Abstract
Pathological glomerular hyposialylation has been implicated in certain unexplained glomerulopathies, including minimal change nephrosis, membranous glomerulonephritis, and IgA nephropathy. We studied our previously established mouse model carrying a homozygous mutation in the key enzyme of sialic acid biosynthesis, N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. Mutant mice died before postnatal day 3 (P3) from severe glomerulopathy with podocyte effacement and segmental glomerular basement membrane splitting due to hyposialylation. Administration of the sialic acid precursor N-acetylmannosamine (ManNAc) led to improved sialylation and survival of mutant pups beyond P3. We determined the onset of the glomerulopathy in the embryonic stage. A lectin panel, distinguishing normally sialylated from hyposialylated glycans, used WGA, SNA, PNA, Jacalin, HPA, and VVA, indicating glomerular hyposialylation of predominantly O-linked glycoproteins in mutant mice. The glomerular glycoproteins nephrin and podocalyxin were hyposialylated in this unique murine model. ManNAc treatment appeared to ameliorate the hyposialylation status of mutant mice, indicated by a lectin histochemistry pattern similar to that of wild-type mice, with improved sialylation of both nephrin and podocalyxin, as well as reduced albuminuria compared with untreated mutant mice. These findings suggest application of our lectin panel for categorizing human kidney specimens based on glomerular sialylation status. Moreover, the partial restoration of glomerular architecture in ManNAc-treated mice highlights ManNAc as a potential treatment for humans affected with disorders of glomerular hyposialylation.
Sialic acids, the most abundant terminal carbohydrates on glycoconjugates of eukaryotic cells, are involved in cellular interactions, signaling, and immune responses, among other processes.1–4 Additionally, sialic acids regulate the polyanionic component of the glomerular glycocalyx, which is integral to permeability selectivity.5,6 Hyposialylation was therefore proposed as a cause of certain unexplained human glomerulopathies; renal biopsies from patients with minimal change nephrosis and membranous glomerulonephritis show decreased sialic acid staining of podocyte membranes.7,8 Biopsies of membranous glomerulonephritis kidneys also exhibit reduced sialic acid staining of the lamina densa externa of the glomerular basement membrane (GBM),8 and serum from patients with IgA nephropathy exhibits hyposialylated circulating IgA1, resulting in the loss of nephrin, a podocyte structural protein.9,10
One of the best characterized glomerular sialoglycoproteins is podocalyxin, a CD-34-related sialomucin highly expressed in podocyte membranes, which contributes to proper foot process and slit diaphragm formation.11–13 Podocalyxin interacts with the actin cytoskeleton via ezrin and the Na+/H+-exchanger regulatory factor 2 (NHERF-2).14,15 This interaction is disrupted in various experimental models of nephrotic syndrome. In mice and rats, removal of sialic acids by sialidase treatment results in foot process effacement.5,15 In rats, reduced sialylation of podocalyxin and consequent loss of normal foot process architecture occurs after injection with the neutralizing agents puromycin aminonucleoside (PAN) or protamine sulfate.16–20
N-Acetylneuraminic acid (Neu5Ac) is the most abundant mammalian sialic acid and is the precursor of all other sialic acids; it is often referred to simply as sialic acid.1,3,4 Intracellular Neu5Ac synthesis is initiated by the bifunctional rate-limiting enzyme uridine diphospho-N-acetylglucosamine (UDP-GlcNAc) 2-epimerase/N-acetylmannosamine (ManNAc) kinase (GNE/MNK), encoded by the GNE gene.21,22 Tight regulation of sialic acid synthesis is provided by feedback inhibition of UDP-GlcNAc 2-epimerase activity by the downstream product cytidine monophosphate-Neu5Ac (CMP-sialic acid).21,23 Human GNE mutations result in hereditary inclusion body myopathy (HIBM; OMIM 600737), an autosomal recessive neuromuscular disorder of adult onset, characterized by slowly progressive muscle weakness and atrophy.22,24,25 More than 500 HIBM patients exist worldwide, harboring >60 different GNE mutations. HIBM patients have recessive (and predominantly missense) mutations in either enzymatic domain of GNE, leading to decreased enzyme activity and, presumably, to decreased sialic acid production.22,26,27 Whether hyposialylation is the main cause of the neuromuscular symptoms in HIBM patients remains uncertain.
We previously generated an HIBM mouse model to study the disease pathology and to test potential sialic acid replacement therapies for HIBM. Because the Gne knockout mouse is embryonic lethal,28 we created a gene-targeted knockin mouse model, mimicking the GNE M712T Persian Jewish HIBM founder mutation.29 Unexpectedly, >90% of homozygous mutant (GneM712T/M712T) mice did not survive beyond postnatal day 3 (P3). At P2, mutant pups displayed severe hematuria, proteinuria, and a glomerulopathy with effacement of the podocyte foot processes and segmental splitting of the GBM, likely due to hyposialylation of specific membrane glycoproteins, including podocalyxin.29 For a possible therapy, we explored dietary supplementation with the uncharged sialic acid precursor N-acetylmannosamine (ManNAc), the intermediate product of the GNE/MNK enzyme. In the absence of MNK activity, ManNAc can be converted to ManNAc-6P by N-acetylglucosamine (GlcNAc) kinase, thus bypassing the kinase domain mutation in our knockin mice.30 ManNAc supplementation increased survival of GneM712T/M712T pups beyond P3 to approximately 50%; survivors showed improved glomerular histology and podocyte ultrastructure, as well as increased podocalyxin sialylation.29
The renal phenotype in our GneM712T/M712T knockin model differed from that of HIBM patients, who have normal urinary laboratory findings and no indications of renal abnormalities.31 This discrepancy may be attributed to differences between species in the relative importance of sialic acid to the kidney, and to the type of sialic acid present. Most mammalian species, including mice, use N-glycolylneuraminic acid (Neu5Gc) as their main sialic acid. Humans, however, rely mainly on Neu5Ac, because humans have evolutionarily lost the ability to synthesize Neu5Gc.32 Protein glycosylation patterns may also differ. For example, the glomerular sialoprotein podocalyxin varies between species in its contingent of N- and O-linked glycosylation sites.33 Further research might elucidate these species-specific glycosylation issues. Although our GneM712T/M712T knockin model did not live long enough to recapitulate the features of the human adult-onset myopathy, the mice illustrated the importance of sialic acid in kidney development and function. To our knowledge, this GneM712T/M712T mouse is the first genetic model of podocyte injury and GBM splitting due to hyposialylation.
In the present study, we extensively characterized the glomerulopathy of the GneM712T/M712T mouse. With ultrastructural, lectin, and antibody analysis of kidney specimens, we established the onset of hyposialylation in the embryonic stage. We designed a lectin staining panel that convincingly determined glomerular hyposialylation of mutant mice. The glomerular glycoprotein nephrin was recognized as a novel marker for glomerular hyposialylation, in addition to the previously recognized podocalyxin. We demonstrate that ManNAc treatment of mutant mice partially rescued their severe albuminuria, their glomerular hyposialylation in general, and specifically their nephrin and podocalyxin hyposialylation. These findings indicate that hyposialylation may underlie unexplained human glomerulopathies; identification of such disorders may aid development of therapies for this relatively unexplored class of renal disorders. Our lectin staining panel can be used to diagnose human renal disorders of hyposialylation, for which ManNAc might be explored as a therapeutic option.
Materials and Methods
Mouse Studies
GneM712T/M712T knockin mice were generated as described previously.29 Animals were housed in a specific pathogen-free facility, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23). All mouse procedures were performed in accordance with protocol G04-3, approved by the Institutional Animal Care and Use Committee of the National Human Genome Research Institute.
ManNAc-treated breeding pairs were fed sterile water supplemented with 5 mg/mL ManNAc (∼1.0 g/kg per day) (New Zealand Pharmaceuticals, Palmerston North, New Zealand). Nursing females continued to receive ManNAc until the pups were euthanized or weaned. Untreated mice were euthanized at P2, and ManNAc-treated mice were euthanized at P5 to P10. Heterozygotic specimens developed no clinical phenotype, normal podocyte foot processes were observed under electron microscopy, and no hyposialylation of podocalyxin was observed with Western blotting. We therefore present the results only of wild-type and homozygous mutant specimens.29
Mouse Kidney Histology
Mouse kidneys were fixed in 4% paraformaldehyde for 48 hours, dehydrated in 70% ethanol, and paraffin-embedded for sectioning. Tissue sections (5 μm) were stained with H&E or with Masson's trichrome according to standard procedures (American Histolabs, Gaithersburg, MD) or were subjected to histochemistry or immunohistochemistry with a variety of antibodies and lectins. Juxtamedullary glomeruli were analyzed in H&E-stained slides of two embryos per genotype (two sections per embryo), and at least 10 untreated animals at P2 per genotype (one section per animal) and 10 ManNAc-treated animals at P5 per genotype (one section per animal). Representative glomeruli were digitally imaged with a Zeiss Axiovert 200M microscope (Zeiss, Thornwood, NY).
