Abstract
Differences in micronutrient status are reported to contribute to racial and ethnic differences in chronic diseases. Diseases related to vitamin K are reported to differ by race and ethnicity, but it is unclear if circulating vitamin K concentrations similarly differ. We examined racial and ethnic differences in serum phylloquionone (K1) in the Multiethnic Study of Atherosclerosis (MESA) (mean ± SD age = 62 ± 10 y; 52% female; 262 white, 180 African American, 169 Hispanic, 93 Chinese American). Overall, 25% had serum K1 <0.1 nmol/L (the lower limit of detection). The prevalence of low serum K1 was 4% in Chinese Americans compared with 24% of whites, 29% of African Americans, and 33% of Hispanics. Compared with whites, Chinese Americans were significantly less likely to have serum K1 <0.1 nmol/L [OR (95% CI): 0.23 (0.09–0.23), adjusted for serum TG, K1 intake, age, sex, BMI, smoking, total cholesterol, site, season, and lipid-lowering medication use]. African Americans and Hispanics had similar odds to whites for having serum K1 <0.1 nmol/L [OR(95% CI): 1.30 (0.79–2.15) and 1.19 (0.66–2.15), respectively; fully adjusted]. In participants with detectable concentrations (n = 523), (natural log) serum K1 was higher in the Chinese Americans compared with whites, African Americans, and Hispanics (geometric mean ± SEM = 2.2 ± 0.1 nmol/L vs. 1.2 ± 0.1 nmol/L, 1.5 ± 0.1 nmol/L, and 1.1 ± 0.1 nmol/L, respectively, adjusted for serum TG, K1 intake, and additional covariates; all P < 0.001). These findings suggest circulating K1 differs by race and ethnicity in U.S. adults, especially among those of Chinese American descent, which merits consideration in the design and interpretation of future population-based and clinical studies of vitamin K and related diseases.
Introduction
Accumulating evidence indicates that low vitamin K status may be a risk factor for chronic diseases, such as cardiovascular disease (CVD)7, bone loss, and osteoarthritis, which are reported to vary by race and ethnicity (1–5). Racial and ethnic differences in nutrient status are reported to account for some of the racial and ethnic variation in other health outcomes (6–8), so it is possible that racial and ethnic differences in vitamin K status may partially account for the observed racial and ethnic differences in health outcomes related to vitamin K.
The primary form of vitamin K in the Western diet is phylloquionone (K1), which is found in green leafy vegetables and vegetable oils, and dietary K1 intake was reported to differ among racial and ethnic groups in NHANES (1999–2004) (9). Two small studies with modest numbers of participants suggested that biomarkers of vitamin K status differed among ethnicities (10, 11); however, these studies collected samples from individuals of different ethnic backgrounds residing in different countries. The majority of population-based studies of vitamin K status have been limited to Caucasians, a group in whom the prevalence of low vitamin K status is reported to be 25–33% (12–16). We used data from the Multiethnic Study of Atherosclerosis (MESA), a large, population-based cohort comprised of white, African American, Chinese American, and Hispanic adults living in the US to determine if concentrations of serum K1 [the predominate form of vitamin K in circulation (17)] differed by race and ethnicity. Understanding the vitamin K status of racial and ethnic groups in the population is important for the design and interpretation of targeted interventions focusing on health outcomes related to vitamin K.
Methods
The MESA study is a large, ongoing, observational study examining the prevalence and determinants of subclinical CVD in a multi-ethnic cohort that began in 2000–2002. The MESA cohort (n = 6814) was recruited from 6 U.S. communities, including: Forsyth County, NC; northern Manhattan and the Bronx, NY; Baltimore County, MD; St. Paul, MN; Chicago and Maywood, IL; and Los Angeles County, CA. The cohort is 38% non-Hispanic white, 28% African American, 22% Hispanic, and 12% Chinese American, all of whom were free of clinically diagnosed CVD at baseline. The study design and methods of MESA have been described in detail (18). MESA was approved the Institutional Review Boards at all 6 study sites (Wake Forest University, Columbia University, John Hopkins University, University of Minnesota, Northwestern University, University of California at Los Angeles) and this substudy was additionally approved by the Institutional Review Boards at Wake Forest University and Tufts University. All participants gave written informed consent.
A randomly chosen subgroup of 780 MESA participants who were not taking warfarin (a vitamin K antagonist) had serum K1 measured from samples obtained at the baseline visit. These participants did not differ from the 6034 participants who did not have serum K1 measured in age, sex, race and ethnicity, BMI, TG, total cholesterol, K1 intake, education, income, season (of blood draw), study site, and smoking status (all P > 0.14). Among the participants whose serum K1 was measured, information on dietary intake and demographic and clinical covariates was available for 704 and they participated in the present analysis [262 white, 180 African American, 169 Hispanic, and 93 Chinese American]. The 76 participants excluded due to missing data more likely to smoke (P = 0.001) and more likely to be African American (n = 55; P < 0.001) compared with the 704 who were included. Otherwise, the 2 groups were similar.
