Abstract
Aim: To assess whether a single urinary spot urinary albumin:creatinine ratio (ACR) can be used to estimate 24-hour urinary protein excretion in women with preeclampsia. Methods: ACR and 24-hour urinary protein excretion were measured in 50 consecutive patients with preeclampsia. ACR was determined in a spot midstream urine sample and the amount of protein excretion was quantified in a 24-hour urine collection performed the following day. The correlation between the spot ACR and 24-hour urine protein excretion was assessed, and the diagnostic value of ACR was expressed in terms of specificity and sensitivity. Receiver operating characteristic curve analysis was used to determine the best cutoff values of the spot ACR for mild preeclampsia (proteinuria ≥ 0.3 g/24 h) and severe preeclampsia (defined in China as proteinuria ≥ 2 g/24 h). Results: A strong correlation was evident between the spot ACR and 24-hour urinary protein excretion (r = .938; P < .001). The optimal spot ACR cutoff point was 22.8 mg/mmol for 0.3 g/24 h of protein excretion (mild preeclampsia) with a sensitivity and specificity of 82.4% and 99.4%, respectively, and 155.6 mg/mmol for 2 g/24 h of protein excretion (severe preeclampsia) with a sensitivity and specificity of 90.6% and 99.6%, respectively. Conclusions: Compared with 24-hour urinary protein excretion, the spot urinary ACR may be a simple, convenient, and accurate indicator of significant proteinuria in women with preeclampsia.
Key words: Urinary albumin:creatinine ratio, Proteinuria, Preeclampsia
Hypertensive disorders complicate 5% to 10% of all pregnancies and are associated with significant maternal morbidity and mortality.1,2 The latest analysis by the World Health Organization suggests that, in developed countries, 16% of maternal deaths in pregnancy were a result of hypertensive disorders.2 In women presenting with elevations in blood pressure in the latter half of pregnancy (defined as a sustained elevation in blood pressure of ≥ 140 mm Hg systolic and/or ≥ 90 mm Hg diastolic), detailed laboratory evaluations are essential to determine whether a patient has a diagnosis of preeclampsia, including assessment of proteinuria, platelet count, and liver and renal function testing. Measurement of protein excretion in a 24-hour urine collection has been the longstanding “gold standard” for the quantitative evaluation of proteinuria in pregnancy. However, 24-hour urine collection is time consuming, inconvenient, and not always reliable because of the difficulty in collecting the sample correctly. A more rapid test capable of accurately predicting the results of a 24-hour urine collection would be valuable.
An alternative method for quantitative evaluation of proteinuria is the measurement of the albumin: creatinine ratio (ACR) in a spot urine sample, which avoids the influence of variations in urinary solute concentration and provides a more convenient and rapid method to assess protein excretion. The usefulness of this method for assessing proteinuria in the nonpregnant population is well substantiated in the literature. Several international organizations—including the International Society for the Study of Hypertension in Pregnancy,3 the Society of Obstetric Medicine of Australia and New Zealand,4 and the Society of Obstetricians and Gynaecologists of Canada5,6—have accepted the spot urine ACR as a reasonable method for the identification of significant proteinuria (> 0.3 g/24 h) during pregnancy, but this is not accepted by all international consensus bodies including the American Congress of Obstetricians and Gynecologists7 and the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy.8 The current study aimed (i) to evaluate the correlation between albuminuria as measured by ACR and the amount of albumin in a 24-hour urine collection in women with preeclampsia, and (ii) to determine the best cutoff values of the spot ACR for mild preeclampsia (proteinuria ≥ 0.3 g/24 h) and severe preeclampsia (defined in China as proteinuria ≥ 2 g/24 h).
Materials and Methods
Patients
Consecutive pregnant women admitted to a single tertiary care obstetric unit at Nanfang Hospital, Southern Medical University, in Guangzhou, China, with a diagnosis of preeclampsia between December 1, 2010 and June 30, 2011 were recruited into the study. The study was approved by the local ethics committee and all patients provided written informed consent. Preeclampsia was defined as a sustained elevation in blood pressure of ≥ 140/90 mm Hg after the 20th week of gestation and a urine protein of ≥ 1+ by dipstick test or chronic hypertension without proteinuria before the 20th week of gestation accompanied by new-onset urine protein of ≥ 1+ by dipstick test. Exclusion criteria included women with known kidney disease or connective tissue disorders, pre-existing diabetes, gestational diabetes, bacteriuria, excessive exercise (defined as > 1 hour of vigorous exercise on the day of urine collection), bed rest > 24 hours, or women who delivered before the 24-hour urinary collection was completed.
