Abstract
Despite a shift from clinical to surgical staging of endometrial cancer in 1988, performance of comprehensive surgical staging for clinically early-stage endometrial cancer remains controversial. Low-, intermediate-, and high-risk groups have been defined pathologically. Herein, we describe the risks and benefits of comprehensive surgical staging. Comprehensive surgical staging is encouraged in high-risk histologies, whereas a method of triage should be used to determine who among the low-grade endometrioid histology may benefit from comprehensive staging.
Key words: Endometrial cancer, Surgical staging, Pelvic lymphadenectomy, Para-aortic lymphadenectomy
Endometrial cancer is the most common gynecologic malignancy in the United States, with 43,470 new cases diagnosed each year; it accounts for 6% of all cancers in women.1 Fortunately, the majority of cases are diagnosed at an early stage, when it may be cured by surgery alone. Patients with localized disease have a 96% 5-year survival rate, which drops to 67% for regional disease and 17% for those with metastatic disease.
Based on International Federation of Gynecologists and Obstetricians (FIGO) criteria, endometrial cancer is surgically staged.2,3 Despite these guidelines, performance of complete surgical staging for endometrial cancer is controversial.
Shift From Clinical to Surgical Staging
Prior to 1988, FIGO staging for endometrial cancer was clinically based.2 Clinical stage I cancer was defined as disease confined to the uterus. In 1984, the Gynecologic Oncology Group (GOG) studied clinicopathologic factors and recurrence patterns in clinical stage I endometrial cancer.4,5 These study results prompted a larger prospective trial (GOG 33) that examined patients with clinical stage I endometrial cancer to further evaluate these factors.6
The shift to surgical staging was due, in part, to the results of GOG 33.6 This study prospectively evaluated 621 patients with clinical stage I endometrial cancer. All patients underwent a standard comprehensive staging procedure, including hysterectomy, bilateral salpingo-oophorectomy, collection of pelvic washings, and a selected pelvic and para-aortic lymph node dissection. Pelvic lymphadenectomy was defined as removal of the lymph-bearing tissues over the external and common iliac vessels and in the obturator fossa above the obturator nerve. Para-aortic lymph node dissection was defined as removal of the fat pad over the inferior vena cava and lower aorta beginning at the bifurcation and extending to the proximity of the renal vessels. Pathologic factors, including histology, grade, depth of invasion, lymphovascular space invasion, and extrauterine involvement, were examined to determine risk of extrauterine involvement and lymph node metastasis. Based on multivariant analysis, three risk categories were defined. Patients with low-risk disease, defined as grade 1 tumor with endometrial involvement only and no intraperitoneal disease, had no pelvic or para-aortic lymph node metastasis. Those with moderate-risk disease, defined as < 50% myometrial invasion and no intraperitoneal disease, had a 3% to 6% incidence of pelvic lymph node metastasis, and a 2% incidence of para-aortic lymph node involvement. High-risk disease was defined by two criteria: deep myometrial invasion and/or intraperitoneal disease. Those with deep myometrial invasion had 18% and 15% incidence of pelvic and para-aortic lymph node metastasis, respectively. Patients with intraperitoneal disease with only < 50% myometrial involvement had a 33% risk of pelvic lymph node metastasis and 8% risk of positive para-aortic lymph nodes. Patients with both high-risk criteria were at the highest risk with 61% pelvic lymph node metastasis and 30% para-aortic lymph node involvement. The results of this study prompted a revision of the FIGO staging for endometrial cancer from clinical to surgical staging.
