Abstract
Consumption of sugar-sweetened beverages (SSB) has been associated with metabolic syndrome (MetS); however, studies conducted on Hispanic adults are scarce. To determine the association between beverages consumed by Hispanic adults and MetS and its components, data were analyzed in 1872 Costa Rican adults who served as controls of a population-based, case-control study of coronary heart disease. Multivariate-adjusted means were calculated for components of MetS by servings (never, ≤1/wk; 2–6/wk, ≥1/d) of 2 traditional fruit-based beverages (“fresco” and freshly-squeezed homemade fruit juice, separately) and 2 SSB (instant drinks and regular sodas, separately and combined). The prevalence ratio (PR) of MetS was calculated for each beverage and the OR was calculated by substituting one serving of homemade fruit juice or water for one of SSB. Significant positive trends were observed for increasing servings of instant drinks with plasma TG and waist circumference and for regular soda with waist circumference (all P-trend < 0.001). Increasing servings of homemade fruit juice were positively associated with HDL cholesterol (P-trend = 0.033). Consuming ≥1 serving/d of instant drinks was associated with a higher PR of MetS [1.42 (95% CI: 1.11, 1.83)] compared with no consumption. Substituting one serving of homemade fruit juice for instant drink was associated with 29% (95% CI: 7, 47%) lower odds of MetS and for regular soda with 30% (95% CI: 1, 50%) lower odds. Substituting water for combined SSB was marginally significant (OR = 0.86 (95% CI: 0.74, 1.00). In conclusion, reducing the consumption of SSB and substituting them with homemade fruit juices in moderation may be a culturally appropriate approach to lower MetS among Hispanic adults.
Introduction
Metabolic syndrome (MetS)6 is a cluster of physiologically dysregulated cardiometabolic parameters, which substantially increase the risk of type 2 diabetes and cardiovascular disease (1). Describing putative contributors to this condition is essential in proposing approaches that may successfully reduce its prevalence and avoid further complications. A large body of evidence has consistently shown an adverse effect of consumption of sugar-sweetened beverages (SSB) on multiple cardiometabolic outcomes (2–6).
In contrast, studies on the role of fruit juice intake on metabolic outcomes are conflicting. Whereas some studies have found an association between higher fruit juice intake and lower fasting glucose (7) and risk of hypertension (3), others report increased risk of type 2 diabetes and weight gain (8–10). The discrepancies may be due to differences in the types of fruit-based beverages, which may vary in nutrient composition and preparation, depending on fruit availability and the preferences of the studied population.
Patterns of beverage consumption have been shown to vary by race/ethnicity in the United States (11), such that middle-aged and elderly Mexican Americans and non-Hispanic blacks tend to consume more fruit drinks and SSB than non-Hispanic whites. However, less is known about specific beverage consumption trends and preferences in Latin America. As most of these countries are undergoing a nutrition transition, shifting toward a more Western-based diet, it is likely that this region has an excessive SSB intake. Specifically in Central America, sugar consumption in general and for soft drinks exclusively has been reported to be higher than in developed countries (12).
Concurrent with such reports, a recent study showed that Costa Ricans adults have a higher mean of nearly every component of MetS compared with the general U.S. population (13). Costa Ricans frequently consume fresh-fruit drinks (14), but less is known about their intake of SSB. Moreover, a link between beverages consumed by Costa Ricans and cardiometabolic markers has not been explored. Thus, we aimed to determine the association of sugar-sweetened and fruit-based beverages traditionally consumed by Costa Rican adults with the components and prevalence of MetS.
Methods
Study population.
Participants were controls from a population-based, case-control study of heart disease conducted in Costa Rica from 1994 to 2004 and who were randomly selected and matched for age, sex, and county of residence (15). Control participants had to be physically and mentally able to participate and without a clinical history of myocardial infarction. All participants provided written informed consent. The Human Subjects Committee of Harvard School of Public Health and University of Costa Rica approved this study.
Data collection and measurements.
Detailed interviews were conducted by trained personnel at the participants’ homes to assess socio-demographic characteristics, lifestyle behaviors, and medical history. Weight, height, waist circumference, and blood pressure (BP) were measured in duplicate and the mean was recorded. BMI was calculated by dividing the weight by the squared height of a participant (kg/m2). The frequency, duration, and intensity of various physical activities were recorded and multiplied; the sum of energy expenditure from all activities was used as the total physical activity score.
Blood samples were collected after an overnight fast in tubes containing 0.1% EDTA and were stored in a cooler at 4°C and transported to the fieldwork station within 4 h. Blood was centrifuged at 1430 × g for 20 min at 4°C to separate the plasma. Plasma samples were stored at −80°C and, within 6 mo of collection, were transported over dry ice to the laboratory for analysis. Plasma TG and HDL cholesterol (HDL-C) were measured enzymatically (Boehringer-Mannheim) using a Roche Cobas Mira Plus autoanalyzer (Roche Diagnostics Systems). Blood glucose was analyzed using an Accu-Check II Blood Glucose Monitor with Chemstrip bG Test Strips (Boehringer-Mannheim). Further methodological details have been described elsewhere (16).
Definitions for dietary exposure and MetS.