Urine Analysis
After providing written informed consent, all patients were asked for a spot mid-stream urine sample followed by a complete 24-hour urine collection. The urinary ACR was determined on the spot urine specimens using a DCA 2000 urinalysis analyzer (Bayer Healthcare LLC, Elkhart, IN) and data was expressed as mg/mmol. The concentration of total protein in the 24-hour urine collection was measured by a Biuret colorimetric assay.
Statistical Analyses
Descriptive statistics were used for the demographic and outcome data and summarized as mean ± standard deviation (SD). The correlation between the ACR in the spot urine samples and urinary protein excretion in the 24-hour collections was assessed using the Pearson correlation test. Receiver operating characteristic (ROC) curve analysis was used, and the area under the curve (AUC) was calculated. Sensitivity, specificity, and various cutoffs for the prediction of significant proteinuria were estimated using the 24-hour urinary protein excretion as the gold standard. SPSS software (version 13.0) was used for the analyses. P value < .05 was considered statistically significant.
Results
A total of 50 pregnant women with preeclampsia met criteria for inclusion in the final analysis. Of these, 28 had mild preeclampsia (proteinuria 300 mg/24 h to 2 g/24 h) and 22 had severe preeclampsia (defined in China as proteinuria > 2 g/24 h). Demographic and clinical variables of the study population are shown inTable 1. Compared with women with mild preeclampsia, women with severe preeclampsia were diagnosed at a significantly earlier gestational age, delivered at a significantly earlier gestational age, and had significantly higher blood pressure measurements and lower concentrations of albumin in the maternal circulation (Table 1). Women with severe preeclampsia also had significantly higher levels of uric acid and cystatin C (a biomarker of preclinical renal dysfunction9) in their urine compared with women with mild preeclampsia as well as higher concentrations of protein and elevated ACR (Table 2).
Table 1.
Demographic and Clinical Variables of the Study Population
| Variable | Mild Preeclampsia | Severe Preeclampsia |
| (n = 28) | (n = 22) | |
| Age (years) | 30.8 ± 3.9 | 32.2 ± 5.7 |
| Gravidity | 2.5 ± 1.5 | 2.9 ± 1.4 |
| Parity | 1.1 ± 0.7 | 0.9 ± 0.6 |
| Gestational age at diagnosis (week)* | 36.2 ± 1.2 | 32.2 ± 5.2 |
| Gestational age at delivery (week)* | 38.2 ± 1.9 | 34.0 ± 5.4 |
| BMI (kg/m2) | 33.1 ± 16.8 | 28.6 ± 16.0 |
| Systolic blood pressure (mm Hg)* | 143.4 ± 16.3 | 174.5 ± 27.2 |
| Diastolic blood pressure (mm Hg)* | 87.0 ± 14.4 | 103.4 ± 15.2 |
| Albumin concentration in maternal serum (g/L)* | 32.6 ± 3.3 | 29.0 ± 6.3 |
Data are presented as mean ± standard deviation.
Significant difference between the two groups (P < .05).
BMI, body mass index.
Table 2.
Urinary Biochemical Analysis in the Study Population
| Variable | Mild Preeclampsia | Severe Preeclampsia |
| (n = 28) | (n = 22) | |
| Uric acid (mmol/L)* | 359.0 ± 113.87 | 450.4 ± 32.2 |
| Urea nitrogen (mmol/L) | 3.6 ± 1.6 | 6.2 ± 4.1 |
| Urine creatinine (mmol/L) | 51.8 ± 8.97 | 77.7 ± 59.4 |
| Cystatin C (mmol/L)* | 1.27 ± 0.26 | 1.63 ± 0.52 |
| Urinary protein (g/24 h)* | 0.70 ± 0.56 | 4.84 ± 4.22 |
| Albumin:creatinine ratio (mg/mmol)* | 72.68 ± 12.4 | 401.2 ± 345.1 |
Data are presented as mean ± standard deviation.
Significant difference between the two groups (P < .05).