FIGO staging for endometrial cancer was once again revised in 2009.3 Stages IA and IB were grouped together to reflect the favorable prognosis of early-stage disease, and stage IIIC was divided into stage IIIC1 for positive pelvic lymphadenopathy and stage IIIC2 for para-aortic lymph node involvement to reflect the poor prognosis with lymphadenopathy. GOG 33 found no para-aortic lymphadenopathy in patients with low-risk disease but found it in up to 30% of patients with high-risk, earlystage disease.6 The rate of positivity of both pelvic and para-aortic lymph nodes ranges from 3% to 6.9%.6,7 Isolated para-aortic lymph node metastases have been found in approximately 1% of patients with early-stage endometrial cancer.8 In the most recent FIGO staging guidelines, emphasis is placed on the site of lymphadenopathy, which reflects a poorer prognosis and identifies patients in need of adjuvant therapy. The 5-year survival of patients with positive para-aortic lymph nodes compared with those with pelvic lymphadenopathy is only 30% to 40% versus 70% to 80%, respectively.9–11
Definition of Comprehensive Surgical Staging
The GOG surgical manual describes comprehensive surgical staging of endometrial cancer as removal of the uterus, cervix, adnexa, and pelvic and para-aortic lymph node tissues, and obtaining pelvic washings.12 GOG defines pelvic lymphadenectomy as removal of the nodal tissue from the distal half of the common iliac arteries, the anterior and medial aspect of the proximal half of the external iliac artery and vein, and the distal half of the obturator fat pad anterior to the obturator nerve; para-aortic lymph node dissection is described as removal of nodal tissue over the distal inferior vena cava from the level of the inferior mesenteric artery to the mid right common iliac artery and removal of the nodal tissue between the aorta and left ureter from the mid inferior mesenteric artery to the mid left common iliac artery.
An adequate nodal dissection requires that lymphatic tissue be excised pathologically from each side (right and left), but no specific nodal counts are required. Thus, some practitioners may opt for selective lymph node sampling rather than a full dissection. However, retrospective data suggest that patients who underwent multiple site sampling had improved survival over those who had limited or no sampling performed.13 The caveat to nodal sampling versus full dissection is that inspection or palpation of nodes has not been shown to be a sensitive method for detection of positive lymph nodes, with fewer than 10% of patients with lymphadenopathy having grossly involved nodes.6
Despite the well-defined criteria for surgical staging, surgeons still debate the extent of lymphadenectomy necessary. Particular controversy exists as to whether to perform bilateral complete paraaortic lymph node dissection in all patients. Para-aortic nodes may be positive in the absence of pelvic lymphadenopathy.7,8 In a large retrospective trial, 734 patients treated at Memorial Sloan-Kettering Cancer Center were identified with isolated para-aortic lymphadenopathy. They reported a rate of 1% to 1.6% of isolated para-aortic lymph node involvement in the setting of negative pelvic lymph nodes and found this to be consistent for both low- and high-grade lesions.8 Therefore, their current practice is to perform surgical staging with pelvic lymphadenectomy, as well as limited intramesenteric paraaortic lymphadenectomy, or offer sentinel node mapping.14,15 Other data suggest para-aortic lymph node dissection may be warranted only in those with high-risk pathology. Mariani and colleagues prospectively examined 281 patients undergoing lymphadenectomy at the time of endometrial cancer staging and found 22% of patients with high-risk disease had lymph node metastases.7 Of these, 51% had both pelvic and para-aortic lymphadenopathy, 33% had positive pelvic lymph nodes only, and 16% had isolated para-aortic lymphadenopathy. In those with para-aortic lymph node involvement, 77% had metastases above the inferior mesenteric artery, and they propose systematic pelvic and extended para-aortic lymphadenectomy up to the renal vessels in patients with high-risk disease.7,16 Conversely, they found that patients with lowgrade disease (ie, grade 1 and 2 endometrioid lesions with myometrial invasion ≤ 50% and tumor size ≤ 2 cm) had no lymphadenopathy and did not benefit from a systematic lymphadenectomy.
