Abstract
Background:
Intra-articular fractures may hasten posttraumatic arthritis in patients who are typically too active and too young for joint replacement. Current orthopaedic treatment principles, including recreating anatomic alignment and establishing articular congruity, have not eliminated posttraumatic arthritis. Additional biomechanical and biological factors may contribute to the development of arthritis. The objective of the present study was to evaluate human synovial fluid for friction-lowering function and the concentrations of putative lubricant molecules following tibial plateau fractures.
Methods:
Synovial fluid specimens were obtained from the knees of eight patients (twenty-five to fifty-seven years old) with a tibial plateau fracture, with five specimens from the injured knee as plateau fracture synovial fluid and six specimens from the contralateral knee as control synovial fluid. Each specimen was centrifuged to obtain a fluid sample, separated from a cell pellet, for further analysis. For each fluid sample, the start-up (static) and steady-state (kinetic) friction coefficients in the boundary mode of lubrication were determined from a cartilage-on-cartilage biomechanical test of friction. Also, concentrations of the putative lubricants, hyaluronan and proteoglycan-4, as well as total protein, were determined for fluid samples.
Results:
The group of experimental samples were obtained at a mean (and standard deviation) of 11 ± 9 days after injury from patients with a mean age of 45 ± 13 years. Start-up and kinetic friction coefficients demonstrated similar trends and dependencies. The kinetic friction coefficients for human plateau fracture synovial fluid were approximately 100% higher than those for control human synovial fluid. Hyaluronan concentrations were ninefold lower for plateau fracture synovial fluid compared with the control synovial fluid, whereas proteoglycan-4 concentrations were more than twofold higher in plateau fracture synovial fluid compared with the control synovial fluid. Univariate and multivariate regression analysis indicated that kinetic friction coefficient increased as hyaluronan concentration decreased.
Conclusions:
Knees afflicted with a tibial plateau fracture have synovial fluid with decreased lubrication properties in association with a decreased concentration of hyaluronan.
Clinical Relevance:
Tibial plateau fractures result in a posttraumatic deficiency in synovial fluid lubrication function.
Traumatic intra-articular fractures, such as those of the tibial plateau, are at risk of joint degeneration and posttraumatic arthritis even when treated according to traditional orthopaedic principles to restore articular congruency and anatomic alignment1-7. The consequences of poor results can be life-changing—painful weight-bearing, restricted activity, and lost time in the work force1,8-10. The costs of posttraumatic arthritis are a component of the estimated >$100 billion annual burden of osteoarthritis on the U.S. economy, with 9.8% of the cases of knee osteoarthritis estimated to be of posttraumatic etiology; furthermore, patients with posttraumatic arthritis and its chronic consequences tend to be young, with an additional impact on employment9. Compared with those without posttraumatic arthritis, patients with posttraumatic arthritis have worse clinical outcomes after arthrodesis or arthroplasty11,12. Posttraumatic arthritis and its consequences have not been eliminated by employing traditional orthopaedic principles, suggesting a possible role for additional biological and biomechanical factors.
The pathogenesis of posttraumatic arthritis is complex and multifactorial. Recent studies have focused on articular chondrocyte death and cartilage damage due to direct trauma, enzyme-mediated cartilage degradation, and the role of reactive oxygen species13-16. Deficient lubrication may also contribute to cartilage deterioration after trauma. Lubrication typically allows articular cartilage to bear a load and slide with low friction and low wear17, and is mediated by high levels of proteoglycan-4 and hyaluronan in synovial fluid18. Synovial fluid lubricant molecules are secreted by chondrocytes and synoviocytes lining the joint, and are concentrated through selective retention by synovium. However, after an intra-articular fracture, soft tissues that normally produce and retain synovial fluid lubricants are damaged, and blood and cellular components from damaged tissues and bone marrow infiltrate the joint space13,19-22. Such alteration of synovial fluid may disrupt its lubrication functions.
Alteration in the lubricating function and lubricant composition of synovial fluid appears to be involved in cartilage deterioration after anterior cruciate ligament (ACL) injury, as well as other injuries of the knee joint. After ACL injury of human knees, the level of proteoglycan-4 in synovial fluid was reduced, while levels of degradative enzymes, cartilage matrix degradation products, and inflammatory markers were increased for up to six to eighteen months after injury15,23. Deficient lubrication after an injury has similarly been detected in the synovial fluid from acutely injured equine joints, in association with a decreased concentration of hyaluronan24. In guinea pig25 and rat26,27 models of knee injury, synovial fluid lubrication function and lubricant levels are also diminished. These studies suggest that human knee trauma, and specifically intra-articular tibial plateau fracture, may lead to a deficiency in synovial fluid lubrication.
The hypothesis tested in the present study was that tibial plateau fractures impair the friction-lowering lubrication function of human synovial fluid in association with changes in the concentrations of proteoglycan-4 and hyaluronan lubricant molecules. The objectives of this study were to compare the synovial fluid from joints after acute tibial plateau fracture and the synovial fluid from normal knees in terms of (1) friction-lowering boundary lubrication function and (2) biochemical composition, including hyaluronan and proteoglycan-4 concentrations. In addition, the possible biomechanical basis for impaired lubricant function was assessed by correlating friction coefficient and lubricant concentration.
