Abstract
Plasma volume expansion has been associated with fetal growth. Our objective was to examine the associations between maternal nutritional status in early pregnancy and extracellular water (ECW), total body water (TBW), and percentage plasma volume change across pregnancy. In a subsample of 377 pregnant women participating in a cluster-randomized trial of micronutrient supplementation, hemoglobin, hematocrit, and multi-frequency bioelectrical impedance were measured at ~10, 20, and 32 wk of gestation. In early pregnancy, women were short (mean ± SD, 148.9 ± 5.3 cm) and thin (19.5 ± 2.5 kg/m2). In mixed-effects multiple regression models, a 1-unit higher BMI at ~10 wk was associated with higher ECW and TBW (0.27 and 0.66 kg per kg/m2, respectively; P < 0.01) at ~10, ~20, and ~32 wk. Height was also positively associated with ECW and TBW at each time point. Early pregnancy BMI was negatively associated with gains in ECW and TBW (−0.06 and −0.14 kg per kg/m2, respectively; P < 0.01) from 10 to 20 wk, but not with 20- to 32-wk gains after accounting for weight gain. BMI was positively associated with percentage changes in plasma volume from 20 to 32 wk (0.57% per kg/m2; P < 0.05). Height was not associated with changes in body water or plasma volume. Women with low BMI and height in early pregnancy have lower ECW and TBW in early, mid, and late pregnancy and lower late pregnancy plasma volume expansion, potentially increasing risk of fetal growth restriction.
Introduction
Pregnancy is a time of substantial physiologic changes to support the growing placenta and fetus. Plasma volume, extracellular water (ECW)5, and total body water (TBW) all increase during the pregnancy as part of this process (1, 2) with plasma volume expansion serving to improve uteroplacental blood flow for nutrient transfer to the fetus (3). Plasma volume is a component of both ECW and TBW; thus, as plasma volume expands, ECW and TBW typically correspondingly increase and allow inferences to be drawn related to plasma volume (1, 4, 5). Invasive tracer methods are best for measuring plasma volume and body water (6–10) but are not feasible for larger studies in rural settings. Multi-frequency bioelectrical impedance analysis (BIA) is a noninvasive technique that is portable, allowing home-based assessments. In high-income countries, BIA has been validated to measure TBW and ECW (11) and has been used to document body water increases from early pregnancy to delivery (2, 12–14). Additionally, we recently validated the use of BIA for the determination of TBW in women of reproductive age in rural Bangladesh (15).
Plasma volume, ECW, and TBW have all been positively associated with birth weight (16–20). In Chile, studies found 10–17% lower plasma volume at early pregnancy and near-term as well as 52% lower plasma volume expansion in mothers with fetal growth restriction compared with those without growth restriction (21–23). Second trimester TBW and ECW (2) and TBW in late gestation (16–18, 24) have similarly been positively associated with fetal growth.
Poor prepregnancy nutritional status has long been known to adversely influence birth weight (25), but patterns of plasma volume, ECW, and TBW change during gestation, in relation to maternal undernutrition at the outset of pregnancy, have not been well studied. In a small study, the late pregnancy plasma volume was 15% lower in underweight than in normal-weight Chilean women, but this may have been explained by lower parity, which is also associated with plasma volume and was more common in underweight women compared with controls (3). Hytten and Paintin (4) found smaller women had lower nonpregnant plasma volumes, but absolute gains were similar for all women. Studies of body water in the US have found 6–10% lower TBW in low-BMI (≤19.8 kg/m2) compared with normal-BMI (19.8–26.0 kg/m2) women during pregnancy, but absolute TBW gains did not differ between these groups (16, 26).
In a rural setting in Bangladesh, where maternal malnutrition and low birth weight are common (27, 28), we examined the independent associations of early pregnancy maternal nutritional status with maternal body water patterns. Specifically, nutritional status was characterized using early pregnancy height, BMI, and weight gain, with TBW, ECW, and their changes during pregnancy, as well as percentage change in plasma volume, as outcomes of interest. The eventual goal is to understand whether such fluid patterns may be related to the high prevalence of low birth weight in this region of the world.