Advantages and Potential Complications of Comprehensive Staging
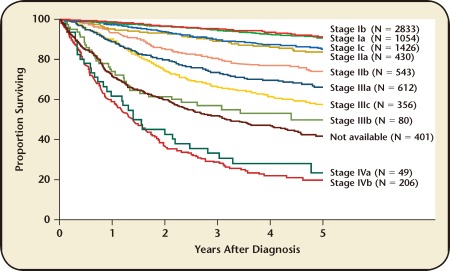
The advantages of comprehensive surgical staging lie in diagnosis, prognosis, and proper triage of patients for adjuvant therapy. FIGO endometrial cancer staging is based on surgical pathology, and comprehensive surgery allows for accurate definition of disease extent. GOG 33 found that 9% of clinically stage I patients had pelvic nodal metastases, 6% had para-aortic lymphadenopathy, 5% had spread to adnexa, and 6% had other extrauterine metastases at the time of surgery.6 Patients with more advanced stage disease have poorer prognoses, which may go unrecognized without comprehensive surgical staging. Figure 1 shows the 5-year overall survival for patients with endometrial cancer based on FIGO surgical substages.17
Figure 1.
Survival by International Federation of Gynecologists and Obstetricians surgical stage for endometrial cancer. Reproduced with permission from Creasman WT et al.17
In addition to defining patients with more advanced stages of endometrial cancer and their need for radiation therapy and/or chemotherapy, patients with stage I disease who should receive further treatment can be identified. GOG 99 defined a high-intermediate risk group of early stage endometrial cancer who benefit from additional therapy in terms of progression-free survival and fewer local recurrences.18 Patients were triaged to pelvic radiation therapy based on age and pathologic factors including grade (2–3), depth of invasion (outer third), and lymphovascular space invasion. In GOG 33, 22% of clinical stage I patients had outer-third myometrial invasion, 71% were grade 2 or 3, and 15% had lymphovascular space invasion and would have been triaged to adjuvant radiation therapy based on age and the number of risk factors present.6 Furthermore, those patients without high-intermediate risk factors can be identified and overtreatment of these patients can be avoided, sparing them from potential complications of radiation therapy.
Comprehensive surgical staging includes pelvic and para-aortic lymphadenectomy, which carries inherent risks. Potential complications of these procedures include injury to major vessels or nerves, lymphedema, and associated cellulitis. Lymphedema occurs in 5% to 38% of patients undergoing pelvic lymph node dissection and can impact quality of life. This can be avoided by limiting the pelvic lymphadenectomy to superior to the circumflex iliac vein, and avoiding removal of the circumflex iliac nodes distal to the external iliac nodes.19,20
Evidence for and Against the Benefits of Surgical Staging
GOG 33 was among the first trials to describe the benefits of surgical staging and presented evidence that clinical stage I disease may pathologically include risk factors warranting adjuvant radiation therapy in 15% to 25% of patients with early-stage disease. In addition, another 5% to 9% of patients may be upstaged by extrauterine involvement, significantly impacting prognosis and plans for adjuvant therapy.6 GOG 99 defined high-intermediate risk factors for recurrence based on surgical pathology in women with stage I cancer. Women with high-intermediate risk factors were randomized to radiation therapy or observation after comprehensive surgery. The incidence of recurrence was 12% in the observation group and 3% in the radiotherapy group, and there was no difference in overall survival.18 Based on these trials, comprehensive surgical staging can identify women at high risk of recurrence, allowing appropriate triage to additional therapy.
Several observational studies have compared outcomes in early-stage endometrial cancer patients with and without systematic lymphadenectomy. Retrospective, single-institution studies advocate lymphadenectomy for all grades of tumor.13,21,22 A large series utilizing a national database supports lymph node dissection for grade 3 tumors only, with no benefit seen in grade 1 or 2 tumors.23 This was also found in an observational study that examined patients with intermediate or high risk factors for recurrence who underwent surgery with pelvic lymphadenectomy with or without para-aortic lymph node dissection. There was a survival benefit for those who had a paraaortic lymphadenectomy compared with those who did not, but this effect was not seen in patients with low-risk cancers.24 Rather than triaging based on risk factors, other studies suggest benefit for lymphadenectomy depends on the number of lymph nodes removed at the time of surgery.25,26 However, there are no randomized trials supporting the benefit of lymphadenectomy in early-stage endometrial cancer.