Serum K1.
At the baseline MESA examination, fasting serum was collected and frozen at −70°C. Serum K1 was measured in thawed samples using reversed-phase HPLC with post-column, solid phase chemical reduction of K1 to its hydroquinone followed by fluorometric detection (19) at the Vitamin K Laboratory at the USDA Human Nutrition Research Center on Aging at Tufts University, where this assay was developed. This laboratory currently participates in the vitamin K external quality assurance scheme (20). The lower limit of detection (LLD) for circulating K1 using this assay with the available sample volume was 0.1 nmol/L; samples with K1 concentrations <0.1 nmol/L were entered as 0.05 nmol/L (19, 21, 22). Low and high control specimens had average values of 0.56 and 3.15 nmol/L, with a total (intra- and inter-assay) percent CV of 15.2 and 10.9%, respectively (21).
Race or ethnicity were self-reported as white, Chinese American, African American, or Hispanic.
Dietary intake.
At the baseline visit, participants’ dietary intake over the previous 12 mo was assessed using a modified 120-item Block FFQ. The MESA FFQ was patterned after the FFQ used in the Insulin Resistance Atherosclerosis Study, which was validated in non-Hispanic white, non-Hispanic black, and Hispanic individuals (23). It was modified to also include unique Chinese foods and culinary practices to accommodate the MESA participant population, as previously described (24, 25). The Nutrition Data Systems for Research database (Nutrition Coordinating Center University of Minnesota) was used to estimate dietary K1 intake by multiplying the number of servings of a particular food times the K1 content per serving size of that food (26). A similar strategy was used to estimate total energy intake.
Covariates.
Height and weight were measured without shoes and BMI was calculated as weight in kg/(height in meters)2. Total cholesterol and TG were measured from fasting serum samples as described (1). Medical history, smoking history, and demographic information were collected using standardized questionnaires.
Statistical analyses.
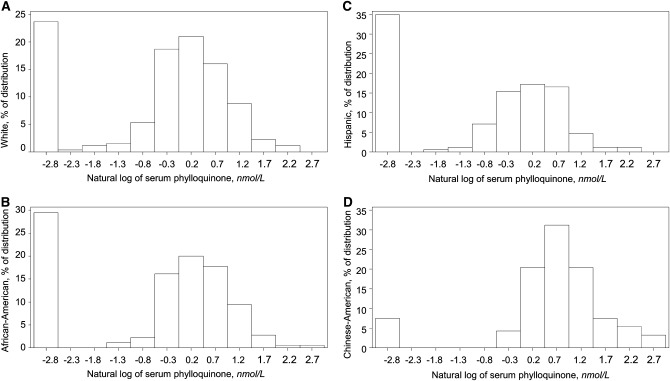
Racial and ethnic differences in participant characteristics were determined using ANOVA (continuous outcomes) or chi-square analyses (categorical outcomes). Education was categorized as less than high school, high school graduate, or college graduate. To reduce skewness, serum K1, K1 intake, and TG were transformed by taking the natural-log (ln). Because 25% of participants had serum K1 <0.1 nmol/L [the LLD for this assay (19)], the (ln)transformed distributions were bimodal (Fig. 1). Therefore, our analytical approach was 2-fold (1): logistic regression was used to determine the OR (95% 95% CI) for having serum K1 <0.1 nmol/L (below the LLD) in African Americans, Hispanics, and Chinese Americans compared with whites. Whites were chosen as the reference group, because they provided the largest sample size and therefore the mean outcome measures for that group were estimated with greater precision (2): we determined the OR(95% CI) for having serum K1 lower than the median among participants with detectable K1 (1.4 nmol/L) in African Americans, Hispanics, and Chinese Americans compared with whites. Racial and ethnic differences in continuous measures of serum K1 were also examined using general linear models. Analysis of serum K1 was adjusted for age, sex, BMI, smoking status, total cholesterol, education, season, study site, and K1 intake. Because serum K1 was natural log-transformed, geometric means were calculated for each racial and ethnic group by back-transforming the unadjusted and adjusted means. SE of the geometric means were estimated using the delta method {SE geometric mean = square root [(geometric mean)2 × (SEM of ln-transformed outcome)2]}. The correlation between (ln) serum K1 and (ln) K1 intakes was calculated using Spearman correlation coefficients adjusted for TG and energy intake. Because McKeown et al. (27) reported the linear relationship between circulating K1 and K1 intake reached a plateau at intakes of 200 μg/d, we subsequently determined the correlations among participants with detectable serum K1 who reported intakes of ≤200 μg/d. Stepwise logistic and linear models were used to determine the percent variability of circulating K1 that is attributable to race and ethnicity, with a P < 0.15 specified for model inclusion. The following variables, chosen based on biological plausibility and a similar analysis of circulating K1 in the Framingham Offspring Study (28), were entered: TG, race (Chinese American, Hispanic, African American), total cholesterol, BMI, age, sex, education, lipid-lowering medication use (yes/no), current smoker (yes/no), study site, and season. All analyses were carried out using SAS v 9.1 and were considered significant when P < 0.05.