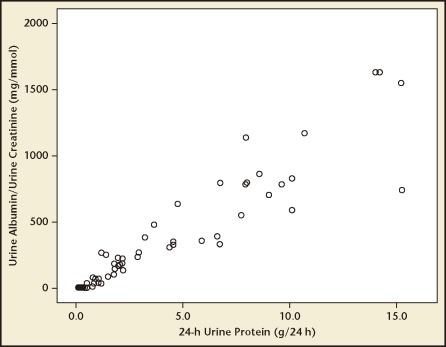
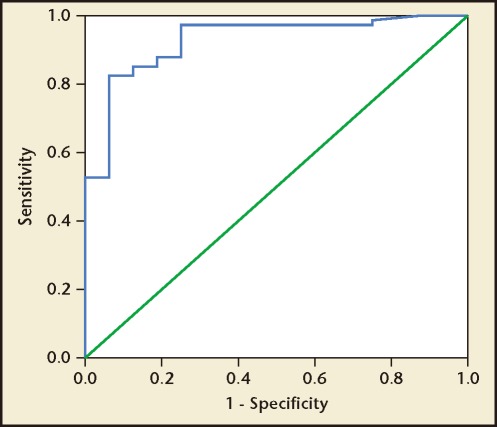
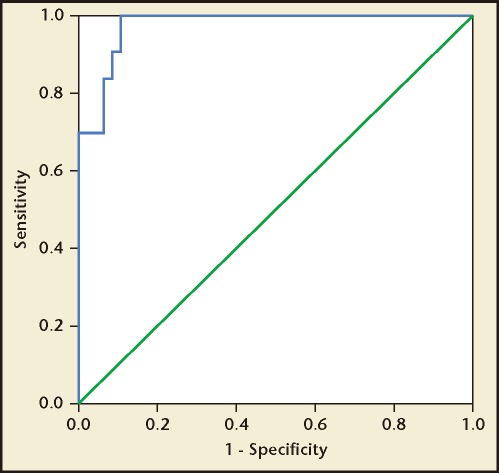
There was a strong positive statistical correlation between the spot ACR and 24-hour urine protein excretion, with a correlation coefficient (r) of .938 (Figure 1). The regression equation can be given as y = .09x + .502 (P < .001) where y indicates urinary protein excretion (g/24 h) and x indicates ACR. According to the ROC curve analysis, an ACR value of 22.8 mg/mmol was identified as the best threshold to detect a urine protein excretion of > .3 g/24 h, with a sensitivity and a specificity of 82.4% and 99.4%, respectively (Figure 2). Similarly, an ACR of 155.6 mg/mmol was identified as the best threshold to detect a urine protein excretion of > 2 g/24 h, with a sensitivity and specificity of 90.6% and 99.6%, respectively (Figure 3). The AUC for > .3 g and > 2.0 g of protein on a 24-hour urine collection was .918 and .956, respectively.
Figure 1.
Correlation between urinary albumin:creatinine ratio (ACR) and 24-hour urine protein estimation in the study population. There was a strong correlation between the spot ACR and the 24-hour urine protein excretion value (r = .938; P < .001).
Figure 2.
Receiver operating characteristic curve analysis in women with preeclampsia. A urinary albumin:creatinine ratio value of 22.8 mg/mmol was identified as the best threshold to detect a urine protein excretion of > 0.3 g/24 h, with a sensitivity and a specificity of 82.4% and 99.4%, respectively. Area under the curve = .918.
Figure 3.
Receiver operating characteristic curve analysis in women with severe preeclampsia. A urinary albumin:creatinine ratio value of 155.6 mg/mmol was identified as the best threshold to detect a urine protein excretion of > 2 g/24 h, with a sensitivity and specificity of 90.6% and 99.6%, respectively. Area under the curve = .956.
