Abstract
Primary cilia are a class of cilia that are typically solitary, immotile appendages present on nearly every mammalian cell type. Primary cilia are believed to perform specialized sensory and signaling functions that are important for normal development and cellular homeostasis. Indeed, primary cilia dysfunction is now linked to numerous human diseases and genetic disorders. Collectively, primary cilia disorders are termed as ciliopathies and present with a wide range of clinical features, including cystic kidney disease, retinal degeneration, obesity, polydactyly, anosmia, intellectual disability, and brain malformations. Although significant progress has been made in elucidating the functions of primary cilia on some cell types, the precise functions of most primary cilia remain unknown. This is particularly true for primary cilia on neurons throughout the mammalian brain. This review will introduce primary cilia and ciliary signaling pathways with a focus on neuronal cilia and their putative functions and roles in human diseases.
Keywords: Primary cilia, Ciliopathy, Neuronal cilia, Ciliary signaling, Somatostatin receptor 3, Serotonin receptor 6, Melanin-concentrating hormone receptor 1, Type 3 adenylyl cyclase
Introduction to cilia
Cilia within the mammalian body are generally classified as either motile or primary. Motile cilia are mainly responsible for generating flow or movement, and include respiratory cilia, ependymal cilia, oviduct cilia, and sperm flagella. Primary cilia are typically immotile and function principally as sensory organelles. These functional classifications are not mutually exclusive, as subsets of primary cilia on the embryonic node are motile and generate flow [1], and motile respiratory cilia also possess sensory functions [2, 3]. All cilia are comprised of a microtubule core called an axoneme that is nucleated by a basal body, which is a specialized centriole that has migrated to the ciliary assembly site, and is linked to the plasma membrane by transition fibers (Fig. 1). In general, the axonemal structure of motile cilia consists of nine outer microtubule doublets and two centrally located microtubule singlets (“9+2”; Fig. 1). The axonemal structure of primary cilia consists of only the nine outer microtubule doublets (“9+0”; Fig. 1). However, there are exceptions, such as “9+0” motile cilia on the embryonic node and “9+2” immotile cilia on olfactory neurons.
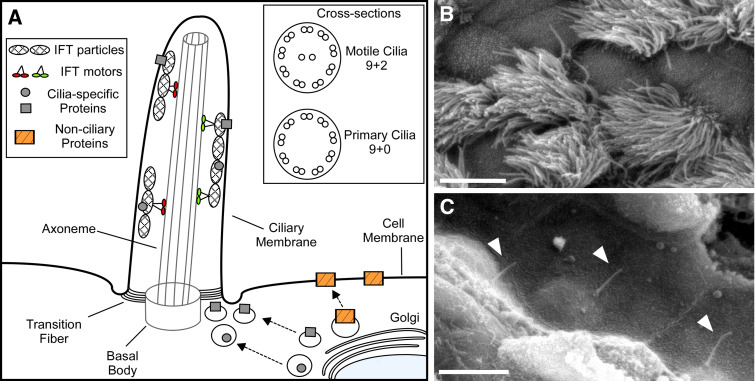
Fig. 1.
Schematic of cilia structure and examples of motile and primary cilia. a Membrane and cytosolic proteins destined for the ciliary compartment are transported in Golgi-derived vesicles and exocytosed at the base of the cilium where they associate with intraflagellar transport (IFT) particles. The transition fibers form a selective barrier to the ciliary compartment and only proteins containing specific ciliary targeting motifs are allowed access. Following entry into the cilium, proteins are transported along the axoneme. Cross-sections show the typical microtubule structures of motile and primary cilia. b Scanning electron micrograph showing numerous motile cilia protruding from epithelial cells of a mouse trachea. Scale bar 5 μm. c Scanning electron micrograph showing solitary primary cilia (arrowheads) projecting from mouse renal epithelial cells lining the nephron. Scale bar 5 μm
Proteins are not synthesized within the cilium. Therefore, the structural proteins required to build and maintain the cilium, as well as the signaling proteins required for cilia function, are synthesized in the cell body and transported into and out of the cilium. This transport is mediated by a highly conserved mechanism known as intraflagellar transport (IFT) [4–6]. IFT is a bidirectional microtubule-based transport process in which complexes of proteins called IFT particles are transported by molecular motors along the axonemal outer doublet microtubules from the base of the cilium to the distal tip and then back to the cell body [4–6]. IFT is required for the formation and maintenance of all mammalian cilia, and defective IFT is associated with severe diseases and developmental defects [7–10].
As the axoneme is assembled, it projects from the cell and becomes ensheathed by a membrane that is continuous with the plasma membrane [11]. The transition fibers act as a selective barrier allowing only specific proteins to localize within the cilium and on the ciliary membrane. It has been proposed that proteins destined for the cilium accumulate at the site where the transition fibers contact the membrane and are then assembled into IFT particles before transport into the cilium [5, 12]. Although this process is not well understood, a number of ciliary targeting sequences have been identified that appear to mediate ciliary localization of specific proteins [13, 14]. Importantly, the functions of cilia are defined by the signaling proteins enriched within the ciliary compartment, and there is diversity in the complement of signaling proteins enriched in cilia on different cell types.