Materials and Methods
Materials
Materials for lubrication testing were obtained as previously described28. The antibody to proteoglycan-4 was that to lubricin from Abcam (Cambridge, Massachusetts); Streptomyces hyaluronidase was from Seikagaku (Tokyo, Japan); SeaKem Gold Agarose (Lonza) was from Fisher Scientific (Pittsburgh, Pennsylvania), and sodium dodecyl sulfate (SDS)-horizontal agarose gel electrophoresis and Western blot materials were from Life Technologies (Carlsbad, California). EDTA-treated adult human blood was purchased from Golden West Biologicals (Temecula, California). EDTA-treated bovine blood was from Animal Technologies (Tyler, Texas).
Patient Samples and Fracture Classification
Samples of synovial fluid were aspirated from the knees of patients after they had provided consent at a level-I trauma center under the auspices of an institutional review board-approved research plan. Patients with an age of twenty-one years or older who were scheduled for surgery after sustaining a closed tibial plateau fracture were asked to be included in the research study prior to the procedure by an orthopaedic surgeon, and eight who consented were subjects for the present study. A particular age range was not sought. Exclusion criteria consisted of open fractures and vulnerable groups including minors, individuals known to be cognitively impaired or institutionalized, patients with known infection with human immunodeficiency virus or hepatitis-C or hepatitis-B virus, and patients unable to provide consent.
Radiographs and computed tomography (CT) scans, if they were available on the basis of routine clinical care, were graded by an attending orthopaedic surgeon using the Schatzker classification system for tibial plateau fractures29.
A total of eleven synovial fluid samples collected from eight patients (twenty-five to fifty-seven years old) were used in the present study. In all patients who had provided consent, attempts were made to aspirate fluid from both the injured knee and the contralateral knee. Synovial fluid samples of sufficient volume (see below) were successfully withdrawn from both the injured knee (fracture synovial fluid) and the contralateral, control knee (control synovial fluid) of three donors with an acute tibial plateau fracture. For two other patients, samples of fracture synovial fluid were obtained, but samples from the contralateral, control knee were not of sufficient volume. Thus, three other samples of control synovial fluid were used from other patients. Radiographs of the contralateral knee were evaluated by radiologists and orthopaedists, and they confirmed that the knee did not have an acute injury. To obtain fluid, after sterilization of the skin overlying each joint, a standard 18-gauge hollow-bore spinal needle (Becton Dickinson, Laguna Hills, California) attached to a 60-mL syringe barrel (Becton Dickinson) was introduced into the joint space and synovial fluid was aspirated. The site of needle introduction into the knee was at the level of the joint line, 1 cm medial or lateral to the patellar tendon on the anterior aspect of the knee30.
Experimental Design
Gross Analysis of Human Synovial Fluid Samples
Samples were initially studied by gross examination for color and clarity. Samples were noted to be straw-colored or bloody. Clarity was described by the degree of opacity as clear or opaque on the basis of the ability to read markings through a distance of approximately 1 cm through the tube. Samples were then centrifuged (3000 g for thirty minutes at 4°C) to obtain discrete fractions. The relative volume of fractions was then estimated directly from the centrifuged tubes. Fluid samples were photographed before and after centrifugation. Following centrifugation, the supernatant and pellet were separated and stored in aliquots at –80°C. Light microscopy analysis of selected supernatants and pellets indicated that the processing was sufficient to separate cells such that they were present in the pellet and absent from the supernatant (data not shown). Samples with adequate volume (1 mL) after processing were selected for use in the study.
Assay Validation for Biochemical Analysis of in Vitro Mixtures of Synovial Fluid and Blood
Because of the blood in the synovial fluid aspirated from patients with a tibial plateau fracture, it was necessary to ensure that the presence of blood components in the synovial fluid did not interfere with the accuracy of the biochemical assays used. Therefore, prior to biochemical analysis of the clinical human synovial fluid samples, an in vitro model of mixtures of fresh EDTA-treated adult human blood (Golden West Biologicals) and human synovial fluid collected during routine arthroscopic surgery was developed, in order to mimic the fracture synovial fluid, and was used to validate the assays for protein, proteoglycan-4, and hyaluronan. First, the macroscopic appearance of in vitro mixtures of human synovial fluid and blood of varying proportions were examined to identify variations after mixing and separation by centrifugation (3000 g for thirty minutes at 4°C). Portions of in vitro human mixtures prior to centrifugation and supernatant and pellet portions of in vitro mixtures after centrifugation were then assayed for the concentrations of total protein, hyaluronan, and proteoglycan-4, relative to the initial mixture volume. To demonstrate the visual appearance of in vitro mixtures, analogous but larger volumes (approximately 5 mL) of bovine blood and bovine synovial fluid (Animal Technologies) were generated and photographed.
Biomechanical and Biochemical Analyses of Human Synovial Fluid Samples from Control and Injured Knees
Portions of control and fracture synovial fluid samples were analyzed by biomechanical lubrication tests for friction-lowering properties, in addition to analysis by biochemical assays for the concentrations of total protein, hyaluronan, and proteoglycan-4.