Electron Microscopy
For transmission electron microscopy, mouse kidney samples were fixed overnight at 4°C in 2% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) and washed with cacodylate buffer. The tissues were then fixed with 2% OsO4 for 2 hours, washed again with 0.1 mol/L cacodylate buffer, washed with water, and placed in 1% uranyl acetate. The tissues were then serially dehydrated in ethanol and propylene oxide and embedded in EMbed 812 resin (Electron Microscopy Sciences, Hatfield, PA). Thin sections (∼80 nm) were obtained using a Leica Ultracut-UCT ultramicrotome (Leica Microsystems, Deerfield, IL), placed onto 300-mesh copper grids, and stained with saturated uranyl acetate in 50% methanol followed by lead citrate. The grids were viewed under a JEM-1200EXII electron microscope (JEOL, Tokyo, Japan) at 80 kV; images were recorded on a XR611M, mid-mounted, 10.5 megapixel CCD camera (Advanced Microscopy Techniques, Danvers, MA). Two embryos per genotype at embryonic day E19 were analyzed (three sections each), and six mutant pups and four wild-type heterozygous pups at P2 (three sections each) and eight ManNAc-treated pups per genotype (three sections each) at different ages beyond P3 (P5, P10, and P19).
Urine Gel Electrophoresis
Mouse urine samples (2 μL, from a one-time urine collection from five mice per genotype before and after ManNAc treatment) were electrophoresed in Laemmli sample buffer supplemented with β-mercaptoethanol (Bio-Rad Laboratories, Hercules, CA) on 4% to 20% Tris-glycine polyacrylamide gradient gels (Novex; Invitrogen, Carlsbad, CA). Pure bovine serum albumin (Amersham; GE Healthcare, Piscataway NJ) was loaded for quantification purposes. Gels were stained with Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories) for 20 minutes and washed with R-250 destaining solution (Bio-Rad Laboratories), according to the manufacturer's protocols. Semiquantitative densitometry was performed on the Coomassie-stained albumin signals.
Lectin Histochemistry
For lectin histochemistry, fluorescein isothiocyanate (FITC) labeled lectins WGA, PNA, Jacalin, HPA, and VVA were purchased from EY Laboratories (San Mateo, CA) and SNA was purchased from Vector Laboratories (Burlingame, CA). Paraffin-embedded sections were deparaffinized [two times for 45 minutes each in Hemo-De solvent (Scientific Safety Solvents, Keller, TX)] and rehydrated (two times for 5 minutes each in 100%, 70%, and 50% ethanol), followed by antigen retrieval (microwaved three times for 5 minutes each in 0.01 mol/L sodium citrate, pH 6.4, at 100%, 50%, and 30% power; Panasonic Genius 1300W microwave), and blocking in carbohydrate-free blocking solution (Vector Laboratories). The slides were incubated at 4°C overnight with each lectin aliquoted in carbohydrate-free blocking solution (FITC-HPA, 5 μg/mL; FITC-SNA, 5 μg/mL; FITC-VVA, 5 μg/mL; FITC-WGA, 15 μg/mL; FITC-PNA, 30 μg/mL; FITC-Jacalin, 50 μg/mL). Washes were performed with 0.1% Triton-X-100 in 1× Tris-buffered saline. The lectin-stained slides were incubated in 0.3% Sudan Black in 70% ethanol solution to reduce autofluorescence. In addition, to verify lectin specificity, each lectin was incubated with its specific inhibitory carbohydrate for 1 hour before overnight incubation on a slide. The inhibitory carbohydrates used were Neu5Ac (Toronto Research Chemicals, North York, ON, Canada) for WGA and SNA, lactose (C-6010-10; EY Laboratories) for PNA, galactose (C-6003-10; EY Laboratories) for Jacalin, and GalNAc (C-6000-10; EY Laboratories) for HPA and VVA. Results of these control experiments, indicating the specificity of each lectin used in this panel, are presented in Supplemental Figure S1 (available at http://ajp.amjpathol.org). All slides were mounted in Vectashield including the nuclear stain DAPI (H-1200; Vector Laboratories).
Fluorescence imaging of multiple juxtamedullary glomeruli per slide was performed under a Zeiss 510 META confocal laser-scanning microscope. All fluorescent images represent collapsed stacks of confocal Z-sections, imaged with a 63× objective. For each lectin, two embryos per genotype at E19 were analyzed (two sections per lectin per genotype), and at least 10 mutant pups and 10 wild-type/heterozygous pups at P2 (three sections per lectin per genotype), as well as more than 10 ManNAc-treated pups per genotype (three sections per lectin per genotype) at different ages beyond P3 (mainly at P5).
Immunoblotting
Frozen kidney specimens were homogenized in CelLytic MT buffer (Sigma-Aldrich, St. Louis, MO) with protease inhibitors (Complete Mini Protease Inhibitor Cocktail; Roche Applied Science, Indianapolis, IN). For the neuraminidase enzymatic treatments, protein homogenates were incubated for 2 hours at 37°C with approximately 1 mU/μg of neuraminidase (P0720S; New England Biolabs, Ipswich, MA). Equal amounts of protein (10 to 30 μg) were electrophoresed in Laemmli sample buffer supplemented with β-mercaptoethanol (Bio-Rad Laboratories) on 4% to 20% Tris-glycine gels (Novex; Invitrogen) and electroblotted onto 0.45-μm Hybond ECL nitrocellulose membranes (GE Healthcare). Membranes were blocked (10% fat-free milk) and incubated with goat-anti-mouse podocalyxin (AF1556; R&D Systems, Minneapolis, MN) or guinea pig-anti-mouse nephrin (GP-N2; PROGEN Biotechnik, Heidelberg, Germany) antibodies, and mouse monoclonal α-tubulin antibodies (loading control; Sigma-Aldrich), followed by horseradish peroxidase-conjugated secondary antibodies [GE Healthcare and Santa Cruz Biotechnology (Santa Cruz, CA)]. Results were visualized with enhanced chemiluminescence (ECL Western blotting detection reagents; GE Healthcare) and exposure to CL-XPosure film (Pierce; Thermo Fisher Scientific, Rockford, IL).
Neuraminidase Expression by Quantitative Real-Time PCR
RNA was extracted from mouse kidneys using an RNeasy mini kit (Qiagen, Valencia, CA). TaqMan primers and probes were ordered as premanufactured assays on demand: mouse lysosomal neuraminidase 1 (Neu1; Mm00456846) and mouse cytosolic neuraminidase 2 (Neu2; Mm00479238) (Applied Biosystems, Foster City, CA). The housekeeping gene B2M (β2 microglobulin, Mm00437762; Applied Biosystems) was used as internal control gene. All quantitative real-time PCR reactions and subsequent analyses were performed on an ABI PRISM 7900 HT sequence detection system (Applied Biosystems). The pre-run thermal cycling conditions were 10 minutes at 95°C to activate the Taq DNA polymerase, followed by 40 cycles of 95°C for 15 seconds and 60°C annealing/extension for 1 minute. Each experiment was performed in triplicate. Within each experiment, reactions were run in triplicate. Relative gene expression levels were determined by the comparative threshold cycle method (ΔΔCT).34 For statistical analysis of the expression data, the independent samples t-test was used.
Results
Kidney Histology and Ultrastructure
H&E-stained kidney specimens of untreated GneM712T/M712T mice and their wild-type littermates were examined at E19 or at P2, because mutant pups die before P3.29 At E19, there were no apparent histological differences between wild-type and mutant mice (see Supplemental Figure S2A at http://ajp.amjpathol.org). This was confirmed at the ultrastructural level, with embryonic glomeruli of mutant mice resembling those of control littermates. Embryonic glomeruli have predominantly cuboidal podocytes (epithelial cells) with occluding junctions. In most podocytes, foot processes have not yet appeared; the basement membrane is slender and immature, and endothelial cells show only sporadic fenestrae (Figure 1A).35,36
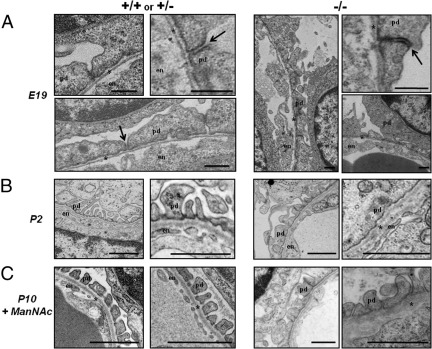
Figure 1.