Ciliary signaling pathways
Primary cilia mediate numerous signaling pathways throughout the mammalian body in response to a diverse set of sensory stimuli. In general, cilia mediate chemo-, photo-, and mechanotransduction. Olfactory cilia on olfactory sensory neurons (OSNs) are an example of chemosensory cilia that directly sense odorants from the environment (Fig. 2a). Each OSN possesses 10–30 olfactory cilia that protrude through the olfactory epithelium into the nasal cavity and contain the signaling molecules and downstream effectors needed for olfaction [15, 16]. The olfactory signal transduction cascade begins with an odorant binding to an olfactory G protein-coupled receptor (GPCR) on the ciliary membrane, which triggers the activation of the stimulatory G protein (Gαolf). The G protein activates type III adenylyl cyclase (ACIII), which increases adenosine 3′, 5′-cyclic monophosphate (cAMP) within the cilium. An increase in cAMP levels leads to the activation and opening of cyclic nucleotide-gated (CNG) channels, allowing for the influx of calcium ions. The increase in intracellular calcium leads to the activation and opening of Ca+2-gated chloride channels, resulting in an efflux of chloride ions further depolarizing the neuron [17, 18]. The absence of cilia on olfactory sensory neurons or the mislocalization of signaling molecules has been implicated in human diseases resulting in an altered sense of smell [15]. For example, the ciliopathies Bardet–Biedl syndrome (BBS) and Leber congenital amaurosis (LCA) can be associated with severely impaired olfactory function [19–21]. Mouse models of these diseases show that lack of BBS proteins leads to disruption of olfactory cilia structure [20], while the lack of CEP290, the protein disrupted in LCA, leads to the selective loss of G proteins in the cilia of olfactory sensory neurons resulting in a lack of signaling [21].
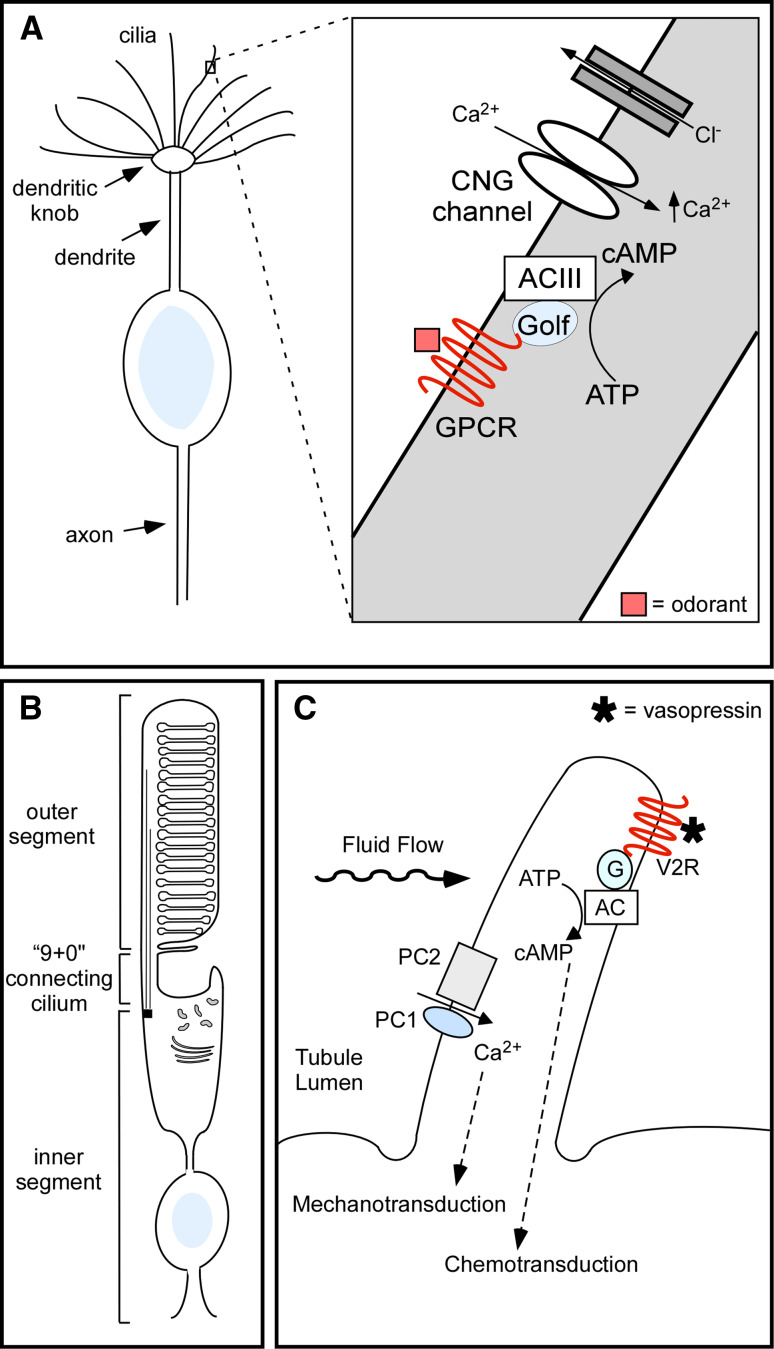
Fig. 2.
Examples of ciliary chemo-, photo-, and mechanotransduction. a Schematic of a single olfactory sensory neuron. Boxed region of interest is magnified and illustrates the ciliary signaling pathway. Odorant activation of olfactory G protein-coupled receptors (GPCRs) results in an increase in cAMP levels, which is mediated by type 3 adenylyl cyclase (ACIII). This results in activation of cyclic nucleotide-gated (CNG) channels leading to an increase in Ca2+ levels, subsequent activation of chloride channels, and depolarization of the neuron. b Schematic of a photoreceptor, which is comprised of an inner and outer segment that are connected by a “9+0” connecting cilium. Proteins are synthesized in the inner segment and transported by IFT across the connecting cilium to the outer segment, which is a highly modified cilium, where they mediate phototransduction. c Schematic of a renal cilium demonstrating Ca2+ signaling mediated by the ciliary proteins polycystin 1 (PC1) and polycystin 2 (PC2) in response to bending of the cilium by fluid flow. Renal cilia may also act as chemosensors by mediating vasopressin activation of the type 2 vasopressin receptor (V2R) on the ciliary membrane, which in turn modulates cAMP-signaling
Phototransduction in the eye is mediated in the outer segments of photoreceptors [22] (Fig. 2b). The outer segment is a highly modified cilium packed with membrane disks full of visual pigments that are composed of a vitamin A-based chromophore and opsin, which is a prototypical GPCR. Upon light activation, opsin activates the G protein transducin, which then stimulates a phosphodiesterase that hydrolyzes cyclic GMP (cGMP) to GMP. This light-induced reduction in cGMP levels causes cGMP-gated channels to close, thereby hyperpolarizing the cell. Similar to other cilia, proteins needed to sense and respond to visual light cues are synthesized in the inner segment and transported along the “9+0” connecting cilium to the outer segment via IFT (Fig. 2b). A unique aspect of signaling in the outer segments is the high turnover of signaling proteins. It has been estimated that 2,000 opsin molecules and 0.1 μm2 of membrane are transported across the connecting cilium every minute [23]. Mouse models have also confirmed that IFT is critical for proper photoreceptor development and maintenance [24, 25]. Thus, photoreceptor maintenance and phototransduction are particularly susceptible to defects in IFT, which may explain why retinal degeneration is such a common feature across the ciliopathies [26].