A semiquantitative FFQ developed and validated specifically for Costa Ricans was used to collect dietary data (17). Categories for frequency of beverage intake during the previous year were: <1 time/mo or never, 1–3 times/mo, 1 time/wk, 2–4 times/wk, 5–6 times/wk, 1 time/d, 2–3 times/d, 4–5 times/d, and ≥6 times/d. The section for beverages included separate items on water, diet and regular carbonated sodas with and without caffeine, instant powdered sugary drinks prepared with water or from a juice box, and “fresco,” a traditional juice from freshly squeezed fruit diluted in water and mixed with sugar, which may be prepared at home or found in food establishments. A section for fruit intake asked participants to indicate frequency of intake of juices from freshly squeezed orange or other fruit that was not mixed with other ingredients. For this analysis, 4 beverage categories were defined as: fresco, homemade fruit juice (100% juice from freshly squeezed fruit only, does not include commercial juices), instant drinks (an SSB comprised of instant sugary powder mix or a juice box), and regular sodas (an SSB comprised of nondiet carbonated beverages with and without caffeine). An SSB category combined instant drinks and regular sodas. The variable for water intake was also used as a beverage. Analysis excluded diet sodas because of low consumption reported by participants (85% never consumed, 1.5% consumed ≥1/d), tendency for reverse causation (18), and lack of reported protective effects on metabolic outcomes to consider as a possible substitute beverage (18, 19). A serving of beverage was defined as 8 ounces (237 mL).
MetS was defined following the Adult Treatment Panel III definition and revised fasting glucose guidelines (20, 21), where a participant must meet ≥3 of the following criteria: abdominal obesity (>102 cm in men, >88 cm in women), elevated BP (>130/85 mm Hg or use of medication), high TG (≥1.69 mmol/L), low HDL-C (<1.03 mmol/L in men and <1.29 mmol/L in women), and impaired fasting glucose (≥5.6 mmol/L).
Statistical analysis.
Of the 2270 control participants with available FFQ data, 328 were excluded for history of diabetes. A total of 1872 control participants with complete data on MetS and potential covariates were included in this analysis. Frequency of intake of beverages was divided into 4 categories: never or <1 serving/mo (referred to as “never”), ≤1 serving/wk, 2–6 servings/wk, and ≥1 serving/d. These frequencies were selected to achieve a uniform distribution of intake across beverages and to denote weekly and daily intakes that would help craft practical recommendations. Individual nutrients were adjusted for total energy intake using the regression residual method. Quintiles were created for continuous covariates. Plasma glucose and TG were log-transformed to achieve normal distribution.
ANOVA was used to determine age-adjusted differences in the distribution of variables by servings of each beverage. Multivariate linear regression was used to obtain adjusted means for components of MetS by servings of beverages after adjusting for age, sex, area of residence, income, alcohol intake, smoking status, physical activity, dietary fiber intake, intake of the ratio of PUFA:SFA, daily teaspoons (4.2 g) of sugar added to food or beverages, caffeine, low-fat milk consumption, and fruit consumption. Each model was adjusted for other beverages as appropriate. Total energy intake and BMI were subsequently added to the models to determine possible mediating effects.
The prevalence ratio (PR) and 95% CI of MetS were estimated from binomial regression models. OR and 95%CI for MetS for the substitution of one serving of water or homemade fruit juice for one serving of each SSB, as well as combined SSB, were estimated from the difference in coefficients of the beverages as continuous variables in the multivariate model. P-trends by increasing categories of each beverage were determined by assigning each participant the median of each category in the respective category of intake, then entering the resulting continuous variable into the model. All tests were considered significant at P < 0.05. Analysis was conducted using SAS software version 9.1 (SAS Institute).
Results
Sociodemographic and dietary characteristics for Costa Rican adults are shown for the lowest and highest categories of servings of beverages (Table 1) and across all 4 categories of intake (Supplemental Tables 1–3). Close to one-half of the participants consumed frescos more than once per day, whereas only 14% consumed homemade fruit juice at the same frequency. Those consuming more fresco and homemade fruit juice tended to earn a higher income, were less likely to smoke, added less sugar to food or beverages, and consumed less caffeine and instant drinks. Higher consumption of fresco was related with a higher intake of fiber, homemade fruit juice, and fruits, whereas higher consumption of homemade fruit juices was related to drinking more water and low-fat milk.
TABLE 1.