Ultrastructural analyses of juxtamedullary glomeruli in mouse kidneys. Representative electron microscopy images of juxtamedullary glomeruli in kidney sections of control (+/+ or +/−) and mutant GneM712T/M712T (−/−) littermate pups. A: At embryonic day 19 (E19), no ultrastructural differences in the glomerular filtration apparatus were observed between control and mutant littermate pups. No endothelial (en) fenestrae have yet formed, and the GBM (asterisk) remains immature. Most podocytes (pd) showed effaced foot processes with occluding junctions (arrows). Scale bars: 500 nm. B: At postnatal day 2 (P2), control glomeruli contain interdigitating foot processes with open filtration slits, an intact GBM and fenestrated endothelial cells (en). In contrast, mutant glomeruli revealed podocyte (pd) foot process effacement with very few filtration slits, segmental splitting of the GBM (asterisk), and seemingly normally fenestrated endothelium (en). Scale bars: 1 μm. C: In ManNAc-supplemented mutant pups at P10 (P10+ManNAc) the podocyte effacement is partially restored with intact filtration slits between foot processes. The littermate control glomeruli showed normal separation of foot processes with patent endothelial fenestrae and an intact GBM. Scale bars: 1 μm.
At P2, kidneys of mutant pups were normal in size but showed surface petechial hemorrhages.29 Histologically, mutant kidneys exhibited increased amounts of embryonic metanephric mesenchyme, compared with wild-type kidneys (see Supplemental Figure S2B at http://ajp.amjpathol.org). Glomeruli were remarkable for mesangial expansion (see Supplemental Figure S3 at http://ajp.amjpathol.org). Focally, tubules were remarkable for nonisometric vacuolization, red blood cell casts, dilation, and atrophic epithelium. Ultrastructurally, podocyte effacement and segmental splitting of the GBM was apparent in mutant kidneys at P2 (Figure 1B), as described previously.29 These severe ultrastructural changes likely account for both the hematuria and proteinuria and the early lethal phenotype.
In GneM712T/M712T mice that were treated with ManNAc, kidneys at P5 demonstrated substantial amounts of uninduced mesenchyme, compared with ManNAc-treated wild-type littermates (see Supplemental Figure S2B at http://ajp.amjpathol.org). In addition, ManNAc-treated mutant pups still manifested increased matrix staining of glomeruli (see Supplemental Figure S2C at http://ajp.amjpathol.org). Focal tubular vacuolization, red blood cell casts, and proteinaceous casts persisted in mutant ManNAc-treated mice, although to a lesser extent than in untreated mice at P2. Ultrastructurally, however, glomeruli of ManNAc-treated GneM712T/M712T mice showed a dramatic improvement, with increased foot process formation and reduced GBM splitting (Figure 1C). These changes were likely responsible for survival of mutant mice beyond P3.29
Urine Gel Electrophoresis
Proteinuria of GneM712T/M712T mice was further examined using urinary gel electrophoresis. It appeared that the major urinary proteins of GneM712T/M712T mice were albumin and a few other higher molecular weight proteins (Figure 2; see also Supplemental Figure S4A at http://ajp.amjpathol.org). Semiquantitative determination of albumin concentrations showed albuminuria of approximately 0.30 μg/μL in untreated mutant mice (at P2), which was reduced by approximately 25% (to ∼0.22 μg/μL) after ManNAc treatment at P5, indicating improved glomerular filtration after treatment (Figure 2; see also Supplemental Figure S4B at http://ajp.amjpathol.org).
Figure 2.
Urine gel electrophoresis. Mouse urine samples (2 μL) were electrophoresed on a 4% to 20% Tris-glycine polyacrylamide gel and stained with Coomassie Brilliant Blue. The major protein band on the gel was albumin (66.7 kDa); for the entire gel image, see Supplemental Figure S4A (available at http://ajp.amjpathol.org). Densitometry quantifications of albumin concentrations in each sample are given below the gel image (see also Supplemental Figure S4B at http://ajp.amjpathol.org).
Lectin Staining Panel
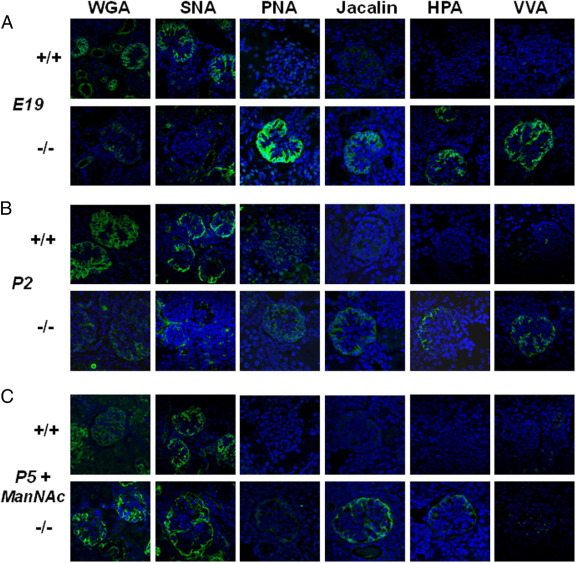
We used a panel of lectins (ie, sugar-binding proteins with ligand specificities for defined carbohydrate sequences37) to examine the sialylation status of the glomerular glycocalyx on paraffin-embedded kidney slides from wild-type and mutant GneM712T/M712T mice before and after ManNAc treatment. We used six lectins: WGA, SNA, PNA, Jacalin, HPA, and VVA (Figure 3).
Figure 3.
Lectin histochemistry of kidney juxtamedullary glomeruli. Representative images of mature juxtamedullary glomeruli in mouse kidneys stained with FITC-labeled lectins WGA, SNA, PNA, Jacalin, HPA, and VVA (green) and the DAPI nuclear dye (blue). Confocal imaging intensity settings were the same across all ages and genotypes per lectin. A: Embryonic kidneys at E19 showed decreased WGA and SNA staining patterns and intensity for mutant (−/−), compared with wild-type (+/+) littermate glomeruli. PNA, Jacalin, HPA, and VVA bind only to mutant glomeruli, indicating hyposialylation of predominantly O-linked glycans in these glomeruli. B: Kidneys from pups at P2 showed decreased WGA and SNA staining intensity in mutant glomeruli, compared with the wild type. Mutant glomeruli bind PNA, Jacalin, HPA, and VVA, indicating hyposialylation of O-linked glycans. The staining intensity at P2 is decreased, compared with embryonic glomeruli, which could be attributed to increased membrane surface at P2 (dilution of the glycan signals) and/or partial amelioration of the hyposialylation status after birth. C: Kidneys from ManNAc-treated pups at P5 showed more intense signals in mutant glomeruli for WGA and SNA, compared with the wild type, suggestive of increased sialylation. After ManNAc treatment, mutant glomeruli retain residual staining for PNA, Jacalin, HPA, and VVA, indicating that the treatment did not fully restore sialylation.
WGA (wheat germ agglutinin from Triticum vulgaris) predominantly recognizes terminal sialic acid (Neu5Ac) and N-acetylglucosamine (GlcNAc) on glycans.37–39 SNA (elderberry bark agglutinin from Sambucus nigra) predominantly recognizes terminal sialic acid (Neu5Ac) in an α(2,6)-linkage with either galactose (prevalent in N-linked glycans) or with N-acetylgalactosamine (GalNAc) (found in O-linked glycans).38,40 WGA and SNA signals were localized primarily to the glomerular epithelial podocytes in E19, P2, and P5 kidneys. Wild-type glomeruli exhibited similar WGA and SNA staining intensities across all ages; mutant glomeruli showed decreased WGA and SNA staining at E19 and P2, compared with the wild type, with recovery of the signals at P5 after ManNAc treatment.
PNA (peanut agglutinin from Arachis hypogaea) preferentially binds to terminal galactose (Gal) linked to GalNAc residues [Gal-β(1,3)-GalNAc],37,38,41 and therefore outlines predominantly hyposialylated O-linked glycans. PNA did not significantly stain wild-type sections, indicating that O-linked glycoconjugates are mostly sialylated, as expected. However, mutant kidneys at E19 and at P2 showed significant PNA signal, outlining mainly the podocytes of the glomerular epithelium and indicating the presence of hyposialylated glycans. In ManNAc-supplemented mutant pups at P5, the PNA signal appeared reduced, reflecting partial glomerular resialylation and/or dilution of the PNA signal consequent to increased membrane surface due to recovery of podocyte foot process formation (Figure 3C).