Mechanosensation is mediated by primary cilia on a variety of cell types, including renal epithelial cells [27, 28], embryonic nodal cells [29], endothelial cells [30, 31], cholangiocytes [32], chondrocytes [33], and smooth muscle cells [34]. In the kidney, bending of renal epithelial cilia by fluid flow generates an intracellular calcium signal [27]. The calcium signal is facilitated by polycystin-1 (PC1) and polycystin-2 (PC2), which localize to primary cilia and form a mechanosensitive Ca2+ channel [35] (Fig. 2c). The absence of cilia on renal tubule cells or the loss of PC1 or PC2 from renal cilia can result in altered ciliary signaling and polycystic kidney disease (PKD) [35–38]. Interestingly, loss of cilia on a kidney cell line results in unregulated calcium entry into the cell [38], suggesting that ciliary localization is necessary for proper regulation of the polycystin complex. There is also evidence that renal cilia mediate chemosensation (Fig. 2c). Recently, the type 2 vasopressin receptor (V2R) was found to localize to cilia on renal epithelial cells [39]. V2R is a GPCR that regulates Na+ and water reabsorption in the mammalian nephron. Interestingly, V2R functionally couples with type V/VI adenylyl cyclase in the cilium and vasopressin treatment of isolated renal cilia results in localized production of cAMP and cAMP-dependent activation of cation-selective channel activity [39]. Thus, in response to vasopressin, renal cilia mediate a cAMP-signaling pathway that targets ciliary channel function. These findings are particularly relevant to polycystic kidney disease as increased renal cAMP levels are a common feature of animal models of PKD [40] and treatment with V2R antagonists inhibits cyst formation [41, 42]. Thus, renal epithelial cilia may act as mechano- and chemosensors and disruption of either signaling pathway results in cystic kidney disease, a common feature across ciliopathies.
Primary ciliary signaling also plays critical roles in a number of important mammalian developmental signal transduction pathways, including Hedgehog (Hh) signaling [43, 44]. The Hh signaling pathway is essential for normal patterning of multicellular embryos and the development of a myriad of tissues and organs [45]. It also functions in postembryonic development and adult tissue homeostasis [45]. Disruptions in Hh signaling can cause severe developmental abnormalities in mice and humans, including neural tube defects, polydactyly, holoprosencephaly, craniofacial defects, and skeletal malformations [46]. The transduction of Hh signaling in cells is mediated by the interactions between the 12-transmembrane Hh receptor patched (Ptc) and the 7-transmembrane GPCR smoothened (Smo). The importance of cilia for Hh signal transduction was first recognized by the discovery that during embryogenesis IFT is required for the specification of ventral cell types in the mouse neural tube [47–49]. Subsequent studies revealed that Ptc localizes to primary cilia in the absence of Hh stimulation and inhibits Smo by preventing it from accumulating in cilia [50]. Upon Hh activation, Ptc leaves the cilium and Smo accumulates in the cilium [50, 51]. Smo then activates signaling at the distal tip of the cilium where the Gli transcription factors are localized [52]. Thus, cilia are required to coordinate the signaling components of the Hh pathway. Cilia-mediated Hh signaling has now been demonstrated in a variety of embryonic and adult cell types. For example, disruption of cilia on the developing limb bud abrogates Hh signaling and leads to alterations in digit number [48, 52, 53], which may explain the presence of polydactyly in ciliopathies such as BBS and Meckel–Gruber syndrome (MKS). In the postnatal brain, cilia-mediated Hh signaling is required for the expansion and establishment of neural precursor cells in the postnatal hippocampus (a process that is thought to be important for learning and memory) [54–56] and cerebellum [54, 57, 58]. Disruption of cilia on cerebellar neural precursors results in severe hypoplasia and abnormal foliation of the cerebellum. Interestingly, several ciliopathies, including Joubert syndrome, MKS, and Oro-facio-digital syndrome 1, are associated with cerebellar malformations [59], suggesting these features are the result of defective cilia-mediated Hh signaling in the developing cerebellum.
There are important conclusions that can be drawn from these examples of ciliary signaling pathways. It is clear that cilia coordinate specialized signaling and that their functions are determined in large part by the specific signaling proteins that are enriched in the ciliary compartment (Fig. 3a). Loss of ciliary signaling proteins or cilia structure disrupts ciliary signaling and can cause diseases (Fig. 3b, c). Finally, although there is great diversity in the functions of different cilia, there is apparent conservation between different ciliary signaling pathways, including the utilization of Ca2+, GPCR, cyclic-nucleotide, and ionic signaling. This conservation may provide great insights into the ciliary signaling pathways mediated by cilia on other cell types, such as post-mitotic neurons in the brain.
