Abstract
Women who cannot negotiate condom use with their partners, often due to socioeconomic factors and sexual abuse, have no means of preventing themselves from acquiring the human immunodeficiency virus (HIV). There is a need to develop HIV-preventive methods initiated and controlled by women. Microbicides and other pre-exposure prophylaxis may help fill that need. Although two decades of research on broad-spectrum microbicides have generally been disappointing, recent trials with HIV-specific agents have yielded promising initial results. A new era of clinical research involves novel biochemical prevention methods, including HIV-specific vaginal microbicides and oral antiretroviral chemoprophylaxis drugs (pre-exposure prophylaxis; PrEP) that may help provide more control for women.
Key words: HIV/AIDS, Pre-exposure prophylaxis, Microbicides, Oral antiretroviral chemoprophylaxis drugs
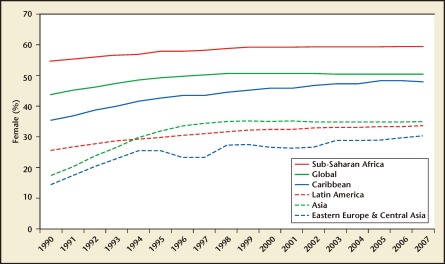
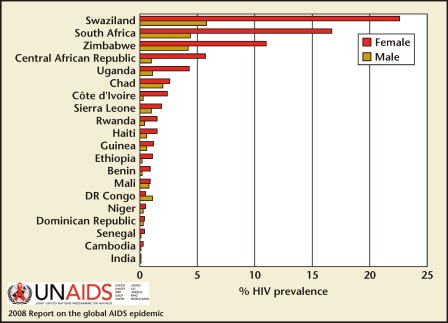
Worldwide, the human immunodeficiency virus (HIV)/AIDS remains the leading cause of death among women of reproductive age (age 15–49 years).1 In highly endemic regions such as sub-Saharan Africa, a woman age 15 to 24 years is three times more likely to be infected than a man of the same age (Figures 1 and 2).1 In developed countries, this discrepancy is often seen among marginalized populations. For example, black and Hispanic women account for 80% of new HIV cases in the United States.2,3
Figure 1.
Percentage of female adults (age 15 years +) living with human immunodeficiency virus (1990-2007). Reproduced with permission from UNAIDS.
Figure 2.
HIV prevalence (%) among 15- to 24-year-olds, by sex, selected countries, 2005–2007. Reproduced with permission from UNAIDS.
Gender Discrepancy
Reasons for this gender discrepancy in HIV acquisition are both biological and socioeconomic. A 7-year longitudinal study of 2200 married adults in Uganda showed that the rate of male-to-female HIV transmission was twice that of female-to-male.4 This may be due to anatomical differences in the surface area of exposed mucosa and reservoirs for bodily fluid, a larger quantity of infectious fluid from semen as compared with vaginal fluid, hormonal differences, and a greater diversity of transmitted viral variants from men to women.5
Additionally, socioeconomic factors often make women less able to negotiate condom use. More than 80% of new infections in women in sub-Saharan Africa occur in the context of marriage or other long-term relationships with a single partner. This makes consistent condom use difficult, as it does not allow for wanted conception and can lead to partner distrust.6 Up to 70% of women experience violence in their lifetime, and studies indicate that the risk of HIV among these women may be three times higher than among those who have not experienced violence.1 Additionally, gender inequities reduce access to education, prevention, and treatment; encourage reliance on men for financial support (often leading to transactional or commercial sex); and increase the likelihood that women will marry at younger ages, often to older, more experienced partners.
The Need for Female-Controlled HIV Prevention Methods
Most HIV infections in women (70%–90%) are spread by heterosexual sex.2 Furthermore, interventions to prevent new infections are largely dependent on male partner initiation and/or participation (ie, male condoms, male circumcision, and abstinence). A new approach to HIV prevention is needed that empowers women to protect themselves against HIV.