FIGURE 1.
Serum K1 concentration distributions in white (A) (n = 262) African American (B) (n = 480), Hispanic (C) (n = 169), and Chinese American (D) (n = 93) adults in the MESA. K1, phylloquinone; MESA, Multiethnic Study of Atherosclerosis.
Results
The overall age of participants was 62 ± 10 y (mean ± SD). The mean BMI among Chinese Americans was in the normal range by U.S. standards, whereas the mean BMI among whites, African Americans, and Hispanics would be considered overweight. Hispanics had the highest mean TG and total cholesterol concentrations compared with the other 3 racial and ethnic groups. Education differed significantly according to race and ethnicity, with over one-half of white participants reporting graduating from college, which was higher than the other groups (Table 1).
TABLE 1.
Participant characteristics1
| White | African American | Hispanic | Chinese American | Overall P value | |
| n | 262 | 180 | 169 | 93 | |
| Female,2 n (%) | 143 (55) | 95 (53) | 83 (49) | 51 (55) | 0.70 |
| Education,2 n (%) | |||||
| <High school | 11 (4) | 24 (12) | 81 (48) | 22 (24) | <0.001 |
| High school graduate | 120 (46) | 88 (49) | 72 (43) | 34 (37) | |
| College graduate | 131 (50) | 72 (43) | 16 (9) | 37 (40) | |
| Current smokers,2 n (%) | 31 (12) | 8 (9) | 24 (15) | 29 (17) | 0.21 |
| Age,3 y | 62 ± 10 | 63 ± 10 | 60 ± 10 | 62 ± 10 | 0.03 |
| BMI,3 kg/m2 | 27.8 ± 5.0b | 29.9 ± 5.3a | 30.0 ± 5.0a | 24.1 ± 3.7c | <0.001 |
| Serum TG,3ndash5 mmol/L | 1.3 ± 0.1b | 1.0 ± 0.1c | 1.6 ± 0.1a | 1.3 ± 0.1b | <0.001 |
| Serum total cholesterol,3,6 mmol/L | 5.0 ± 0.1a | 4.8 ± 0.1b | 5.1 ± 0.1a | 4.9 ± 0.1a,b | 0.03 |
| Vitamin K measures | |||||
| K1 intake,3,4 μg/d | 98 ± 5a | 111 ± 7a | 77 ± 5b | 100 ± 8a,b | <0.001 |
| Energy-adjusted7 | 99 ± 4a | 103 ± 4a | 73 ± 6b | 119 ± 5a | <0.001 |
| Fully adjusted8 | 96 ± 4a | 97 ± 4a | 73 ± 5b | 97 ± 6a | <0.001 |
| n9 | 200 | 127 | 110 | 86 | |
| Serum K1,2,3 nmol/L | 1.3 ± 0.1b,c | 1.5 ± 0.1b | 1.2 ± 0.1c | 2.4 ± 0.2a | <0.001 |
| TG-adjusted10 | 1.2 ± 0.1c | 1.6 ± 0.1b | 1.1 ± 0.1c | 2.3 ± 0.2a | <0.001 |
| Fully adjusted11 | 1.2 ± 0.1b | 1.5 ± 0.1b | 1.1 ± 0.1b | 2.2 ± 0.2a | <0.001 |
Values are means ± SD unless indicated otherwise. Values in a row with superscripts without a common letter differ, < 0.05 (Tukey's Honestly Significant Difference test). K1, phylloquinone.
value based on chi-square analysis.
value is for overall F-statistic, based on unadjusted ANOVA.
The outcome was transformed using natural log; means presented are geometric means ± SEM.
To convert mmol/L to mg/dL, multiply by 88.57.
To convert mmol/L to mg/dL, multiply by 38.67.
value is for overall F-statistic for race and ethnicity based on ANCOVA using natural log-transformed outcome, adjusted for kcal/d; means presented are geometric, least-square means ± SEM.
Further adjusted for education, age, sex, BMI, current smoker, season, and study site.
Analysis limited to participants with detectable serum K1 (≥0.1 nmol/L, = 523).
value is for overall F-statistic for race and ethnicity based on ANCOVA using natural log-transformed outcome, adjusted for TG; means presented are geometric, least-square means ± SEM.