Participants and Methods
This study involved a subsample of pregnant women (n = 500) participating in a double-masked, cluster-randomized controlled trial (n = 45,000) to reduce infant mortality by providing daily a 15 vitamin and mineral supplement compared with iron and folic acid (standard of care). The supplement being tested is similar to the formulation recommended by UNICEF and WHO (29) but is designed to meet the current RDA set by the Institute of Medicine. This trial is being conducted in an ~435 km2 area of rural northwestern Bangladesh where the study area was divided into 596 sectors of comparable size that were used as units of randomization. Prior to enrollment, nonpregnant women in the study area were visited every 5 wk by a study worker and offered a urine-based pregnancy test if they were amenorrheic for 30 d. Women testing positive were enrolled into the trial. Local field workers tracked participants weekly for follow-up and dosing with micronutrient supplements from enrollment to 3 mo postpartum.
A substudy was conducted to assess the changes in vitamin and mineral status due to supplementation and to conduct a more enhanced assessment of women during pregnancy. The substudy area was selected to be representative of the parent trial across a range of factors, including sociodemographic and geographic variations, which were evaluated during an earlier trial in the same area (30). The substudy area included 64 sectors (~10% of total study area) that were balanced by supplement allocation. Approximately one-half of the substudy area (31 sectors) was selected for an intensive cord blood and placenta investigation, with the sample size based on the number of cord blood samples required to detect differences in micronutrient status between supplementation groups. From February 2009 to March 2010, newly pregnant women in the substudy area were asked for consent to the additional cord blood and placental measurements after consenting to the parent study. Gravida were identified and enrolled at a median of 9.6 (IQR = 7.7, 11.7) wk of gestation. Those >28 wk of gestation at the time of enrollment were admitted to the larger trial but were excluded from the current study.
Of the 510 pregnant women approached for consent, 10 declined and 500 were enrolled. Two women refused the mid-pregnancy visit after enrollment. Sixty-nine pregnancies ended in miscarriage or induced abortion and 6 in stillbirths (15% total pregnancy loss). At mid- and late pregnancy, 19 and 21 women were missed, respectively, typically due to extended travel away from their homes. Participants who delivered twins (n = 3) and those with a BMI >29 kg/m2 (extreme outliers, n = 3) were excluded from analysis, leaving 377 women with live singleton births for this analysis.
Women were measured in their homes by technicians trained in anthropometry and phlebotomy; visits were scheduled just after enrollment (early pregnancy), at 20 wk (mid-pregnancy), and at 32 wk (late pregnancy). A single weight reading was taken to the nearest 0.1 kg using a digital scale provided by UNICEF (Seca Scales). At the first visit only, standing height was measured in triplicate to the nearest 0.1 cm by standard procedures (31) using a Harpenden pocket stadiometer (Cromwell) and the median was used for analysis. The early pregnancy height was used to calculate ECW and TBW at all 3 time points, assuming a negligible change in stature during pregnancy.
The QuadScan 4000 bioelectrical impedance analyzer (Bodystat) was used to measure full-body bioelectrical impedance (Z) at frequencies of 5, 50, 100, and 200 kHz at the 3 pregnancy visits. Each day before field use, the analyzers were tested at 5, 50, 100, and 200 kHz frequencies using standard resistors from the manufacturer at 500 ohms; Z values were within the acceptable limits of 496–503 ohms. During the home visits, women were guided to lie flat on their back on a nonconductive surface with arms extended at a 30-degree angle from the body and legs at a 60-degree angle from each other; shoes and jewelry were removed. Two electrodes were placed at a minimum distance of 3 cm from each other on the right wrist and hand and on the right ankle and foot. Z values were recorded directly from the analyzer. One irreconcilable error due to software malfunction was later found in the recorded data and set to missing for 1 participant at early pregnancy.
At ~10- and ~32-wk visits, venous blood (~7 mL) was collected from the left arm for hemoglobin (Hb) and hematocrit (Hct) measurements and for subsequent assessment of plasma micronutrients and other biochemical analytes. At the ~20-wk visit, blood was collected by fingerstick in the smaller substudy to assess Hb and Hct. Hb was measured on the spot with a HemoCue photometer (Hb-301, HemoCue). Hct was measured by packed cell volume after microcapillary tubes were centrifuged. Laboratory technicians read the tubes in duplicate on a chart-type Critocaps Micro-Hematocrit Capillary Tube Reader (8889–111004, McCormick Scientific) and the mean was used for analysis.