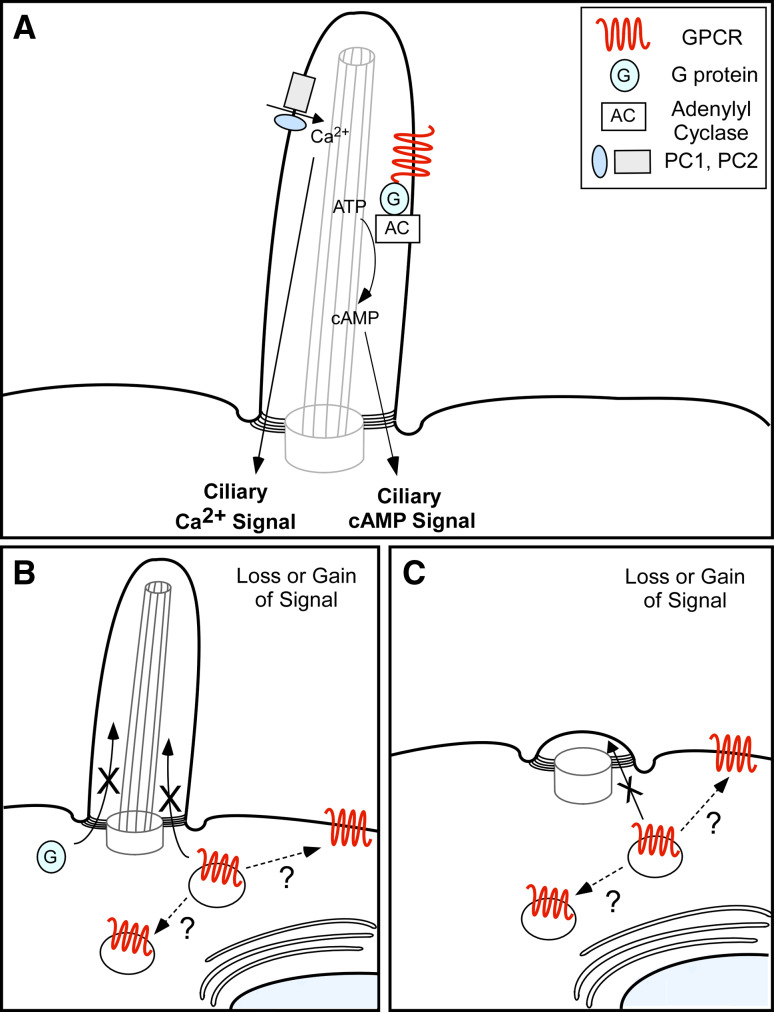
Fig. 3.
Model of ciliary signaling disruption. a Signaling proteins are enriched in the cilium where they coordinate cilia-specific signaling that is transmitted to the cell. Two examples of ciliary signaling pathways are illustrated; PC1- and PC2-mediated Ca2+ signaling and GPCR-mediated cAMP-signaling. b Disruption in trafficking of signaling proteins (indicated by X) into the ciliary compartment leads to loss of a cilia-specific signal. Ciliary receptors that are not trafficked to the cilium may accumulate in intracellular vesicles or mislocalize on the cell membrane (indicated by ?), possibly resulting in a gain of signal. c Defects in cilia structure prevents proper localization of ciliary signaling proteins (indicated by X) and leads to loss of a cilia-specific signal. Ciliary receptors that are not trafficked to the cilium may accumulate in intracellular vesicles or mislocalize on the cell membrane (indicated by ?), possibly resulting in a gain of signal
Cilia on central neurons
It has been more than 50 years since ultrastructural studies identified “9+0” cilia connecting the inner and outer segments of photoreceptors [60]. This was the first example of neuronal primary cilia and was followed shortly thereafter by observations of primary cilia on adult neurons in the preoptic nucleus of the goldfish [61] and granular neurons in the rat cerebral cortex [62]. At the time, it was hypothesized that neuronal cilia were either sensory organelles analogous to connecting cilia or vestigial appendages that were formed simply because of an innate tendency of centrioles to form cilia [62]. The first hint as to a function for neuronal cilia arose from the discovery just over 10 years ago that somatostatin receptor subtype 3 (Sstr3) selectively localizes to neuronal cilia throughout the rat brain [63]. The presence of a particular receptor enriched on the neuronal ciliary membrane suggested at least some neuronal cilia function as chemosensors of the extracellular milieu. Indeed, this was further supported by the subsequent findings that serotonin receptor 6 [64, 65] and melanin-concentrating hormone receptor 1 [66, 67] are enriched on neuronal cilia. The discovery of Sstr3 ciliary localization also provided the first marker for selectively labeling neuronal cilia. The marker for cilia on most cell types, acetylated tubulin, is not specific for neuronal cilia and labels neuronal processes and cell bodies. Labeling for Sstr3 revealed for the first time the abundance and widespread distribution of neuronal cilia (Fig 4). Indeed, recent immunohistological studies support the assertion that most neurons in the mammalian brain possess a primary cilium [63, 68, 69]. The recognition that neuronal cilia were widespread and enriched for specific receptors, combined with the link between cilia dysfunction and human disease, sparked a renewed interest in understanding the functions of these organelles in the brain [69, 70].
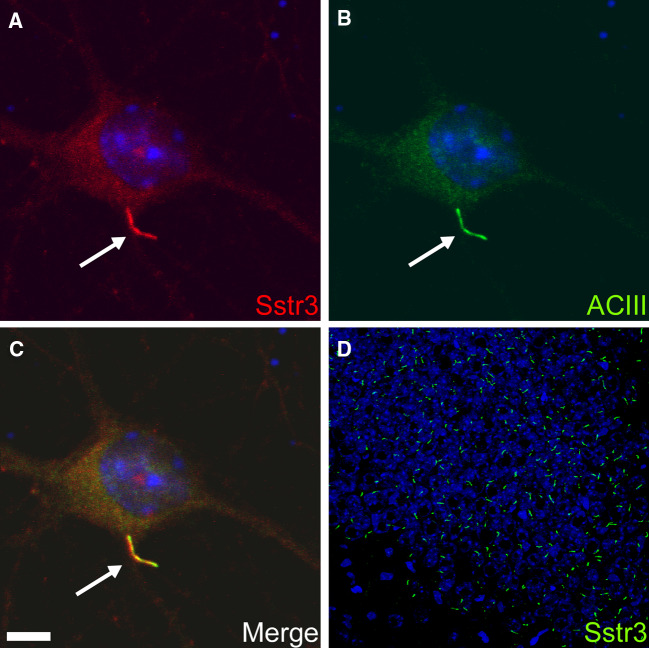
Fig. 4.