Further adjusted for K1 intake, education, age, sex, BMI, total cholesterol, lipid-lowering medication use, current smoker, season, and study site.
The prevalence of serum K1 <0.1 nmol/L was 24% in whites, 29% in African Americans, 33% in Hispanics, and 4% in Chinese American (Fig. 1). In fully adjusted analysis, Chinese American had 0.23-fold lower odds of having serum K1 <0.1 nmol/L than whites, whereas the odds for African Americans and Hispanics did not differ from whites (Table 2). Among participants with detectable serum K1, Chinese American and African Americans were significantly less likely than whites to have serum K1 below the median level (1.4 nmol/L) in adjusted models (Table 2).
TABLE 2.
OR (95% CI) for low serum K1 in African American, Hispanic, and Chinese American participants compared with white participants of the MESA1
| White | African American | Hispanic | Chinese American | P value2 | |
| Among all participants | |||||
| n | 262 | 180 | 169 | 93 | |
| Serum K1 <0.1 nmol/L (LLD3) | |||||
| n (%) | 62 (24) | 53 (29) | 59 (33) | 7 (4) | |
| Unadjusted | 1.00 | 1.35 (0.88–2.07) | 1.73 (1.13–2.65) | 0.26 (0.12–0.60) | <0.001 |
| Model 24 | 1.00 | 1.17 (0.75–1.82) | 1.95 (1.25–3.04) | 0.26 (0.11–0.59) | <0.001 |
| Model 35 | 1.00 | 1.30 (0.79–2.15) | 1.19 (0.66–2.15) | 0.23 (0.09–0.23) | 0.005 |
| Among those with detectable serum K1 | |||||
| n | 200 | 127 | 110 | 86 | |
| Serum K1 <1.4 nmol/L (median) | |||||
| n (%) | 111 (56) | 57 (45) | 63 (57) | 16 (19) | |
| Unadjusted | 1.00 | 0.81 (0.55–1.20) | 1.34 (0.88–2.04) | 0.17 (0.10–0.29) | <0.001 |
| Model 24 | 1.00 | 0.48 (0.30–0.77) | 1.32 (0.79–2.20) | 0.16 (0.09–0.30) | <0.001 |
| Model 35 | 1.00 | 0.48 (0.29–0.80) | 1.05 (0.53–2.06) | 0.14 (0.07–0.29) | <0.001 |
K1, phylloquinone; LLD, lower limit of detection; MESA, Multiethnic Study of Atherosclerosis.
Based on logistic regression chi-square statistic.
For serum K1 using this HPLC assay (19).
Adjusted for serum TG and K1 intake.
Adjusted for serum TG, K1 intake, education, age, sex, BMI, smoking status, total cholesterol, site, season, and lipid-lowering medication use.
Energy-adjusted K1 intakes were significantly lower among Hispanics compared with the 3 other racial and ethnic groups (Table 1) and these differences persisted following adjustment for education, age, sex, BMI, current smoker, season, and study site. Among participants with detectable serum K1, Chinese Americans had higher concentrations compared with whites, African Americans, and Hispanics. Serum K1 remained significantly higher among Chinese Americans compared with whites, African Americans, and Hispanics after adjustment for TG (Table 1). The racial and ethnic differences were similar after further adjustment for K1 intake, education, age, sex, BMI, cholesterol, lipid-lowering medication use, current smoking, season, and study site.
The energy-adjusted K1 intake of participants with serum K1 <0.1 nmol/L was 80 ± 4 μg/d (geometric mean ± SEM), less than among participants with detectable serum K1, 102 ± 3 μg/d (P < 0.01). Overall among participants with detectable serum K1, (ln) serum K1 positively correlated with (ln) K1 intake (r = 0.20; P < 0.001 adjusted for TG and energy intake). However, the correlation between serum K1 and K1 intake was not significant among African Americans (r = 0.04; P = 0.64), Hispanics (r = 0.14; P = 0.13), and Chinese Americans (r = 0.01; P = 0.90) but was significant in whites (r = 0.21; P = 0.003). When participants who reported dietary K1 intake >200 μg/d were excluded (n = 104), the correlations between (ln) serum K1 and (ln) K1 intake were not appreciably changed (white: n = 158, r = 0.23, P = 0.004; African American: n = 92, r = 0.03, P = 0.78; Hispanic: n = 96, r = 0.18, P = 0.07; Chinese American: n = 73, r = 0.14, P = 0.23).
Using stepwise linear regression of participants with detectable serum K1, Chinese Americans and African American race and ethnicity and TG were significant predictors of serum K1 (Table 3), which along with K1 intake and BMI explained 21% of the variability in serum K1 in this group. When the outcome of having serum K1 less than the LLD (considered dichotomously) was similarly analyzed, only 10% of the variability in the outcome was explained by the included predictors. In this analysis, Chinese American ethnicity, TG, K1 intake, total cholesterol, lipid-lowering medication use, season, and study site were identified as significant predictors of serum K1.
