Abstract
Morphine increases the susceptibility to opportunistic infection by attenuating bacterial clearance through inhibition of Fcγ receptor (FcgR)–mediated phagocytosis. Mechanisms by which morphine inhibits this process remain to be investigated. Actin polymerization is essential for FcgR-mediated internalization; therefore, disruption of the signaling mechanisms involved in this process is detrimental to the phagocytic ability of macrophages. To our knowledge, this study is the first to propose the modulation of actin polymerization and upstream signaling effectors [cAMP, Rac1-GTP, and p38 mitogen-activated protein kinase (MAPK)] as key mechanisms by which morphine leads to inhibition of pathogen clearance. Our results indicate that long-term morphine treatment in vitro and in vivo, through activation of the μ-opioid receptor, leads to an increase in intracellular cAMP, activation of protein kinase A, and inhibition of Rac1-GTPase and p38 MAPK, thereby attenuating actin polymerization and reducing membrane ruffling. Furthermore, because of long-term morphine treatment, FcgR-mediated internalization of opsonized dextran beads is also reduced. Morphine's inhibition of Rac1-GTPase activation is abolished in J774 macrophages transfected with constitutively active pcDNA3-EGFP-Rac1-Q61L plasmid. Dibutyryl-cAMP inhibits, whereas H89 restores, activation of Rac-GTPase and abolishes morphine's inhibitory effect, implicating cAMP as the key effector in morphine's modulation of actin polymerization. These findings indicate that long-term morphine treatment, by increasing intracellular cAMP and activating protein kinase A, leads to inhibition of Rac1-GTPase and p38 MAPK, causing attenuation of actin polymerization, FcgR-mediated phagocytosis, and decreased bacterial clearance.
Opioid use and abuse steadily increased through the 1990s and has continued to increase over recent years.1,2 The 2004 National Survey on Drug Use and Health shows that between 1999 and 2001, the annual incidence of opioid analgesic abuse increased from 628,000 initiates in 1990 to 2.4 million initiates in 2001.3 It is well established that long-term opioid use or abuse results in severe immunosuppression and increased susceptibility to infection.4–6 Long-term morphine use has modulated the innate immune system through a decrease in the proliferative capacity of macrophage progenitor cells and lymphocytes7 by inhibiting macrophage phagocytic8,9 and migratory capabilities,10 leading to increased risk of sepsis in mice.5,6 Similar studies11 show that morphine's inhibition of pro-inflammatory cytokines can be overcome by addition of untreated macrophages, suggesting that morphine-induced immunosuppression is due to a deficit in macrophage function. Despite these deleterious consequences of opioid abuse, morphine and other opioid-based pain relievers remain widely prescribed and abused worldwide.12 There is clearly an urgent need to delineate the underlying cellular and molecular mechanisms by which long-term opiate use or abuse increases susceptibility to bacterial infection. Understanding these mechanisms will allow for the development of novel approaches to treat and prevent bacterial infection in the population abusing opiates and in patients who receive opioids for pain management.
Innate immunity has a crucial role in clearance of bacterial infections. Phagocytes, such as macrophages, play an essential role as the first line of defense against microbial pathogens by using their phagocytic abilities to facilitate bacterial clearance and elimination. To internalize a wide variety of pathogens, macrophages have evolved a diverse set of phagocytic receptors, such as the mannose receptor, the scavenger receptor, the complement receptor, and Fcγ receptors (FcgRs). FcgRs are the most important Fc receptors for inducing phagocytosis of opsonized microbes.13 They facilitate internalization of opsonized extracellular bacteria by recognizing the Fc region of the IgG antibody coating (opsonizing) the surface of the pathogen. Cross-linking of Fcγ-activating receptors by immune complexes leads to tyrosine phosphorylation and a signal transduction cascade, ultimately resulting in activation of Rho GTPases, which are molecular switches that control the organization of the actin cytoskeleton and are essential for actin polymerization, membrane extension, and pathogen engulfment.14 Interestingly, opioid abuse has increased the incidence of extracellular bacterial infections, such as Streptococcus pneumoniae15 and Enterococcus faecalis16; however, mechanisms underlying this phenomenon remain to be explored. We tested the hypothesis that morphine treatment causes inhibition of actin polymerization, leading to attenuated FcgR-mediated phagocytosis and diminished clearance of invading bacterial pathogens. We also investigated the molecular mechanisms by which morphine modulates actin polymerization, leading to inhibition of FcgR-mediated phagocytosis. Signaling pathways involving cAMP, protein kinase A (PKA), Rho GTPases, and mitogen-activated protein kinases (MAPKs) were examined as potential mechanisms of morphine's action.
Intracellular cAMP plays an important role in mediating signaling downstream from the μ-opioid receptor (MOR). Long-term morphine treatment activates G proteins that lead to increased levels of cAMP.17 Although elevation of cAMP in lymphocytes leads to immune suppression by a PKA-dependent pathway,18 the effects of morphine induction of cAMP on actin polymerization in macrophages have not been investigated. Therefore, we examined if the morphine-mediated increase in cAMP is instrumental to inhibition of actin polymerization and phagocytosis. Furthermore, recent studies19 show that MAPKs, such as p38 and the related extracellular signal–regulated kinase 1/2 (ERK1/2), are activated and play an important role during neutrophil phagocytosis. We examined the role of these kinases in macrophage phagocytosis and whether morphine modulates p38 MAPK and ERK1/2 phosphorylation to inhibit actin polymerization. Our results indicate that morphine, by elevating cAMP, acts through a PKA-dependent mechanism to inhibit activation of Rac1-GTP, causing inhibition of p38 MAPK and ultimately attenuating actin polymerization and opsonophagocytosis.
Materials and Methods
Reagents
Heat-killed Escherichia coli particles [E2861: E. coli (K-12 strain) BioParticles, fluorescin conjugate (excitation, 494 nm; emission, 518 nm)], opsonizing reagent (E2870), dextran beads (1-μm yellow-green fluorescent Fluo-Spheres; F8852 (Molecular Probes, Eugene, OR); excitation, 488 nm; emission, 518 nm), and rhodamine phalloidin (R415) were obtained from Molecular Probes. DAPI, cytochalasin D (CytD), H89, DB-cAMP, anisomycin, PD98059, and SB203580 were obtained from Sigma-Aldrich (St. Louis, MO). Antibodies used in fluorescence-activated cell sorter (FACS) analysis included the following: anti-mouse CD64 a and b (phosphatidylethanolamine conjugated, catalogue number 558455; BD Pharmingen, Sparks, MD), anti-mouse F4/80 [fluorescein isothiocyanate (FITC) conjugated, catalogue number 11–4801; eBioscience, San Diego, CA], and anti-mouse CD16/CD32 FITC-conjugated (catalogue number 11-0161; eBioscience). A cAMP detection kit from R&D Systems, Minneapolis, MN (catalogue number KGE002), Rac-GTP G-protein ELISA kits (catalogue number BK125), and the Rac-GTP pull-down kit (catalogue number BK035) were obtained from Cytoskeleton (Denver, CO). Morphine HCl powder and 75-mg slow-release pellets were a gift from the National Institute on Drug Abuse.
Cells
The macrophage cell line J774.1 was obtained from American Type Culture Collection (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin (all from Gibco, Grand Island, NY) under standard conditions for cell growth.
Animals
MOR knockout (MORKO) mice (C57BL/6 × 129/Ola genetic background) were produced as previously described by Loh and colleagues.20 Briefly, a XhoI/XbaI fragment, which spans exons 2 and 3, was replaced with a Neor cassette, followed by the ligation of a thymidine kinase expression cassette to the 3′ end of this segment. Wild-type (WT) mice (B6129PF1/J), aged 8 weeks, were obtained from the Jackson Laboratory (Bar Harbor, ME). Animal studies have been reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Primary Macrophages
Primary peritoneal macrophages were obtained from WT or MORKO male mice aged 6 to 8 weeks; peritoneum was lavaged. The cells were collected and plated in 96-well plates in serum-free media for 30 minutes; nonadherent cells were washed with PBS; and remaining adherent macrophages were maintained in enriched DMEM, as previously described, with or without 1 μmol/L morphine, where appropriate (to avoid morphine withdrawal), and used for further experimentation.
Long-Term Morphine Treatment
For all in vitro experiments, 1 μmol/L morphine HCl was added overnight (18 hours). For studies involving morphine treatment in vivo, mice administered morphine were implanted with 75 mg of slow-release morphine or placebo pellets for 72 hours. During the extraction of peritoneal cells from morphine-treated WT or MORKO mice, 1 μmol/L morphine was maintained in all PBS and media used in the experiment to prevent withdrawal. Concentrations used in the in vitro paradigm were chosen to closely replicate morphine plasma levels (11 to 1440 ng/mL), which are present in patients undergoing morphine sulfate treatment (2.5 to 90 mg every 4 hours),21 as well as mice after 72-hour implantation with 75-mg morphine pellets.