Age-adjusted sociodemographic and dietary characteristics by servings of beverages among Costa Rican adults1
| Homemade fruit juice2 |
Fresco3 |
Instant drink4 |
Regular soda5 |
SSB6 |
||||||
| Never7 | ≥1/d | Never | ≥1/d | Never | ≥1/d | Never | ≥1/d | Never | ≥1/d | |
| n (%) | 811 (43.3) | 254 (13.6) | 251 (13.4) | 901 (48.1) | 1049 (56.0) | 263 (14.1) | 1131 (60.4) | 87 (4.7) | 705 (37.7) | 345 (18.4) |
| Socio-demographics | ||||||||||
| Age, y | 59.1 ± 10.5 | 58.5 ± 11.5 | 59.0 ± 11.0 | 57.3 ± 11.2 | 58.9 ± 10.7 | 55.0 ± 11.4* | 59.7 ± 10.6 | 53.0 ± 11.6* | 60.7 ± 10.0 | 54.6 ± 11.4* |
| Sex, % female | 24.2 | 27.0 | 24.2 | 23.7 | 25.5 | 21.6 | 27.7 | 15.9* | 29.6 | 20.8* |
| Area of residence, % rural | 38.0 | 46.4* | 32.9 | 40.5 | 42.6 | 38.1* | 39.7 | 40.6 | 43.2 | 40.9 |
| Income, $/wk | 475 ± 15 | 828 ± 26* | 557 ± 28 | 599 ± 14* | 596 ± 13 | 536 ± 27* | 575 ± 13 | 570 ± 47 | 584 ± 17 | 555 ± 24 |
| Physical activity, METs8 | 37.1 ± 0.6 | 33.4 ± 1.0* | 36.9 ± 1.1 | 36.9 ± 0.6 | 36.3 ± 0.5 | 37.0 ± 1.0 | 35.6 ± 0.7 | 37.0 ± 1.8 | 35.7 ± 0.6 | 37.1 ± 0.9 |
| Current smoker, % | 26.5 | 16.6* | 31.5 | 20.9* | 22.5 | 25.2 | 22.6 | 20.4 | 20.5 | 25.7 |
| Dietary factors | ||||||||||
| Total energy, kcal/d | 2380 ± 27 | 2610 ± 47* | 2237 ± 47 | 2624 ± 25* | 2424 ± 24 | 2620 ± 47* | 2376 ± 22 | 3049 ± 80* | 2318 ± 29 | 2705 ± 40* |
| Alcohol,9 g/d | 5.7 ± 0.5 | 7.0 ± 0.9 | 7.5 ± 0.9 | 5.8 ± 0.5 | 6.8 ± 0.4 | 4.5 ± 0.9* | 6.4 ± 0.4 | 6.9 ± 1.5 | 6.6 ± 0.5 | 5.7 ± 0.8 |
| Total carbohydrates,9 g/d | 344 ± 1.6 | 343 ± 2.9 | 331 ± 2.8 | 353 ± 1.5* | 343 ± 1.4 | 346 ± 2.9 | 341 ± 1.4 | 342 ± 5.0 | 342 ± 1.8 | 344 ± 2.5 |
| Dietary fiber,9 g/d | 23.4 ± 0.2 | 23.1 ± 0.4 | 22.2 ± 0.4 | 23.3 ± 0.2* | 23.4 ± 0.2 | 21.8 ± 0.4* | 23.7 ± 0.2 | 18.5 ± 0.6* | 24.0 ± 0.2 | 21.0 ± 0.3* |
| PUFA:SFA ratio | 0.59 ± 0.01 | 0.63 ± 0.02 | 0.60 ± 0.02 | 0.62 ± 0.01 | 0.61 ± 0.01 | 0.59 ± 0.02 | 0.60 ± 0.01 | 0.60 ± 0.03 | 0.60 ± 0.01 | 0.59 ± 0.02 |
| Caffeine,9 g/d | 355 ± 6.6 | 264 ± 11.8* | 371 ± 11.9 | 308 ± 6.3* | 324 ± 5.9 | 336 ± 12.0 | 328 ± 5.7 | 269 ± 20.4* | 323 ± 7.3 | 325 ± 10.3 |
| Fresco, servings/d | 0.9 ± 0.04 | 1.1 ± 0.1 | — | — | 1.2 ± 0.03 | 0.58 ± 0.1* | 1.0 ± 0.03 | 0.79 ± 0.1 | 1.1 ± 0.1 | 0.63 ± 0.1* |
| Homemade fruit juice, servings/d | — | — | 0.21 ± 0.03 | 0.34 ± 0.02* | 0.33 ± 0.02 | 0.26 ± 0.03* | 0.31 ± 0.01 | 0.37 ± 0.05 | 0.33 ± 0.02 | 0.29 ± 0.03 |
| Instant drinks, servings/d | 0.34 ± 0.02 | 0.25 ± 0.04* | 0.52 ± 0.04 | 0.20 ± 0.02* | — | — | 0.28 ± 0.02 | 0.48 ± 0.07* | — | — |
| Regular soda, servings/d | 0.14 ± 0.02 | 0.17 ± 0.03 | 0.19 ± 0.03 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.21 ± 0.03* | — | — | — | — |
| Low-fat milk, servings/d | 0.22 ± 0.02 | 0.39 ± 0.04* | 0.27 ± 0.04 | 0.28 ± 0.02 | 0.31 ± 0.02 | 0.18 ± 0.04* | 0.26 ± 0.02 | 0.28 ± 0.07 | 0.30 ± 0.03 | 0.20 ± 0.04 |
| Water, servings/d | 2.7 ± 0.1 | 3.2 ± 0.1* | 3.0 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.1 | 2.6 ± 0.1 | 3.0 ± 0.1 | 2.6 ± 0.2* | 3.0 ± 0.1 | 2.6 ± 0.1* |
| Sugar added,10 tsp/d | 6.8 ± 0.2 | 3.6 ± 0.3* | 6.4 ± 0.3 | 5.3 ± 0.2* | 5.5 ± 0.2 | 6.3 ± 0.3* | 5.6 ± 0.2 | 4.5 ± 0.6 | 5.4 ± 0.2 | 5.9 ± 0.3 |
| Fruits, servings/d | 3.8 ± 0.1 | 4.0 ± 0.2 | 3.3 ± 0.2 | 4.5 ± 0.1* | 4.1 ± 0.1 | 3.5 ± 0.2* | 4.0 ± 0.1 | 4.4 ± 0.3 | 4.2 ± 0.1 | 3.7 ± 0.2* |
| Biomarkers | ||||||||||
| BMI, kg/m2 | 26.0 ± 0.1 | 26.5 ± 0.3* | 26.1 ± 0.3 | 26.2 ± 0.1 | 26.0 ± 0.1 | 26.7 ± 0.3 | 26.1 ± 0.1 | 27.6 ± 0.4* | 25.9 ± 0.2 | 26.6 ± 0.2* |
| Systolic BP, mm Hg | 135 ± 0.7 | 134 ± 1.2 | 136 ± 1.3 | 135 ± 0.7 | 135 ± 0.6 | 135 ± 1.2 | 135 ± 0.6 | 137 ± 2.2 | 135 ± 0.8 | 135 ± 1.1 |