There are two randomized trials that provide evidence against surgical staging. Benedetti Panici and colleagues randomized 514 women with clinical stage I endometrial cancer to either systematic pelvic lymphadenectomy or no lymph node dissection and found no improvement in disease-free or overall survival between the two groups.27 This was followed by the Adjuvant External Beam Radiotherapy in the Treatment of Endometrial Cancer (ASTEC) trial, a large, multicenter, European trial that randomized 1408 women with clinical stage I endometrial cancer to staging surgery with or without pelvic lymphadenectomy.28 Those women with early-stage disease with intermediate or high risk factors for recurrence were then randomized, independent of lymph node status, to the ASTEC radiotherapy trial. They found no difference in progression-free or overall survival and recommended against routine pelvic lymphadenectomy in presumed early-stage endometrial cancer.
Despite these randomized trials showing no benefit to comprehensive surgical staging, controversy still exists. This is due, in part, to the criticisms of the ASTEC trial, which include a high rate of crossover to radiotherapy and selection bias. Patients were secondarily randomized to radiation therapy based on uterine pathology only, leaving some patients with lymphadenopathy untreated by radiotherapy. One benefit of nodal dissection is triage to adjuvant therapy. However, the clinical value of triage to treatment in this trial was obscured because only half of the patients with high-risk disease were randomized to adjuvant therapy. Furthermore, 7% to 9% of low-risk patients and 53% to 61% of those with advanced disease excluding lymph node involvement were not randomized to adjuvant therapy but did receive some radiotherapy. In addition, the lymphadenectomy versus no dissection arms were unbalanced in terms of high-risk criteria. There were 3% more high-risk histologies, 3% more high-grade lesions, 3% more lymphovascular space invasion, and 10% more deep myometrial invasions in the lymphadenectomy arm despite randomization. This difference may appear small, but could have affected the power of the study to detect differences in survival.28,29 The ASTEC trial also does not provide information regarding the usefulness of pelvic lymphadenectomy for guiding adjuvant treatment because patients were secondarily randomized to radiotherapy without factoring in lymph node status. Additionally, the benefit of paraaortic lymph node dissection is not addressed because patients underwent para-aortic node palpation and selective sampling rather than systemic dissection.
The Way Forward
Despite FIGO surgical staging, several observational studies, and two large, randomized trials, comprehensive surgical staging for endometrial cancer is still controversial. For early-stage disease, there are proponents of no lymphadenectomy, pelvic lymphadenectomy only, and complete pelvic and para-aortic lymph node dissection, but no clear consensus. Table 1 describes several studies in which some patients were completely surgically staged and some were incompletely surgically staged with either omission of lymphadenectomy or optional nodal dissection.32 Acknowledging the limitations of cross-trial comparisons, the overall survival across these studies is similar, regardless of the surgical procedures performed.
Table 1.
Comparison of Surgically and Clinically Staged Endometrial Cancer Trials
| Trial | n | Stage | Surgery | Randomization | Vaginal and/or Pelvic Relapse | Overall Survival |
| GOG 99 (2004)18 | 392 | IB, IC, occult II | TAH-BSO and lymphadenectomy | No additional therapy vs 50.4 Gy to pelvis | 12% vs 3% at 2 years (P = .007) | 86% vs 92% at 4 years (P = .557) |
| ASTEC (2009)28 | 906 | IA, IB, IC, IIA | TAH-BSO ± lymphadenectomy | Brachytherapy vs vaginal brachytherapy + 40–46 Gy to pelvis | 6.1% vs. 3.2% (P = .02) | 84% vs 84% at 5 years (P = NS) |
| PORTEC (2004)30 | 714 | IB grade 2/3, IC grade 1/2 | TAH-BSO only | No additional therapy vs 46 Gy to pelvis | 10%–14% vs 1%–5% at 5 years (P < .001) | 70%–91% vs 58%–83% at 5 years (P = NS) |
| PORTEC 2 (2010)31 | 427 | I or II, high-intermediate risk* | TAH-BSO, lymphadenectomy optional | 46 Gy to pelvis vs vaginal brachytherapy | 2.1% vs 5.1% at 5 years (P = .17) | 79.6% vs 82.7% at 5 years (P = .57) |
High-intermediate risk criteria: (1) age ≥ 60 and stage IC grade 1/2; (2) age ≥ 60 and stage IB grade 3; or (3) any age and stage IIA grade 1/2 or grade 3 with < 50% myometrial invasion.