We calculated TBW using an equation developed with 2H2O dilution using postpartum participants enrolled from the same study area (15) and ECW using an equation developed with bromide dilution as the gold standard in healthy volunteers in The Netherlands (11):
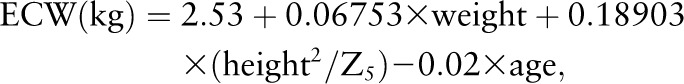 |
where weight is in kg, height is in cm, age is in y, and Z50 and Z5 are impedance at 50 and 5 kHz, respectively. According to the respective authors, the prediction equation for TBW explained 76% of the total variance in actual TBW, with a predicted residual sums of squares statistic determined by an internal validation approach of 1.35 kg and prediction residuals unrelated to the amount of TBW (15), and the ECW prediction equation explained 86% of the total variance in ECW and overestimated ECW by 1.1 ± 0.8 kg with a CV of 5% (11).
In the absence of an invasive, direct measure of plasma volume, we used the Strauss equation (32) for estimating the percent change in plasma volume:
where Hb is in g/dL, Hct is a percentage, and 1 denotes the earlier time and 2 the later time of measurement. Greenleaf et al. (33) found the Strauss equation acceptable for the estimation of percentage change in plasma volume when change in plasma osmolality was <13 mosmol/kg (i.e., participant not dehydrated). Because it was developed for assessing plasma volume change across short time intervals in which repeated dilution methods were not possible, the accuracy based on gold standard methods is not available to the authors’ knowledge. The Strauss equation has been used to assess short-term changes in plasma volume in studies of stress, fainting, and exercise (33–36) but has not previously been used in pregnancy. Due to the indirect estimation of percentage change in plasma volume, we were not able to assess actual plasma volume at the 3 visits.
Shortly after enrollment into the trial (~10 wk), data on maternal education, age, previous pregnancy history, and numerous household socioeconomic factors were collected by trained interviewers. For socioeconomic status, we created a living standards scale by factor analysis to capture the common variance in 13 assets and dwelling characteristic and thus estimate “living standard” as a single latent variable. Included variables were based on a living standard index previously created for our first trial in this population (37). At both ~10 and ~32 wk of gestation, women were visited by the same interviewers to ascertain maternal morbidities, strenuous work, diet, and tobacco and betel nut habits. Infant sex was recorded at birth.
Gestational age was determined by crown-rump length (SonoSite Titan, SonoSite) at <15 wk by study ultrasound technicians (n = 326) or, when ultrasound was not possible, by date of last menstrual period (LMP; n = 51) reported by women every month as part of the pregnancy surveillance. No sex determination was done. In cases in which LMP differed from the ultrasound estimation by >4 wk, the ultrasound measurements (crown-rump length, biparietal diameter, or femur length) ≥15 wk were used for gestational age estimation (n = 9) or LMP was set to missing (n = 1). Gestational age at birth ≥44 wk (n = 6; LMP estimates) was considered biologically implausible and set to missing.
Statistical analyses.
Nutritional status was grouped by categories of BMI (<18.5 or ≥18.5 kg/m2) and height (<145 or ≥145 cm) to define underweight and short stature, respectively. The Kruskal-Wallis test was used to examine differences in ECW and TBW at each time point and ANOVA was used for changes in ECW and TBW plus percentage change in plasma volume, by nutritional status category. Next, early pregnancy height and BMI as continuous variables were used as independent predictors in mixed-effects linear regression models (to allow for random intercept by individual and random slope by gestational age) with repeated ECW and TBW values as dependent variables. Regression models were built by first modeling gestational age at each measurement as a continuous variable. A quadratic term was significant for TBW (and retained) but not for ECW. A random slope for gestational age was added (tested by log likelihood and SD of the residual) to allow women to differ in their overall rate of change for outcomes (38). Because the exact gestational age at a visit can be different for each woman, an unstructured variance-covariance structure of the random effects was used. After modeling gestational age, BMI and height were added to the model, which improved the fit (P < 0.0001) by the likelihood ratio test. For changes in body water, we used linear regression models; we examined BMI and height, and BMI, height, and weight gain to mid-pregnancy as independent variables for changes to mid-pregnancy and changes to late-pregnancy as outcomes, respectively.
Potential confounders were infant sex, maternal age, parity, anemia status, education, strenuous work, chewing betel nut, chewing tobacco, and the living standard scale, but none acted statistically as a confounder by changing adjusted coefficients by >10%. Also, participants were enrolled in a randomized micronutrient trial and received daily supplementation, so treatment effect (still blinded due to the ongoing trial) was tested as a confounder. It showed no association with outcomes, nor did it change associations of interest in extended models and was excluded from the final model. Thus, based on evidence from published studies, we forced the following confounders into all final models: woman’s age (y), woman’s education (y), parity (0 vs. ≥1), infant sex, and the living standard scale.