Localization of somatostatin receptor subtype 3 (Sstr3) to neuronal cilia. a,b Images of a day 7 mouse hippocampal neuron immunolabeled with antibodies to Sstr3 (red) and type 3 adenylyl cyclase (ACIII; green). c Merged image demonstrating colocalization of Sstr3 and ACIII to neuronal cilia. Nuclei were stained with DRAQ5 (blue) and the cilium is indicated with arrows. Scale bar 5 μm. d Adult mouse brain section corresponding to the CA3 region of the hippocampus immunolabeled with an antibody to Sstr3 (green). Note the abundance of Sstr3-positive cilia. Nuclei were stained with DRAQ5 (blue). (All images courtesy of Nicolas Berbari, University of Alabama, Birmingham, AL, USA)
The ligand for Sstr3, somatostatin, is a widely distributed neurotransmitter and modulator of neural activity that can affect many physiological processes in the brain, such as motor activity and cognitive function [71–74]. Altered somatostatin signaling has been implicated in depression, dementia, and general cognitive impairment [74]. The effects of somatostatin are mediated by at least five somatostatin receptor subtypes (Sstr1-5) that are all GPCRs. Interestingly, only Sstr3 localizes to neuronal cilia, even in neurons coexpressing Sstr3 and other somatostatin receptors [75]. Furthermore, Sstr3 appears to be restricted to neuronal cilia, with the exception of the cerebellar cortex where Sstr3 localization to neuronal processes is seen. This suggests that Sstr3 signaling is predominantly mediated by the cilium. However, it is possible that Sstr3 also localizes to the cell membrane, but it is difficult to detect because it is present at such a low level or is diffusely localized. Nevertheless, given the prevalence and distribution of Sstr3 ciliary localization in the brain, somatostatin certainly binds to Sstr3 on the ciliary membrane. However, signaling within neuronal cilia has not yet been shown.
Ciliary localization of Sstr3 is also developmentally dynamic. Sstr3-positive cilia are rare in the embryonic and neonatal rodent brain, although they have been found in the developing cerebral cortex of E13 mice [76]. In the neonatal rodent hippocampus, Sstr3-positive cilia are scarce and restricted to the CA3 region [77, 78]. By P7, there are abundant Sstr3-positive cilia in the CA3 region and the appearance of some Sstr3-positive cilia in the CA1 region and dentate gyrus [77, 78]. Interestingly, in the rat hippocampus, the density of Sstr3-positive cilia is highest in the CA1 and CA3 regions in juvenile animals (P20) and then decreases in adulthood, while the density in the dentate gyrus increases and is maintained into adulthood [78]. Thus, there appear to be regional and developmental differences in Sstr3 ciliary signaling. It is important to note that the lack of Sstr3-positive cilia does not necessarily reflect the absence of cilia. Nevertheless, these results indicate that ciliary localization, and likely signaling, of Sstr3 is regionally and developmentally regulated.
Although it has been known for more than 10 years that Sstr3 localizes to neuronal cilia, remarkably we still do not know whether Sstr3 signals on the ciliary membrane and how this affects neuronal function. However, recent studies utilizing Sstr3 knockout mice have begun to elucidate potential ciliary somatostatin signaling. Specifically, an investigation of the role of Sstr3 signaling in learning and memory revealed that Sstr3 is critical for object recognition memory but not spatial memory [79]. Furthermore, the authors found that regulation of cAMP-signaling is disrupted in Sstr3 knockout mice or wild-type mice treated with an Sstr3 antagonist. Given that Sstr3 is highly enriched in cilia, the authors suggest Sstr3 mediates cAMP-signaling within cilia and impacts cognition. However, further studies are necessary to confirm that Sstr3 modulates cAMP levels within the cilium and whether loss of cilia on Sstr3-expressing neurons similarly impacts object recognition memory and cAMP-signaling.
As opposed to Sstr3, serotonin receptor 6 (5-HT6) ciliary localization in the rodent brain is confined to the striatum, nucleus accumbens, olfactory tubercle and islands of Calleja [64, 65]. Moreover, 5-HT6 also localizes to dendrites in the striatum and hippocampus. Thus, 5-HT6 may function at the synapse or cilium, depending on the brain region or even the subset of neurons within the region. Nevertheless, it is interesting that 5-HT6 can be targeted to different neuronal compartments. One possibility is that 5-HT6 localizes to dendrites when it is expressed in a neuron lacking a cilium. Alternatively, certain neurons may possess mechanisms for overriding ciliary localization in order to target the receptor to the dendrite. In the brain, serotonin modulates neural activity and affects virtually all human behavioral processes. Specifically, 5-HT6 is thought to be involved in anxiety and depression [80], eating disorders [81, 82], and learning and memory [83, 84]. Additional studies are required to dissect the precise roles of 5-HT6 on the ciliary membrane. Unfortunately, studies of ciliary 5-HT6 are hindered by the lack of a specific 5-HT6 antibody.