Analytical Methods
Lubrication Test
Portions of individual samples of human synovial fluid from control and injured knees were analyzed for static and kinetic friction coefficients in the boundary lubrication mode on articulating cartilage surfaces (one friction test per synovial fluid specimen) as described previously28,31. Intact articular surfaces were in the form of osteochondral cores and anuli from adult bovine knees, and were stored in phosphate-buffered saline solution (PBS) supplemented with protease inhibitors (2 mM Na-EDTA, 1 mM PMSF [phenylmethanesulfonyl fluoride], 5 mM Benz-HCl [benzamidine hydrochloride], and 10 mM NEM [N-ethylmaleimide]) at –80°C. For lubrication testing, cartilage samples were thawed at 4°C and then bathed in approximately 0.5 mL of the subsequent test lubricant supplemented with protease inhibitors, with the cartilage completely immersed for sixteen to twenty-four hours at 4°C prior to lubrication testing at room temperature. The lubricant sample and cartilage were then tested at room temperature by preconditioning, compressing to 18% of the total cartilage thickness, and allowing thirty minutes for stress relaxation and interstitial fluid depressurization. Then samples were rotated at an effective velocity of 0.3 mm/s with pre-sliding durations (Tps; the duration the sample is stationary prior to rotation) of 120, 12.0, and 1.2 s. Friction coefficients (μ) were calculated from the expression μ = τ/(Reff × Neq), where τ is torque, Neq is the equilibrium axial load after thirty minutes of stress relaxation, and Reff is the effective radius of the cartilage, as described previously28,31. Briefly, a static friction coefficient (μstatic) was calculated with use of the peak | τ |, measured immediately after (within 10° of) the start of rotation, and Neq. A kinetic friction coefficient (μkinetic) was calculated with use of both the | τ | averaged during the second complete revolution of the test sample and also Neq.
Consistent with previous results31 comparing friction coefficients with increasing Tps, all test lubricants demonstrated little variation in kinetic friction coefficients with values at Tps = 1.2 s remaining within 11% of values at Tps = 120 s; therefore, for brevity and clarity μkinetic data are presented as the average at all pre-sliding durations. As expected, the mean static and kinetic friction coefficients measured for PBS were >0.20 for all Tps, consistent with previous results31, so PBS data were not analyzed further.
Biochemical Analysis
Portions of synovial fluid samples were analyzed biochemically for absolute concentrations of lubricant molecules hyaluronan and proteoglycan-4, as well as for total protein. A minimum number of two replicate samples per specimen were used for concentration measurements.
Hyaluronan
The concentration of hyaluronan in synovial fluid samples was determined by an enzyme-linked immunosorbent assay (ELISA)-like assay with use of hyaluronan binding protein (Corgenix, Broomfield, Colorado).
Proteoglycan-4
The concentration of proteoglycan-4 in human synovial fluid samples was quantified by Western blot with use of antibody to lubricin after SDS-horizontal agarose gel electrophoresis on 2% 3-mm-thick agarose gels and transfer to PVDF (polyvinylidene fluoride) membrane (100 mA, overnight). The immunoreactive proteins were visualized by Amersham ECL Plus detection and digital scanning with a STORM 840 imaging system (GE Healthcare, Piscataway, New Jersey). ImageQuant (GE Healthcare) was used to generate densitometric scans. The proteoglycan-4 in human synovial fluid was quantified with use of standards of proteoglycan-4 that were purified32 from conditioned medium of human cartilage explants and run on the same gels.
Statistical Analysis
Continuous variables are presented as the mean and the standard error of the mean. The effects of joint injury (trauma versus control) on synovial fluid properties (μkinetic and the concentration of lubricants) were assessed by analysis of variance (ANOVA). The effects of joint injury (trauma versus control, as a fixed factor) and Tps (1.2, 12.0, and 120 s, as a repeated factor) on synovial fluid static friction coefficient (μstatic) were assessed by two-level ANOVA. Planned comparisons for μstatic were performed between trauma and control groups at each Tps value. The dependencies of friction coefficients on the concentrations of putative lubricants (hyaluronan and proteoglycan-4) were analyzed by univariate and multivariate regression, with both the absolute and log10 value of hyaluronan concentrations (since it varied by several orders of magnitude). Statistical analysis was performed with use of Systat software (version 10.2; Systat, Richmond, California).
Source of Funding
This study was supported by grants from the Orthopaedic Trauma Association and the National Institutes of Health (NIH R01 AR055637 and T35 HL007491), a National Science Foundation Graduate Research Fellowship, and an award under the Howard Hughes Medical Institute Professors Program.
Results
Patient Demographics
A total of eleven synovial fluid samples from eight patients were used in the present study (see Appendix). For these patients, the tibial plateau fracture types ranged from Schatzker type II to type VI and from Orthopaedic Trauma Association (OTA) class 41B2.2 to 41C3.333,34. The control group of six normal samples was from patients with a mean age (and standard deviation) of 42 ± 16 years, with a mean interval after the injury and before synovial fluid acquisition of 11 ± 7 days. The injury group of five experimental samples was from patients with a mean age of 45 ± 13 years, with a mean interval after the injury of 11 ± 9 days. Of the eight patients, six were male and two were female; both control and experimental synovial fluid samples were successfully obtained from the two female patients.