TABLE 3.
Predictors of serum K1 in the MESA1
| (ln) Serum K12 |
Serum K1 <0.1 nmol/L (yes/no)34 |
|||||
| Outcome | Parameter estimate | P value | Variability, % | OR (95% CI) | P-value | Variability, % |
| n | 523 | 704 | ||||
| Race (compared with whites) | ||||||
| Chinese American | 0.37 | <0.001 | 9.4 | 0.23 (0.09–0.53) | <0.001 | 3.2 |
| Hispanic | — | — | ||||
| African American | 0.19 | <0.001 | 2.7 | — | ||
| (ln) serum TG, mmol/L | 0.29 | <0.001 | 7.4 | 0.51 (0.34–0.74) | <0.001 | 1.6 |
| BMI, kg/m2 | −0.01 | 0.01 | 1.0 | — | ||
| (ln) K1 intake, μg/d | 0.04 | 0.11 | 0.4 | 0.74 (0.59–0.94) | 0.01 | 0.8 |
| Serum total cholesterol, mmol/L | — | 0.99 (0.98–1.00) | 0.02 | 0.7 | ||
| Lipid-lowering medication use | — | 0.46 (0.26–0.79) | 0.005 | 0.7 | ||
| Season (compared with January–March) | — | 1.2 | ||||
| April–June | 0.58 (0.37–0.93) | 0.02 | ||||
| July–September | 0.49 (0.29–0.83) | 0.008 | ||||
| October–December | 0.58 (0.35–0.98) | 0.04 | ||||
| Site (compared with St. Paul, MN) | — | 2.7 | ||||
| Los Angeles, CA | 0.53 (0.29–0.97) | 0.04 | ||||
| Forsyth County, NC | 0.44 (0.24–0.81) | 0.01 | ||||
| New York, NY | 0.79 (0.44–1.43) | 0.43 | ||||
| Chicago, IL | 0.36 (0.19–0.69) | 0.002 | ||||
| Baltimore County, MD | 0.35 (0.18–0.67) | 0.001 | ||||
| Total variability explained by model, % | 20.9 | 10.8 | ||||
K1, phylloquinone; LLD, lower limit of detection; MESA, Multiethnic Study of Atherosclerosis.
Limited to participants with detectable serum K1 and based on stepwise linear regression, the following exposures were entered: serum TG, race and ethnicity (Chinese American, Hispanic, African American), total cholesterol, BMI, age, sex, education (category), lipid-lowering medication use, current smoker, season, and study site; entry < 0.15. Dash, did not meet P < 0.15 entry criteria.
Based on stepwise logistic regression, the following exposures were entered: TG, race and ethnicity (Chinese American, Hispanic, African American), total cholesterol, BMI, age, sex, education (category), lipid-lowering medication use, current smoker, season, and study site; entry < 0.15.
LLD for serum K1 using HPLC assay is 0.1 nmol/L (19).
Discussion
The overall distributions of serum K1 were right-skewed and did not normalize following a log transformation, unlike other nutrient biomarkers, which are normalized by such transformation (29, 30). Upon inspection, the basis appeared to be the strong bimodal distribution of serum K1 and the large proportion of persons (25%) with concentrations <0.1 nmol/L (the LLD for this assay), which has been used to measure circulating K1 in other population-based studies (13, 21). Although participants with <0.1 nmol/L circulating K1 reported consuming slightly less dietary K1 (80 vs. 103 μg/d), it is unlikely that this observed difference in intake fully accounted for having circulating K1 <0.1 nmol/L for 2 reasons. Outcomes of dietary depletion-repletion studies found step-wise repletion using up to 450 μg/d K1 did not increase circulating K1 concentrations to within the normal range for adults (31). Results of our stepwise models found self-reported K1 intake explained 0.8% of the variability of having <0.1 nmol/L of serum K1. Overall, we only accounted for 10% of the variability in having serum K1 <0.1 nmol/L, suggesting much of the variability in circulating K1 remains to be explained. Because evidence from warfarin studies suggests genetic factors may be important (32), future research using multiple cohorts (to obtain sufficient statistical power to detect genetic associations) may clarify the genetic contribution to vitamin K status, hence its role in chronic diseases.