Jacalin (jackfruit agglutinin from Artocarpus integrifolia), like PNA, binds to terminal Gal-β(1,3) -GalNAc residues, but can also recognize sialylated O-linked glycans (sialic acid-Gal-GalNAc).42,43 Jacalin and PNA staining patterns appeared similar for the embryonic and P2 specimens (ie, increased staining in mutant glomeruli, compared with the wild type). In ManNAc-treated mutant glomeruli, the PNA signal appeared more attenuated than the Jacalin signal (Figure 3C).
HPA (edible snail agglutinin from helix pomatia) and VVA (hairy vetch agglutinin from Vicia villosa) predominantly bind GalNAc O-linked to serine or threonine residues of proteins.38,44–46 Staining with HPA was absent from all wild-type glomeruli, as expected, but mutant kidneys at all ages showed a glomerular epithelial staining pattern similar to that of PNA and Jacalin. The HPA and VVA signals were greatly diminished after ManNAc treatment.
The lectin staining panel established the onset of hyposialylation in mutant glomeruli at the embryonic stage (Figure 3A). Compared with staining of mutant glomeruli at E19, the intensity of PNA, Jacalin, HPA, and VVA signals was greatly reduced at P2 (Figure 3B). This change may be attributed to the larger surface area of the growing and interdigitating epithelial cells at P2, compared with E19, or may reflect a partial increase of O-linked sialylation after birth in mutant glomeruli. ManNAc supplementation appeared to attenuate sialylation status in mutant glomeruli at P5 (Figure 3C).
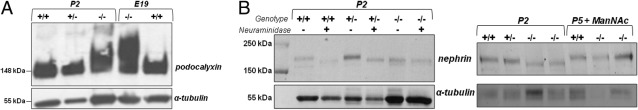
Immunoblotting of Podocalyxin and Nephrin
Immunoblotting of kidney extracts showed hyposialylation of the glomerular sialoprotein podocalyxin, as evidenced by an upward shifted protein band due to reduced negative charge, not only in mutant pups at P2, as previously reported,29 but also in embryonic mutant kidneys (Figure 4A).
Figure 4.
Immunoblotting of podocalyxin and nephrin. A: Immunoblot of kidney extracts (ages P2 and E19) labeled with antibodies against podocalyxin (∼140 to 150 kDa) and α-tubulin (∼55 kDa, loading control). Wild-type (+/+) (lanes 1 and 5) and heterozygote (+/−) (lane 2) specimens show normal migration. Slower hyposialylated podocalyxin migration (160 to 180 kDa) in GneM712T/M712T (−/−) is present in utero (E19; lane 4) and continues postnatally (P2, lane 3). B: Immunoblots of kidney extracts labeled with antibodies against nephrin (∼180 kDa) and α-tubulin (∼55 kDa, loading control). Left: Lane 1: Molecular weight ladder. Wild-type (+/+) (lanes 2 and 3) and heterozygous Gne+/M712T (+/−) (lanes 4 and 5) kidney extracts were left untreated or treated for desialylation by neuraminidase, resulting in nephrin migrating faster than untreated samples. Mutant GneM712T/M712T (−/−) (lanes 6 and 7) kidney extracts showed the faster running desialylated nephrin signal, which did not change after neuraminidase treatment in mutant kidneys. Right: Wild-type (+/+) (lane 1), heterozygous Gne+/M712T (+/−) (lane 2), and mutant (−/−) (lanes 3 and 4) kidney extracts at P2 demonstrate hyposialylation of nephrin in mutant (−/−) kidney extracts. Nephrin recovered after ManNAc treatment in kidney extracts of mutants (−/−) at P5 (P5+ManNAc) (lanes 6 and 7); also shown are ManNAc-treated wild-type (+/+) (lane 5) kidney extracts at P5.
Immunoblotting for the glomerular glycoprotein nephrin demonstrated hyposialylation of nephrin in kidney extracts of mutant mice at P2, as evidenced by a lower molecular weight band similar in size to that of kidney extracts of wild-type and heterozygous mice at P2 that were desialylated by neuraminidase treatment (Figure 4B). After ManNAc treatment at P5, the molecular weight and sialylation of nephrin in mutant kidneys was restored to that of wild-type kidneys (Figure 4B).
Discussion
Various hereditary forms of proteinuria and/or hematuria can be attributed to defects in genes coding for structural proteins of the glomerular filtration apparatus; most such disorders involve GBM splitting and/or podocyte effacement.47 Some unexplained forms of congenital proteinuria and hematuria have recently been attributed to novel genetic mechanisms, such as defects in signaling pathways48,49 or micro-RNAs.50,51 However, few studies have addressed the consequences of glomerular glycocalyx defects. The glomerular glycocalyx is enriched in glycoconjugates, including proteoglycans, glycosaminoglycans, and sialoglycoproteins; it contributes to the size- and charge-selectivity of the glomerular filtration barrier. All three components of the filtration barrier (fenestrated endothelial cells,52 GBM53,54 and epithelial podocytes55) contain a glycocalyx.
Sialic acids, which are terminal, negatively charged residues on most glycoconjugates, play crucial roles in formation, maintenance, and function of the glomerular glycocalyx. Experimental evidence indicated that removal of glomerular sialic acids by infusion of sialidase,5 or charge neutralization of sialic acids by polycations,18,56 resulted in foot process effacement and proteinuria. In addition, experimental desialylation of podocalyxin, the major glomerular sialoglycoprotein, resulted in foot process effacement and proteinuria.16
In the present study, we further characterized the unique glomerulopathy of our previously described GneM712T/M712T mouse model, which appeared lethal between birth and P3.29 We now have established that the glomerulopathic process begins in the embryonic stage, because lectin staining demonstrated hyposialylation (Figure 3) and immunoblotting showed hyposialylation of podocalyxin (Figure 4) in GneM712T/M712T embryonic kidneys. This occurred even though no histological or ultrastructural abnormalities were identified in GneM712T/M712T embryonic kidneys, compared with wild-type littermates (Figure 1A; see also Supplemental Figure S2A at http://ajp.amjpathol.org). We surmise that hyposialylation permits some maturation of the glomerular filtration apparatus in utero, allowing GneM712T/M712T mice to survive the embryonic stage, in contrast to complete Gne knockout mice that were embryonic lethal.28 Nevertheless, at P2, GneM712T/M712T kidneys showed a delayed maturation of glomeruli and a failure to form podocyte foot processes. Administration of the sialic acid precursor ManNAc to GneM172T/M712T mice partially rescued this phenotype and allowed for the survival of mutant pups beyond P3.29
We previously reported increased urinary protein (>500 mg/dL, measured by dipstick) in mutant GneM712T/M712T mice.29 In the present study, we characterized the proteinuria by gel electrophoresis of mouse urine. The major urinary proteins of GneM712T/M712T mice were albumin and a few other higher molecular weight proteins (Figure 2; see also Supplemental Figure S4 at http://ajp.amjpathol.org). These findings indicate glomerular injury rather than tubular defects in GneM712T/M712T mice.57 Semiquantitative determination of albumin concentrations showed albuminuria of approximately 0.30 μg/μL in untreated mutant mice (at P2), which was reduced by approximately 25% (to ∼0.22 μg/μL) after ManNAc treatment in GneM712T/M712T mice at P5, indicating improved (but still affected) glomerular filtration after treatment. Note that untreated wild-type and heterozygous mice at P2 also have minor albuminuria (∼0.06 μg/μL), likely due to immature glomerular development at this age, which was not present in urine of wild-type mice at P5.
The initiation signals for development of podocyte foot processes and formation of slit diaphragms are largely unknown. Increased blood pressure58 and the insertion of podocalyxin in the apical portion of the podocyte membrane11,59 have been suggested as triggers. In fact, we documented hyposialylated podocalyxin in GneM172T/M712T kidneys, and ManNAc supplementation restored podocalyxin sialylation as well as podocyte foot process and slit diaphragm formation. Of note, podocalyxin knockout mice, in contrast to our GneM712T/M712T mice, do not have abnormalities of the GBM.11 This suggests that reduced sialylation of other sialoproteins may account for the GBM splitting seen in our mice. Podocytes are linked to the GBM through a number of sialylated anchoring molecules, such as α1β3 integrin and α/β-dystroglycans.60–62 These molecules facilitate crosstalk between the GBM and the podocytes and determine podocyte structure and possibly also the composition of the GBM. Altered sialylation of these molecules or other anchoring proteins may account for the observed GBM changes, through altered signaling and dysregulation of GBM synthesis.