In Vivo Phagocytosis Assay
Mice were treated in vivo with morphine (as previously described), and 30 minutes before sacrifice, they were injected with heat-killed, FITC-labeled E. coli BioParticles. Macrophages were collected from peritoneal lavage (as previously described) and washed with 50% trypan blue to extinguish fluorescence of noninternalized particles. Cells were plated in 96-well plates, left to adhere for 30 minutes in serum-free DMEM, washed, stained with DAPI, and quantified using the fluorometric assay.
Fluorometric Assay
Cells were plated in 96-well plates (10,000 cells per well), treated with morphine, and cultured overnight in standard growing conditions (37°C, 10% CO2, 80% Rh). The following day, fluorescent (FITC-conjugated) opsonized dextran (OPDex) beads or heat-killed opsonized (HKO) E. coli was added (1:20, cell:bacteria/bead ratio). Beads or bacteria were opsonized with IgG opsonizing reagent (Invitrogen) for 1 hour at 37°C, according to the manufacturer's instructions. Phagocytosis was conducted at varied time points and was stopped by addition of trypan blue, which extinguishes fluorescence of noninternalized particles. Cells were washed two times with PBS and stained with DAPI. Fluorescence was recorded using a fluorescence plate reader (FLUOstar Omega; BMGLabtech, Offenburg, Germany) at an excitation of 485 nm and an emission of 520 nm (FITC) and an excitation of 355 nm and an emission of 460 nm (DAPI). Data were quantified as follows: Phagocytic Index = FITC/DAPI [both given in relative fluorescence units (RFUs)], indicative of particle fluorescence per cell. In actin polymerization experiments, cells were treated similar to that previously described, washed, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained with rhodamine phalloidin (Invitrogen), according to the manufacturer's protocol. After rhodamine, cells were stained with DAPI and fluorescence was measured using the fluorescence plate reader. Actin polymerization was quantified as ratio of rhodamine (RFU) (excitation, 544 nm; emission, 590 nm)/DAPI (RFU), indicative of actin polymerization per cell. Data were expressed as percentage of vehicle control.
Confocal Microscopy
Following the same treatment as previously described, cells were stained with rhodamine phalloidin according to the manufacturer's instructions. Images were taken using a Nikon inverted confocal microscope (model Ti-E eclipse 100) and a Roper camera (model Cool-snap HQQ) (both from Nikon Instuments, Elgin, IL) at ×60, with additional digital magnification. Images shown are the flattened sum of 15 cross sections.
Pull-Down and GLISA Assays
The J774 cells were plated at 500,000 cells per 10 mL of supplemented DMEM (10-cm Petri dish) and cultured for 2 days. On the second day, morphine was added; on the third day, cells were treated with OPDex beads (1:20 ratio) for 30 minutes. Cells were washed, collected, and analyzed according to the manufacturer's instructions. Briefly, cell lysates were incubated with PAK-PBD beads and allowed to pull down the PAK-PBD/GTP-Rac complex. The amount of activated Rac1 is determined by using Western blot analysis with a Rac1-specific antibody. GTPγS is a nonhydrolyzable GTP analog used as a positive control, and GDP is used as a negative control. Samples analyzed via GLISA assay were prepared as previously described. Protein, 0.7 μg/μL, was added per well; Rac-control protein, 0.2 μg/μL, was used as a positive control.
Plasmid Transfection
Addgene plasmid 12981:pcDNA3-EGFP-Rac1-Q61L and pcDNA3-Flag MKK6(glu) were obtained from Addgene (Cambridge, MA), donated by Dr. Gary Bokoch (Scripps Research Institute, La Jolla, CA)22 and Dr. Roger Davis (University of Massachusetts Medical School, Worcester, MA),23 respectively. The plasmids were transfected using Fugene reagent (catalogue number 04-709-705-001; Roche, Basel, Switzerland), according to the manufacturer's protocol. The next day after the transfection, cells were collected, plated, and treated with 1 μmol/L morphine overnight and HKO E. coli (Texas Red), as previously described.
Results
Long–Term Morphine Inhibits Macrophage Phagocytosis in Vitro and in Vivo
Macrophages play a crucial role in morphine–induced immunosuppression.11 Because morphine has been implicated in increased susceptibility to infections by extracellular pathogens and FcgR is the key phagocytic receptor for clearance of extracellular pathogens, we investigated how morphine modulates FcgR-mediated phagocytosis.
To determine the effects of long-term morphine treatment on bacterial clearance mediated by FcgR, macrophages (cell line J774) undergoing long-term morphine treatment (1 μmol/L overnight) were allowed to phagocytose live opsonized green fluorescent protein (GFP)–tagged E. coli for 30 minutes. After extensive washing, fixation (4% paraformaldehyde), and permeabilization (acetone), cells were stained with rhodamine phalloidin (red, actin) and DAPI (blue, nucleus); analyzed via confocal microscopy (Figure 1A); and quantified for pixel density (Figure 1B). Confocal microscopy demonstrates that J774 cells undergoing long-term morphine treatment display a significant inhibition of internalization (≥50% inhibition) of live opsonized GFP-tagged E. coli (at 30 minutes) when compared with the vehicle-treated cells.
Figure 1.
Long-term morphine leads to inhibition of FcgR-mediated phagocytosis by the J774 macrophage cell line. A: After undergoing long-term morphine treatment (1 μmol/L overnight) and 30-minute incubation with live opsonized GFP-tagged E. coli, J774 cells were fixed, stained, and analyzed via confocal microscopy using Nikon EZ-C1 3.90 software. Original magnification, ×60. Scale bar = 10 μm. Red indicates rhodamine (actin polymerization); GFP, E. coli; DAPI, nucleus. Images were quantified for pixel density using ImageJ software. B: Phagocytic index = GFP pixel density/DAPI pixel density. C: J774 cells were treated with 10 μmol/L naltrexone (Sigma-Aldrich) 2 hours before overnight morphine treatment; both were maintained overnight, and phagocytosis was examined the next day using fluorometric analysis, after a 60-minute incubation with OPDex beads. D: After 72 hours of in vivo morphine treatment, in vivo phagocytosis was examined 30 minutes after the i.p. injection of HKO E. coli (WT and MORKO mice). The data illustrate the average phagocytic index for five mice for each treatment. To avoid withdrawal, morphine was maintained in washes and media were used during the macrophage extraction process (for mice treated with morphine in vivo). Phagocytosis was assessed using fluorometric analysis. E and F: Cells undergoing morphine/vehicle treatment and phagocytosis of HKO E. coli (Texas Red conjugated), recorded in Supplemental Video S1 (available at http://ajp.amjpathol.org), were quantified for internalization, where the phagocytic index is defined as the intensity of red fluorescence per cell (and expressed as percentage of vehicle control) (E) or cell elongation (defined as change in length = longest length - shortest length observed in 30 minutes of phagocytosis) (F). Fifteen cells per treatment were recorded using Nikon NIS Elements AR 3.2 software. G and H: FACS analysis of morphine modulation of FcgR1 a/b (anti-mouse CD64 a and b–phosphatidylethanolamine conjugated; BD Pharmingen) and macrophage marker anti-mouse F4/80 (FITC conjugated; eBioscience) (G) and FcgR2 and FcgR3 surface expression (anti-mouse CD16/CD32 FITC; eBioscience) (H). Left panels: Vehicle treatment. Middle panels: Morphine treatment. Right panels: Graphical representation of receptor expression in three independent experiments. Data were collected using Guava EasyCyte and quantified using Guava cell plus software. Significance was determined using the Student's t-test. **P < 0.01 and ***P < 0.001.
To study the mechanisms involved in this process, without the potential confounding effects of bacterial toxins and lipopolysaccharides, IgG-opsonized FITC-labeled dextran (OPDex) beads were used to mimic internalized pathogen. Similar to phagocytosis with E. coli, long-term morphine treatment led to a decrease in internalization of OPDex beads, as demonstrated through a reduction in the phagocytic index in a time-dependent manner (see Supplemental Figure S1A at http://ajp.amjpathol.org), with maximal inhibition at 60 minutes (Figure 1C). Morphine's attenuation of internalization was limited to opsonized particles because phagocytosis of unopsonized particles was not altered with morphine treatment (see Supplemental Figure S1B at http://ajp.amjpathol.org). In addition, morphine's inhibitory effect was abolished in cells pretreated with naltrexone (10 μmol/L) 2 hours before the addition of morphine (Figure 1C). These findings indicate that morphine's inhibitory effect in J774 cells is mediated by classic opioid receptors.