The third neuronal ciliary GPCR identified, melanin-concentrating hormone receptor 1 (Mchr1), is notable for two reasons. First, we identified Mchr1 as a ciliary GPCR based on the presence of a putative ciliary localization sequence that we identified in Sstr3 and 5-HT6 [66], suggesting that some ciliary GPCRs are targeted to the ciliary membrane by a similar mechanism and that ciliary targeting sequences can be used to predict novel ciliary GPCRs. Second, Mchr1 and its ligand, melanin-concentrating hormone (MCH), are important regulators of feeding behavior. Injection of MCH into the brains of mice induces a rapid increase in feeding behavior [85], while injection of Mchr1 antagonists reduces feeding behavior [86]. Transgenic mice overexpressing MCH [87] are obese and mice lacking expression of either MCH [88] or Mchr1 [89] are lean. Interestingly, Mchr1 localizes to cilia in regions of the brain known to regulate feeding and reward behavior, suggesting ciliary localization of Mchr1 may be important for signaling through the receptor and proper regulation of these processes. Neuronal cilia have been implicated in the regulation of feeding behavior. Mice lacking cilia in the brain or specifically on pro-opiomelanocortin-expressing cells in the hypothalamus are hyperphagic and become obese [90]. Further, obesity is a hallmark of some ciliopathies, including Alstrom syndrome and BBS. Notably, mouse models of BBS lack ciliary localization of Mchr1 [67]. Together, these results suggest neuronal cilia mediate signaling that is required for proper regulation of feeding behavior.
The signaling protein that is enriched in the largest proportion of neuronal cilia is ACIII [68]. ACIII colocalizes with Sstr3 (Fig. 4) [67, 77] and Mchr1 [66, 67] on subsets of cilia, suggesting neuronal cilia can mediate cAMP-signaling. ACIII is 1 of 10 known mammalian isoforms of adenylyl cyclase that function to convert ATP to cyclic cAMP in response to activation by a variety of hormones, neurotransmitters, and other regulatory molecules [91]. Cyclic AMP, in turn, activates several other target molecules to control a broad range of intracellular processes. As discussed above, ACIII is enriched in cilia of olfactory sensory neurons [92] where it couples activation of odorant GPCRs to increased cAMP levels in olfactory cilia [93]. It is possible that ACIII in neuronal cilia acts analogously to ACIII in olfactory cilia to affect membrane potential and alter neuronal firing rates. Intriguingly, ACIII knockout mice become obese as they age due to hyperphagia, reduced physical activity, and leptin insensitivity [94], suggesting that cAMP-signaling within neuronal cilia is important for proper regulation of food consumption and energy balance. Notably, colabeling for ACIII and Sstr3 reveals the majority of neuronal cilia are positive for ACIII but negative for Sstr3 [77], suggesting that Sstr3-negative neurons may express other unknown ciliary receptors.
In summary, neuronal cilia are abundant and widely distributed in the brain, suggesting that they possess important functions. Yet, their precise functions are not known. It is likely that neuronal cilia provide specialized non-synaptic sensory and signaling functions that affect neuronal function [69, 70]. This organelle contains a subset of signaling receptors and second messengers important for many signal transduction pathways that may be critical for such processes as learning and eating behavior. The absence of neuronal cilia or the mislocalization of ciliary signaling machinery may result in altered non-synaptic/ciliary signaling and disease phenotypes such as cognitive deficits and obesity. Further studies are necessary to dissect the molecular mechanisms of ciliary receptor activation and signal transduction within these specialized signaling organelles.
Neuronal cilia functions: challenges and opportunities
A critical step in determining the functions of neuronal cilia is to identify the signaling proteins present within the cilium. Although proteomic analyses of mammalian photoreceptor outer segments and olfactory cilia are available [95, 96], and methods for the isolation of primary cilia from cells have been described [97–100], the prospect of selectively isolating cilia from central neurons in sufficient quantity for proteomic analysis is daunting. However, there are various approaches for identifying novel ciliary signaling proteins. One approach is to make predictions based on the conservation of signaling pathways between cilia on different cell types. For example, subsets of neuronal cilia contain GPCRs and ACIII, suggesting they are functionally coupled as in olfactory cilia. Thus, it is possible they share additional signaling components, such as G proteins, CNGs and chloride channels, a hypothesis that can be readily tested. A second approach is to identify ciliary localization sequences and use these sequences to predict novel ciliary signaling proteins. We recently identified a putative GPCR ciliary localization sequence in the third intracellular loops of Sstr3 and 5-HT6 and, using a computational approach combined with prioritization based on known biological functions, predicted that Mchr1 would localize to neuronal cilia. Expression of fluorescently-tagged Mchr1 in a ciliated renal cell line and labeling for endogenous Mchr1 in mouse brain sections confirmed that Mchr1 is enriched in neuronal cilia [66]. Interestingly, the i3 consensus sequence is sufficient for GPCR ciliary localization but is not required, suggesting ciliary GPCRs contain additional sequences that mediate localization to cilia. There are approximately 950 GPCRs in the human genome, with 500 of those coding for odorant or taste receptors [101]. It is likely a significant percentage of the other 450 GPCRs localize to cilia on cell types throughout the body, including central neurons. Further studies to identify and characterize GPCR ciliary localization sequences could allow for a systematic identification of the complete subset of ciliary GPCRs.
There are also experimental approaches that can be utilized to identify novel ciliary signaling proteins. Ciliary localization sequences can be utilized in screens, such as yeast two-hybrid analysis, to identify the proteins that mediate ciliary localization. Such proteins can then subsequently be used to identify interacting proteins, subsets of which are likely ciliary proteins. This approach could be particularly fruitful given that it is likely that the trafficking mechanism of ciliary signaling proteins is conserved across populations of cells while the precise signaling components vary. Thus, ciliary localization mediators could be used to identify a wide range of signaling proteins across different cell populations.