ASTEC, Adjuvant External Beam Radiotherapy in the Treatment of Endometrial Cancer; GOG, Gynecologic Oncology Group; NS, not significant; PORTEC, Postoperative Radiation Therapy in Endometrial Carcinoma; TAH-BSO, total abdominal hysterectomy-bilateral salpingo-oophorectomy.
Adapted from Diavolitsis V et al.32
One approach to resolution may be to triage patients prior to surgery to lymphadenectomy versus no lymphadenectomy based on endometrial biopsy pathology. This approach would distinguish between low-grade endometrioid cancer from more aggressive histologies and this information can guide the need for comprehensive staging. Table 2 describes the 5-year overall survival for histologic subtypes of endometrial cancer.17 Grade 3 endometrioid, papillary serous, clear cell, undifferentiated, and squamous histologies have a poorer prognosis33 and may be triaged to lymphadenectomy. The advantages of comprehensive staging outweigh the disadvantages in these high-risk histologies.
Table 2.
Survival by Histology for Patients With Uterine Cancer
| Histology Sample Type | 5-Year Overall Survival |
| Endometrioid | 80% |
| Adenosquamous | 79% |
| Mucinous | 73% |
| Papillary | 54% |
| Clear cell | 63% |
| Squamous | 64% |
| Other | 65% |
Adapted from Creasman WT et al.17
Preoperative tumor grade may also aid in triage of patients to lymphadenectomy. Low-grade endometrioid cancer accounts for the majority of endometrial cancers and is the most controversial group when it comes to surgical therapy. Many studies advocate lymphadenectomy for all grades of endometrial cancers.13,21,22 Several observational studies have found no benefit to lymphadenectomy in low-grade tumors.23,24,34,35 A large, multi-institutional study utilized a central pathology review and included only patients with preoperative grade 1 endometrioid endometrial cancer with and without lymphadenectomy. They found no difference in recurrence-free or overall survival,35 consistent with other observational studies that showed no benefit to comprehensive staging in low-grade disease. Thus, in patients with grade 1 endometrioid histologies, there may be no advantage to comprehensive staging.
Intraoperative pathology may also be used to triage patients to lymphadenectomy.7,36 Mariani and colleagues prospectively used frozen section to determine whether to perform lymphadenectomy in patients with early-stage endometrial cancer. Frozen section was used to determine depth of myometrial invasion, primary tumor diameter, and grade and endometrioid histology. Patients with low-risk disease (grade 1 or 2, endometrioid histology, myometrial invasion ≤ 50%, and primary tumor diameter ≤ 2 cm) on frozen section were not required to undergo lymphadenectomy and no benefit was shown for those patients with low-risk disease who did undergo lymphadenectomy.7 Conversely, Case and colleagues performed a prospective, blinded study of the accuracy of frozen section in endometrial cancer surgery and found that grade and depth of invasion on frozen section correlated poorly with final pathology.37 However, this study only included 60 patients, whereas Mariani and colleagues studied 422 patients with triage by frozen section.