We next investigated the effect of in vivo long-term morphine treatment on phagocytosis using an in vivo phagocytosis assay (as described in Materials and Methods). WT and MORKO mice were implanted with slow-releasing 75-mg morphine or placebo pellets. Thirty minutes before sacrifice, mice were i.p. injected with HKO E. coli. Peritoneal lavage cells were washed in trypan blue to extinguish fluorescence of noninternalized HKO E. coli, and peritoneal macrophages were isolated using standard protocols. Phagocytosis of HKO E. coli was inhibited in primary peritoneal macrophages isolated from morphine-treated WT mice. This effect was abolished in peritoneal macrophages harvested from MORKO mice (Figure 1D). These data indicate that the phagocytic ability of primary peritoneal macrophages is significantly reduced in mice undergoing in vivo long-term morphine treatment and is mediated by MOR, as shown by the lack of morphine-induced inhibition in macrophages extracted from the MORKO mice. These findings were further confirmed using time-lapse imaging, during which we observed that, over 60 minutes of phagocytosis of Texas Red–labeled HKO E. coli (Invitrogen), J774 cells that underwent long-term morphine treatment displayed significantly impaired bacterial clearance (Figure 1E; see also Supplemental Video S1 at http://ajp.amjpathol.org) and reduced cell elongation (Figure 1F; see also Supplemental Video S1 at http://ajp.amjpathol.org).
To examine if morphine's attenuation of phagocytosis is due to modulation of FcgR surface expression, we conducted FACS analysis of morphine-treated J774 macrophages (Figure 1, G and H). FACS analysis determined that expression of key phagocytic receptors, such as FcgR1 a/b (Figure 1G), FcgR2, and FcgR 3 (Figure 1H), was not altered in J774 cells after morphine treatment, indicating that morphine modulation of FcgR is not involved in morphine- induced inhibition of phagocytosis. Based on this evidence, we speculate that cross talk between the MOR and FcgR signaling pathways must be downstream from the FcgRs. Considering recent reports24–26 that indicate that morphine induces macrophage apoptosis, the effects of morphine on cell viability were investigated. An MTT assay was used to quantify cell viability after morphine treatment or OPDex bead exposure (see Supplemental Figure S1C at http://ajp.amjpathol.org). The data indicate that, in this model, there were no changes in cell viability with morphine or OPDex bead treatments.
Together, these findings show that morphine-induced inhibition of phagocytosis observed in the macrophage cell line J774 can be replicated in primary macrophages, and that morphine's inhibitory effects on phagocytosis are not due to changes in FcgR expression or changes in cell viability. Furthermore, we also demonstrate, via QT-PCR, that primary and J774 macrophages display comparable MOR and FcgR expression levels, expressed as ratio of MOR/FcgR (see Supplemental Figure S1H at http://ajp.amjpathol.org). Therefore, all subsequent mechanistic studies were conducted in J774 cells using OPDex beads.
Long-Term Morphine Treatment Inhibits Phagocytosis by Inhibiting Actin Polymerization
Remodeling of the actin cytoskeleton is a prerequisite for FcgR-mediated phagocytosis.27,28 Actin polymerization enables formation of the phagocytic cup, leading to the subsequent internalization of the phagocytic target and phagosome maturation.28 In our initial studies using time-lapse imaging (see Supplemental Video S1 at http://ajp.amjpathol.org), we observed that cells undergoing long-term morphine treatment, in addition to a reduced ability to internalize bacteria, displayed profound defects in cell motility and elongation (Figure 1F) during phagocytosis of HKO E. coli. When exposed to long-term morphine treatment, cells were more rounded and had decreased motility when compared with the vehicle-treated cells, which were more spindlelike and had greater cell motility (see Supplemental Video S1 at http://ajp.amjpathol.org). These observations of changes in phagocytosis and cell elongation indicate that morphine may play a role in modulation of actin polymerization.
To examine the function of actin polymerization in morphine-mediated attenuation of phagocytosis, we used CytD, a known inhibitor of actin polymerization. As expected, pretreatment by CytD (10 μmol/L) abolished J774 macrophage phagocytosis of both vehicle- and morphine-treated cells (Figure 2A). The dramatic decrease in the phagocytic rate after CytD treatment confirms the essential role of actin in FcgR-mediated phagocytosis.
Figure 2.
Long-term morphine treatment of the J774 cells inhibits actin polymerization. A: Cells were treated overnight with 1 μmol/L morphine and with CytD (10 μmol/L) 10 minutes before the addition of OPDex particles. The phagocytic index was assayed using fluorometry, as previously described. B: Confocal microscopic analysis after long-term morphine (1 μmol/L) and phagocytosis of OPDex beads (60 minutes), fixation (4% paraformaldehyde), permeabilization (0.01% Triton X-100), and staining for actin using rhodamine phalloidin (Invitrogen), according to the manufacturer's instructions. The arrows indicate changes in lamellipodia; arrowheads, changes in membrane ruffling. The black-and-white image was inverted from the rhodamine stain. Scale bar = 10 μm. C: Fluorometric analysis of actin polymerization in J774 cells treated with naltrexone and morphine, followed by 60 minutes of phagocytosis of OPDex beads. D and E: Actin polymerization in primary macrophages from WT (D) or MORKO (E) mice treated in vivo with morphine/placebo (72-hour slow-release pellet) and phagocytosis of HKO E. coli ex vivo. F and G: J774 cells were treated as previously described, after the addition of OPDex bead phagocytosis was synchronized by centrifugation (3 minutes at 0.6 g) and analyzed in a time course for phagocytosis (F) and actin polymerization (G). Each time point was stopped by the addition of trypan blue, washing, and paraformaldehyde. Actin polymerization (%) = % actin polymerization index compared with vehicle control (at 0 minutes). Actin polymerization index = rhodamine (RFU)/DAPI (RFU). Significance was determined using the Student's t-test. *P < 0.01, **P < 0.001, and ***P < 0.0001.
Confocal microscopic analysis of cells undergoing phagocytosis after long-term morphine treatment exhibited a reduction in membrane ruffling (Figure 2B), as well as changes in lamellipodial protrusions (Figure 2B) compared with vehicle controls. Furthermore, morphine-treated cells displayed a reduction in overall intensity of rhodamine phalloidin staining (Figure 2B), suggesting a decrease in actin polymerization. A similar inhibition with morphine treatment was observed in fluorometric analysis, in J774 cells treated with morphine for a long time (see Supplemental Figure S1D at http://ajp.amjpathol.org) exposed for 60 minutes to OPDex beads (Figure 2C). Morphine modulation of actin polymerization was only observed with opsonized particles (see Supplemental Figure S1E at http://ajp.amjpathol.org), and antagonized by naltrexone pretreatment (Figure 2C). The role of MOR in morphine's inhibitory effect on actin polymerization was further confirmed using macrophages isolated from MORKO mice. Morphine's inhibition of actin polymerization, observed in WT macrophages (Figure 2D), was abolished in primary macrophages extracted from MORKO mice (Figure 2E). These data indicate the crucial involvement of classic opioid receptors (namely, MOR) in modulation of actin polymerization.
Phagocytosis was also analyzed after synchronization using cytospin, which allowed for phagocytosis to begin at a fixed time. Cells were treated with morphine (previously described), and immediately after the addition of OPDex beads, they were centrifuged to synchronize the beginning of phagocytosis (with the starting point determined as time 0 minutes after centrifugation). Actin polymerization is rapidly increased at early time points after synchronization. Results show that morphine does not merely delay, but rather inhibits, the process of phagocytosis (Figure 2F) and actin polymerization (Figure 2G) at each time point.
Long-Term Morphine Inhibits Actin Polymerization by Modulating Activation of Rac-GTPases
Rho GTPases, such as Rac and Cdc42, play an essential role in actin polymerization by modulating membrane ruffling and lamellipodial protrusions.29 Because morphine leads to defects in cell elongation (Figure 1F) and membrane ruffling (Figure 2B), we investigated if long-term morphine inhibits activation of Rho GTPases. Long-term morphine treatment significantly inhibits activation of Rac1-GTPase in cells undergoing phagocytosis of OPDex beads, as shown in the pull-down assay (Figure 3A). In contrast, Cdc42 (see Supplemental Figure S1F at http://ajp.amjpathol.org) was only marginally affected by long-term morphine treatment; therefore, we focused on Rac1-GTPase in all of our subsequent studies.
Figure 3.
Constitutive expression of Rac-1 GTP abolishes morphine-mediated inhibition of actin polymerization and phagocytosis. A: Analysis of Rac1-GTPase activation using the pull-down assay. Cells undergoing long-term morphine treatment were exposed to OPDex beads for 30 minutes. Following the manufacturer's protocol, they were washed, lysed, and analyzed for Rac1-GTP activation. GDP, negative control; GTPγS, positive control; densitometry analysis of Rac1-GTP/total Rac-1 (input) expressed as % control. Top row, pull-down Rac1-GTP; middle row, Western blot (WB)–total Rac1-input; bottom row, WB loading control for input. The data shown indicate representative graphs from three independent experiments. B–D: The J774 cells were transfected with constitutively active pcDNA-EGFP-Rac-1-GTP or pcDNA3 empty vector control using Fugene reagent (Roche). Second day cells were treated with morphine overnight (1 μmol/L), and third day cells were treated with opsonized HKO E. coli (Texas Red conjugated) for 30 minutes (B) or opsonized or unopsonized dextran bead for 60 minutes (C and D), fixed and stained with DAPI, and analyzed for phagocytosis using fluorescence microscopy (scale bar = 10 μm) (B) or quantified for phagocytosis (C) or actin polymerization (D) using fluorometric analysis. Microscope Nikon EZ-C1 3.90 software. Original magnification, ×120. Fluorophore, GFP-Rac1–transfected cells; rhodamine, actin polymerization; Texas Red, HKOP E. coli; DAPI, nucleus. Data shown indicate representative graphs from three independent experiments. Significance was determined using the Student's t-test. **P < 0.01, ***P < 0.001.