Chinese American participants were less likely to have serum K1 concentrations lower than the LLD. Similar results were seen among participants with detectable serum K1; Chinese American participants were significantly less likely to have concentrations less than the median (1.4 nmol/L) compared with the other racial and ethnic groups. Likewise, Chinese American participants had higher serum K1 when analyzed as a continuous outcome in those with detectable concentrations, and African Americans had higher concentrations than whites or Hispanics. The higher circulating K1 concentration we observed in Chinese Americans is consistent with 2 previous small studies that compared circulating K1 across racial and ethnic groups from different countries. Postmenopausal Chinese women had higher plasma K1 compared with Caucasian women from Great Britain and Gambian women (10), and older adults from China had higher plasma K1 compared with those from Great Britain (11). One possible explanation for this difference was a 2-fold higher K1 intake among those in China compared with those in Great Britain [geometric mean (95% CI) intakes: 247 (226, 270) vs. 103 (94, 112) μg/d) (11). The self-reported K1 intake among Chinese American participants in MESA was significantly higher than that of Hispanics, but it did not significantly differ from whites or African Americans in MESA, yet the Chinese Americans had higher circulating concentrations compared with all racial and ethnic groups. In addition, our primary analysis included adjustment for intake, together suggesting that differences in self-reported dietary intakes did not entirely explain the differences in circulating K1 concentrations among racial and ethnic groups.
We explored the determinants of serum K1 in MESA following a stepwise approach that included race and ethnicity as a potential predictor, because previous similar analyses were racially homogeneous (28). Race and ethnicity (Chinese American and African American) accounted 3–9% of the variability, suggesting it should be considered in future population-based studies of vitamin K status, especially in cohorts that include persons of Chinese American descent. Of interest, MESA participants from Minnesota were more likely to have low circulating K1 compared with most of the other sites. No other studies to our knowledge have reported on regional differences in serum K1 in the US. Forty-three percent of participants from Minnesota were Hispanic and the remainder were white. When we excluded Minnesota participants (n = 121) from this analysis, Hispanic ethnicity became significantly associated with having serum K1 <0.1 nmol/L [OR(95% CI): 3.72(1.79–5.43)], whereas site no longer met the P < 0.15 model inclusion criteria. It is plausible this regional difference we detected is a reflection of Hispanics and whites having lower serum K1.
Circulating K1 and K1 intake were modestly correlated among white MESA participants but were not correlated within the other racial and ethnic groups. This may be due to one or a combination of the following. The MESA FFQ has been validated for macronutrient intake (22) but not specifically for vitamin K intake. It may lack the sensitivity to quantify K1 intake precisely enough to correlate with serum measures. The correlations between serum measures and intakes differ by race and ethnicity because of dietary pattern differences. Even though the FFQ was modified to include foods more commonly consumed by the racial and ethnic groups in MESA (23, 24), it is plausible the FFQ did not sufficiently query specific foods that contribute to vitamin K status in racial and ethnic groups. Serum K1 reflects intakes over the previous 12 h, whereas the FFQ queries usual intakes over the previous year. Hence, FFQ-estimated intakes may not necessarily be reflected by serum measures from one time point. Racial and ethnic differences in circulating K1 may be related to genetic factors that influence absorption, storage, and/or metabolism of vitamin K. Indirect evidence that vitamin K metabolism differs by race and ethnicity is provided by population-based studies of oral anticoagulant stability in response to the vitamin K antagonist, warfarin. Asian Americans are reported to require a lower warfarin dose to maintain coagulation within therapeutic range, which suggests that the enzyme required for recycling of vitamin K is more sensitive to warfarin disruption. In addition to warfarin sensitivity, disease conditions related to vitamin K differ according to racial and ethnic groups (1–5). For example, Chinese American participants in MESA who had higher serum K1 were found to be at lower risk for coronary calcium progression compared with other racial and ethnic groups (5). It is uncertain if circulating vitamin K concentrations are implicated in racial and ethnic differences in warfarin response and disease conditions for which a role of vitamin K is reported (12, 14–16).