Gross Appearance of Human Synovial Fluid Samples from Control and Injured Knees
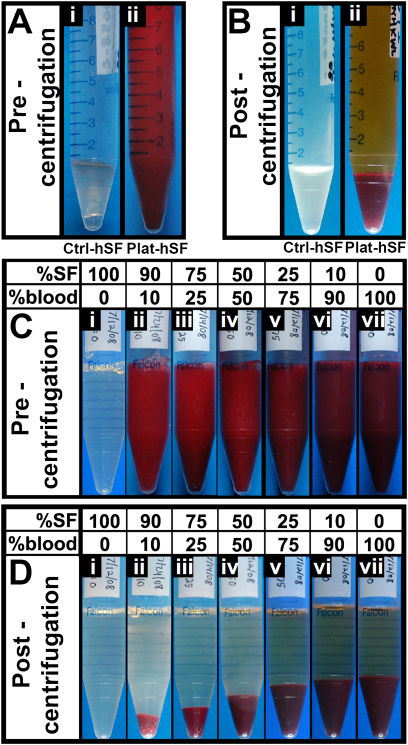
The synovial fluid samples from the control and injured joints appeared markedly different. Before centrifugation, the control synovial fluid showed only traces of blood, whereas the plateau fracture synovial fluid was grossly bloody (Fig. 1-A). After centrifugation, the supernatant of control synovial fluid samples was clarified and appeared clear or straw-colored (Fig. 1-B). In contrast, the synovial fluid samples from the injured joints separated into a yellow supernatant and a dark red pellet of varying relative volumes (Fig. 1-B).
Fig. 1.
Gross images of human synovial fluid collected following tibial plateau fracture (Figs. 1-A and 1-B) and mixtures of bovine synovial fluid (SF) and blood (Figs. 1-C and 1-D).The supernatant in Figures 1-B and 1-D is the fluid portion, including synovial fluid, and the bottom layer is the cell pellet. Human synovial fluid was collected from either a normal, noninjured knee (Ctrl-hSF; Fig. 1-A-i and Fig. 1-B-i) or a knee with tibial plateau fracture (Plat-hSF; Fig. 1-A-ii and Fig. 1-B-ii). This specimen was obtained approximately seven days following injury. Bovine synovial fluid and blood were mixed in designated proportions (Fig. 1-C and Fig. 1-D). Images are shown for fluids before (Fig. 1-A and Fig. 1-C) and after (Fig. 1-B and Fig. 1-D) centrifugation.
Biochemical Assay Validation with in Vitro Mixtures of Synovial Fluid and Blood
The in vitro mixtures of bovine synovial fluid and bovine blood appeared similar to the range of control and plateau fracture human synovial fluid samples. Before centrifugation, mixtures with an increasing proportion of blood were of an increasingly darker shade of red (Fig. 1-C). After centrifugation, samples separated into a supernatant that was increasingly yellow and a pellet that was increasing in size as the proportion of blood increased (Fig. 1-D). The in vitro mixtures of human synovial fluid and human blood appeared to separate similarly, although they were more difficult to visualize because of the small volumes available.
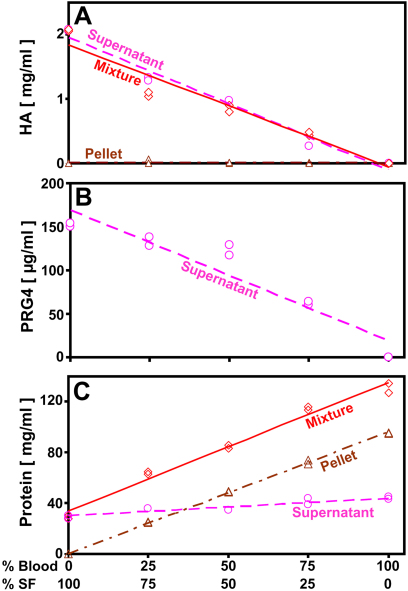
Biochemical analysis of the in vitro mixtures of human synovial fluid and blood, as well as the supernatants and pellets, revealed that the hyaluronan and proteoglycan-4 constituents, when present, separated primarily into the supernatant fraction (Figs. 2-A and 2-B), indicating they were maintained in the fluid phase even when synovial fluid was mixed with blood. In contrast, protein separated between the pellet and supernatant according to the concentrations of protein in the plasma and cells of whole human blood, and the concentration of protein in human synovial fluid (Fig. 2-C). The biochemical assays were confirmed to be sensitive and specific to the target components by mixture and spiking studies (data not shown). Consistent with this finding, the total amounts of hyaluronan (Fig. 2-A) and proteoglycan-4 (Fig. 2-B) in the supernatant fractions were similar (p = 0.25 to 1.00) to those of the corresponding mixtures before centrifugation. Also, the total amount of protein (Fig. 2-C) in the supernatant and pellet fractions, together, was similar (p = 0.38) to that of the corresponding mixtures before centrifugation. In addition, the supernatant hyaluronan, supernatant proteoglycan-4, and total protein in the in vitro mixtures were similar to the proportionate amounts based on the pure synovial fluid and blood preparations. Thus, supernatant fractions could be readily analyzed biochemically for the putative lubricants, hyaluronan and proteoglycan-4.
Fig. 2.
Effect of mixing human synovial fluid and blood and subsequent centrifugation on the distribution of hyaluronan (HA) (Fig. 2-A), proteoglycan-4 (PRG4) (Fig. 2-B), and protein (Fig. 2-C). Samples were analyzed as mixtures before centrifugation and as supernatants and pellets after centrifugation. Proteoglycan-4 was detected only in supernatants. Linear regression fits are indicated. Circles represent the supernatant fraction; diamonds represent the mixture; triangles represent the pellet fraction. SF = synovial fluid.