| Diastolic BP, mm Hg | 81.3 ± 0.4 | 82.3 ± 0.7 | 81.2 ± 0.7 | 81.7 ± 0.4 | 81.5 ± 0.3 | 81.7 ± 0.7 | 81.2 ± 0.3 | 83.7 ± 1.2* | 80.8 ± 0.4 | 81.8 ± 0.6 |
| Waist circumference, cm | 89.7 ± 0.4 | 90.7 ± 0.6 | 89.8 ± 0.6 | 90.5 ± 0.3 | 89.5 ± 0.3 | 91.7 ± 0.6* | 89.7 ± 0.3 | 94.8 ± 1.1* | 88.9 ± 0.4 | 91.9 ± 0.5* |
| HDL-C, mmol/L | 1.04 ± 0.01 | 1.08 ± 0.01* | 1.06 ± 0.01 | 1.05 ± 0.01 | 1.06 ± 0.01 | 1.04 ± 0.01 | 1.06 ± 0.01 | 1.03 ± 0.02 | 1.06 ± 0.01 | 1.04 ± 0.01 |
| Plasma TG, mmol/L | 2.36 ± 0.1 | 2.52 ± 0.1 | 2.39 ± 0.1 | 2.44 ± 0.1 | 2.32 ± 0.04 | 2.58 ± 0.1* | 2.37 ± 0.4 | 2.67 ± 0.2 | 2.25 ± 0.1 | 2.60 ± 0.1* |
| Fasting plasma glucose, mmol/L | 4.24 ± 0.04 | 4.15 ± 0.08 | 4.30 ± 0.08 | 4.22 ± 0.04 | 4.20 ± 0.04 | 4.32 ± 0.07 | 4.19 ± 0.04 | 4.44 ± 0.13 | 4.18 ± 0.05 | 4.35 ± 0.07* |
| MetS, % | 29.1 | 26.9 | 31.4 | 28.4 | 26.9 | 35.9* | 29.2 | 36.7 | 26.9 | 34.0 |
Data are mean ± SD or percentage, = 1872. *P-trend < 0.05. BP, blood pressure; HDL-C, HDL cholesterol; MetS, metabolic syndrome; SSB, sugar-sweetened beverage.
Homemade fruit juice: 100% juice from freshly squeezed orange or other fruit, not mixed with other ingredients. Does not include commercial juices.
Fresco: a traditional juice from freshly squeezed fruit diluted in water and mixed with sugar.
Instant drink: SSB comprised of instant sugary powder mix prepared with water, or a juice box.
Regular soda: SSB comprised of nondiet, carbonated beverages with or without caffeine.
SSB: combined instant drink and regular soda.
Never or <1 serving/mo.
METs calculated as the sum of energy expenditure from all activities recorded, based on the frequency, duration, and intensity of various physical activities.
Energy adjusted using the residuals method.
Sugar added to food or beverages (1 tsp = 4.2 g).
Younger participants tended to consume more SSB (instant drinks and regular soda). Participants consumed instant drinks more frequently than regular soda (14.1 vs. 4.7% at ≥1 serving/d). Those consuming more instant drinks were less likely to live in a rural area, earned less income, added more sugar to food or beverages, and consumed less alcohol, fiber, fresco and homemade fruit juice, and fruits but more regular soda. Those consuming more regular soda were more likely to be male and consume less fiber but more instant drinks.
Multivariate-adjusted models for cardiometabolic parameters by type of beverage were tested (Supplemental Tables 4–6); the means are shown for the fully-adjusted model (Table 2). After adjustment for putative confounders, higher intake of homemade fruit juice was associated with a significant trend for higher HDL-C (P-trend = 0.033). This association remained significant after adjusting for total energy intake and BMI as possible mediators (data not shown). Higher intake of fresco was associated with lower plasma glucose, but this association was no longer observed after including other beverages in the fully adjusted model. A higher frequency of intake of instant drinks and combined SSB were associated with a trend for higher waist circumference (P ≤ 0.001) and higher TG (P ≤ 0.001). In addition to the overall trends, those consuming ≥1 serving/d of instant drink and combined SSB had higher waist circumference (P ≤ 0.001) and TG (P ≤ 0.001) than those never consuming them. Adjusting for total energy intake and BMI attenuated these results (data not shown). A trend for higher waist circumference was observed for those consuming regular soda more frequently (P-trend = 0.0004), with those in the highest category having a mean 90.9 cm compared with 87.2 cm for those never consuming instant drinks (P = 0.005). This association remained significant after adjusting for potential mediators.
TABLE 2.