Other sialoproteins may also play a role in the glomerular pathology of our mouse model. We previously tested a series of glomerular proteins by immunoblotting for sialylation status in GneM172T/M712T kidneys [including laminin-1, podocin, laminin β1, desmin, and VSMA (α-SMA)], but did not find any abnormal intensity or size difference, compared with wild-type kidneys.29 Some other candidate proteins may arise from mouse models with defects in genes encoding proteins residing in podocytes or slit diaphragms (eg, angiopoietin-like 4 protein,63 integrin β1,61 Neph1,64 or CD2AP55); these model mice exhibit effaced podocytes leading to nephrosis and death hours to weeks after birth. Whether these proteins are sialylated and affected in our mice remains unknown.
Because nephrin-deficient mice65 and human nephrin-deficiency [resulting in congenital nephrotic syndrome of the Finnish type (NPHS1; OMIM 256300)]47,66 showed a similar renal phenotype as our GneM172T/M712T mice, we investigated nephrin sialylation in kidney extracts of our mice. Surprisingly, immunoblotting demonstrated hyposialylated nephrin in GneM172T/M712T mice (Figure 4B), consistent with in vitro studies of nephrin deglycosylation.67 Kidney extracts from ManNAc-treated mutant mice at P5 showed recovery of nephrin sialylation after treatment. Nephrin is a transmembrane glycoprotein expressed by podocytes and is a structural component of the glomerular slit diaphragm. Nephrin molecules from adjacent foot processes are thought to interact in the middle of the slit to form a filtering structure.68 Nine of 10 potential N-linked glycosylation sites in nephrin were experimentally proven to be modified by N-linked glycosylation.67 O-linked nephrin glycosylation has not been experimentally examined, but database searches predict five potential O-glycosylation sites (NetOGlyc 3.1 server; http://www.cbs.dtu.dk/services/NetOGlyc). Future research may reveal which glycan structures on nephrin are hyposialylated in our GneM172T/M712T mice. N-linked glycosylation on nephrin was shown to be critical for folding and localization of the protein, and thus has been suggested to be an important factor in the pathogenesis of glomerular diseases.69
Rather than investigating each podocyte and slit diaphragm-associated candidate protein individually, we used a lectin panel to determine overall glomerular glycosylation status in our mice. We demonstrated hyposialylation of predominantly O-linked glycans in the glomerular glycocalyx of GneM712T/M712T mice by showing increased binding of the lectins PNA, Jacalin, HPA, and VVA to GneM712T/M712T glomeruli. Although ManNAc treatment did not appear to completely restore the overall hyposialylation pattern, it is likely that sialylation of certain sialoproteins is restored on ManNAc supplementation, as was demonstrated for podocalyxin and nephrin by immunoblotting (Figure 4). It is possible that sialyltransferases have different affinities for CMP-sialic acid70–72 and that, with a paucity of sialic acid, certain glycans may be preferentially sialylated. Sialyltransferase expression itself may also be regulated by sialic acid availability and/or the amount of Gne mRNA.73 However, we encountered normal Gne mRNA expression levels in GneM712T/M712T mice.29
Increased neuraminidase expression and activity was previously suggested to play a role in glomerular hyposialylation of certain renal diseases.60,74 We therefore determined mRNA expression of lysosomal (Neu1) and cytoplasmic (Neu2) neuraminidase in our mouse kidneys (see Supplemental Figure S5 at http://ajp.amjpathol.org). We found a significant decrease of both Neu1 and Neu2 expression in mutant kidneys, compared with wild-type kidneys at P2, perhaps indicating a possible down-regulation of neuraminidase expression in response to glomerular hyposialylation in mutant kidneys at P2 due to the Gne defect. After ManNAc treatment, mutant and wild-type kidneys showed similar Neu1 and Neu2 expression rates, consistent with recovery of sialylation status in mutant mice. Note the high expression of Neu1 and Neu2 mRNA in wild-type kidneys at P2, compared with P5, which may be related to the high demand for sialic acid in the first few days after birth.75
In humans, hyposialylation has been suggested in several unexplained glomerulopathies, including minimal change nephrosis,7,8 membranous glomerulonephritis8 and IgA nephropathy.9,10 Other disorders involving proteinuria and/or hematuria for which the etiology is unknown may also be related to changes in glomerular sialylation. These include certain forms of focal and segmental glomerulosclerosis,76 membranous glomerulonephritis,77 lupus nephritis,78, and other idiopathic nephritic syndromes.79 Our lectin staining panel can be applied as a simple diagnostic tool to identify human renal disorders of hyposialylation. Moreover, if the human hyposialylation pattern proves to be similar to that in our mouse model, ManNAc might be explored as a therapeutic option.6,80 One must keep in mind, however, that untreated GneM712T/M712T mice had a severe congenital glomerular phenotype and did not survive the postnatal days, unless treated throughout the embryonic stage with ManNAc, and humans likely would also not survive the postnatal period with such a severe phenotype. Nevertheless, human glomerulopathies with hyposialylation, whether congenital or acquired later in life, remain good candidates for ManNAc therapy.
The use of ManNAc for the treatment of HIBM patients is being pursued. Other suggested therapies to increase sialylation involve intravenous immunoglobulins as a source of sialic acid31 or administration of acute phase reactants (α-1-acid glycoprotein or orosomucoid), which improved nephritic syndrome in rats.81,82 Testing various drug delivery mechanisms for efficient delivery to kidney glomeruli will be useful for future therapeutic development. Caution is required, with attention paid to the difference between humans and other mammals used to test therapies, because most mammalian species use the sialic acid Neu5Gc (N-glycolylneuraminic acid), but humans have lost the ability to synthesize Neu5Gc and rely on Neu5Ac as their main sialic acid.32 In addition, protein glycosylation patterns vary among tissues and/or species; in particular, the contingent of O- and N-linked glycosylation sites in podocalyxin differs widely among species.33
Acknowledgments
We thank Theresa Calhoun and Kevin Jackson for their skilled assistance with mouse maintenance and thank Heidi Dorward, Lisa Vincent, Katherine Patzel, Maggie Lin, Adrian Astiz-Martinez, and Mark Ziats for their expert laboratory work.
Footnotes
Supported by the Intramural Research Programs of the National Human Genome Research Institute, the National Dental and Craniofacial Research Institute, and the National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, MD.
S.K. and T.Y. contributed equally to this work.
This work was performed in partial fulfillment of the requirements for Ph.D. degree of T.Y., Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Disclosures: M.H., I.M., E.D.K., and W.A.G. are co-inventors on patent PCT/US2008/006895 “N-acetyl mannosamine as a therapeutic agent.”
Supplemental material for this article can be found on http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.12.023.
Supplementary data
Control panel for lectin specificity. Representative images of mouse juxtamedullar glomeruli stained with each substrate-inhibited lectin (green) and with the nuclear dye DAPI (blue). Each FITC-labeled lectin was incubated with its specific inhibitory carbohydrate (i.e., Neu5Ac for WGA and SNA, galactose for Jacalin, lactose for PNA, GalNAc for HPA and VVA) prior to overnight incubation on kidney slides of untreated wild-type (+/+) and GneM712T/M712T (-/-) pups at E19 (A), P2 (B), as well as ManNAc treated pups at P5 (C). The images show a greatly reduced or absent fluorescent signal for each lectin, when compared to Figure 3, for which no inhibitory carbohydrates were added. This indicates specificity of each lectin used in this panel.
Histological kidney analyses of H&E stained sections by light microscopy. Representative H&E stained kidney sections of wild type (+/+) and mutant GneM712T/M712T (-/-) littermate pups are shown at different magnifications. Left panels show overall cortex (c) and medullary (m) structures (Scale bars: 200 μm); middle panels show developing glomeruli (g) in the superficial cortex region where nephrogenesis occurs (Scale bars: 50 μm); and right panels show high powered images of representative mature juxtamedullary glomeruli (Scale bars: 20 μm). A: At embryonic day 19 (E19), no obvious developmental or structural differences in cortex, medulla, tubules or glomeruli were observed between mutant and wild-type pups. B: At postnatal day 2 (P2) mutant kidneys demonstrated vacuolated tubular epithelial cells (asterisk), infiltration of red blood cells (plus sign) and proteinaceous material (arrow) in Bowman's space and tubular lumens. C: In ManNAc supplemented mutant pups at postnatal day 5 (P5+ManNAc), the kidney histology considerably improved. Red cell casts and proteinaceous casts were markedly reduced but remained in some nephrons (asterisk).
