxygen studies in retrolental fibroplasia. Am J Ophthalmol. 1953;36:1511–1522. [PubMed] [Google Scholar]
- 41.Michaelson I.C. The mode of development of the vascular system of the retina: With some observations on its significance for certain retinal diseases. Trans Ophthal Soc UK. 1948;68:137–180. [Google Scholar]
- 42.Ashton N., Ward B., Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–430. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aiello L.P., Pierce E.A., Foley E.D., Takagi H., Chen H., Riddle L., Ferrara N., King G.L., Smith L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartnett M.E., Martiniuk D.J., Byfield G.E., Geisen P., Zeng G., Bautch V.L. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49:3107–3114. doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartoli M., Platt D., Lemtalsi T., Gu X., Brooks S.E., Marrero M.B., Caldwell R.B. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 2003;17:1562–1564. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- 46.Brown M.S., Baron A.E., France E.K., Hamman R.F. Association between higher cumulative doses of recombinant erythropoietin and risk for retinopathy of prematurity. J AAPOS. 2006;10:143–149. doi: 10.1016/j.jaapos.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Chen J., Connor K.M., Aderman C.M., Willett K.L., Aspegren O.P., Smith L.E. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yahata Y., Shirakata Y., Tokumaru S., Yamasaki K., Sayama K., Hanakawa Y., Detmar M., Hashimoto K. Nuclear translocation of phosphorylated STAT3 Is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem. 2003;278:40026–40031. doi: 10.1074/jbc.M301866200. [DOI] [PubMed] [Google Scholar]
- 49.Samardzija M., Wenzel A., Aufenberg S., Thiersch M., Reme C., Grimm C. Differential role of Jak-STAT signaling in retinal degenerations. FASEB J. 2006;20:2411–2413. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- 50.Rawlings J.S., Rosler K.M., Harrison D.A. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 51.Mintz-Hittner H.A., Kuffel R.R., Jr Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008;28:831–838. doi: 10.1097/IAE.0b013e318177f934. [DOI] [PubMed] [Google Scholar]
- 52.Budd S., Byfield G., Martiniuk D., Geisen P., Hartnett M.E. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res. 2009;89:718–727. doi: 10.1016/j.exer.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sone H., Kawakami Y., Kumagai A.K., Okuda Y., Sekine Y., Honmura S., Segawa T., Suzuki H., Yamashita K., Yamada N. Effects of intraocular or systemic administration of neutralizing antibody against vascular endothelial growth factor on the murine experimental model of retinopathy. Life Sci. 1999;65:2573–2580. doi: 10.1016/s0024-3205(99)00526-3. [DOI] [PubMed] [Google Scholar]