Multivariate-adjusted means for metabolic risk factors by servings of beverages consumed by Costa Rican adults1
| Waist circumference | Systolic BP | Diastolic BP | Plasma HDL-C | Plasma TG | Fasting plasma glucose | |
| cm | mm Hg | mm Hg | mmol/L | mmol/L | mmol/L | |
| Homemade fruit juice2 | ||||||
| Never3 | 87.6 ± 0.4 | 132 ± 0.9 | 79.2 ± 0.5 | 1.07 ± 0.01 | 2.09 (2.00, 2.19) | 4.06 (3.98, 4.15) |
| ≤1 /wk | 87.1 ± 0.6 | 133 ± 1.2 | 79.1 ± 0.7 | 1.08 ± 0.01 | 2.07 (1.94, 2.19) | 4.02 (3.90, 4.13) |
| 2–6/wk | 88.1 ± 0.5 | 134 ± 1.2 | 79.8 ± 0.6 | 1.10 ± 0.01 | 2.20 (2.09, 2.33) | 4.05 (3.94, 4.15) |
| ≥1/d | 87.3 ± 0.7 | 131 ± 1.5 | 79.4 ± 0.8 | 1.10 ± 0.02 | 2.15 (2.00, 2.29) | 3.95 (3.83, 4.08) |
| P-trend | 0.935 | 0.436 | 0.584 | 0.033 | 0.229 | 0.152 |
| Fresco4 | ||||||
| Never | 87.0 ± 0.7 | 133 ± 1.4 | 78.9 ± 0.7 | 1.08 ± 0.02 | 2.04 (1.91, 2.18) | 4.11 (3.98, 4.24) |
| ≤1 /wk | 87.6 ± 0.7 | 131 ± 1.5 | 78.6 ± 0.8 | 1.08 ± 0.02 | 2.06 (1.92, 2.20) | 4.10 (3.97, 4.24) |
| 2–6/wk | 87.4 ± 0.5 | 134 ± 1.1 | 79.9 ± 0.6 | 1.09 ± 0.01 | 2.11 (2.00, 2.22) | 4.04 (3.94, 4.14) |
| ≥1/d | 87.9 ± 0.4 | 132 ± 0.9 | 79.3 ± 0.5 | 1.08 ± 0.01 | 2.17 (2.08, 2.26) | 3.99 (3.91, 4.08) |
| P-trend | 0.231 | 0.480 | 0.716 | 0.812 | 0.052 | 0.052 |
| Instant drinks5 | ||||||
| Never | 86.8 ± 0.4 | 133 ± 0.9 | 79.1 ± 0.5 | 1.09 ± 0.01 | 2.03 (1.95, 2.12) | 4.00 (3.92, 4.09) |
| ≤1 /wk | 88.9 ± 0.7* | 133 ± 1.5 | 80.3 ± 0.8 | 1.09 ± 0.02 | 2.16 (2.01, 2.32) | 4.07 (3.94, 4.21) |
| 2–6/wk | 87.7 ± 0.6 | 133 ± 1.3 | 79.3 ± 0.7 | 1.09 ± 0.01 | 2.19 (2.07, 2.33) | 4.04 (3.96, 4.15) |
| ≥1/d | 89.3 ± 0.7* | 132 ± 1.4 | 79.4 ± 0.7 | 1.07 ± 0.02 | 2.33 (2.18, 2.48)* | 4.12 (3.99, 4.25) |
| P-trend | 0.001 | 0.636 | 0.852 | 0.333 | 0.0001 | 0.131 |
| Regular soda6 | ||||||
| Never | 87.2 ± 0.4 | 132 ± 0.9 | 78.9 ± 0.5 | 1.08 ± 0.01 | 2.08 (2.00, 2.17) | 4.02 (3.83, 4.10) |
| ≤1 /wk | 87.4 ± 0.6 | 132 ± 1.2 | 80.2 ± 0.6 | 1.10 ± 0.01 | 2.20 (2.08, 2.33) | 4.07 (3.96, 4.19) |
| 2–6/wk | 88.5 ± 0.7 | 134 ± 1.4 | 79.5 ± 0.8 | 1.08 ± 0.02 | 2.11 (1.98, 2.26) | 4.03 (3.90, 4.16) |
| ≥1/d | 90.9 ± 1.1* | 134 ± 2.3 | 80.7 ± 1.2 | 1.06 ± 0.03 | 2.34 (2.10, 2.61) | 4.05 (3.85, 4.27) |
| P-trend | 0.0004 | 0.207 | 0.156 | 0.365 | 0.099 | 0.842 |
| SSB7 | ||||||
| Never | 86.3 ± 0.5 | 132 ± 1.0 | 78.5 ± 0.5 | 1.08 ± 0.01 | 5.18 (5.13, 5.22) | 4.28 (4.26, 4.30) |
| ≤1 /wk | 87.7 ± 0.6 | 133 ± 1.3 | 80.5 ± 0.7* | 1.11 ± 0.01 | 5.18 (5.13, 5.22) | 4.28 (4.26, 4.30) |
| 2–6/wk | 88.0 ± 0.5* | 133 ± 1.1 | 79.7 ± 0.6 | 1.08 ± 0.01 | 5.18 (5.13, 5.22) | 4.28 (4.26, 4.30) |
| ≥1/d | 89.2 ± 0.6* | 132 ± 1.3 | 79.4 ± 0.7 | 1.07 ± 0.01 | 5.18 (5.13, 5.22)* | 4.28 (4.26, 4.30) |
| P-trend | <0.0001 | 0.736 | 0.482 | 0.265 | <0.0001 | 0.066 |
Data are adjusted mean ± SEM or adjusted geometric mean (95% CI) for back-transformed values of plasma TG and fasting glucose, = 1872. *Significantly different from lowest category, P < 0.05. Adjusted for age, sex, area of residence, income, alcohol intake, smoking, physical activity, dietary fiber (quintiles), PUFA:SFA ratio (quintiles), sugar added to food or beverages (quintiles), caffeine (quintiles), low-fat milk (servings/d) and fruit consumption (servings/d), and other beverages depending on the model (fresco, homemade fruit juice, instant drinks, regular soda, water). BP, blood pressure; HDL-C, HDL cholesterol; SSB, sugar-sweetened beverage.