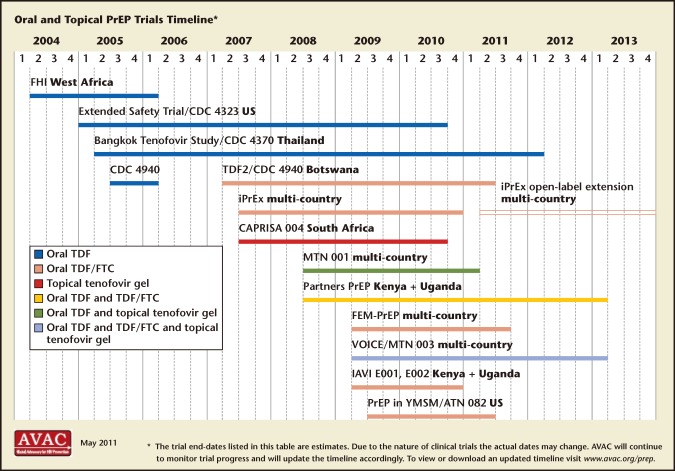
Female-controlled prevention has been proposed as an option to fill this gap. Microbicides are compounds applied inside the vagina or rectum to protect against sexually transmitted infections (STIs), including HIV. Two decades of research on microbicides have yielded great lessons but few successes. Last year, however, brought new hope to the field of female-controlled chemoprophylaxis with the success of the antiretroviral-containing microbicide, 1% tenofovir gel.7 Two other placebo-controlled trials found daily tenofovir (TDF) and emtricitabine (FTC) protective in heterosexual women (Partners PrEP and TDF2), but conflicting results were obtained from the VOICE and Femprep trials. Many other HIV-specific pre-exposure prophylaxis (PrEP) agents using already-marketed antiretroviral drugs (ARVs) are in development and hold further promise (Figure 3). Most trials include TDF and FTC due to superior penetration into the vaginal or rectal mucosa.
Figure 3.
Pre-exposure prophylaxis (PrEP) trials timeline. FTC, emtricitabine; TDF, tenofovir. Reproduced with permission from AVAC: Global Advocacy for HIV Prevention.
What Are Microbicides and Pre-Exposure Prophylaxis?
The Optimal Microbicide
The optimal microbicide is one that is affordable, effective, safe, broadly acceptable and accessible, and allows for a pregnancy when desired. They can be formulated as gels, creams, films, suppositories, vaginal rings, or probiotics. Though there are many types, they fall into three general categories: broad-spectrum, HIV-specific, and contraceptive.
Prior Failures
Broad-spectrum microbicides aim to provide universal protection against several STIs, including HIV. They act by several mechanisms including surfactants, polyanionic entry inhibitors, and vaginal milieu protectors. They could provide the greatest global impact, as they may protect against other infections such as herpes simplex virus (HSV), bacterial vaginosis, and human papillomavirus, all of which are relevant to the HIV epidemic as well as many other important health issues affecting women. However, large, randomized, control trials (RCTs) comparing the five most promising non-HIV-specific microbicides to placebo found no protection from HIV infection.8–11 A large, phase II RCT of 9000 women testing the microbicide, PRO2000, did show a 30% reduction in HIV acquisition; however, this did not reach statistical significance. Subsequent phase III trials showed no protective benefit.10 The most well-known vaginal microbicide, nonoxynol-9, may even increase HIV transmission, possibly due to an increase in genital ulcerations and vulvitis.8,12
New Successes
Vaginal Microbicides
The recent landmark RCT by the Centre for the AIDS Program of Research in South Africa (CAPRISA 004) proved that a topical vaginal microbicide containing 1% tenofovir antiretroviral gel reduced the risk of acquiring HIV by 39% and HSV-2 by 51% among 889 HIV-uninfected women between the ages of 18 and 40.7 These researchers estimated from their data, that “once confirmed and implemented, tenofovir gel has the potential to alter the HIV epidemic … this gel could prevent 1.3 million new HIV infections and over 800,000 deaths in South Africa alone.”7
Oral PrEP
Subsequently, researchers were able to prove that an oral ARV can prevent HIV infection in uninfected people when taken on a regular basis. The Pre-Exposure Prophylaxis Initiative (iPrEx) study showed that, compared with placebo, TDF/FTC (Truvada®; Gilead Sciences, Inc., Foster City, CA) decreased HIV acquisition by 44% in 2499 HIV-seronegative men or transgender women between the ages of 18 and 67.13