Discussion
Preeclampsia (gestational proteinuric hypertension) remains a major cause of maternal morbidity and mortality worldwide.1,2 In Latin America and the Caribbean, hypertensive disorders are responsible for almost 26% of maternal deaths, whereas in Africa and Asia they contribute to only 9% of deaths. Even in developed countries such as the United Kingdom and the United States, although the absolute risk of maternal mortality is far lower, around 16% of maternal deaths can be attributed to hypertensive disorders.7,10
Prior studies have shown that proteinuric hypertension (preeclampsia) has worse maternal and fetal outcomes than nonproteinuric hypertension (gestational hypertension) in pregnancy.11–13 Significant proteinuria is one of the prerequisites for the diagnosis of preeclampsia.7 For many years, a 24-hour urine collection has been regarded as the gold standard for proteinuria, but it is cumbersome for both patients and staff and is subject to error due to inaccurate timing and/or incompleteness. Waiting for the results of protein estimation in a 24-hour urine collection can delay the diagnosis of preeclampsia unnecessarily and potentially put the mother and fetus at risk.14,15 As such, the ability to substitute a spot urine ACR for a 24-hour urine collection could have significant clinical implications, including the facilitation of prompt clinical decision making and more expeditious delivery. Such an approach could also impact healthcare costs and improve patient outcome and satisfaction.16
With easier collection and results available within hours, a spot ACR would be a more efficient test than a 24-hour collection for proteinuria assessment. Moreover, because, by definition, the ACR corrects urinary protein concentrations for creatinine, it is independent of the degree of dilution of the urine. There is extensive literature in the nonpregnant population suggesting that a spot ACR performs just as well at assessing proteinuria as a 24-hour urine collection in patients with systemic lupus erythematosus (SLE), underlying glomerular disease, and following renal transplant.17–19 Indeed, the US National Kidney Foundation has suggested that spot urine samples rather than 24-hour urine collections be used to detect and monitor proteinuria in both children and adults.20 Such recommendations have not as yet been made by US consensus organizations with regard to proteinuria in pregnancy.7,8
In our study, we found a strong correlation (r = .938) between the spot ACR ratio and the 24-hour urine protein estimation. According to the ROC curve analysis, an ACR of 22.8 mg/mmol was identified as the best threshold to detect urine protein excretion of > 0.3 g/24 h, with a sensitivity and specificity of 82.4% and 99.4%, respectively. An ACR ratio of 155.6 mg/mol was identified as the best threshold to detect urine protein excretion of > 2 g/24 h (which constitutes severe preeclampsia in China), with a sensitivity and specificity of 90.6% and 99.6%, respectively. These findings support the use of proteinuria measured as spot ACR as a substitute for 24-hour urine collection in women with preeclampsia. Although the absolute numbers may differ (because the ACRs in this article are reported as mg/mmol, whereas they are typically reported as mg/g in other studies), these data are consistent with prior publications suggesting that spot ACR measurements may be useful in the diagnosis of preeclampsia (summarized in two meta-analyses of 7 and 13 studies21,22). Interestingly, recent publications have suggested that ACR measurements in the first trimester23 or early second trimester24 may predict the development of preeclampsia weeks or months before the clinical onset of the disease. Weaknesses of this study include a relatively small sample size and different criteria for the diagnosis of severe preeclampsia (in the United States, severe preeclampsia by proteinuria is defined as > 5.0 g/24 h,7 whereas it is > 3.0 g/24 h in the United Kingdom, and > 2.0 g/24 h in China).
Conclusions
Compared with the measurement of total protein in a 24-hour urine collection, a spot ACR in a midstream urine specimen may be a simple, convenient, and accurate indicator of significant proteinuria in women with preeclampsia. Consideration should be given to replacing 24-hour urine collections for protein excretion with spot ACR in the evaluation of women with suspected preeclampsia.
Main Points.
Albumin:creatinine ratio (ACR) and 24-hour urinary protein excretion were measured in 50 consecutive patients with preeclampsia. ACR was determined in a spot midstream urine sample and the amount of protein excretion was quantified in a 24-hour urine collection performed the following day. The correlation between the spot ACR and 24-hour urine protein excretion was assessed, and the diagnostic value of ACR was expressed in terms of specificity and sensitivity. Receiver operating characteristic (ROC) curve analysis was used to determine the best cutoff values of the spot ACR for mild preeclampsia (proteinuria ≥ 0.3 g/24 h) and severe preeclampsia (defined in China as proteinuria ≥ 2 g/24 h).
A strong correlation was evident between the spot ACR and 24-hour urinary protein excretion (r = .938; P < .001). The optimal spot ACR cutoff point was 22.8 mg/mmol for 0.3 g/24 h of protein excretion (mild preeclampsia) with a sensitivity and specificity of 82.4% and 99.4%, respectively, and 155.6 mg/mmol for 2 g/24 h of protein excretion (severe preeclampsia) with a sensitivity and specificity of 90.6% and 99.6%, respectively.