Histological kidney analyses of Masson Trichrome stained kidney sections by light microscopy. Representative Masson Trichrome stained kidney sections of wild-type (+/+) and mutant GneM712T/M712T (-/-) littermate pups (Scale bars: 500 μm). At E19, glomeruli (g) appear normal in architecture for both wild type and mutant specimens. At P2, a representative glomerulus in wild type appears normal. However, mesangial expansion is evident in the mutant glomerulus as seen by increased blue stain. Tubular lumens contain proteinaceous debris (arrow), suggesting glomerular leakage of protein, and the tubular epithelial cells show vacuolization (asterisk). There is apparent improvement in mutant glomeruli at P5 after ManNAc administration (P5+ManNAc).
Urine gel electrophoresis and albumin quantitation. A: Image of the whole Coomassie Blue stained gel, loaded with mouse urines (2 μL), partly displayed in Figure 2. Note that albumin is the major protein present in mutant mice urines and that lower molecular weight proteins are greatly absent, indicating a renal glomerular defect, rather than a tubular defect in this mouse model. Molecular weight ladder (lane 1); pure bovine serum albumin, 3.0 μg (lane 2) and 0.3μg (lane 3); urines of untreated pups at P2, wild type (lane 4), heterozygote (lane 5) and two different mutant pups (lanes 6 and 7); urines of ManNAc treated pups at P5, wild type (lane 8) and two different mutant pups (lanes 9 and 10). B: Graphic display of the semi-quantitative determined albumin concentration in 2 μL urine of each mouse.
Expression of neuraminidases Neu1 and Neu2 in mouse kidneys. Quantitative real-time PCR results of mouse sialidases Neu1 (lysosomal) and Neu2 (cytoplasmic) mRNA expression levels in mouse kidneys. Displayed values represent the relative quantification (RQ) normalized to B2M, with expression in wild type (+/+) at P2 set to 1 for each Taqman probe. *P = 0.0001–0.05; **P < 0.0001. At age P2, mutant mice (-/-) kidneys displayed significantly decreased Neu1 and Neu2 mRNA expression compared to wild type mice (+/+) at the same age. At age P5, both Neu1 and Neu2 mRNA expression in wild type mice (+/+) kidneys decreased significantly, which likely reflects an age-dependent effect (Note that no untreated mutant mice kidneys at P5 could be assessed since these mice die before P3 without treatment). After ManNAc supplementation at P5 (P5 + ManNAc), both wild type and mutant mice kidneys showed Neu1 and Neu2 levels similar to each other and to untreated kidneys at P5.
References
- 1.Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 2.Keppler O.T., Hinderlich S., Langner J., Schwartz-Albiez R., Reutter W., Pawlita M. UDP-GlcNAc 2-epimerase: a regulator of cell surface sialylation. Science. 1999;284:1372–1376. doi: 10.1126/science.284.5418.1372. [DOI] [PubMed] [Google Scholar]
- 3.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelberg H., Healy L., Whiteley H., Miller L.A., Vimr E. In vivo enzymatic removal of alpha 2–>6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab Invest. 1996;74:907–920. [PubMed] [Google Scholar]
- 6.Quaggin S.E. Sizing up sialic acid in glomerular disease. J Clin Invest. 2007;117:1480–1483. doi: 10.1172/JCI32482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blau E.B., Haas J.E. Glomerular sialic acid and proteinuria in human renal disease. Lab Invest. 1973;28:477–481. [PubMed] [Google Scholar]
- 8.Quatacker J., Praet M., Matthys E. Ultrastructural alterations in the sialic acid distribution in minimal change disease and membranous glomerulonephritis. Pathol Res Pract. 1987;182:188–194. doi: 10.1016/s0344-0338(87)80103-6. [DOI] [PubMed] [Google Scholar]
- 9.Coppo R., Amore A. Aberrant glycosylation in IgA nephropathy (IgAN) Kidney Int. 2004;65:1544–1547. doi: 10.1111/j.1523-1755.2004.05407.x. [DOI] [PubMed] [Google Scholar]
- 10.Coppo R., Fonsato V., Balegno S., Ricotti E., Loiacono E., Camilla R., Peruzzi L., Amore A., Bussolati B., Camussi G. Aberrantly glycosylated IgA1 induces mesangial cells to produce platelet-activating factor that mediates nephrin loss in cultured podocytes. Kidney Int. 2010;77:417–427. doi: 10.1038/ki.2009.473. [DOI] [PubMed] [Google Scholar]
- 11.Doyonnas R., Kershaw D.B., Duhme C., Merkens H., Chelliah S., Graf T., McNagny K.M. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerjaschki D., Sharkey D.J., Farquhar M.G. Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98:1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Li Y., Brophy P.D., Kershawt D.B. Gene structure and alternative splicing of murine podocalyxin: a member of the CD34 sialomucin family. DNA Seq. 2001;12:407–412. doi: 10.3109/10425170109084466. [DOI] [PubMed] [Google Scholar]
- 14.Orlando R.A., Takeda T., Zak B., Schmieder S., Benoit V.M., McQuistan T., Furthmayr H., Farquhar M.G. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol. 2001;12:1589–1598. doi: 10.1681/ASN.V1281589. [DOI] [PubMed] [Google Scholar]
- 15.Takeda T., McQuistan T., Orlando R.A., Farquhar M.G. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerjaschki D., Vernillo A.T., Farquhar M.G. Reduced sialylation of podocalyxin—the major sialoprotein of the rat kidney glomerulus—in aminonucleoside nephrosis. Am J Pathol. 1985;118:343–349. [PMC free article] [PubMed] [Google Scholar]
- 17.Kurihara H., Anderson J.M., Kerjaschki D., Farquhar M.G. The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol. 1992;141:805–816. [PMC free article] [PubMed] [Google Scholar]
- 18.Seiler M.W., Rennke H.G., Venkatachalam M.A., Cotran R.S. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab Invest. 1977;36:48–61. [PubMed] [Google Scholar]
- 19.Andrews P.M. Scanning electron microscopy of the kidney glomerular epithelium after treatment with polycations in situ and in vitro. Am J Anat. 1978;153:291–303. doi: 10.1002/aja.1001530208. [DOI] [PubMed] [Google Scholar]
- 20.Quatacker J. Alterations in the sialic acid content of the rat glomerular filter in aminonucleoside nephrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;50:237–247. doi: 10.1007/BF02889904. [DOI] [PubMed] [Google Scholar]
- 21.Hinderlich S., Stasche R., Zeitler R., Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver: Purification and characterization of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. J Biol Chem. 1997;272:24313–24318. doi: 10.1074/jbc.272.39.24313. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg I., Avidan N., Potikha T., Hochner H., Chen M., Olender T., Barash M., Shemesh M., Sadeh M., Grabov-Nardini G., Shmilevich I., Friedmann A., Karpati G., Bradley W.G., Baumbach L., Lancet D., Asher E.B., Beckmann J.S., Argov Z., Mitrani-Rosenbaum S. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- 23.Seppala R., Lehto V.P., Gahl W.A. Mutations in the human UDP-N-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am J Hum Genet. 1999;64:1563–1569. doi: 10.1086/302411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Argov Z., Yarom R. “Rimmed vacuole myopathy” sparing the quadriceps: A unique disorder in Iranian Jews. J Neurol Sci. 1984;64:33–43. doi: 10.1016/0022-510x(84)90053-4. [DOI] [PubMed] [Google Scholar]
- 25.Griggs R.C., Askanas V., DiMauro S., Engel A., Karpati G., Mendell J.R., Rowland L.P. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–713. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi S., Keira Y., Murayama K., Ogawa M., Fujita M., Kawahara G., Oya Y., Imazawa M., Goto Y., Hayashi Y.K., Nonaka I., Nishino I. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J Biol Chem. 2004;279:11402–11407. doi: 10.1074/jbc.M313171200. [DOI] [PubMed] [Google Scholar]