Initial results of oral PrEP in women have so far yielded mixed results. In 2011, two major studies, the FEM PrEP HIV Prevention Study and Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial, prematurely discontinued their TDF/FTC arm after an interim review showed that the trial would be unable to demonstrate the effectiveness of daily oral TDF/FTC in preventing HIV infection. However, other studies have been more promising. The Partners PrEP trial compared the safety and efficacy of two different oral PrEP strategies—once-daily tenofovir and once-daily TDF/FTC—in reducing the risk of HIV transmission among 4758 heterosexual couples in Kenya and Uganda in which one partner was HIV positive and the other HIV negative. Early data showed that both tenofovir and TDF/FTC taken daily can reduce the risk of HIV transmission among both men and women. Daily oral TDF reduced HIV risk by 62% and daily oral TDF/FTC reduced HIV risk by ∼73% when compared with placebo. Further data from the US Centers for Disease Control and Prevention TDF2 study in Botswana of sexually active men and women found a 62% risk reduction of HIV infection in men and women taking TDF/FTC as compared with placebo. Table 1 summarizes these and other ongoing studies of microbicides and other PrEP. Conflicting findings between trials may reflect differences in study characteristics including adherence rates and other unrecognized factors.
Table 1.
Pre-Exposure Prophylaxis (PrEP) and Antiretroviral Drug-Based Microbicide Trials
| Trial | Phase | Product Tested | Population | Status/Findings |
| CAPRISA 004 | Phase IIB | 1% tenofovir gel | 889 women in South Africa | Results reported July 2010; tenofovir gel reduced risk of HIV infection by 39% |
| iPrEx | Phase III | Oral TDF/FTC | 2499 gay men and other men who have sex with men and transgender women in Brazil, Ecuador, Peru, South Africa, Thailand, and the US | Results reported November 2010. TDF/FTC reduced risk of HIV by an average of 42% |
| FEM-PrEP | Phase III | Oral TDF/FTC | 1951 heterosexual women in Kenya, South Africa, and Tanzania | Stopped for futility in April 2011, with 28 HIV infections in each arm. Full results expected early 2012 |
| Partners PrEP | Phase III | Oral TDF and oral TDF/FTC | 4758 scrodiscordant heterosexual couples in Kenya and Uganda | DSMB review in July 2011 showed daily TDF reduced risk of HIV by an average of 62%; daily TDF/FTC reduced risk of HIV by an average of 73%. As a result, placebo arms discontinued but the trial is ongoing. Additional data expected 2013 |
| TDF2 | Phase II | Oral TDF/FTC | 1219 heterosexual men and women in Botswana | Results released July 2011. TDF/FTC reduced risk of HIV infection by an average of 63% |
| VOICE | Phase IIB | Oral TDF, oral TDF/FTC and 1% tenofovir gel | 5029 women in South Africa, Uganda, and Zimbabwe | Oral TDF arm dropped for futility based on data from DSMB review. Other arms continuing. Full results expected early 2013 |
| Bangkok Tenofovir | Phase II/III | Oral TDF | 2400 injecting drug users in Bangkok, Thailand | Results expected 2012 |
| iPrEx OLE | Openlabel extension | Oral TDF/FTC | Enrolling participants from iPrEx and ATN 082 | Results expected 2013 |
| FACTS 001 | Phase III | 1% tenofovir gel | Will enroll 3150 women in South Africa | Expected to begin in Q4 2011; Results expected 2013 |
DSMB, Data and Safety Monitoring Board; FTC, emtricitabine; TDF, tenofovir.
Reproduced with permission from AVAC: Global Advocacy for HIV Prevention.