To further establish the role of Rac1-GTPase in this process, we examined the effects of morphine on J774 cells expressing constitutively active Rac1. Cells transfected with Rac1-Q61L, in the absence of phagocytic stimuli, displayed a distinct increase in cell spreading and actin polymerization, which was absent in the pcDNA3 control (see Supplemental Figure S1G at http://ajp.amjpathol.org). These observations are in accordance with the literature because Rac1-GTPase activation has been implicated in increased formation of lamellipodia and membrane ruffling.29 Therefore, it was not surprising that constitutive activation of Rac1-GTPase resulted in increased actin polymerization, despite the absence of phagocytic stimuli.
By using both fluorescence microscopy (Figure 3B) and fluorometry (Figure 3C), we demonstrate that morphine treatment resulted in inhibition of opsonophagocytosis in control pcDNA3-transfected cells (Figure 3B), whereas cells transfected with constitutively active Rac1-GTPase (pcDNA3-EGFP-Rac1-Q61L plasmid) rescued morphine's inhibitory effect (Figure 3B). Similarly, the transfection of constitutively active Rac1-Q61L plasmid abolished morphine-mediated inhibition of actin polymerization (Figure 3D).
Because Rac1-Q61L expression can also increase particle uptake through macropinocytosis, independent of FcgR, we examined if our findings are potentially confounded by this process. The role of macropinocytosis was tested by comparison of internalization of opsonized with unopsonized particles by cells expressing Rac1-Q61L plasmid. Cells phagocytizing unopsonized particles were not affected by long-term morphine treatment in either Rac-Q61L– or pcDNA3-transfected cells (Figure 3, C and D). This finding indicates that macropinocytosis is not contributing to morphine's inhibitory effect and not confounding our conclusion that Rac overexpression rescues morphine's effect on phagocytosis.
Because constitutive expression of Rac1-GTP overrode morphine's inhibition of actin polymerization and phagocytosis, we conclude that morphine attenuates actin polymerization by inhibiting Rac1-GTPase activation, thus leading to inhibition of phagocytosis.
Inhibition of Actin Polymerization by Long-Term Morphine Treatment Is Mediated by cAMP and PKA
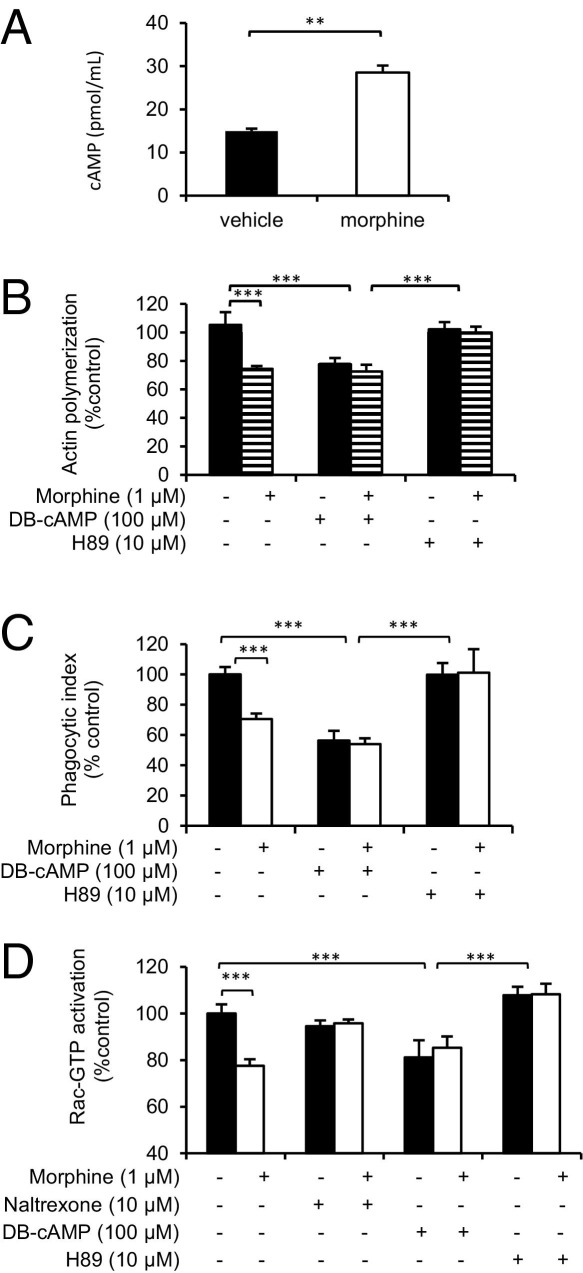
We have previously shown that morphine up-regulates cAMP levels in macrophages.30 Therefore, we investigated cAMP as a potential mechanism by which morphine modulates actin polymerization and phagocytosis. As expected, long-term morphine treatment of macrophage cell line J774 led to a significant increase in cAMP (Figure 4A). To directly observe the effects of elevated cAMP, we used DB-cAMP, a cell-permeable cAMP analog. After a 15-minute incubation with DB-cAMP (100 μmol/L) before the addition of OPDex beads, both actin polymerization (Figure 4B) and phagocytosis (Figure 4C) of OPDex beads were inhibited in vehicle and morphine treatments, further indicating that morphine is modulating FcgR phagocytosis via a cAMP-dependent mechanism.
Figure 4.
Long-term morphine treatment leads to an increase in intracellular cAMP, resulting in inhibition of actin polymerization and phagocytosis. A: J774 cells were treated overnight with morphine, washed in PBS, centrifuged, resuspended at a final density of 1 × 107 cells/mL, and lysed in cell lysis buffer. The supernatant of lysed cells was analyzed using a cAMP assay kit (R&D Systems). To determine the effects of the morphine-induced increase in cAMP and PKA on phagocytosis, cells were treated with DB-cAMP (100 μmol/L) (B–D) for 10 minutes after long-term morphine treatment before the addition of dextran beads; H89 (10 μmol/L, PKA inhibitor) (B–D) was added 2 hours before long-term morphine treatment. Actin polymerization (B) and phagocytosis (C) were assessed using fluorometric analysis, and activation of Rac-GTP (D) was quantified using a GLISA assay (Cytokine, Denver, CO). D: J774 cells were grown for 3 days to confluence of 70% and treated with morphine, DB-cAMP, H89, and OPDex beads, as previously described. Samples were collected (0.7 mg/mL of protein/well) and processed per manufacturer's protocol and assessed for Rac1 activation using Rac1,2,3- GLISA. Significance was determined using the Student's t-test. **P < 0.01, and ***P < 0.001.
To examine downstream targets of cAMP and their role in this process, we used H89, a known inhibitor of PKA. Pretreating J774 cells with H89 (10 μmol/L) 2 hours before the addition of morphine abolished morphine's inhibitory effect on actin polymerization (Figure 4B) and phagocytosis of OPDex beads (Figure 4C).
The GLISA assay (Figure 4D) further supports our finding that morphine inhibits Rac-GTPase activation and that it does so in a naltrexone-reversible manner, indicating involvement of classic opioid receptors in inhibition of Rac-GTPase. Furthermore, DB-cAMP treatment of cells before OPDex phagocytosis led to inhibition of Rac-GTPase activity in both morphine- and vehicle-treated cells, as confirmed by the GLISA assay (Figure 4D). In addition, cells pretreated with H89 (previously described) displayed an absence of morphine's suppression of Rac-GTPase activation, indicating that H89 pretreatment, or inhibition of PKA, abolishes morphine's inhibition of Rac-GTPase activation. Therefore, morphine, through MOR activation, activates adenylyl cyclase, increases cAMP, and activates PKA, leading to inhibition of Rac-GTPase activation, actin polymerization, and subsequent phagocytosis.
Inhibition of Actin Polymerization by Long-Term Morphine Is Mediated by p38 MAPK
p38 MAPK is a modulator of actin polymerization downstream of Rac1 and plays an important role in regulation of macrophage phagocytosis.31 Therefore, morphine modulation of this pathway may be one of the mechanisms by which morphine treatment leads to inhibition of actin polymerization and phagocytosis.