- 27.Huizing M., Krasnewich D.M. Hereditary inclusion body myopathy: a decade of progress. Biochim Biophys Acta. 2009;1792:881–887. doi: 10.1016/j.bbadis.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzkopf M., Knobeloch K.P., Rohde E., Hinderlich S., Wiechens N., Lucka L., Horak I., Reutter W., Horstkorte R. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galeano B., Klootwijk R., Manoli I., Sun M., Ciccone C., Darvish D., Starost M.F., Zerfas P.M., Hoffmann V.J., Hoogstraten-Miller S., Krasnewich D.M., Gahl W.A., Huizing M. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117:1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinderlich S., Berger M., Keppler O.T., Pawlita M., Reutter W. Biosynthesis of N-acetylneuraminic acid in cells lacking UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. Biol Chem. 2001;382:291–297. doi: 10.1515/BC.2001.036. [DOI] [PubMed] [Google Scholar]
- 31.Sparks S., Rakocevic G., Joe G., Manoli I., Shrader J., Harris-Love M., Sonies B., Ciccone C., Dorward H., Krasnewich D., Huizing M., Dalakas M.C., Gahl W.A. Intravenous immune globulin in hereditary inclusion body myopathy: a pilot study. BMC Neurol. 2007;7:3. doi: 10.1186/1471-2377-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou H.H., Takematsu H., Diaz S., Iber J., Nickerson E., Wright K.L., Muchmore E.A., Nelson D.L., Warren S.T., Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kershaw D.B., Beck S.G., Wharram B.L., Wiggins J.E., Goyal M., Thomas P.E., Wiggins R.C. Molecular cloning and characterization of human podocalyxin-like protein: Orthologous relationship to rabbit PCLP1 and rat podocalyxin. J Biol Chem. 1997;272:15708–15714. doi: 10.1074/jbc.272.25.15708. [DOI] [PubMed] [Google Scholar]
- 34.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 35.Abrahamson D.R. Structure and development of the glomerular capillary wall and basement membrane. Am J Physiol. 1987;253:F783–F794. doi: 10.1152/ajprenal.1987.253.5.F783. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy K.J. Morphogenesis of the glomerular filter: the synchronous assembly and maturation of two distinct extracellular matrices. Microsc Res Tech. 1997;39:233–253. doi: 10.1002/(SICI)1097-0029(19971101)39:3<233::AID-JEMT4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. J Biol Chem. 2007;282:2753–2764. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- 38.Iskratsch T., Braun A., Paschinger K., Wilson I.B. Specificity analysis of lectins and antibodies using remodeled glycoproteins. Anal Biochem. 2009;386:133–146. doi: 10.1016/j.ab.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Kronis K.A., Carver J.P. Specificity of isolectins of wheat germ agglutinin for sialyloligosaccharides: a 360-MHz proton nuclear magnetic resonance binding study. Biochemistry. 1982;21:3050–3057. doi: 10.1021/bi00256a003. [DOI] [PubMed] [Google Scholar]
- 40.Shibuya N., Goldstein I.J., Broekaert W.F., Nsimba-Lubaki M., Peeters B., Peumans W.J. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 41.Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea) J Biol Chem. 1975;250:8518–8523. [PubMed] [Google Scholar]
- 42.Hortin G.L., Trimpe B.L. Lectin affinity chromatography of proteins bearing O-linked oligosaccharides: application of jacalin-agarose. Anal Biochem. 1990;188:271–277. doi: 10.1016/0003-2697(90)90605-9. [DOI] [PubMed] [Google Scholar]
- 43.Tachibana K., Nakamura S., Wang H., Iwasaki H., Maebara K., Cheng L., Hirabayashi J., Narimatsu H. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology. 2006;16:46–53. doi: 10.1093/glycob/cwj038. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez J.F., Lescar J., Chazalet V., Audfray A., Gagnon J., Alvarez R., Breton C., Imberty A., Mitchell E.P. Biochemical and structural analysis of Helix pomatia agglutinin: A hexameric lectin with a novel fold. J Biol Chem. 2006;281:20171–20180. doi: 10.1074/jbc.M603452200. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher U., Mitchell B.S., Brooks S.A., Delpech B., Leathem A.J. Does the lectin Helix pomatia agglutinin bind to hyaluronic acid in breast and colon cancer? Acta Histochem. 1996;98:435–440. doi: 10.1016/s0065-1281(96)80010-9. [DOI] [PubMed] [Google Scholar]
- 46.Puri K.D., Gopalakrishnan B., Surolia A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4) FEBS Lett. 1992;312:208–212. doi: 10.1016/0014-5793(92)80937-c. [DOI] [PubMed] [Google Scholar]
- 47.Tryggvason K., Patrakka J., Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 48.Wei C., Möller C.C., Altintas M.M., Li J., Schwarz K., Zacchigna S., Xie L., Henger A., Schmid H., Rastaldi M.P., Cowan P., Kretzler M., Parrilla R., Bendayan M., Gupta V., Nikolic B., Kalluri R., Carmeliet P., Mundel P., Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 49.Faul C., Donnelly M., Merscher-Gomez S., Chang Y.H., Franz S., Delfgaauw J., Chang J.M., Choi H.Y., Campbell K.N., Kim K., Reiser J., Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harvey S.J., Jarad G., Cunningham J., Goldberg S., Schermer B., Harfe B.D., McManus M.T., Benzing T., Miner J.H. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi S., Yu L., Chiu C., Sun Y., Chen J., Khitrov G., Merkenschlager M., Holzman L.B., Zhang W., Mundel P., Bottinger E.P. Podocyte-selective deletion of dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol. 2008;19:2159–2169. doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeansson M., Haraldsson B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol. 2006;290:F111–F116. doi: 10.1152/ajprenal.00173.2005. [DOI] [PubMed] [Google Scholar]
- 53.Barnes J.L., Radnik R.A., Gilchrist E.P., Venkatachalam M.A. Size and charge selective permeability defects induced in glomerular basement membrane by a polycation. Kidney Int. 1984;25:11–19. doi: 10.1038/ki.1984.2. [DOI] [PubMed] [Google Scholar]
- 54.Charonis A.S., Wissig S.L. Anionic sites in basement membranes: Differences in their electrostatic properties in continuous and fenestrated capillaries. Microvasc Res. 1983;25:265–285. doi: 10.1016/0026-2862(83)90018-3. [DOI] [PubMed] [Google Scholar]
- 55.Pavenstädt H. The charge for going by foot: modifying the surface of podocytes. Exp Nephrol. 1998;6:98–103. doi: 10.1159/000020511. [DOI] [PubMed] [Google Scholar]
- 56.Caulfield J.P., Reid J.J., Farquhar M.G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis: Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976;34:43–59. [PubMed] [Google Scholar]
- 57.D'Amico G., Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003;63:809–825. doi: 10.1046/j.1523-1755.2003.00840.x. [DOI] [PubMed] [Google Scholar]
- 58.Tóth-Heyn P., Drukker A., Guignard J.P. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol. 2000;14:227–239. doi: 10.1007/s004670050048. [DOI] [PubMed] [Google Scholar]
- 59.Schnabel E., Dekan G., Miettinen A., Farquhar M.G. Biogenesis of podocalyxin—the major glomerular sialoglycoprotein—in the newborn rat kidney. Eur J Cell Biol. 1989;48:313–326. [PubMed] [Google Scholar]
- 60.Vogtländer N.P., van der Vlag J., Bakker M.A., Dijkman H.B., Wevers R.A., Campbell K.P., Wetzels J.F., Berden J.H. Expression of sialidase and dystroglycan in human glomerular diseases. Nephrol Dial Transplant. 2010;25:478–484. doi: 10.1093/ndt/gfp465. [DOI] [PubMed] [Google Scholar]
- 61.Kanasaki K., Kanda Y., Palmsten K., Tanjore H., Lee S.B., Lebleu V.S., Gattone V.H., Jr, Kalluri R. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313:584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogtländer N.P., Dijkman H., Bakker M.A., Campbell K.P., van der Vlag J., Berden J.H. Localization of alpha-dystroglycan on the podocyte: from top to toe. J Histochem Cytochem. 2005;53:1345–1353. doi: 10.1369/jhc.4A6596.2005. [DOI] [PubMed] [Google Scholar]
- 63.Clement L.C., Avila-Casado C., Mace C., Soria E., Bakker W.W., Kersten S., Chugh S.S. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donoviel D.B., Freed D.D., Vogel H., Potter D.G., Hawkins E., Barrish J.P., Mathur B.N., Turner C.A., Geske R., Montgomery C.A., Starbuck M., Brandt M., Gupta A., Ramirez-Solis R., Zambrowicz B.P., Powell D.R. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Putaala H., Soininen R., Kilpeläinen P., Wartiovaara J., Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Kestilä M., Lenkkeri U., Männikkö M., Lamerdin J., McCready P., Putaala H., Ruotsalainen V., Morita T., Nissinen M., Herva R., Kashtan C.E., Peltonen L., Holmberg C., Olsen A., Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 67.Khoshnoodi J., Hill S., Tryggvason K., Hudson B., Friedman D.B. Identification of N-linked glycosylation sites in human nephrin using mass spectrometry. J Mass Spectrom. 2007;42:370–379. doi: 10.1002/jms.1170. [DOI] [PubMed] [Google Scholar]