Never or <1 serving/mo.
Homemade fruit juice: 100% juice from freshly squeezed orange or other fruit, not mixed with other ingredients. Does not include commercial juices.
Fresco: a traditional juice from freshly squeezed fruit diluted in water and mixed with sugar.
Instant drink: an SSB comprised of instant sugary powder mix prepared with water, or a juice box.
Regular soda: an SSB comprised of nondiet, carbonated beverages with or without caffeine.
SSB: combined instant drink and regular soda.
A significant association with MetS was observed for instant drink consumption, with those consuming ≥1 serving/d having a PR = 1.42 (95% CI: 1.11, 1.83) for higher likelihood of having MetS than those who never consume instant drinks (Table 3). A trend for a higher PR of MetS was observed with higher frequency of instant drinks (P-trend = 0.011). Similar results were obtained for combined SSB, with 1.39 (95% CI: 1.08, 1.80) higher odds of MetS for those consuming ≥1 serving/d and an overall higher trend with increasing frequency of intake (P-trend = 0.009).
TABLE 3.
PR (95% CI) of MetS by frequency of consumption of beverages in Costa Rican adults1
| Reference (never)2 | ≤1/wk | 2–6/wk | ≥1/d | P-trend | |
| Water | 1.00 | 1.36 (0.74, 2.48) | 1.20 (0.75, 1.93) | 1.19 (0.87, 1.62) | 0.507 |
| Homemade fruit juice3 | 1.00 | 0.87 (0.68, 1.11) | 1.03 (0.83, 1.28) | 0.80 (0.61, 1.06) | 0.263 |
| Fresco4 | 1.00 | 1.00 (0.72, 1.38) | 0.92 (0.69, 1.22) | 0.93 (0.71, 1.21) | 0.571 |
| Instant drink5 | 1.00 | 1.18 (0.90, 1.54) | 1.10 (0.86, 1.40) | 1.42 (1.11, 1.83) | 0.011 |
| Regular soda6 | 1.00 | 1.03 (0.82, 1.29) | 0.98 (0.74, 1.30) | 1.29 (0.86, 1.92) | 0.370 |
| SSB7 | 1.00 | 1.07 (0.82, 1.41) | 1.18 (0.94, 1.47) | 1.39 (1.08, 1.80) | 0.009 |
Adjusted for age, sex, area of residence, income, alcohol intake, smoking, physical activity, dietary fiber, PUFA:SFA ratio, sugar added to food or beverages, caffeine, low-fat milk, fruit consumption, and other beverages depending on the model, = 1872. MetS, metabolic syndrome; PR, prevalence ratio; SSB, sugar-sweetened beverage.
Never or <1 serving/mo.
Homemade fruit juice: 100% juice from freshly squeezed orange or other fruit, not mixed with other ingredients. Does not include commercial juices.
Fresco: a traditional juice from freshly squeezed fruit diluted in water and mixed with sugar.
Instant drink: SSB comprised of instant sugary powder mix prepared with water, or a juice box.
Regular soda: SSB comprised of nondiet, carbonated beverages with or without caffeine.
SSB: combined instant drink and regular soda.
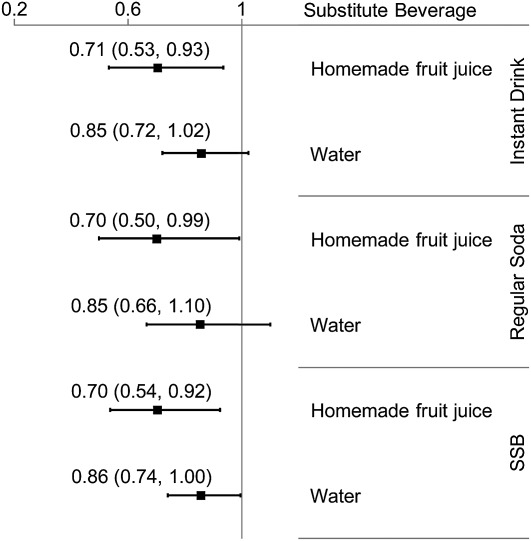
Substituting one serving of homemade fruit juice for one serving of instant drink was associated with 29% (95% CI: 7, 47%) lower odds of MetS and for regular soda with 30% (95% CI: 1, 50%) lower odds (Fig. 1). These associations were slightly attenuated after adjusting for total energy intake and BMI (data not shown). Substituting water for either SSB was associated with a nonsignificant reduction in odds of MetS [OR = 0.85 (95% CI: 0.72, 1.02); P = 0.07 for instant drinks and OR = 0.85 (95% CI: 0.66, 1.10); P = 0.21 for regular soda)]. When combining SSB, replacing with homemade fruit juice was associated with a 30% (95% CI: 8, 56%) reduction in MetS, whereas substituting water was marginally significant [OR = 0.86 (95% CI: 0.74, 1.00)].
FIGURE 1.