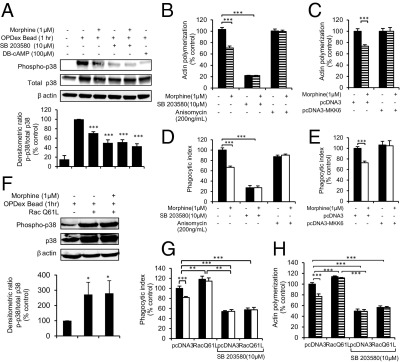
To study if long-term morphine treatment modulates activation of p38 MAPK, J774 cells were pretreated with 10 μmol/L SB203580 (a p38 MAPK inhibitor) for 4 hours before overnight morphine (1 μmol/L) incubation. SB203580 was maintained overnight, along with morphine, followed by OPDex bead treatment for 60 minutes the next day. Western blot analysis (Figure 5A) indicates that phagocytosis of OPDex beads increases phosphorylation of p38 MAPK. Morphine treatment significantly reduced, whereas SB203580 further decreased, phosphorylation of p38 MAPK in both vehicle- and morphine-treated cells. Furthermore, DB-cAMP treatment (previously described) reduced p38 MAPK phosphorylation, indicating that p38 is downstream and that morphine treatment, by increasing intracellular cAMP, leads to inhibition of p38 MAPK. In addition, J774 cells were assessed for actin polymerization (Figure 5B) and phagocytosis (Figure 5D), following the same treatment as previously described. The addition of SB203580 led to inhibition of actin polymerization (Figure 5B) and phagocytosis (Figure 5D). The inhibition of p38 MAPK reduced phagocytic levels to those of cells treated with CytD (20% of control, Figure 2A), indicating strong attenuation of actin polymerization. This highlights the crucial role of p38 MAPK in actin polymerization and phagocytosis. To examine if morphine acts through p38 MAPK to inhibit actin polymerization, we treated cells with anisomycin, a known activator of p38 MAPK.32 Anisomycin activated p38 MAPK and overrode morphine's inhibition of actin polymerization (Figure 5B) and phagocytosis (Figure 5D). These findings were further confirmed by transfection of constitutively active MKK6 [pcDNA3-Flag MKK6(glu)], an upstream activator of p38 MAPK. As expected, the activation of p38 MAPK by transfection of constitutively active MKK6 overrode the inhibitory effect of morphine on actin polymerization (Figure 5C) and phagocytosis (Figure 5E). Taken together, these data indicate that morphine, by inhibiting p38 MAPK phosphorylation, leads to the inhibition of actin polymerization and macrophage pathogen internalization.
Figure 5.
Long-term morphine inhibits phagocytosis and actin polymerization by inhibiting p38 MAPK phosphorylation. A: Western blot analysis of p38 MAPK activation. The bar graph indicates densitometry analysis of the blot below comparing the ratio between phosphorylated p38 and total p38. Densitometry analysis of the Western blot represents the average of three independent experiments. B–E: Graphs indicate fluorometric analysis of actin polymerization (B and C) after phagocytosis of OPDex beads (60 minutes) in the J774 macrophage cell line (D and E). B and D: Cells were treated with SB203580 (4 hours) or anisomycin (2 hours), followed by overnight morphine treatment and 60 minutes of phagocytosis of OPDex beads. C and E: Cells were transfected with pcDNA3-FlagMKK6(glu) plasmid using Fugene reagent (Roche). On day 2, they were treated with morphine and OPDex beads, fixed, stained, and analyzed using fluorometric analysis. Cells were transfected with pcDNA-EGFP-Rac-1-GTP or pcDNA3 empty vector control using Fugene reagent (Roche). On the second day, cells were treated with SB203580 (4 hours) before morphine (1 μmol/L) treatment overnight and OPDex beads, as previously described. On the third day after phagocytosis, cells were either collected for Western blot analysis or fixed and stained for fluorometric analysis (F) of phagocytosis (G) or actin polymerization (H). Densitometry analysis (using ImageJ software) represents the average of three independent experiments. Significance was determined using the Student's t-test. *P < 0.05, **P < 0.01, and ***P < 0.001.
To determine whether p38 MAPK is downstream to Rac1, we transfected J774 cells with constitutively active Rac1-Q61L plasmid and treated them with SB2035580 before addition of morphine and OPDex beads (previously described). Cells were analyzed for p38 MAPK activation by using Western blot analysis (Figure 5F), whereas phagocytosis (Figure 5G) and actin polymerization (Figure 5H) were quantified using fluorometric analysis. Western blot analysis results (Figure 5F) indicate that p38 MAPK phosphorylation is significantly increased in cells transfected with Rac1-Q61L plasmid in both vehicle- and morphine-treated cells. Rac1-Q61L overexpression overrides morphine-mediated inhibition of p38 MAPK phosphorylation (Figure 5F). Rac1-Q61L transfection also increased levels of total p38 MAPK, while increasing phosphorylated p38 MAPK. Despite the increase in total p38 MAPK, the densitometric ratio of phospho-p38/total p38 was unaltered by morphine in cells transfected with Rac1-Q61L, indicating that constitutive activation of Rac1-GTPase abolishes morphine-mediated inhibition of p38 MAPK activation.
Furthermore, p38 MAPK inhibitor SB 203580 (10 μmol/L) abolished the Rac1-Q61L–induced increase in phagocytosis and actin polymerization (Figure 5, G and H, respectively) in vehicle- and morphine-treated cells, indicating that p38 MAPK is downstream to Rac1-GTPase. Taking these findings into consideration, we conclude that cAMP, by inhibiting Rac1-GTPase, leads to inhibition of p38 MAPK activation and, therefore, inhibition of actin polymerization and phagocytosis.
Inhibition of Actin Polymerization by Long-Term Morphine Is Not Mediated by ERK1/2
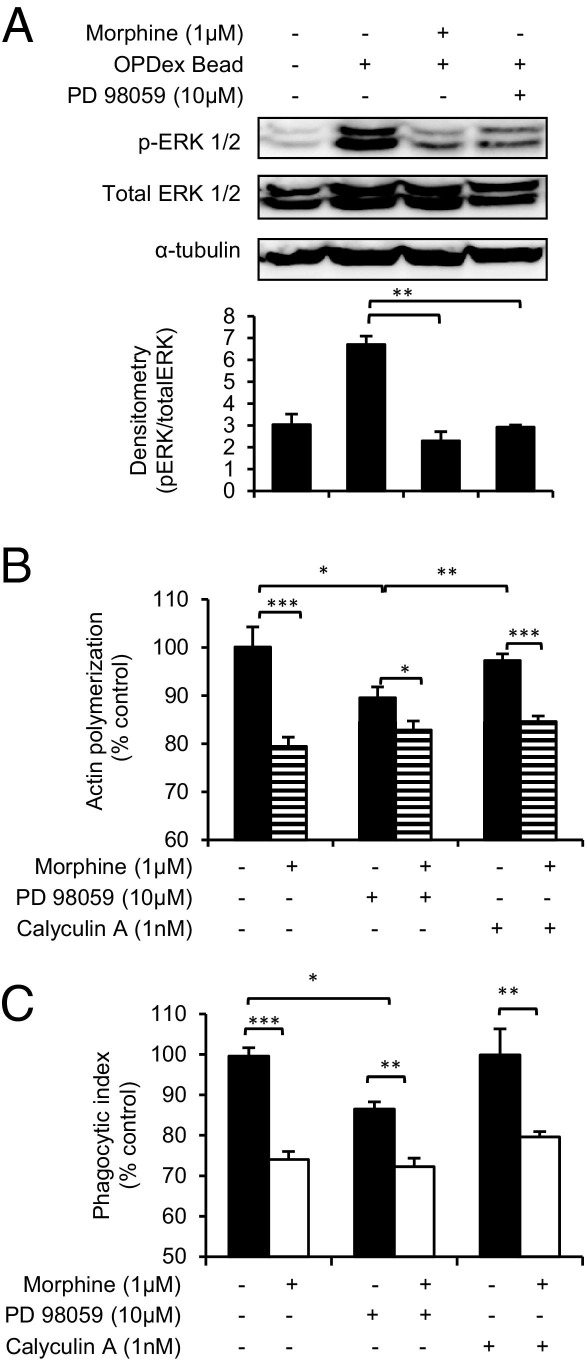
We next investigated the role of ERK1/2, another MAPK that plays a role in phagocytosis. To study the role of ERK1/2 in morphine's modulatory pathway, phosphorylation of ERK1/2 was inhibited by PD98059, a known inhibitor of ERK1/2 phosphorylation. Western blot analysis results (Figure 6A) showed that phosphorylation of ERK1/2 was enhanced with the addition of OPDex beads. Morphine treatment inhibited ERK1/2 phosphorylation, similar to PD98059-treated cells. Macrophage pretreatment with 10 μmol/L PD98059 (before the addition of morphine and OPDex beads) led to the inhibition of actin polymerization (Figure 6B) and phagocytosis (Figure 6C) of vehicle but not to morphine-treated macrophages. This can be explained by the fact that morphine already reached maximal inhibition of actin polymerization and, therefore, the additional inhibition of polymerization by PD98059 was not seen in PD98059+ morphine-treated cells. Although morphine alone inhibits phosphorylation of ERK1/2 (Figure 6A), the addition of PD98059 does not further potentiate morphine's inhibition of phagocytosis, indicating that the mechanism involving ERK1/2 is partially involved in phagocytosis but is not a part of the major modulatory pathway. This was further confirmed by the addition of a known inhibitor of protein phosphatase 2A, calyculin A,33 which inhibits protein phosphatase 2–mediated dephosphorylation of ERK1/2.34,35 By inhibiting ERK1/2 dephosphorylation, calyculin A leads to enhanced ERK1/2 activity. Enhanced ERK1/2 activation via calyculin A (1 nmol/L) pretreatment was not able to abolish morphine-mediated inhibition of actin polymerization and phagocytosis, further supporting our previous conclusion that ERK1/2, although modulated by morphine, does not play a major role in morphine-mediated attenuation of actin polymerization and macrophage phagocytosis.