- 68.Tryggvason K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol. 1999;10:2440–2445. doi: 10.1681/ASN.V10112440. [DOI] [PubMed] [Google Scholar]
- 69.Yan K., Khoshnoodi J., Ruotsalainen V., Tryggvason K. N-linked glycosylation is critical for the plasma membrane localization of nephrin. J Am Soc Nephrol. 2002;13:1385–1389. doi: 10.1097/01.asn.0000013297.11876.5b. [DOI] [PubMed] [Google Scholar]
- 70.Takashima S. Characterization of mouse sialyltransferase genes: their evolution and diversity. Biosci Biotechnol Biochem. 2008;72:1155–1167. doi: 10.1271/bbb.80025. [DOI] [PubMed] [Google Scholar]
- 71.Takashima S., Tsuji S., Tsujimoto M. Comparison of the enzymatic properties of mouse beta-galactoside alpha2,6-sialyltransferases, ST6Gal I and II. J Biochem. 2003;134:287–296. doi: 10.1093/jb/mvg142. [DOI] [PubMed] [Google Scholar]
- 72.Kono M., Ohyama Y., Lee Y.C., Hamamoto T., Kojima N., Tsuji S. Mouse beta-galactoside alpha 2,3-sialyltransferases: comparison of in vitro substrate specificities and tissue specific expression. Glycobiology. 1997;7:469–479. doi: 10.1093/glycob/7.4.469. [DOI] [PubMed] [Google Scholar]
- 73.Wang Z., Sun Z., Li A.V., Yarema K.J. Roles for UDP-GlcNAc 2-epimerase/ManNAc 6-kinase outside of sialic acid biosynthesis: modulation of sialyltransferase and BiP expression: GM3 and GD3 biosynthesis, proliferation, and apoptosis, and ERK1/2 phosphorylation. J Biol Chem. 2006;281:27016–27028. doi: 10.1074/jbc.M604903200. [DOI] [PubMed] [Google Scholar]
- 74.Cohen-Forterre L., Mozere G., Andre J., Sternberg M. Studies on kidney sialidase in normal and diabetic rats. Biochim Biophys Acta. 1984;801:138–145. doi: 10.1016/0304-4165(84)90222-8. [DOI] [PubMed] [Google Scholar]
- 75.Duncan P.I., Raymond F., Fuerholz A., Sprenger N. Sialic acid utilisation and synthesis in the neonatal rat revisited. PLoS One. 2009;4:e8241. doi: 10.1371/journal.pone.0008241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daskalakis N., Winn M.P. Focal and segmental glomerulosclerosis. Cell Mol Life Sci. 2006;63:2506–2511. doi: 10.1007/s00018-006-6171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ronco P., Debiec H. New insights into the pathogenesis of membranous glomerulonephritis. Curr Opin Nephrol Hypertens. 2006;15:258–263. doi: 10.1097/01.mnh.0000222692.99711.02. [DOI] [PubMed] [Google Scholar]
- 78.Molino C., Fabbian F., Longhini C. Clinical approach to lupus nephritis: recent advances. Eur J Intern Med. 2009;20:447–453. doi: 10.1016/j.ejim.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 79.Vats A.N. Genetics of idiopathic nephrotic syndrome. Indian J Pediatr. 2005;72:777–783. doi: 10.1007/BF02734151. [DOI] [PubMed] [Google Scholar]
- 80.Topham P., Barratt J., Feehally J. A spoonful of sugar helps the proteinuria go down? Nephrol Dial Transplant. 2008;23:813–815. doi: 10.1093/ndt/gfm720. [DOI] [PubMed] [Google Scholar]
- 81.Muchitsch E., Pichler L., Schwarz H.P., Ulrich W. Effects of human alpha-1-acid glycoprotein on aminonucleoside-induced minimal change nephrosis in rats. Nephron. 1999;81:194–199. doi: 10.1159/000045276. [DOI] [PubMed] [Google Scholar]
- 82.Hjalmarsson C., Lidell M.E., Haraldsson B. Beneficial effects of orosomucoid on the glomerular barrier in puromycin aminonucleoside-induced nephrosis. Nephrol Dial Transplant. 2006;21:1223–1230. doi: 10.1093/ndt/gfk050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control panel for lectin specificity. Representative images of mouse juxtamedullar glomeruli stained with each substrate-inhibited lectin (green) and with the nuclear dye DAPI (blue). Each FITC-labeled lectin was incubated with its specific inhibitory carbohydrate (i.e., Neu5Ac for WGA and SNA, galactose for Jacalin, lactose for PNA, GalNAc for HPA and VVA) prior to overnight incubation on kidney slides of untreated wild-type (+/+) and GneM712T/M712T (-/-) pups at E19 (A), P2 (B), as well as ManNAc treated pups at P5 (C). The images show a greatly reduced or absent fluorescent signal for each lectin, when compared to Figure 3, for which no inhibitory carbohydrates were added. This indicates specificity of each lectin used in this panel.
Histological kidney analyses of H&E stained sections by light microscopy. Representative H&E stained kidney sections of wild type (+/+) and mutant GneM712T/M712T (-/-) littermate pups are shown at different magnifications. Left panels show overall cortex (c) and medullary (m) structures (Scale bars: 200 μm); middle panels show developing glomeruli (g) in the superficial cortex region where nephrogenesis occurs (Scale bars: 50 μm); and right panels show high powered images of representative mature juxtamedullary glomeruli (Scale bars: 20 μm). A: At embryonic day 19 (E19), no obvious developmental or structural differences in cortex, medulla, tubules or glomeruli were observed between mutant and wild-type pups. B: At postnatal day 2 (P2) mutant kidneys demonstrated vacuolated tubular epithelial cells (asterisk), infiltration of red blood cells (plus sign) and proteinaceous material (arrow) in Bowman's space and tubular lumens. C: In ManNAc supplemented mutant pups at postnatal day 5 (P5+ManNAc), the kidney histology considerably improved. Red cell casts and proteinaceous casts were markedly reduced but remained in some nephrons (asterisk).
Histological kidney analyses of Masson Trichrome stained kidney sections by light microscopy. Representative Masson Trichrome stained kidney sections of wild-type (+/+) and mutant GneM712T/M712T (-/-) littermate pups (Scale bars: 500 μm). At E19, glomeruli (g) appear normal in architecture for both wild type and mutant specimens. At P2, a representative glomerulus in wild type appears normal. However, mesangial expansion is evident in the mutant glomerulus as seen by increased blue stain. Tubular lumens contain proteinaceous debris (arrow), suggesting glomerular leakage of protein, and the tubular epithelial cells show vacuolization (asterisk). There is apparent improvement in mutant glomeruli at P5 after ManNAc administration (P5+ManNAc).
Urine gel electrophoresis and albumin quantitation. A: Image of the whole Coomassie Blue stained gel, loaded with mouse urines (2 μL), partly displayed in Figure 2. Note that albumin is the major protein present in mutant mice urines and that lower molecular weight proteins are greatly absent, indicating a renal glomerular defect, rather than a tubular defect in this mouse model. Molecular weight ladder (lane 1); pure bovine serum albumin, 3.0 μg (lane 2) and 0.3μg (lane 3); urines of untreated pups at P2, wild type (lane 4), heterozygote (lane 5) and two different mutant pups (lanes 6 and 7); urines of ManNAc treated pups at P5, wild type (lane 8) and two different mutant pups (lanes 9 and 10). B: Graphic display of the semi-quantitative determined albumin concentration in 2 μL urine of each mouse.
Expression of neuraminidases Neu1 and Neu2 in mouse kidneys. Quantitative real-time PCR results of mouse sialidases Neu1 (lysosomal) and Neu2 (cytoplasmic) mRNA expression levels in mouse kidneys. Displayed values represent the relative quantification (RQ) normalized to B2M, with expression in wild type (+/+) at P2 set to 1 for each Taqman probe. *P = 0.0001–0.05; **P < 0.0001. At age P2, mutant mice (-/-) kidneys displayed significantly decreased Neu1 and Neu2 mRNA expression compared to wild type mice (+/+) at the same age. At age P5, both Neu1 and Neu2 mRNA expression in wild type mice (+/+) kidneys decreased significantly, which likely reflects an age-dependent effect (Note that no untreated mutant mice kidneys at P5 could be assessed since these mice die before P3 without treatment). After ManNAc supplementation at P5 (P5 + ManNAc), both wild type and mutant mice kidneys showed Neu1 and Neu2 levels similar to each other and to untreated kidneys at P5.