Lubrication Function of Human Synovial Fluid Samples from Control and Injured Knees
The boundary mode friction coefficients were higher for the fracture synovial fluid than the control synovial fluid (Figs. 3-A and 3-B). The μstatic varied with test lubricant (p < 0.001) and pre-spin duration (p < 0.001) without an interaction effect (p = 0.25; Fig. 3-A). The average μstatic values were 49% to 120% higher for fracture synovial fluid than for control synovial fluid, with the percentage difference increasing as the pre-spin duration decreased from 120 s to 1.2 s. At the short pre-spin duration of 1.2 s, the μstatic values approached the μkinetic values (Figs. 3-A and 3-B). The average μkinetic value for fracture synovial fluid was double that of control synovial fluid (0.044 versus 0.022, p < 0.001; Fig. 3-B).
Fig. 3.
Effect of tibial plateau fracture on the boundary lubrication of articular cartilage by human synovial fluid (SF). Static (Fig. 3-A) and kinetic (Fig. 3-B) friction coefficients for synovial fluid from six control knees (Ctrl-hSF) and from five knees with a fracture (Plat-hSF). Data are given as the mean and the standard error of the mean. *P < 0.05). **P < 0.001.
Biochemical Analysis of Human Synovial Fluid Samples from Control and Injured Knees
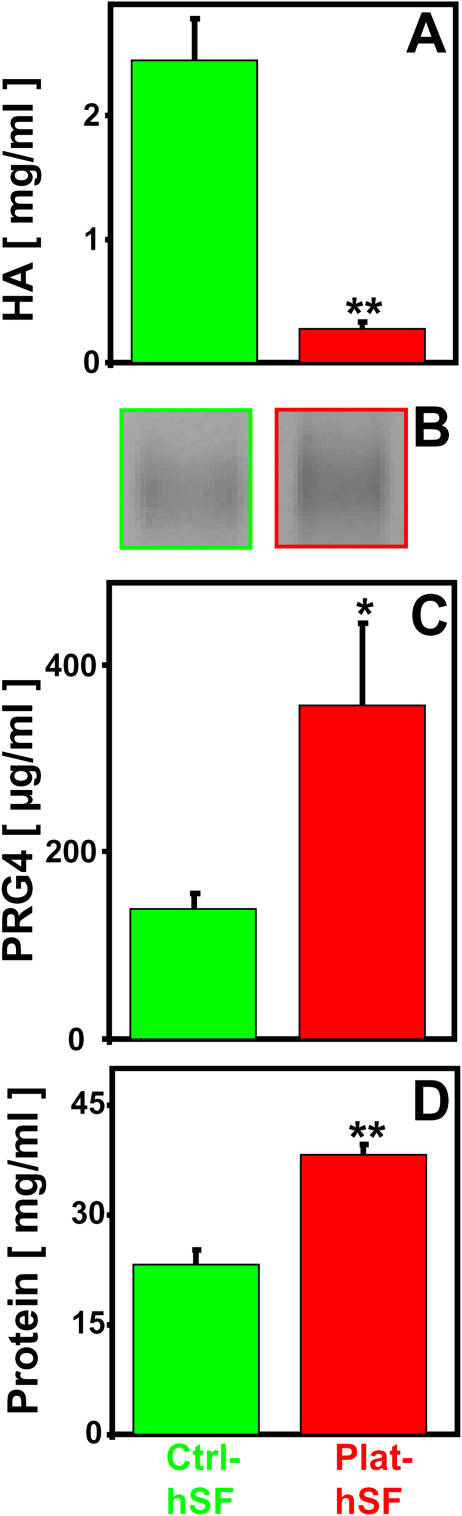
The concentrations of lubricant molecules were different for control and plateau fracture synovial fluid (Fig. 4). The concentration of hyaluronan (Fig. 4-A) was markedly lower in fracture synovial fluid than in control synovial fluid (0.27 mg/mL versus 2.53 mg/mL; an 89% decrease, p < 0.001). On the basis of Western blot analysis, which identified a specific immunoreactive band at >460 kDa in synovial fluid samples (Fig. 4-B), the concentration of proteoglycan-4 (Fig. 4-C) was markedly higher in fracture synovial fluid than control synovial fluid (356 versus 139 μg/mL; an increase of 156%, p < 0.05). The concentration of protein (Fig. 4-D) was somewhat higher in fracture synovial fluid than control synovial fluid (38 mg/mL versus 22 mg/mL; an increase of 72%, p < 0.001).
Fig. 4.
Effect of tibial plateau fracture on hyaluronan (HA) (Fig. 4-A), proteoglycan-4 (PRG4) (Figs. 4-B and 4-C), and protein (Fig. 4-D). Figs. 4-A, 4-C, and 4-D Concentrations were determined for human synovial fluid from six control knees (Ctrl-hSF) and from five knees with a fracture (Plat-hSF). Data are given as the mean and the standard error of the mean. *P < 0.05. **P < 0.001. Fig. 4-B Representative Western blots of synovial fluid samples from control knees and knees with a fracture probed for proteoglycan-4.