Compared with 24-hour urinary protein excretion, the spot urinary ACR may be a simple, convenient, and accurate indicator of significant proteinuria in women with preeclampsia.
References
- 1.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2008. Natl Vital Stat Rep. 2010;59:1. [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 3.Lowe SA, Brown MA, Dekker GA, et al. Society of Obstetric Medicine of Australia and New Zealand. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust N Z J Obstet Gynaecol. 2009;49:242–246. doi: 10.1111/j.1479-828X.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- 4.Magee LA, Helewa M, Moutquin JM, von Dadelszen P. Hypertension Guideline Committee; Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;3(suppl 3):S1–S48. doi: 10.1016/S1701-2163(16)32776-1. [DOI] [PubMed] [Google Scholar]
- 5.Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens. 2008;2:484–494. doi: 10.1016/j.jash.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Lindheimer MD, Taler SJ, Cunningham FG. ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich) 2009;11:214–225. doi: 10.1111/j.1751-7176.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ACOG Committee on Practice Bulletins-Obstetrics, authors. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 8.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 9.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Duley L, Meher S, Abalos E. Management of preeclampsia. BMJ. 2006;332:463–468. doi: 10.1136/bmj.332.7539.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua S, Redman CW. Prognosis for pre-eclampsia complicated by 5 g or more of proteinuria in 24 hours. Eur J Obstet Gynecol Reprod Biol. 1992;43:9–12. doi: 10.1016/0028-2243(92)90236-r. [DOI] [PubMed] [Google Scholar]
- 12.Chan P, Brown M, Simpson JM, Davis G. Proteinuria in pre-eclampsia: how much matters? BJOG. 2005;112:280–285. doi: 10.1111/j.1471-0528.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 13.Boler L, Zbella EA, Gleicher N. Quantitation of proteinuria in pregnancy by the use of single voided urine samples. Obstet Gynecol. 1987;70:99–100. [PubMed] [Google Scholar]
- 14.Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848–852. doi: 10.1067/s0002-9378(03)00849-4. [DOI] [PubMed] [Google Scholar]
- 15.Al RA, Baykal C, Karacay O, et al. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol. 2004;104:367–371. doi: 10.1097/01.AOG.0000134788.01016.2a. [DOI] [PubMed] [Google Scholar]
- 16.Boulware LE, Jaar BG, Tarver-Carr ME, et al. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 17.Christopher-Stine L, Petri M, Astor BC, Fine D. Urine protein-to-creatinine ratio is a reliable measure of proteinuria in lupus nephritis. J Rheumatol. 2004;31:1557–1559. [PubMed] [Google Scholar]
- 18.Chitalia VC, Kothari J, Wells EJ, et al. Cost-benefit analysis and prediction of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin Nephrol. 2001;55:436–447. [PubMed] [Google Scholar]
- 19.Xin G, Wang M, Jiao LL, et al. Protein-to-creatinine ratio in spot urine samples as a predictor of quantitation of proteinuria. Clin Chim Acta. 2004;350:35–39. doi: 10.1016/j.cccn.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation, authors. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 21.Papanna R, Mann LK, Kouides RW, Glantz JC. Protein/creatinine ratio in preeclampsia: a systematic review. Obstet Gynecol. 2008;112:135–144. doi: 10.1097/AOG.0b013e3181778cfc. [DOI] [PubMed] [Google Scholar]
- 22.C\ oté, Brown MA, Lam E, et al. Diagnostic accuracy of urinary spot protein : creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ. 2008;336:1003–1006. doi: 10.1136/bmj.39532.543947.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poon LC, Kametas N, Bonino S, et al. Urine albumin concentration and albumin-to-creatinine ratio at 11(10) to 13(16) weeks in the prediction of pre- eclampsia. BJOG. 2008;115:866–873. doi: 10.1111/j.1471-0528.2007.01650.x. [DOI] [PubMed] [Google Scholar]
- 24.Baweja S, Kent A, Masterson R, et al. Prediction of pre-eclampsia in early pregnancy by estimating the spot urinary albumin : creatinine ratio using high-performance liquid chromatography. BJOG. 2011;118:1126–1132. doi: 10.1111/j.1471-0528.2011.02960.x. [DOI] [PubMed] [Google Scholar]