Although the knowledge of the signaling proteins present in cilia itself provides important insight into the potential functions of neuronal cilia, another challenge will be observing signaling within the cilium. As mentioned above, the cilium is a restricted compartment, and commonly used reagents for signaling assays, such as cAMP reporter probes, are excluded. Combined with the fact that ciliary receptors may not be exclusively on the ciliary membrane but may also localize to the cell membrane, it is difficult to distinguish between signaling within the cilium and in the cell body. Studies are also needed to test whether signaling in neuronal cilia affects membrane potential. Although it is possible to use electrophysiology to measure changes in membrane potential of the cell body as a downstream measure of ciliary signaling, this approach has the same drawback of not distinguishing between signaling generated in the cilium and in the cell. Ideally, changes in potential across the ciliary membrane should be measured. Electrophysiological approaches have been used to analyze channel activity on olfactory cilia [102] and cilia isolated from renal epithelial cells [100]. Yet the application of these approaches to the analysis of neuronal cilia will require the development of novel methods to visualize and perhaps isolate cilia from central neurons.
Another approach that has proven successful for determining the functions of cilia is the generation of mouse lines with cilia defects. Indeed, seminal studies utilizing a mouse mutant with a partial defect in IFT88 first established the connection between cilia dysfunction and human disease [37, 103, 104]. IFT88 is required for the formation and maintenance of all cilia and flagella, and complete loss of the protein results in developmental defects and is embryonic lethal. However, mouse lines carrying conditional IFT alleles can be used to ablate cilia on specific cell types or at various stages of development in order to elucidate the roles of cilia in cellular homeostasis and disease. This approach provided the first demonstration that neuronal cilia are required for proper regulation of feeding behavior [90]. Conditional knockout mice can be used to dissect the physiological functions of neuronal cilia in complex neural systems.
Interestingly, neuronal cilia are especially abundant in certain brain regions, including the hippocampus, amygdala, and nucleus accumbens [68]. It will be exciting to test how ablation of cilia, specifically in these regions, affects complex behaviors, such as cognition, fear and anxiety, and reward.
Conclusions
Primary cilia are specialized subcellular compartments that provide important sensory and signaling functions. Seminal studies in several organ systems have elegantly demonstrated how this organelle has been adapted to perform specialized functions and the striking physiological consequences that result from loss of cilia function. Yet the precise functions of the vast majority of primary cilia remain unknown. In the brain, neuronal cilia appear to contribute to somatostatin, serotonin, and MCH signaling. The abundance of neuronal cilia and enrichment of signaling machinery, including GPCRs and ACIII, suggest that these tiny organelles play a specialized role in surveying the extracellular mileu and provide non-synaptic signaling.
This supplementary signaling mechanism surely adds to the complex circuitry of the brain and provides an additional way neuromodulators can regulate physiological processes. Thus, the study of neuronal cilia offers tremendous potential for making fundamental discoveries into the roles of neuronal ciliary signaling in neural function and disease.
Acknowledgments
This work was supported in part by the Systems and Integrative Biology Training Grant T32 GM068412 (J.G.) and R01 GM083120 (K.M.) from the NIH/National Institute of General Medical Sciences.
References
- 1.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/S0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 2.Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–509. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 3.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 6.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 7.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 8.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest. 2005;85:452–463. doi: 10.1038/labinvest.3700253. [DOI] [PubMed] [Google Scholar]
- 10.Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- 11.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 12.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/S0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 13.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–149. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins PM, McEwen DP, Martens JR. Olfactory cilia: linking sensory cilia function and human disease. Chem Senses. 2009;34:451–464. doi: 10.1093/chemse/bjp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen DP, Jenkins PM, Martens JR. Olfactory cilia: our direct neuronal connection to the external world. Curr Top Dev Biol. 2008;85:333–370. doi: 10.1016/S0070-2153(08)00812-0. [DOI] [PubMed] [Google Scholar]
- 17.Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 2008;33:839–859. doi: 10.1093/chemse/bjn048. [DOI] [PubMed] [Google Scholar]
- 18.Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannaccone A, Mykytyn K, Persico AM, Searby CC, Baldi A, Jablonski MM, Sheffield VC. Clinical evidence of decreased olfaction in Bardet–Biedl syndrome caused by a deletion in the BBS4 Gene. Am J Med Genet A. 2005;132A:343–346. doi: 10.1002/ajmg.a.30512. [DOI] [PubMed] [Google Scholar]
- 20.Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 21.McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, Lopez I, Swaroop A, Martens JR. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci USA. 2007;104:15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramamurthy V, Cayouette M. Development and disease of the photoreceptor cilium. Clin Genet. 2009;76:137–145. doi: 10.1111/j.1399-0004.2009.01240.x. [DOI] [PubMed] [Google Scholar]
- 23.Wolfrum U, Schmitt A. Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil Cytoskeleton. 2000;46:95–107. doi: 10.1002/1097-0169(200006)46:2<95::AID-CM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/S0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- 25.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- 27.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 28.Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–520. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 29.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/S0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 30.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 34.Lu CJ, Du H, Wu J, Jansen DA, Jordan KL, Xu N, Sieck GC, Qian Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008;31:171–184. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289:F978–F988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 37.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol. 2006;290:F1320–F1328. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