Regression Analysis of Lubrication Properties of Human Synovial Fluid from Control and Injured Knees with Biochemical Constituents
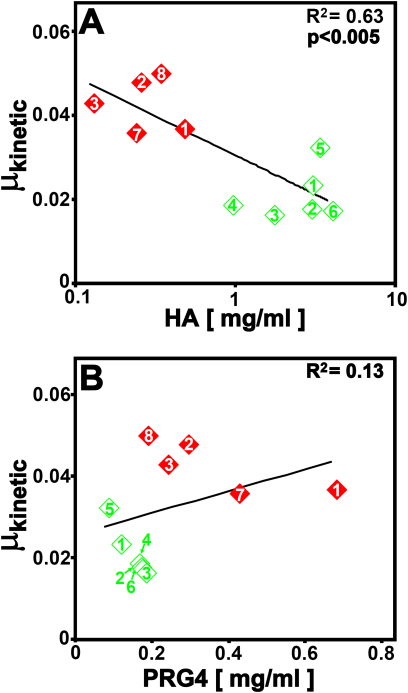
Regression analysis indicated certain correlations between friction coefficients and concentrations of lubricant molecules. Univariate regression showed a significant negative correlation between μkinetic and hyaluronan concentration (in milligrams per milliliter) (slope = −0.0067, r2 = 0.44, p < 0.05) as well as between μkinetic and the natural log of the hyaluronan concentration (in milligrams per millimeter) (slope = −0.0081, r2 = 0.63, p < 0.005; Fig. 5-A), and no significant correlation between μkinetic and proteoglycan-4 concentration (in milligrams per milliliter) (slope = 0.027, r2 = 0.13, p = 0.28; Fig 5-B). Similar correlation trends were also observed for static friction coefficients (data not shown). Multivariate regression yielded similar results (elimination of variation due to proteoglycan-4, leaving only correlation with hyaluronan concentration).
Fig. 5.
Correlation of kinetic friction coefficients with concentrations of hyaluronan (HA) (Fig. 5-A) and proteoglycan-4 (PRG4) (Fig. 5-B) in human synovial fluid samples from control knees (open green diamonds) and knees with a fracture (closed red diamonds). Each sample is labeled with the patient donor number corresponding to the first column in the Appendix.
Discussion
The present study identified marked alterations in the native lubrication function and lubricant composition of synovial fluid from patients with intra-articular tibial plateau fractures in the initial stages of treatment. Compared with control human synovial fluid, synovial fluid from knees with intra-articular fractures had markedly decreased lubrication ability (an increase of 100% in μkinetic; 0.022 versus 0.044) in the acute postinjury time period that was studied. Concomitantly, tibial plateau fractures led to changes in the concentration of putative lubricant molecules, with a decrease in hyaluronan (a decrease of 89%; 2.53 mg/mL to 0.27 mg/mL) and an increase in proteoglycan-4 (an increase of 156%; 139 μg/mL to 356 μg/mL). Regression analysis indicated that poorly lubricating synovial fluid was associated with diminished hyaluronan concentration.
The experimental approach of the present study involved a number of considerations. Lubrication testing of the human synovial fluid samples was performed with normal adult bovine cartilage surfaces, minimizing effects of variation in cartilage surface properties. The test configuration facilitates boundary lubrication by apposed articular cartilage surfaces, maintaining possible interactions between synovial fluid and the articular surfaces, and also minimizing confounding factors such as ploughing28. Adult bovine cartilage appears to be lubricated similarly by normal bovine synovial fluid (μkinetic = 0.025)31 and by control human synovial fluid (μkinetic = 0.022; Fig. 3). Also, human synovial fluid was obtained only during surgical procedures with the patient under general anesthesia. These procedures occurred at various time points up to thirty days after the injury, so the long-term and time-dependent effects of tibial plateau fracture on synovial fluid properties were not determined. Additionally, the number of samples was limited because of the relatively small number of patients meeting the inclusion criteria and consenting to participate in the study. Furthermore, the control samples were not only from contralateral knees because of the difficulty in obtaining such fluid.
The present study expands on previous research on the effects of human knee injuries on synovial fluid lubricant properties by focusing on synovial fluid after tibial plateau fracture. In a previous study, synovial fluid aspirated from patients seen in the emergency department who had mono-articular knee effusions and no radiographic abnormalities also exhibited poor lubrication relative to saline solution (Δμ = –0.045) compared with control human synovial fluid relative to saline solution (Δμ = –0.095)35. Although the lubrication test methods were different, with the former study using a glass-on-latex arthrotripsometer compared with articulating cartilage in the present study, the synovial fluid from intra-articular tibial plateau fractures also resulted in a kinetic coefficient of friction approximately double that of the controls (Fig. 3). With attention to sample preparation and a previously validated boundary-mode friction test protocol28, subsequent characterization of friction properties of synovial fluid at a cartilage-cartilage interface can be obtained with low variability in measured friction coefficient with a coefficient of variation of 12% to 28%. However, the amount of variability in the composition of the fluid from the injured knees may be due to a number of factors, including donor age, severity of the injury, number of days after the injury that the sample was collected, and assay sensitivity. Thus, the lubrication properties of human knee synovial fluid may be disrupted in a variety of scenarios of acute injury or inflammation.