Although biomarker-based estimates are not without shortfalls, they are strengthened by their ability to reflect nutrient absorption and metabolism (which may be affected by nondietary factors) and are not susceptible to the biases inherent to dietary intake questionnaires (33, 34). The intra-individual variability for circulating K1 is high and because serum K1 was measured at one time point, it may not accurately reflect long-term status (35). However, it is an acceptable measure for ranking individuals across a range of levels (27). The concentration of serum K1 that is considered sufficient has not been defined. Although 0.5 nmol/L is reported to be the lower limit of normal (36), it has been suggested that a concentration ≥1.0 nmol/L is associated with lower risk of chronic diseases for which a role for vitamin K is suggested (14). We repeated our analysis using these thresholds for low serum K1 and the results were not substantively changed. Currently, there is no biomarker of vitamin K nutrition that has a demonstrated dose-response risk for a chronic disease other than coagulation time for coagulation abnormalities. Therefore, the dietary requirements for vitamin K, particularly as they relate to extra-hepatic tissues, are not yet established (37). It is plausible that optimal serum K1 concentrations needed to be sufficient for physiological functions of vitamin K differ by race and ethnicity. This would need to be confirmed in larger clinical trials, with measurements of biomarkers of vitamin K function, such as the degree of carboxylation of osteocalcin or other extra-hepatic vitamin K-dependent proteins. Undercarboxylated osteocalcin (considered a functional measure of vitamin K status of bone) is reported to differ between ethnic groups in between-country comparisons (10, 11). Furthermore, other forms of vitamin K exist, such as menaquinone (vitamin K2). Menaquinones 4–10 [which are found in smaller amounts in meats and dairy-based foods (38)] do not appear to have an important role in the typical U.S. diet (17) but may be present in different amounts in diets consumed by certain racial and ethnic groups. Although inclusion of additional vitamin K measures in MESA would have provided further insight into racial and ethnic differences in vitamin K status in the US, K1 is the primary dietary form of vitamin K and circulating K1 is considered a global indicator of vitamin K status. As novel roles for K1 and menaquinones are emerging, examining whether or not different forms of vitamin K vary by race and ethnicity merits future study. The correlation between circulating K1 and K1 intake among whites in MESA was modest but similar to what has been reported using a single blood draw and adjusted for TG in other Caucasian adults (28, 35), suggesting it may be a suitable for ranking white adults according to K1 intake. The FFQ appeared to be less accurate in estimating K1 across other racial and ethnic groups. However, our primary aim was not to describe racial and ethnic differences in K1 intake and we acknowledge that vitamin K intake may have accounted for more of the variability in serum K1 if a more precise dietary measure was available. Dietary assessment tools that estimate vitamin K intake across different ethnic groups need to be developed and validated. Compared with other population-based studies of nutrient biomarkers (39–43), our sample size was modest. There were fewer Chinese American participants compared with the other groups and a disproportionate number of participants excluded due to incomplete data were African American.
This is the first report to our knowledge that the circulating K1 concentrations differ by race and ethnicity among adults living in the United States, especially among persons of Chinese American descent. Racial and ethnic differences in serum K1 persisted after adjustment for K1 intake, suggesting the differences we observed were not entirely dependent on dietary intakes. As novel roles for vitamin K in health and disease are emerging (12), the racial and ethnic differences in serum K1 we found may have implications for the design and interpretation of intervention studies focused on health-outcomes related to vitamin K. The extent to which the differences in circulating K1 influence racial and ethnic differences in vitamin K-related health outcomes merits future investigation.
Acknowledgments
M.K.S. designed the study, performed the statistical analyses, and drafted the manuscript; S.L.B., J.A.N., G.L.B., H.C., and S.B.K. contributed to the design of the analyses, the interpretation of the data, and writing of the manuscript; and SLB was also responsible for laboratory analyses. All authors read and approved the final manuscript.
Footnotes
Supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by the National Institute of Aging (P30AG021332-08), the AHA (09CRP2070013), the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), and the USDA, Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707. J.A.N. is supported by a K01 from the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (5K01DK082729).
Author disclosures: M. K. Shea, S. L. Booth, J. A. Nettleton, G. L. Burke, H. Chen, and S. B. Kritchevsky, no conflicts of interest.
Abbreviations used: CVD, cardiovascular disease; K1, phylloquinone; LLD, lower limit of detection; MESA, Multiethnic Study of Atherosclerosis.