OR (95% CI) of MetS by substituting one serving of instant drink, regular soda, or combined SSB for either homemade fruit juice or water among Costa Rican adults. Instant drink: instant sugary powdered mix prepared with water, or a juice box; regular soda: nondiet, carbonated beverages with or without caffeine; combined SSB: instant drink and regular soda; homemade fruit juice: 100% juice from freshly squeezed fruit, not mixed with other ingredients, does not include commercial juices. Model was adjusted for age, sex, area of residence, income, alcohol intake, smoking, physical activity, dietary fiber, PUFA:SFA ratio, sugar added to food or beverages, caffeine, low-fat milk, fruit consumption, and other beverages depending on the model, n = 1872. MetS, metabolic syndrome; SSB, sugar-sweetened beverage.
Discussion
This study showed that consuming homemade 100% fruit juices is associated with a better cardio-metabolic profile, whereas intake of instant drinks and combined SSB is associated with higher likelihood of MetS. Moreover, substituting one serving of homemade fruit juice for one serving of SSB may help maintain lower odds of MetS. Specifically, we found that homemade fruit juice consumption is positively associated with HDL-C. This supports results from an intervention study showing that higher intake of orange juice improves lipid profiles, including increased HDL-C (22). The authors propose that the flavonoid content in fruit may directly affect lipoprotein regulatory pathways in the liver.
Contrasting the favorable role of fruit juice intake in HDL-C observed here, some studies have shown that it may increase the risk of type 2 diabetes (8–10). Bazzano et al. (8) reported such results in women for higher intake of both fruit juice and fruit punch, yet whole fruit consumption was inversely associated with the risk of type 2 diabetes. In a similar but younger cohort, fruit punch, but not fruit juice, increased type 2 diabetes risk (10). Odegaard et al. (9) did not specify the type of juice in their study in Chinese adults, but it likely included fast food-like sugary drinks. It is possible that processing in commercial fruit juices may contribute to a higher sugar concentration (thus higher glycemic index) and loss of protective phytochemicals, which may lead to the reported inverse associations. Conversely, as in our study, juice extracted at home from the fruit in its natural state may retain a slight amount of fiber (from the pulp and peel, depending on the fruit and preparation) and flavonoid content, which renders it beneficial. Fresco, another fruit-based beverage, was not associated with any metabolic outcome. This traditional and frequently consumed beverage is prepared by diluting the juice of the fruit in water and adding sugar. This practice may counterbalance any potential benefits of the fruit on fresco. Because fresco did not show a clear directionality with metabolic components, even after adjusting for sugar added to food or beverages, we did not consider it a possible healthy substitute but neither an SSB that may need replacement. Even so, because fresco had no negative association with metabolic components, it may still be a healthier traditional alternative to SSB, provided individuals limit the amount of sugar added to the beverage. Further studies should consider the type and preparation of fruit beverage and explore the inconsistencies of associations with specific metabolic markers and disease outcomes.
We observed that the most commonly consumed commercial SSB among Costa Ricans adults were instant drinks rather than regular sodas. Moreover, some associations were observed for both types of commercial SSB, whereas others were observed for only instant drinks. Instant drinks and regular soda, individually and combined, were associated with higher waist circumference, which supports multiple studies on abdominal obesity, overall obesity, and weight gain (3, 5, 6, 10, 23). Proposed mechanisms for this association include lower satiety from SSB and higher energy intake, because liquid energy adds to the energy from solid food in excess of energy requirements (23). The high fructose content in SSB may also contribute to increased hepatic de novo lipogenesis and accumulation of visceral fat compared with the effect of glucose (23–25). In our study, the associations between SSB and waist circumference was attenuated after adjusting for energy and BMI, suggesting these factors may mediate the relationship. Similar observations have been reported on the association between SSB and type 2 diabetes (2).
We observed a positive trend between higher intake of instant drinks and combined SSB, but not regular soda alone, and increased plasma TG and MetS. Other studies have shown similar associations for commercial SSB (3, 5, 6); however, it is difficult to draw comparisons with our study, because most of those studies combined several types of SSB. Fructose, a major component of instant drinks, has been strongly linked to elevated TG (24). Dysregulated TG and abdominal obesity may then culminate in MetS. Additionally, and contrary to regular sodas, many powdered instant drinks contain regular sugar in addition to, or instead of, fructose. Sucrose increases the postprandial TG to a similar extent as fructose alone (26) and glucose-, but not fructose-, sweetened beverage consumption increases fasting plasma TG concentrations after a diet intervention (25). Alternatively, it is possible that we could not detect significant results with regular soda because of the small frequency of consumption reported for this SSB. Because the results of combined SSB are parallel to those observed for instant drinks alone, it is likely that this beverage, and not regular soda, may be driving the associations reported for combined SSB. It may also be possible that consumption of both types of SSB strengthens the unfavorable association with metabolic outcomes. We did not observe associations between any SSB and BP or plasma glucose, although they have been reported by others (3–6). Differences in types of SSB or in the study populations, such as patterns of intake or baseline characteristics, may explain the lack of replication.
Notably, we report that substituting one serving of homemade fruit juice for one serving of either or both SSB may help lower the odds of MetS by 30%. This may occur in 2 ways: reducing added fructose and/or glucose by restricting SSB may limit adverse metabolic effects, and the high content of phytochemicals and antioxidants in homemade fruit juice may decrease oxidative stress and inflammation that leads to MetS. Ghanim et al. (27) reported that adding water or a glucose drink to a high-fat, high-carbohydrate diet increased oxidative and inflammatory stress, whereas orange juice prevented those meal-induced responses. Water may be a possible alternative, because it is metabolically inert and does not provide energy; in this study, we found a nonsignificant reduction in odds of MetS by substituting water for either instant drinks or regular soda. The association was strengthened, but remained nonsignificant, when analyzing the substitution of water for combined SSB, suggesting that water may be an appropriate alternative for those consuming a higher frequency of both types of SSB.