The present study is also consistent with and adds to information on the concentration of lubricant molecules in joint synovial fluid from humans and animals. The decreased concentration of hyaluronan is consistent with effects of disease and injury of human knees36,37. At the time of total knee arthroplasty, the hyaluronan concentration of synovial fluid from osteoarthritic knees was 1.3 mg/mL38. Compared with these values and the control value of 2.53 mg/mL in the present study, hyaluronan concentrations in synovial fluid from knees with tibial plateau fractures were markedly lower at 0.27 mg/mL. In horses, joint injury has also been associated with a decreased concentration of hyaluronan24,39. Studies of synovial fluid lubrication in experimental animal injury models have focused on proteoglycan-425,27 but not hyaluronan. The correlation between decreased lubrication properties and decreased hyaluronan concentration in the present study suggests the importance of diminished hyaluronan concentration in posttraumatic human synovial fluid.
The reported effects of injury and osteoarthritis on proteoglycan-4 concentration in humans and animal models are quite varied, with some studies indicating an injury-associated decrease in concentration of proteoglycan-415,23,35 and others indicating an increase24,40. In osteoarthritic knees, increasing proteoglycan-4 concentration correlated with worsening lubrication and osteoarthritis severity40. Differences in the reported concentration of proteoglycan-4 in human synovial fluid from osteoarthritic knees (151 μg/mL) and knees after traumatic injury (356 μg/mL) may be due to a difference in analytical methods, including standards, or a difference in patient populations. Total protein levels were also markedly different with 27 mg/mL for synovial fluid from end-stage osteoarthritic knees at the time of total knee arthroplasty38 compared with 38 mg/mL in knees after a traumatic injury and 22 mg/mL in control knees in the present study. This may be due to differences in the pathologic processes since, after trauma, the joint space is compromised and synovial fluid may be diluted by extra-articular contents and infiltrated by protein in blood. Although the specimens were processed to remove red blood cells, it is likely that a considerable amount of blood components, such as serum proteins, remained and were present in the samples analyzed for both chemical constituents and lubrication properties.
Further studies on the components of the fracture synovial fluid samples would be useful to clarify the mechanism of altered lubrication. The structure of hyaluronan and proteoglycan-4 in intra-articular fracture conditions may affect their roles in the boundary lubrication of articular cartilage after joint injury. Although proteoglycan-4 levels were higher in the samples of tibial fracture synovial fluid, this elevated concentration may not have been sufficient to compensate for the alterations in lubrication function after joint injury. Concurrently, the structural quality of hyaluronan and proteoglycan-4 may have been compromised during injury, such as having a reduction in the concentration of high molecular weight hyaluronan in the fracture synovial fluid. Blood-derived components may also have contributed to deficient lubrication in the plateau fracture synovial fluid.
The consequences of increased friction coefficient, in the context of intra-articular fracture and other joint injuries, remain to be fully elucidated. Increased friction and wear appear to be related. It is possible that even a short period of deficient synovial fluid lubrication, in the setting of soft-tissue or weight-bearing loads, is sufficient to initiate damage that can have longer-term consequences. Studying early time points is the first step to investigating and identifying a longer-term effect.
The collective results in the present study indicate that lubricant molecules in synovial fluid after a tibial plateau fracture are acutely altered with diminished hyaluronan concentration and elevated concentrations of proteoglycan-4 and total protein compared with controls. The correlation of impaired lubrication of fracture synovial fluid with diminished hyaluronan and elevated proteoglycan-4 remains to be evaluated further. The proteoglycan-4 that is elevated may not be functional alone, or functional in a manner dependent on high concentrations of hyaluronan. The presence of blood in the knee joint may lead to permanent derangement of the articular cartilage22,41,42. The results and conclusions of the present study may also be pertinent to other intra-articular fractures20. Posttraumatic changes in synovial fluid may lead to prolonged changes in synovial joint biomechanical function as well as biological function15. In view of these results, correction of lubrication and other pathologic changes may offer an opportunity to protect and preserve articular cartilage, facilitate lubrication for early range of motion activities, and ultimately modulate the development of posttraumatic arthritis.
Appendix
A table showing demographic data on the donors is available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors thank Vedant Kulkarni, MD, Jonah Hulst, MD, Hugo Sanchez, MD, and Suzanne Steinman, MD, University of California, San Diego, for contributing to clinical sample acquisition for this study.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, one or more of the authors has had another relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72:634-8 [DOI] [PubMed] [Google Scholar]
- 2.Marsh JL, Buckwalter J, Gelberman R, Dirschl D, Olson S, Brown T, Llinias A. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002;84:1259-71 [PubMed] [Google Scholar]
- 3.Rasmussen PS. Tibial condylar fractures. Impairment of knee joint stability as an indication for surgical treatment. J Bone Joint Surg Am. 1973;55:1331-50 [PubMed] [Google Scholar]
- 4.Jensen DB, Rude C, Duus B, Bjerg-Nielsen A. Tibial plateau fractures. A comparison of conservative and surgical treatment. J Bone Joint Surg Br. 1990;72:49-52 [DOI] [PubMed] [Google Scholar]