Figure 6.
Role of ERK1/2 phosphorylation in morphine-mediated inhibition of phagocytosis and actin polymerization. J774 cells were treated with PD98059 for 4 hours or with calyculin A 2 hours before overnight morphine (1 μmol/L) treatment; phagocytosis of OPDex beads was conducted the next day, and cells were assessed via Western blot analysis (A) or fluorometric analysis (B and C) for actin polymerization (B) and phagocytosis (C), as previously described. A: After PD98059, morphine treatment and phagocytosis of OPDex bead cells were collected, and whole cell lysates were analyzed for ERK1/2 phosphorylation using Western blot analysis. A densitometry graph was generated using UltraLum software (images generated by the Omega UltraLum System), and it compares the ratio of phosphorylated ERK1/2 (phosphorylated p44/p42) pixel density/total ERK1/2 (p44/p42). Densitometry analysis represents the average of three independent experiments. Significance was determined using the Student's t-test. *P < 0.01, **P < 0.001, and ***P < 0.0001.
Discussion
This study shows, for the first time to our knowledge, that morphine-induced inhibition of FcgR-mediated phagocytosis occurs through attenuation of actin polymerization by an increase in intracellular cAMP, activation of PKA, inhibition of Rac1-GTPase, and inhibition of p38 MAPK. These findings are supported by several lines of evidence. First, we show that long-term morphine treatment inhibits IgG opsonophagocytosis by inhibiting actin polymerization through a Rac1-GTPase–dependant mechanism. Second, morphine modulates macrophage phagocytic ability and actin polymerization by increasing intracellular cAMP, which activates PKA to inhibit actin polymerization by inhibiting activation of Rac1-GTPase and p38 MAPK. Finally, third, overexpression of Rac1-GTPase rescues morphine's inhibitory effect on p38 MAPK activation, suggesting that Rac1-GTPase is upstream to p38 MAPK.
The prevalence of opioid use extends beyond the drug abuse population to the clinical setting. Morphine-mediated suppression of innate and adaptive immunity is an established phenomenon that is often indicated by an increase in frequency of bacterial infections.4,36,37 Although morphine-mediated immune suppression is well investigated, the mechanisms involved in the modulation of innate immunity are not yet fully understood. Macrophages play an essential role in bacterial clearance, and morphine has been implicated in inhibiting their phagocytic function.38,39 Because of the important role that macrophages play in the elimination of pathogens and the significance of deleterious effects caused by the disruption of macrophage homeostasis by opioids, we examined the mechanisms by which morphine modulates key macrophage functions, such as actin polymerization and phagocytosis.
Our results show that morphine treatment in vitro and in vivo inhibits FcgR-mediated phagocytosis in the primary peritoneal and J774 macrophage cell lines. Morphine, through MOR, inhibits actin polymerization and phagocytosis without affecting FcgR expression or cell viability. This indicates that the point of convergence between FcgR and MOR occurs further downstream in the signaling cascade. FcgR-mediated phagocytosis is dependent on actin polymerization and Rac-GTPases that lead to formation of lamellipodia and membrane ruffles.29 There are three isoforms of Rac (1, 2, and 3) in mammals, but little is known about the relative contributions of each isoform to Rac-dependent responses. We chose to study Rac1 because it is the most extensively studied isoform; in addition, it plays an important part in macrophage phagocytosis.40,41 The roles of Rac2 and Rac3 in phagocytosis are not well explored. Rac1 is essential in actin polymerization and dynamics42,43; any modulation of Rac1-GTPase alters downstream functions, such as actin polymerization and phagocytosis. In addition to previous mechanisms, morphine inhibits actin polymerization via inhibition of Rac1-GTPase. This was further confirmed by the observation that overexpression of constitutively activated Rac1-GTPase overrode morphine's inhibitory effect. We show, for the first time to our knowledge, that morphine causes inhibition of actin polymerization and that it does so by inhibiting activation of Rac1-GTPase. Although Rac1 plays an essential role in macrophage function and cytoskeletal reorganization, it is also involved in many other mechanisms, such as cell growth, vesicle trafficking, and epithelial differentiation,42 that can potentially be modulated by opioids in other model systems and lead to disruption of homeostasis.
Researchers8,9,30 have correlated long-term morphine-mediated increases in intracellular cAMP to inhibition of phagocytosis. Our research8,9 supports findings by Tomei and Renaud, showing that cAMP plays an essential role in morphine-mediated modulation of phagocytosis. Our previously published work,30 and this study, shows that cAMP levels remain elevated after 18 hours of 1 μmol/L morphine treatment and that this increase in cAMP leads to inhibition of phagocytosis. This study adds to the current understanding in the field by implying that a pharmacological increase in cAMP via DB-cAMP leads to a significant decrease in actin polymerization, leading to the attenuation of phagocytosis. Furthermore, inhibition of PKA, via H89, restored activation of Rac1-GTPase, actin polymerization, and phagocytic rates to the baseline level, indicating that cAMP acts through PKA to inhibit Rac1-GTPase activation and, ultimately, actin polymerization and phagocytosis.
The role of PKA and cAMP on modulation of actin polymerization and Rac-GTPase has been controversial. In some systems, such as endothelial cells, activation of PKA inhibits Rac1-GTPase activity, leading to inhibition of endothelial cell migration in vitro and angiogenesis in vivo.44 cAMP-mediated Rac1-GTP inhibition has also been observed in thyroid cells,45 and in THP-1 monocyte cell line, in which lipoxins, via elevation of cAMP, lead to inhibition of Rho and Rac GTPases.46 In addition, several groups have shown that cAMP inhibits Rac-GTPase in serum-induced cell migration, by inhibiting the formation of lamellipodia at the leading edge of migrating fibroblasts47 or by disrupting cytoskeletal dynamics.48–50 Our data show that DB-cAMP inhibits Rac-GTPase, whereas the PKA antagonist, H89, rescues morphine-mediated inhibition of Rac-GTP activation, indicating that, in our model system, elevation of cAMP may be a major player in inhibiting Rac activation. The positive and negative roles of PKA-cAMP in GTPase signaling, cytoskeletal dynamics, and cell migration have been extensively described in the review by Howe.51 Considering the literature provided herein, there is evidence to prove that PKA can lead to both activation and inhibition of actin polymerization in multiple cell types and that our model further supports the cAMP-PKA inhibitory role in these processes.
In addition to elevating cAMP, morphine plays an important role in the modulation of MAPKs. Three MAPK cascades have been identified in mammalian cells; the well-characterized MAPK cascade results in the activation of extracellular response kinases or ERKs (ERK1/2, also called p42/p44 MAPK). Opioids have differentially modulated MAPK.52 Because ERK1/2 and p38 MAPKs may play a role in neutrophil phagocytosis,19 we examined their role in morphine's inhibitory effects on macrophage phagocytosis and actin polymerization. Initially, we found that ERK1/2 is involved in the FcgR-mediated actin polymerization and phagocytic pathway. However, the role of ERK1/2 in morphine-mediated inhibition of phagocytosis seemed to be minor because the inhibition of ERK1/2 by PD98059 resulted in inhibition of actin polymerization in vehicle-treated, but not in morphine-treated, cells. Furthermore, the activation of ERK1/2 by calyculin A was unable to abolish morphine's attenuation of actin polymerization, indicating that ERK1/2 is not involved in the modulatory pathway. On the other hand, our results indicate that morphine inhibits p38 MAPK phosphorylation and that inhibition of p38 MAPK by SB203580 leads to a reduction in phagocytosis. The activation of p38 MAPK by anisomycin or overexpression of MKK6 abolished morphine-mediated inhibition, suggesting that p38 MAPK is essential for this process. Inhibition of p38 MAPK activation had a much more detrimental effect on macrophage phagocytosis than inhibition of ERK1/2, indicating that, although both are involved, p38 MAPK plays a more significant role in FcgR-mediated phagocytic mechanisms.
In the proposed diagram in Figure 7, we depict a summary of the current literature and our findings. Morphine inhibits Rac1-GTPase through activation of cAMP and PKA. Rac-GTPase inhibition negatively regulates p38 MAPK, ultimately leading to decreased actin polymerization and phagocytosis. To our knowledge, this study is the first to propose mechanisms of cross talk between the MOR and FcgR. Our findings are supported by several studies53,54 that suggest cAMP leads to inhibition of MAPKs, such as p38 and ERK1/2. However, although a decrease in p38 MAPK is observed after morphine treatment, the effect is antagonized after overexpression of constitutively active Rac1-GTPase. This result implies that the inhibition of p38 MAPK observed in our studies is a consequence of reduced Rac1-GTPase activation and not the result of a parallel inhibitory pathway activated by morphine. These data also support our conclusion that p38 MAPK is downstream from Rac1 and that morphine, by increasing intracellular cAMP and activating PKA, inhibits Rac1-GTP, leading to inhibition of p38 MAPK directly via inhibition of Rac1-GTPase.