Interactions for infant sex and parity with maternal BMI and height were tested and were not significant (P > 0.05). Because weight and height are included in prediction equations for ECW and TBW and we were interested in the association of BMI and height with these outcomes, we repeated regression models with impedance alone and height2/impedance (each at 5 or 50 kHz) as outcomes.
Analysis was conducted using STATA version 11.1 (StataCorp) and associations were considered significant at P < 0.05. Results in the text are mean ± SD.
The Institutional Review Board at the Johns Hopkins School of Public Health, Baltimore, MD and the Bangladesh Medical Research Council, Dhaka, Bangladesh granted ethical approval for this study and the parent trial in which it is nested.
Results
Characteristics did not differ between those included and excluded among those with live births (assuming women with pregnancy loss would be different), except fewer excluded women had paid employment (data not shown). Women were young (22.8 ± 5.2 y), with over one-half in their 20s and over one-third delivering their first child (Table 1). Maternal education was 5.6 ± 3.8 y in this predominantly Muslim (93%) population. A large proportion of women were anemic (Hb <110 g/L), of short stature (<145 cm), or underweight (<18.5 kg/m2). In this rural setting, less than one-fifth of households had electricity. Women delivered at 39.2 ± 1.7 wk of gestation and 54.6% of infants were male.
TABLE 1.
Individual and household characteristics of women in early pregnancy (~10 wk of gestation), n = 377
| n | % | |
| Maternal | ||
| Parity | ||
| 0 | 142 | 38 |
| 1 | 133 | 35 |
| ≥2 | 102 | 27 |
| Age, y | ||
| <20 | 118 | 31 |
| 20–29 | 216 | 57 |
| ≥30 | 43 | 11 |
| Height | ||
| Short (<145 cm) | 87 | 23 |
| Normal (≥145 cm) | 290 | 77 |
| BMI, kg/m2 | ||
| Underweight (<18.5) | 146 | 39 |
| Normal (18.5–24.9) | 225 | 60 |
| Overweight (>24.9) | 6 | 2 |
| Anemic | ||
| Hb <110 g/L | 58 | 15 |
| Hb ≥110 g/L | 319 | 85 |
| Education, y | ||
| 0 | 81 | 21 |
| 1–7 | 148 | 39 |
| ≥8 | 148 | 39 |
| Chewed tobacco | ||
| Never | 353 | 94 |
| Any amount | 24 | 6 |
| Household | ||
| Owns clock1 | 269 | 72 |
| Owns television | 51 | 14 |
| Owns cabinet | 145 | 38 |
| Owns bicycle | 142 | 38 |
| Owns mobile phone | 131 | 35 |
| Owns 2 or more beds | 224 | 59 |
| Has electricity | 58 | 15 |
| Has 2 or more living rooms | 163 | 43 |
| Toilet is water sealed/slab | 291 | 77 |
| Wall construction | ||
| Thatch, grass, sticks | 64 | 17 |
| Bamboo, wood, mud | 51 | 14 |
| Tin/wood plank | 237 | 63 |
| Cement | 25 | 7 |
| Kitchen facility | ||
| No separate room2 | 109 | 29 |
| Outside home, not enclosed | 15 | 4 |
| Outside home, enclosed | 253 | 67 |
One missing value. Hb, hemoglobin.
One reported separate room within house.
Maternal weight, ECW, and TBW increased from 10 to 20 and 20 to 32 wk, with greater late compared with early pregnancy increases for weight and TBW (Table 2). TBW as a percentage of body weight was 55.1 ± 3.6% at 10 wk and decreased by ~1% (P < 0.001) between each visit. ECW and TBW increased by ~1 kg, or 4.0–4.5% (P < 0.001), between each visit; however, the plasma volume increased much more from early to mid-pregnancy (P < 0.001) and then decreased from mid- to late pregnancy (P < 0.001). We observed characteristic decreases in Hb and Hct from early to mid-pregnancy and then small increases from mid- to late pregnancy (all P < 0.001). In early pregnancy, BMI was positively correlated with ECW (r = 0.61; P < 0.001) and TBW (r = 0.65; P < 0.001) and height was also correlated with ECW and TBW (r = 0.59 and r = 0.57, respectively; P < 0.001).