- 5.Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma 1995;9:273-7 [DOI] [PubMed] [Google Scholar]
- 6.Duwelius PJ, Connolly JF. Closed reduction of tibial plateau fractures. A comparison of functional and roentgenographic end results. Clin Orthop Relat Res. 1988;230:116-26 [PubMed] [Google Scholar]
- 7.Lucht U, Pilgaard S. Fractures of the tibial condyles. Acta Orthop Scand 1971;42:366-76 [DOI] [PubMed] [Google Scholar]
- 8.Stevens DG, Beharry R, McKee MD, Waddell JP, Schemitsch EH. The long-term functional outcome of operatively treated tibial plateau fractures. J Orthop Trauma. 2001;15:312-20 [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 2004;427 Suppl:S6-15 [DOI] [PubMed] [Google Scholar]
- 10.Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9:273-7 [DOI] [PubMed] [Google Scholar]
- 11.Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85:218-21 [DOI] [PubMed] [Google Scholar]
- 12.Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93:143-7 [DOI] [PubMed] [Google Scholar]
- 13.Furman BD, Olson SA, Guilak F. The development of posttraumatic arthritis after articular fracture. J Orthop Trauma. 2006;20:719-25 [DOI] [PubMed] [Google Scholar]
- 14.Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O'Keefe RJ. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421-30 [DOI] [PubMed] [Google Scholar]
- 15.Lohmander LS, Roos H, Dahlberg L, Hoerrner LA, Lark MW. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Orthop Res. 1994;12:21-8 [DOI] [PubMed] [Google Scholar]
- 16.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517-21 [PubMed] [Google Scholar]
- 17.Ateshian GA, Mow VC. Friction, lubrication, and wear of articular cartilage and diarthrodial joints. : Mow VC, Huiskes R, Basic orthopaedic biomechanics and mechano-biology. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 447-94 [Google Scholar]
- 18.Swanson SAV. Friction, wear, and lubrication. : Freeman MAR, editor Adult articular cartilage. Wells, England: Pitman Medical; 1979. p 415-60 [Google Scholar]
- 19.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17-26 [DOI] [PubMed] [Google Scholar]
- 20.Dirschl DR, Marsh JL, Buckwalter JA, Gelberman R, Olson SA, Brown TD, Llinias A. Articular fractures. J Am Acad Orthop Surg. 2004;12:416-23 [DOI] [PubMed] [Google Scholar]
- 21.Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg Am. 1977;59:1068-76 [PubMed] [Google Scholar]
- 22.Hooiveld M, Roosendaal G, Vianen M, van den Berg M, Bijlsma J, Lafeber F. Blood-induced joint damage: longterm effects in vitro and in vivo. J Rheumatol. 2003;30:339-44 [PubMed] [Google Scholar]
- 23.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, Shalvoy R, Jay GD. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonacci JM, Schmidt TA, Serventi LA, Shu YL, Gastelum NS, Schumacher BL, McIlwraith CW, Sah RL. Effects of joint injury on synovial fluid and boundary lubrication of cartilage. Trans Orthop Res Soc. 2007;32:156 [Google Scholar]
- 25.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, Mechrefe AP. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26:231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermúdez MA, Blanchet T, Gleghorn JP, Bonassar LJ, Bendele AM, Morris EA, Glasson SS. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840-7 [DOI] [PubMed] [Google Scholar]
- 27.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, Teeple E, Waller KA, Elsaid KA. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62:2382-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15:35-47 [DOI] [PubMed] [Google Scholar]
- 29.Schatzker J, McBroom R, Bruce D. The tibial plateau fracture. The Toronto experience 1968—1975. Clin Orthop Relat Res. 1979;138:94-104 [PubMed] [Google Scholar]
- 30.Pascual E, Doherty M. Aspiration of normal or asymptomatic pathological joints for diagnosis and research: indications, technique and success rate. Ann Rheum Dis. 2009;68:3-7 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882-91 [DOI] [PubMed] [Google Scholar]
- 32.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144-52 [DOI] [PubMed] [Google Scholar]
- 33.Fracture and dislocation compendium Orthopaedic Trauma Association Committee for Coding and Classification. J Orthop Trauma. 1996;10 Suppl 1: 1-154 [PubMed] [Google Scholar]
- 34.Müller ME, Nazarian S, Koch P, Schatzker J. The comprehensive classification of fractures of long bones. 1st ed. New York: Springer; 1990 [Google Scholar]
- 35.Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, Chichester CO. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557-564 [PubMed] [Google Scholar]
- 36.Asari A, Miyauchi S, Sekiguchi T, Machida A, Kuriyama S, Miyazaki K, Namiki O. Hyaluronan, cartilage destruction and hydrarthrosis in traumatic arthritis. Osteoarthritis Cartilage. 1994;2:79-89 [DOI] [PubMed] [Google Scholar]
- 37.Praest BM, Greiling H, Kock R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clin Chim Acta. 1997;266:117-28 [DOI] [PubMed] [Google Scholar]
- 38.Mazzucco D, Scott R, Spector M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: correlation with flow properties. Biomaterials. 2004;25:4433-45 [DOI] [PubMed] [Google Scholar]
- 39.Saari H, Konttinen YT, Tulamo RM, Antti-Poika I, Honkanen V. Concentration and degree of polymerization of hyaluronate in equine synovial fluid. Am J Vet Res. 1989;50:2060-3 [PubMed] [Google Scholar]
- 40.Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62:2680-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooiveld M, Roosendaal G, Wenting M, van den Berg M, Bijlsma J, Lafeber F. Short-term exposure of cartilage to blood results in chondrocyte apoptosis. Am J Pathol. 2003;162:943-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen NW, Roosendaal G, Wenting MJ, Bijlsma JW, Theobald M, Hazewinkel HA, Lafeber FP. Very rapid clearance after a joint bleed in the canine knee cannot prevent adverse effects on cartilage and synovial tissue. Osteoarthritis Cartilage. 2009;17:433-40 [DOI] [PubMed] [Google Scholar]