Figure 7.
Mechanistic diagram of morphine-mediated inhibition of actin polymerization. MOR activation during long-term morphine treatment leads to superactivation of adenylyl cyclase and elevation of cAMP. After activation of FcgR by IgG OPDex beads, a Rac1-GTP signaling cascade is activated. During phagocytosis in cells undergoing long-term morphine treatment, an MOR-mediated increase in cAMP activates PKA to inhibit activation of Rac1-GTPase, which then leads to inhibition of the downstream signaling cascade. This finally leads to inhibition of p38 MAPK phosphorylation. Therefore, morphine treatment by inhibiting Rac-GTPase and, therefore, the signaling cascade that follows results in inhibition of actin polymerization, which ultimately leads to inhibition of phagocytosis.
Several groups55 have examined the role of Rac-GTPases in p38 MAPK activation. p38 MAPK has regulated actin filament formation through the downstream kinases MAPK-APK2/3 or MAPK-APK5 [PRAK (p38-regulated/activated kinase)] and subsequently through heat-shock protein 25/27. To further our understanding of morphine-mediated inhibition of actin polymerization and phagocytosis, future studies investigating the role of effectors downstream of p38 MAPK in modulating phagocytosis and actin polymerization would be useful.
The significance of our findings presented herein is not limited solely to macrophage phagocytosis, because similar modulations of actin may be occurring in different cell types, resulting in additional deleterious effects, such as inhibition of leukocyte migration and trafficking. Our observations emphasize the broad scope of morphine's effects on modulation of diverse mechanisms significant to macrophage function. This study highlights several essential pathways of morphine's immunomodulation that signify the importance and need for discoveries of new therapeutic agents used in pain management that would minimize these immunosuppressive effects.
Acknowledgments
We thank Dr. Subhas Das, Dr. Santanu Banerjee, Dr. Raini Dutta, and Haidong Yu for their technical advice and support.
Footnotes
Supported by grants from the NIH (NIH/National Institute on Drug Abuse 5F31-DA026264 and T32 DA07097 to J.N.; RO1 DA12104, RO1 DA022935, KO2 DA015349, and P50 DA11806 to S.R.).
CME Disclosure: None of the authors disclosed any relevant financial relationships.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.11.034.
Supplementary data
A–F: J774 cells were treated with long-term morphine treatment (1 μmol/L overnight) and the next day were allowed to phagocytose opsonized (A, C, D, and F) or unopsonized (B and E) dextran beads for various times. After internalization, cells were quantified for cell viability via an MTT assay according to standard protocols (C). Others were assayed for actin polymerization (D and E), as previously described. Cdc42 activation was determined using the GLISA assay (F), in which absorbance values were recorded and reported as percentage from unstimulated vehicle control. G: Fluorescence microscopy illustrating baseline changes in actin polymerization in unstimulated macrophages after transfection with pcDNA3-EGFP-Rac1-Q61L or pcDNA3. Scale bar = 20 μm. H: QT-PCR analysis of FcgR and MOR expression shown as the ratio of MOR/FcgR in J774 and in primary macrophages from WT mice. Significance was determined using the Student's t-test. *P < 0.01, **P < 0.001, and ***P < 0.0001.
Time-lapse imaging of J774 cells internalizing HKO E. coli. Time-lapse recording of J774 macrophages phagocytizing HKO E. coli (conjugated with Texas Red). Cells were treated overnight with vehicle or 1 μmol/L morphine. The next day, cells were allowed to phagocytose HKO E. coli (1:20 cell:bacteria ratio) for 60 minutes. Time-lapse microscopy was recorded by Nikon Biostation IM Cell Incubator and Imaging. Images were taken every 30 seconds, and the video shows a merged image of bright-field and red fluorescence with vehicle-treated cells (left panel) and morphine-treated cells (right panel).
References
- 1.Nora D., Volkow M.D. 2010. Scientific Research on Prescription Drug Abuse, Before the Subcommittee on Crime and Drugs, Committee on the Judiciary and the Caucus on International Narcotics Control United States Senate. 2008. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration, Department of Health and Human Services . 2007. The NSDUH Report: Patterns and Trends in Nonmedical Prescription Pain Reliever Use: 2002 to 2005. [Google Scholar]
- 3.Compton W.M., Volkow N.D. Abuse of prescription drugs and the risk of addiction. Drug Alcohol Depend. 2006;83(Suppl 1):S4–S7. doi: 10.1016/j.drugalcdep.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Friedman H., Eisenstein T.K. Neurological basis of drug dependence and its effects on the immune system. J Neuroimmunol. 2004;147:106–108. doi: 10.1016/j.jneuroim.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Roy S., Cain K.J., Charboneau R.G., Barke R.A. Morphine accelerates the progression of sepsis in an experimental sepsis model. Adv Exp Med Biol. 1998;437:21–31. doi: 10.1007/978-1-4615-5347-2_3. [DOI] [PubMed] [Google Scholar]
- 6.Feng P., Truant A.L., Meissler J.J., Jr, Gaughan J.P., Adler M.W., Eisenstein T.K. Morphine withdrawal lowers host defense to enteric bacteria: spontaneous sepsis and increased sensitivity to oral Salmonella enterica serovar Typhimurium infection. Infect Immun. 2006;74:5221–5226. doi: 10.1128/IAI.00208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy S., Wang J., Kelschenbach J., Koodie L., Martin J. Modulation of immune function by morphine: implications for susceptibility to infection. J Neuroimmune Pharmacol. 2006;1:77–89. doi: 10.1007/s11481-005-9009-8. [DOI] [PubMed] [Google Scholar]
- 8.Tomassini N., Renaud F., Roy S., Loh H.H. Morphine inhibits Fc-mediated phagocytosis through mu and delta opioid receptors. J Neuroimmunol. 2004;147:131–133. doi: 10.1016/j.jneuroim.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Tomei E.Z., Renaud F.L. Effect of morphine on Fc-mediated phagocytosis by murine macrophages in vitro. J Neuroimmunol. 1997;74:111–116. doi: 10.1016/s0165-5728(96)00213-5. [DOI] [PubMed] [Google Scholar]
- 10.Malik A.A., Radhakrishnan N., Reddy K., Smith A.D., Singhal P.C. Morphine-induced macrophage apoptosis modulates migration of macrophages: use of in vitro model of urinary tract infection. J Endourol. 2002;16:605–610. doi: 10.1089/089277902320913314. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein T.K., Bussiere J.L., Rogers T.J., Adler M.W. Immunosuppressive effects of morphine on immune responses in mice. Adv Exp Med Biol. 1993;335:41–52. doi: 10.1007/978-1-4615-2980-4_7. [DOI] [PubMed] [Google Scholar]
- 12.Manchikanti L., Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(Suppl):S63–S88. [PubMed] [Google Scholar]
- 13.Fridman W.H. Fc receptors and immunoglobulin binding factors. FASEB J. 1991;5:2684–2690. doi: 10.1096/fasebj.5.12.1916092. [DOI] [PubMed] [Google Scholar]
- 14.Bokoch G.M. Regulation of cell function by Rho family GTPases. Immunol Res. 2000;21:139–148. doi: 10.1385/IR:21:2-3:139. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Barke R.A., Charboneau R., Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- 16.Hilburger M.E., Adler M.W., Truant A.L., Meissler J.J., Jr, Satishchandran V., Rogers T.J., Eisenstein T.K. Morphine induces sepsis in mice. J Infect Dis. 1997;176:183–188. doi: 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- 17.Childers S.R. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48:1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- 18.Bryn T., Mahic M., Enserink J.M., Schwede F., Aandahl E.M., Tasken K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol. 2006;176:7361–7370. doi: 10.4049/jimmunol.176.12.7361. [DOI] [PubMed] [Google Scholar]
- 19.McLeish K.R., Klein J.B., Coxon P.Y., Head K.Z., Ward R.A. Bacterial phagocytosis activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades in human neutrophils. J Leukoc Biol. 1998;64:835–844. [PubMed] [Google Scholar]
- 20.Roy S., Barke R.A., Loh H.H. MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 21.Aherne G.W., Piall E.M., Twycross R.G. Serum morphine concentration after oral administration of diamorphine hydrochloride and morphine sulphate. Br J Clin Pharmacol. 1979;8:577–580. doi: 10.1111/j.1365-2125.1979.tb01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subauste M.C., Von Herrath M., Benard V., Chamberlain C.E., Chuang T.H., Chu K., Bokoch G.M., Hahn K.M. Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. J Biol Chem. 2000;275:9725–9733. doi: 10.1074/jbc.275.13.9725. [DOI] [PubMed] [Google Scholar]