Conclusions
Based on FIGO staging guidelines, clinically early-stage endometrial cancer patients should undergo comprehensive surgical staging. However, the disadvantages of surgical staging may outweigh the risks in patients with low-grade endometrioid tumors. In this subset of patients, intraoperative frozen pathology may be used as a method of triaging patients to lymphadenectomy. In higher risk disease, such as grade 3 endometrioid, papillary serous, clear cell, and undifferentiated histologies, the benefits of complete surgical staging outweigh any potential disadvantages of lymphadenectomy.
Main Points.
Endometrial cancer is the most common gynecologic malignancy in the United States. Patients with localized disease have a 96% 5-year survival rate, which drops to 67% for regional disease and 17% for those with metastatic disease.
Despite the well-defined criteria for surgical staging, surgeons still debate the extent of lymphadenectomy necessary. Particular controversy exists as to whether to perform bilateral complete para-aortic lymph node dissection in all patients.
The advantages of comprehensive surgical staging lie in diagnosis, prognosis, and proper triage of patients for adjuvant therapy.
Based on FIGO staging guidelines, clinically early-stage endometrial cancer patients should undergo comprehensive surgical staging. However, the disadvantages of surgical staging may outweigh the risks in patients with low-grade endometrioid tumors. In this subset of patients, intraoperative frozen pathology may be used as a method of triaging patients to lymphadenectomy. In higher risk disease, such as grade 3 endometrioid, papillary serous, clear cell, and undifferentiated histologies, the benefits of complete surgical staging outweigh any potential disadvantages lymphadenectomy. of lymphadenectomy.
Footnotes
The authors report no real or apparent conflicts of interest.
References
- 1.Jemal A, Siegel R, Xu K, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT. Announcement, FIGO stages: 1988 revisions. Gynecol Oncol. 1989;35:125–127. [Google Scholar]
- 3.Mutch DG. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol Oncol. 2009;115:325–328. [Google Scholar]
- 4.Boronow RC, Morrow CP, Creasman WT, et al. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63:825–832. [PubMed] [Google Scholar]
- 5.DiSaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in stage I endometrial carcinoma. Am J Obstet Gynecol. 1984;151:1009–1015. doi: 10.1016/0002-9378(85)90371-0. [DOI] [PubMed] [Google Scholar]
- 6.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer: a Gynecologic Oncology Group study. Cancer. 1987;60(8 suppl):2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Rustum NR, Gomez JD, Alektiar KM, et al. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol. 2009;15:236–238. doi: 10.1016/j.ygyno.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Hirahatake K, Hareyama H, Sakurgai N, et al. A clinical and pathologic study on para-aortic lymph node metastasis in endometrial carcinoma. J Surg Oncol. 1997;65:82–87. doi: 10.1002/(sici)1096-9098(199706)65:2<82::aid-jso3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Mariani A, Webb MJ, Keeney GL, et al. Stage IIIC endometrioid corpus cancer includes distinct subgroups. Gynecol Oncol. 2002;87:112–117. doi: 10.1006/gyno.2002.6789. [DOI] [PubMed] [Google Scholar]
- 11.Todo Y, Kato H, Minobe S, et al. A validation study of the new revised FIGO staging system to estimate prognosis for patients with stage IIIC endometrial cancer. Gynecol Oncol. 2011;121:126–130. doi: 10.1016/j.ygyno.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Gynecologic Oncology Group., authors Surgical Procedures Manual. Buffalo, NY: Gynecologic Oncology Group; 2007. [Accessed April 2, 2012]. http://www.gog.org. [Google Scholar]