Literature Cited
- 1.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–20 [DOI] [PubMed] [Google Scholar]
- 2.Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, Beck TJ, Ensrud KE. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53:183–9 [DOI] [PubMed] [Google Scholar]
- 3.Nam HS, Shin MH, Zmuda JM, Leung PC, Barrett-Connor E, Orwoll ES, Cauley JA. Race/ethnic differences in bone mineral densities in older men. Osteoporos Int. 2010;21:2115–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, Fang F, Schwartz TA, Abbate LM, Callahan LF, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–80 [PubMed] [Google Scholar]
- 5.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722–30 [DOI] [PubMed] [Google Scholar]
- 6.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8 [DOI] [PubMed] [Google Scholar]
- 7.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–9 [DOI] [PubMed] [Google Scholar]
- 8.Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. Am J Clin Nutr. 2010;92:1344–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Y, Jackson RT. Dietary phylloquinone intakes and metabolic syndrome in US young adults. J Am Coll Nutr. 2009;28:369–79 [DOI] [PubMed] [Google Scholar]
- 10.Beavan SR, Prentice A, Stirling DM, Dibba B, Yan L, Harrington DJ, Shearer MJ. Ethnic differences in osteocalcin gamma-carboxylation, plasma phylloquinone (vitamin K1) and apolipoprotein E genotype. Eur J Clin Nutr. 2005;59:72–81 [DOI] [PubMed] [Google Scholar]
- 11.Yan L, Zhou B, Greenberg D, Wang L, Nigdikar S, Prynne C, Prentice A. Vitamin K status of older individuals in northern China is superior to that of older individuals in the UK. Br J Nutr. 2004;92:939–45 [DOI] [PubMed] [Google Scholar]
- 12.McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr. 2009;90:889–907 [DOI] [PubMed] [Google Scholar]
- 13.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neogi T, Booth SL, Zhang YQ, Jacques PF, Terkeltaub R, Aliabadi P, Felson DT. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006;54:1255–61 [DOI] [PubMed] [Google Scholar]
- 15.Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203:489–93 [DOI] [PubMed] [Google Scholar]
- 16.Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB, Sr, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Kathiresan S, Keaney JF, Jr, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;128:785–8 [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81 [DOI] [PubMed] [Google Scholar]
- 19.Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–21 [DOI] [PubMed] [Google Scholar]
- 20.Card DJ, Shearer MJ, Schurgers LJ, Harrington DJ. The external quality assurance of phylloquinone (vitamin K(1)) analysis in human serum. Biomed Chromatogr. 2009;23:1276–82 [DOI] [PubMed] [Google Scholar]
- 21.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904–9 [DOI] [PubMed] [Google Scholar]
- 22.McLean RR, Booth SL, Kiel DP, Broe KE, Gagnon DR, Tucker KL, Cupples LA, Hannan MT. Association of dietary and biochemical measures of vitamin K with quantitative ultrasound of the heel in men and women. Osteoporos Int. 2006;17:600–7 [DOI] [PubMed] [Google Scholar]
- 23.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol. 1999;9:314–24 [DOI] [PubMed] [Google Scholar]
- 24.Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi-Ethnic Study of Atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr. 2009;102:1220–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DR., Jr Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2006;83:1369–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKeown NM, Rasmussen HM, Charnley JM, Wood RJ, Booth SL. Accuracy of phylloquinone (vitamin K-1) data in 2 nutrient databases as determined by direct laboratory analysis of diets. J Am Diet Assoc. 2000;100:1201–4 [DOI] [PubMed] [Google Scholar]
- 27.McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PW, Booth SL. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr. 2002;132:1329–34 [DOI] [PubMed] [Google Scholar]
- 28.Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D'Agostino RB, Sr, Ordovas JM, O'Donnell CJ, Dawson-Hughes B, Vasan RS, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shea MK, Houston DK, Tooze JA, Davis CC, Johnson MA, Hausman DB, Cauley JA, Bauer DC, Tylavsky F, Harris TB, et al. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in black and white older adults: the health, aging and body composition study. J Am Geriatr Soc. 2011;59:1165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel S, Contois JH, Tucker KL, Wilson PW, Schaefer EJ, Lammi-Keefe CJ. Plasma retinol and plasma and lipoprotein tocopherol and carotenoid concentrations in healthy elderly participants of the Framingham Heart Study. Am J Clin Nutr. 1997;66:950–8 [DOI] [PubMed] [Google Scholar]
- 31.Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133:2565–9 [DOI] [PubMed] [Google Scholar]
- 32.Limdi NA, Veenstra DL. Warfarin pharmacogenetics. Pharmacotherapy. 2008;28:1084–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125:507–25 [DOI] [PubMed] [Google Scholar]
- 34.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr. 2003;133 Suppl 3:875S–80S [DOI] [PubMed] [Google Scholar]
- 35.Booth SL, Tucker KL, McKeown NM, Davidson KW, Dallal GE, Sadowski JA. Relationships between dietary intakes and fasting plasma concentrations of fat-soluble vitamins in humans. J Nutr. 1997;127:587–92 [DOI] [PubMed] [Google Scholar]
- 36.Sadowski JA, Hood SJ, Dallal GE, Garry PJ. Phylloquinone in plasma from elderly and young adults: factors influencing its concentration. Am J Clin Nutr. 1989;50:100–8 [DOI] [PubMed] [Google Scholar]
- 37.Food and Nutrition Board IoM. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001 [PubMed] [Google Scholar]
- 38.Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. Vitamin K contents of meat, dairy, and fast food in the U.S. diet. J Agric Food Chem. 2006;54:463–7 [DOI] [PubMed] [Google Scholar]
- 39.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–92 [DOI] [PubMed] [Google Scholar]
- 41.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(4 Suppl 5):S5–101 [PubMed] [Google Scholar]
- 42.Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr. 2006;84:375–83 [DOI] [PubMed] [Google Scholar]
- 43.Kant AK, Graubard BI. Ethnicity is an independent correlate of biomarkers of micronutrient intake and status in American adults. J Nutr. 2007;137:2456–63 [DOI] [PubMed] [Google Scholar]