- 23.Raingeaud J., Whitmarsh A.J., Barrett T., Derijard B., Davis R.J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhat R.S., Bhaskaran M., Mongia A., Hitosugi N., Singhal P.C. Morphine-induced macrophage apoptosis: oxidative stress and strategies for modulation. J Leukoc Biol. 2004;75:1131–1138. doi: 10.1189/jlb.1203639. [DOI] [PubMed] [Google Scholar]
- 25.Singhal P.C., Sharma P., Kapasi A.A., Reddy K., Franki N., Gibbons N. Morphine enhances macrophage apoptosis. J Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- 26.Singhal P.C., Bhaskaran M., Patel J., Patel K., Kasinath B.S., Duraisamy S., Franki N., Reddy K., Kapasi A.A. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J Immunol. 2002;168:4025–4033. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]
- 27.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 28.Araki N. Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis. Front Biosci. 2006;11:1479–1490. doi: 10.2741/1897. [DOI] [PubMed] [Google Scholar]
- 29.Wells C.M., Walmsley M., Ooi S., Tybulewicz V., Ridley A.J. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- 30.Kelschenbach J., Ninkovic J., Wang J., Krishnan A., Charboneau R., Barke R.A., Roy S. Morphine withdrawal inhibits IL-12 induction in a macrophage cell line through a mechanism that involves cAMP. J Immunol. 2008;180:3670–3679. doi: 10.4049/jimmunol.180.6.3670. [DOI] [PubMed] [Google Scholar]
- 31.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33(Pt 5):891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 32.Wang X., Mader M.M., Toth J.E., Yu X., Jin N., Campbell R.M., Smallwood J.K., Christe M.E., Chatterjee A., Goodson T., Jr, Vlahos C.J., Matter W.F., Bloem L.J. Complete inhibition of anisomycin and UV radiation but not cytokine induced JNK and p38 activation by an aryl-substituted dihydropyrrolopyrazole quinoline and mixed lineage kinase 7 small interfering RNA. J Biol Chem. 2005;280:19298–19305. doi: 10.1074/jbc.M413059200. [DOI] [PubMed] [Google Scholar]
- 33.Resjo S., Oknianska A., Zolnierowicz S., Manganiello V., Degerman E. Phosphorylation and activation of phosphodiesterase type 3B (PDE3B) in adipocytes in response to serine/threonine phosphatase inhibitors: deactivation of PDE3B in vitro by protein phosphatase type 2A. Biochem J. 1999;341(Pt 3):839–845. [PMC free article] [PubMed] [Google Scholar]
- 34.Silverstein A.M., Barrow C.A., Davis A.J., Mumby M.C. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci U S A. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das S., Kelschenbach J., Charboneau R., Barke R.A., Roy S. Morphine withdrawal stress modulates lipopolysaccharide-induced interleukin 12 p40 (IL-12p40) expression by activating extracellular signal-regulated kinase 1/2, which is further potentiated by glucocorticoids. J Biol Chem. 2011;286:29806–29817. doi: 10.1074/jbc.M111.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boschini A., Smacchia C., Di Fine M., Schiesari A., Ballarini P., Arlotti M., Gabrielli C., Castellani G., Genova M., Pantani P., Lepri A.C., Rezza G. Community-acquired pneumonia in a cohort of former injection drug users with and without human immunodeficiency virus infection: incidence, etiologies, and clinical aspects. Clin Infect Dis. 1996;23:107–113. doi: 10.1093/clinids/23.1.107. [DOI] [PubMed] [Google Scholar]
- 37.Roy S., Ninkovic J., Banerjee S., Charboneau R.G., Das S., Dutta R., Kirchner V.A., Koodie L., Ma J., Meng J., Barke R.A. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6:442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenstein T.K., Hilburger M.E. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol. 1998;83:36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- 39.Eisenstein T.K., Rogers T.J., Meissler J.J., Jr, Adler M.W., Hilburger M.E. Morphine depresses macrophage numbers and function in mouse spleens. Adv Exp Med Biol. 1998;437:33–41. doi: 10.1007/978-1-4615-5347-2_4. [DOI] [PubMed] [Google Scholar]
- 40.Castellano F., Montcourrier P., Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J Cell Sci. 2000;113(Pt 17):2955–2961. doi: 10.1242/jcs.113.17.2955. [DOI] [PubMed] [Google Scholar]
- 41.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 42.Ridley A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Niedergang F., Chavrier P. Regulation of phagocytosis by Rho GTPases. Curr Top Microbiol Immunol. 2005;291:43–60. doi: 10.1007/3-540-27511-8_4. [DOI] [PubMed] [Google Scholar]
- 44.Bakre M.M., Zhu Y., Yin H., Burton D.W., Terkeltaub R., Deftos L.J., Varner J.A. Parathyroid hormone-related peptide is a naturally occurring, protein kinase A-dependent angiogenesis inhibitor. Nat Med. 2002;8:995–1003. doi: 10.1038/nm753. [DOI] [PubMed] [Google Scholar]
- 45.Fortemaison N., Miot F., Dumont J.E., Dremier S. Regulation of H2O2 generation in thyroid cells does not involve Rac1 activation. Eur J Endocrinol. 2005;152:127–133. doi: 10.1530/eje.1.01815. [DOI] [PubMed] [Google Scholar]
- 46.Maderna P., Cottell D.C., Berlasconi G., Petasis N.A., Brady H.R., Godson C. Lipoxins induce actin reorganization in monocytes and macrophages but not in neutrophils: differential involvement of rho GTPases. Am J Pathol. 2002;160:2275–2283. doi: 10.1016/S0002-9440(10)61175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Zhang J.J., Huang X.Y. cAMP inhibits cell migration by interfering with Rac-induced lamellipodium formation. J Biol Chem. 2008;283:13799–13805. doi: 10.1074/jbc.M800555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandulache V.C., Parekh A., Li-Korotky H., Dohar J.E., Hebda P.A. Prostaglandin E2 inhibition of keloid fibroblast migration, contraction, and transforming growth factor (TGF)-beta1-induced collagen synthesis. Wound Repair Regen. 2007;15:122–133. doi: 10.1111/j.1524-475X.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 49.Hirakawa M., Karashima Y., Watanabe M., Kimura C., Ito Y., Oike M. Protein kinase A inhibits lysophosphatidic acid-induced migration of airway smooth muscle cells. J Pharmacol Exp Ther. 2007;321:1102–1108. doi: 10.1124/jpet.106.118042. [DOI] [PubMed] [Google Scholar]
- 50.Lorenowicz M.J., Fernandez-Borja M., Hordijk P.L. cAMP signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2007;27:1014–1022. doi: 10.1161/ATVBAHA.106.132282. [DOI] [PubMed] [Google Scholar]
- 51.Howe A.K. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Barke R.A., Charboneau R., Loh H.H., Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278:37622–37631. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- 53.Feng W.G., Wang Y.B., Zhang J.S., Wang X.Y., Li C.L., Chang Z.L. cAMP elevators inhibit LPS-induced IL-12 p40 expression by interfering with phosphorylation of p38 MAPK in murine peritoneal macrophages. Cell Res. 2002;12:331–337. doi: 10.1038/sj.cr.7290135. [DOI] [PubMed] [Google Scholar]
- 54.Zhu N., Cui J., Qiao C., Li Y., Ma Y., Zhang J., Shen B. cAMP modulates macrophage development by suppressing M-CSF-induced MAPKs activation. Cell Mol Immunol. 2008;5:153–157. doi: 10.1038/cmi.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paliga A.J., Natale D.R., Watson A.J. p38 Mitogen-activated protein kinase (MAPK) first regulates filamentous actin at the 8-16-cell stage during preimplantation development. Biol Cell. 2005;97:629–640. doi: 10.1042/BC20040146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A–F: J774 cells were treated with long-term morphine treatment (1 μmol/L overnight) and the next day were allowed to phagocytose opsonized (A, C, D, and F) or unopsonized (B and E) dextran beads for various times. After internalization, cells were quantified for cell viability via an MTT assay according to standard protocols (C). Others were assayed for actin polymerization (D and E), as previously described. Cdc42 activation was determined using the GLISA assay (F), in which absorbance values were recorded and reported as percentage from unstimulated vehicle control. G: Fluorescence microscopy illustrating baseline changes in actin polymerization in unstimulated macrophages after transfection with pcDNA3-EGFP-Rac1-Q61L or pcDNA3. Scale bar = 20 μm. H: QT-PCR analysis of FcgR and MOR expression shown as the ratio of MOR/FcgR in J774 and in primary macrophages from WT mice. Significance was determined using the Student's t-test. *P < 0.01, **P < 0.001, and ***P < 0.0001.
Time-lapse imaging of J774 cells internalizing HKO E. coli. Time-lapse recording of J774 macrophages phagocytizing HKO E. coli (conjugated with Texas Red). Cells were treated overnight with vehicle or 1 μmol/L morphine. The next day, cells were allowed to phagocytose HKO E. coli (1:20 cell:bacteria ratio) for 60 minutes. Time-lapse microscopy was recorded by Nikon Biostation IM Cell Incubator and Imaging. Images were taken every 30 seconds, and the video shows a merged image of bright-field and red fluorescence with vehicle-treated cells (left panel) and morphine-treated cells (right panel).