Future Directions
Current research has shown that microbicides and other PrEP may be safe in women. However, the safety and efficacy of use in adolescents, pregnant women, or in the rectum are unknown, and further studies to confirm the results of recent trials are needed. Newer trials are under way to determine the optimal vehicle for PrEP (vaginal ring, cap, diaphragm, or other gel and pill formulations) as well as optimal dosing. Furthermore, although the field has moved toward HIV-specific PrEP, identifying a broad-spectrum microbicide still remains the supreme goal for prevention of HIV as well as other STIs that may affect HIV transmission.
It is unlikely that a single micro-bicide or other PrEP will provide 100% protection against HIV. However, with encouraging results from recent vaccine trials in Thailand, male circumcision, and behavioral programs to decrease risk factors for acquisition, a combination of preventive tools that includes chemoprevention methods may bring us closer to our goal for full prevention. Current results are promising that microbicides and other PrEP may soon provide new options to empower women to protect themselves against HIV infection and end—or at least slow—this debilitating epidemic.
Main Points.
Most human immunodeficiency virus (HIV) infections in women (70%–90%) are spread by heterosexual sex. Furthermore, interventions to prevent new infections are largely dependent on male partner participation (ie, male condoms, male circumcision, and abstinence). A new approach to HIV prevention is needed that empowers women to protect themselves against HIV.
The recent landmark randomized, controlled trial by the Centre for the AIDS Program of Research in South Africa (CAPRISA 004) proved that a topical vaginal microbicide containing 1% tenofovir antiretroviral gel reduced the risk of acquiring HIV by 39% and herpes simplex virus 2 by 51% among 889 HIV-uninfected women between the ages of 18 and 40.
Current research has shown that microbicides and other pre-exposure prophylaxis are safe and acceptable for vaginal use. However, the safety and efficacy of use in adolescents, pregnant women, or in the rectum is unknown, and further studies to confirm the results of recent trials are needed.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS), authors Fact Sheet: Women, Girls, and HIV. [Accessed March 27, 2012]. http://data.unaids.org/pub/FactSheet/2010/20100302_fs_womenhiv_en.pdf.
- 2.HIV Infection in Women. National Institute of Allergy and Infectious Diseases (NIAID) Web site, authors. [Accessed March 27, 2012]. http://www.niaid.nih.gov/topics/hivaids/understanding/population%20specific%20information/pages /womenhiv.aspx. Updated September 10, 2008.
- 3.HIV Surveillance by Race/Ethnicity (through 2010). Centers for Disease Control and Prevention Web site, authors. [Accessed March 27, 2012]. http://www.cdc.gov/hiv/topics/surveillance/resources/slides/race-ethnicity/index.htm. Updated March 23, 2012.
- 4.Carpenter LM, Kamali A, Ruberantwari A, et al. Rates of HIV-1 transmission within marriage in rural Uganda in relation to the HIV sero-status of the partners. AIDS. 1999;13:1083–1089. doi: 10.1097/00002030-199906180-00012. [DOI] [PubMed] [Google Scholar]
- 5.Long EM, Martin HL , Jr, Kreiss JK, et al. Gender differences in HIV-1 diversity at the time of infection. Nat Med. 2000;6:71–75. doi: 10.1038/71563. [DOI] [PubMed] [Google Scholar]
- 6.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 7.AbdoolKarim Q, AbdoolKarim S. Safety and effectiveness of 1% tenofovir vaginal microbicide gel in South African women: results of the CAPRISA 004 trial.; Presented at: XVIII International AIDS Society Conference; July 18–23, 2012; Vienna, Austria. [Google Scholar]
- 8.Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase 2 Study Group. AIDS. 2000:14. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 9.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 10.Roehr B. Microbicide offers no protection against HIV infection. BMJ. 2009;339:b5538. doi: 10.1136/bmj.b5538. [DOI] [PubMed] [Google Scholar]
- 11.Van Damme L, Govinden R, Mirembe FM, et al. CS Study Group. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008:359. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 12.Kreiss J, Ngugi E, Holmes K, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268:477–482. [PubMed] [Google Scholar]
- 13.Grant RM, Lama JR, Anderson PL, et al. iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]