- 39.Raychowdhury MK, Ramos AJ, Zhang P, McLaughin M, Dai XQ, Chen XZ, Montalbetti N, Del Rocio Cantero M, Ausiello DA, Cantiello HF. Vasopressin receptor-mediated functional signaling pathway in primary cilia of renal epithelial cells. Am J Physiol Renal Physiol. 2009;296:F87–F97. doi: 10.1152/ajprenal.90509.2008. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Ward CJ, Harris PC, Torres VE. Cyclic nucleotide signaling in polycystic kidney disease. Kidney Int. 2010;77:129–140. doi: 10.1038/ki.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 42.Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH., 2nd Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 43.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 46.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/S0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 47.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 48.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 49.Huangfu D, Anderson KV. Cilia and hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 51.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 52.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 54.Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 56.Whitfield JF, Chakravarthy BR. The neuronal primary cilium: driver of neurogenesis and memory formation in the hippocampal dentate gyrus? Cell Signal. 2009;21:1351–1355. doi: 10.1016/j.cellsig.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han YG, Alvarez-Buylla A Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20:58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjostrand FS. The ultrastructure of the innersegments of the retinal rods of the guinea pig eye as revealed by electron microscopy. J Cell Physiol. 1953;42:45–70. doi: 10.1002/jcp.1030420104. [DOI] [PubMed] [Google Scholar]
- 61.Palay SL. The fine structure of secretory neurons in the preoptic nucleus of the goldish (Carassius auratus) Anat Rec. 1960;138:417–443. doi: 10.1002/ar.1091380404. [DOI] [PubMed] [Google Scholar]
- 62.Dahl HA. Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikrosk Anat. 1963;60:369–386. doi: 10.1007/BF00336612. [DOI] [PubMed] [Google Scholar]
- 63.Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/S0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 64.Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. doi: 10.1016/S0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- 65.Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Verge D. Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology. 1999;21:68S–76S. doi: 10.1016/S0893-133X(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 66.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bishop GA, Berbari NF, Lewis JS, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs JL, Schwark HD. Neuronal primary cilia: a review. Cell Biol Int. 2004;28:111–118. doi: 10.1016/j.cellbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Whitfield JF. The neuronal primary cilium—an extrasynaptic signaling device. Cell Signal. 2004;16:763–767. doi: 10.1016/j.cellsig.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20:255–264. doi: 10.1385/ENDO:20:3:255. [DOI] [PubMed] [Google Scholar]
- 72.Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 73.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 74.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286:75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Schulz S, Handel M, Schreff M, Schmidt H, Hollt V. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J Physiol Paris. 2000;94:259–264. doi: 10.1016/S0928-4257(00)00212-6. [DOI] [PubMed] [Google Scholar]
- 76.Miyoshi K, Onishi K, Asanuma M, Miyazaki I, Diaz-Corrales FJ, Ogawa N. Embryonic expression of pericentrin suggests universal roles in ciliogenesis. Dev Genes Evol. 2006;216:537–542. doi: 10.1007/s00427-006-0065-8. [DOI] [PubMed] [Google Scholar]
- 77.Berbari NF, Bishop GA, Askwith CC, Lewis JS, Mykytyn K. Hippocampal neurons possess primary cilia in culture. J Neurosci Res. 2007;85:1095–1100. doi: 10.1002/jnr.21209. [DOI] [PubMed] [Google Scholar]
- 78.Stanic D, Malmgren H, He H, Scott L, Aperia A, Hokfelt T. Developmental changes in frequency of the ciliary somatostatin receptor 3 protein. Brain Res. 2009;1249:101–112. doi: 10.1016/j.brainres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 79.Einstein EB, Patterson CA, Hon BJ, Regan KA, Reddi J, Melnikoff DE, Mateer MJ, Schulz S, Johnson BN, Tallent MK. Somatostatin signaling in neuronal cilia is critical for object recognition memory. J Neurosci. 2010;30:4306–4314. doi: 10.1523/JNEUROSCI.5295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Svenningsson P, Tzavara ET, Qi H, Carruthers R, Witkin JM, Nomikos GG, Greengard P. Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J Neurosci. 2007;27:4201–4209. doi: 10.1523/JNEUROSCI.3110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heal DJ, Smith SL, Fisas A, Codony X, Buschmann H. Selective 5-HT6 receptor ligands: progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol Ther. 2008;117:207–231. doi: 10.1016/j.pharmthera.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 82.Fisas A, Codony X, Romero G, Dordal A, Giraldo J, Merce R, Holenz J, Vrang N, Sorensen RV, Heal D, Buschmann H, Pauwels PJ. Chronic 5-HT6 receptor modulation by E-6837 induces hypophagia and sustained weight loss in diet-induced obese rats. Br J Pharmacol. 2006;148:973–983. doi: 10.1038/sj.bjp.0706807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–333. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 84.Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- 85.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 86.Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 87.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 89.Chen Y, Hu C, Hsu CK, Zhang Q, Bi C, Asnicar M, Hsiung HM, Fox N, Slieker LJ, Yang DD, Heiman ML, Shi Y. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology. 2002;143:2469–2477. doi: 10.1210/en.143.7.2469. [DOI] [PubMed] [Google Scholar]
- 90.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol. 2000;279:F400–F416. doi: 10.1152/ajprenal.2000.279.3.F400. [DOI] [PubMed] [Google Scholar]
- 92.Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- 93.Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/S0896-6273(00)00060-X. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z, Li V, Chan GC, Phan T, Nudelman AS, Xia Z, Storm DR. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One. 2009;4:e6979. doi: 10.1371/journal.pone.0006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr, Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayer U, Kuller A, Daiber PC, Neudorf I, Warnken U, Schnolzer M, Frings S, Mohrlen F. The proteome of rat olfactory sensory cilia. Proteomics. 2009;9:322–334. doi: 10.1002/pmic.200800149. [DOI] [PubMed] [Google Scholar]
- 97.Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–G509. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- 98.Mitchell KA, Gallagher BC, Szabo G, Otero Ade S. NDP kinase moves into developing primary cilia. Cell Motil Cytoskeleton. 2004;59:62–73. doi: 10.1002/cm.20025. [DOI] [PubMed] [Google Scholar]
- 99.Narita K, Kawate T, Kakinuma N, Takeda S. Multiple primary cilia modulate the fluid transcytosis in choroid plexus epithelium. Traffic. 2010;11:287–301. doi: 10.1111/j.1600-0854.2009.01016.x. [DOI] [PubMed] [Google Scholar]
- 100.Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem. 2005;280:34718–34722. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- 101.Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/S0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 102.Kleene SJ, Gesteland RC. Transmembrane currents in frog olfactory cilia. J Membr Biol. 1991;120:75–81. doi: 10.1007/BF01868593. [DOI] [PubMed] [Google Scholar]
- 103.Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The oak ridge polycystic kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- 104.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]