Our study has some limitations. Establishing causality is limited because of the cross-sectional design. In addition, we did not have specific information on beverage preparation, combinations or patterns of intake of foods and beverages, or type of fruit for the juices. Thus, the results of this study may be generalized to other ethnic groups who consume similar beverages. Although we considered several possible confounders in our models, residual confounding cannot be completely discarded.
A major strength of this study is that it distinguished between types of beverages and detected distinct associations that may help focus dietary advice. For example, in Costa Rica, more emphasis should be given to reducing the consumption of instant drinks while maintaining a low intake of regular soda, especially at younger ages. Also, by considering the preferences in beverages of Costa Ricans and analyzing possible substitutes for SSB, we propose appropriate and practical alternatives for this population. Our results may provide the groundwork for interventions on beverage intake that aim to prevent MetS and type 2 diabetes in Hispanic ethnic subgroups. They may also support current efforts for nutritional education and public health initiatives on limiting the availability and intake of SSB, while substituting with a healthy beverage.
In conclusion, reducing the consumption of instant sugary drinks and regular soda and substituting them with homemade 100% fruit juices, in moderation, may be a culturally appropriate approach to lower MetS among Hispanics. Water may be another healthy replacement for SSB. Given the high prevalence of MetS among this growing ethnic group, these results become paramount in the search for dietary strategies to help prevent and control metabolic conditions.
Supplementary Material
Acknowledgments
J.M. designed the research, analyzed data, wrote the manuscript, interpreted the results, and revised the manuscript; H.C. designed the research, interpreted the results, revised the manuscript, and had primary responsibility for final content; and V.M. and F.B.H. contributed to the analytical approach, interpreted the results, and revised the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grants HL60692 and HL49086.
Author disclosures: J. Mattei, V. Malik, F. B. Hu, and H. Campos, no conflicts of interest.
Supplemental Tables 1 through 6 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: BP, blood pressure; HDL-C, HDL cholesterol; MetS, metabolic syndrome; PR, prevalence ratio; SSB, sugar-sweetened beverage.
Literature Cited
- 1.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program-Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13 [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR, Jr, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2010;92:954–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Caballero B, Mitchell DC, Loria C, Lin PH, Champagne CM, Elmer PJ, Ard JD, Batch BC, Anderson CA, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation. 2010;121:2398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denova-Gutiérrez E, Talavera JO, Huitron-Bravo G, Mendez-Hernandez P, Salmeron J. Sweetened beverage consumption and increased risk of metabolic syndrome in Mexican adults. Public Health Nutr. 2010;13:835–42 [DOI] [PubMed] [Google Scholar]
- 6.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–8 [DOI] [PubMed] [Google Scholar]
- 7.Yoshida M, McKeown NM, Rogers G, Meigs JB, Saltzman E, D'Agostino R, Jacques PF. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr. 2007;137:2121–7 [DOI] [PubMed] [Google Scholar]
- 8.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31:1311–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odegaard AO, Koh WP, Arakawa K, Yu MC, Pereira MA. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol. 2010;171:701–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34 [DOI] [PubMed] [Google Scholar]
- 11.Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc. 2006;106:1992–2000 [DOI] [PubMed] [Google Scholar]
- 12.Ismail AI, Tanzer JM, Dingle JL. Current trends of sugar consumption in developing societies. Community Dent Oral Epidemiol. 1997;25:438–43 [DOI] [PubMed] [Google Scholar]
- 13.Rehkopf DH, Dow WH, Rosero-Bixby L. Differences in the association of cardiovascular risk factors with education: a comparison of Costa Rica (CRELES) and the USA (NHANES). J Epidemiol Community Health. 2010;64:821–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmuth C. Culture and customs of Costa Rica. Westport (CT): Greenwood Press; 2000 [Google Scholar]
- 15.Campos H, Siles X. Siesta and the risk of coronary heart disease: results from a population-based, case-control study in Costa Rica. Int J Epidemiol. 2000;29:429–37 [PubMed] [Google Scholar]
- 16.Truong H, DiBello JR, Ruiz-Narvaez E, Kraft P, Campos H, Baylin A. Does genetic variation in the Delta6-desaturase promoter modify the association between alpha-linolenic acid and the prevalence of metabolic syndrome? Am J Clin Nutr. 2009;89:920–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabagambe EK, Baylin A, Allan DA, Siles X, Spiegelman D, Campos H. Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am J Epidemiol. 2001;154:1126–35 [DOI] [PubMed] [Google Scholar]
- 18.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR., Jr Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2009;32:688–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 21.Hanley AJ, Karter AJ, Williams K, Festa A, D'Agostino RB, Jr, Wagenknecht LE, Haffner SM. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation. 2005;112:3713–21 [DOI] [PubMed] [Google Scholar]
- 22.Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piche LA, Serratore P. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr. 2000;72:1095–100 [DOI] [PubMed] [Google Scholar]
- 23.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121:1356–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tappy L, Le KA, Tran C, Paquot N. Fructose and metabolic diseases: new findings, new questions. Nutrition. 2010;26:1044–9 [DOI] [PubMed] [Google Scholar]
- 25.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88:S1733–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91:940–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.