- 13.Kilgore LC, Partridge EE, Alvarez RD, et al. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Rustum NR, Khoury-Collado F, Pandit-Taskar N, et al. Sentinel lymph node mapping for grade 1 endometrial cancer: is it the answer to the surgical staging dilemma? Gynecol Oncol. 2009;113:163–169. doi: 10.1016/j.ygyno.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury-Collado F, Murray MP, Hensley ML, et al. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol Oncol. 2001;122:251–254. doi: 10.1016/j.ygyno.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Dowdy SC, Aletti G, Cliby WA, et al. Extra-peritoneal laparoscopic para-aortic lymphadenectomy-a prospective cohort study of 293 patients with endometrial cancer. Gynecol Oncol. 2008;111:418–424. doi: 10.1016/j.ygyno.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 18.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Rustum NR, Alektiar K, Iasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol. 2006;103:714–718. doi: 10.1016/j.ygyno.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Todo Y, Yamamoto R, Minobe S, et al. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol Oncol. 2010;119:60–64. doi: 10.1016/j.ygyno.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Mohan DS, Samuels MA, Selim MA, et al. Long-term outcomes of therapeutic pelvic lymphadenectomy for stage I endometrial adenocarcinoma. Gynecol Oncol. 1998;70:165–171. doi: 10.1006/gyno.1998.5098. [DOI] [PubMed] [Google Scholar]
- 22.Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 23.Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998;71:340–343. doi: 10.1006/gyno.1998.5254. [DOI] [PubMed] [Google Scholar]
- 24.Todo Y, Kato H, Kaneucki M, et al. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 25.Lutman CV, Havrilesky LJ, Cragun JM, et al. Pelvic lymph node count is an important prognostic variable for FIGO stage I and II endometrial carcinoma with high-risk histology. Gynecol Oncol. 2006;102:92–97. doi: 10.1016/j.ygyno.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Chan JK, Cheung MK, Huh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: a study of 12,333 patients. Cancer. 2006;107:1823–1830. doi: 10.1002/cncr.22185. [DOI] [PubMed] [Google Scholar]
- 27.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 28.Kitchener H, Swart AM, Qian Q, Parmar M ASTEC Study Group, authors. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creasman WT, Mutch DE, Herzog TJ. ASTEC lymphadenectomy and radiation therapy studies: are conclusions valid? Gynecol Oncol. 2010;116:293–294. doi: 10.1016/j.ygyno.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 30.Creutzberg CL, van Putten WL, Wárlám-Rodenhuis CC, et al. Postoperative Radiation Therapy in Endometrial Carcinoma Trial. O Outcome of high-risk stage IC, Grade 3, compared with stage I endometrial carcinoma patients: the Postoperative Radiation Therapy in Endometrial Carcinoma Trial. J Clin Oncol. 2004;22:1234–1241. doi: 10.1200/JCO.2004.08.159. [DOI] [PubMed] [Google Scholar]
- 31.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 32.Diavolitsis V, Boyle J, Singh DK, Small W., Jr. The role of adjuvant radiation in endometrial cancer. Oncology (Williston Park) 2009;23:342–349. [PubMed] [Google Scholar]
- 33.Wilson TO, Podratz KC, Gaffey TA, et al. Evaluation of unfavorable histologic subtypes in endometrial adenocarcinoma. Am J Obstet Gynecol. 1990;162:418–423. doi: 10.1016/0002-9378(90)90399-r. discussion 423–426. [DOI] [PubMed] [Google Scholar]
- 34.Mariani A, Webb MJ, Keeney GL, et al. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506–1519. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 35.Bernardini MQ, May T, Khalida MA, et al. Evaluation of two management strategies for preoperative grade 1 endometrial cancer. Obstet Gynecol. 2009;114:7–15. doi: 10.1097/AOG.0b013e3181aa97fc. [DOI] [PubMed] [Google Scholar]
- 36.Mariani A, El-Nashar SA, Dowdy SC. Lymphadenectomy in endometrial cancer: which is the right question? Int J Gynecol Cancer. 2010;20(11 suppl 2):S52–S54. doi: 10.1111/IGC.0b013e3181f60d0f. [DOI] [PubMed] [Google Scholar]
- 37.Case AS, Rocconi RP, Straughn JM, Jr, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006;108:1375–1379. doi: 10.1097/01.AOG.0000245444.14015.00. [DOI] [PubMed] [Google Scholar]