TABLE 2.
Maternal anthropometry, body composition, and plasma volume changes during pregnancy1
| 10 wk | 20 wk | 32 wk | Δ10–20 wk | Δ20–32 wk | |
| Gestational age at each visit, wk | 9.7 (7.9, 11.9) | 20.4 (20.0, 21.4) | 32.1 (31.7, 32.7) | ||
| Height, cm | 149 ± 5.3 | ||||
| BMI, kg/m2 | 19.0 (18.0, 20.8) | ||||
| Weight, kg | 43.1 ± 5.9 | 45.7 ± 5.5* | 48.9 ± 5.9* | 2.56 ± 2.00#x2020; | 3.19 ± 1.93*#x2020 |
| ECW, kg | 10.5 ± 1.0 | 10.9 ± 1.0* | 11.4 ± 1.1* | 0.44 ± 0.49#x2020; | 0.49 ± 0.57#x2020; |
| TBW, kg | 23.6 ± 2.3 | 24.5 ± 2.2* | 25.6 ± 2.5* | 0.90 ± 1.00#x2020; | 1.08 ± 1.12*#x2020 |
| ECW, % Δ | 4.36 ± 4.86#x2020; | 4.51 ± 5.28#x2020; | |||
| TBW, % Δ | 3.94 ± 4.34#x2020; | 4.48 ± 4.61#x2020; | |||
| Plasma volume, % Δ | 16.6 ± 15.4#x2020; | −4.1 ± 10.8*#x2020 | |||
| Hb, g/L | 119 ± 11 | 109 ± 11* | 112 ± 11* | −10.4 ± 11.0#x2020; | 2.9 ± 9.6*#x2020; |
| Hematocrit | 0.36 ± 0.030 | 0.33 ± 0.029* | 0.34 ± 0.033* | −0.034 ± 0.0284 | 0.015 ± 0.026*#x2020; |
| Systolic blood pressure, mm Hg | 108 ± 9.4 | 107 ± 9.1 | −0.97 ± 9.72 | ||
| Diastolic blood pressure, mm Hg | 65 ± 7.8 | 65 ± 9.5 | 0.38 ± 10.32 |
Values are mean ± SD or median (IQR), = 377, except values were missing for gestational age (n = 7), ECW and TBW at 10 wk (n = 1), plasma volume Δ10–20 wk (n = 12), plasma volume Δ20–32 wk (n = 16), Hb at 20 wk (n = 5) and 32 wk (n = 3), Hct at 10 wk (n = 4), 20 wk (n = 5), and 32 wk (n = 9). *Different from earlier visit or change (t test), P < 0.01; †different from 0 (t test), P < 0.01. ECW, extracellular water; Hb, hemoglobin; Hct, hematocrit; TBW, total body water.
Change from 10 to 32 wk.
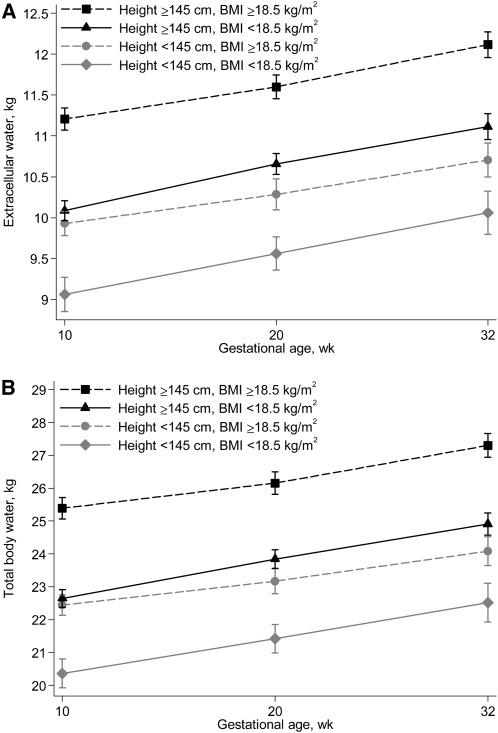
In unadjusted analysis, women who were both short (<145 cm) and underweight (<18.5 kg/m2) had the lowest ECW and TBW throughout pregnancy, whereas those who were either short or underweight had comparable intermediary values compared with those with no evidence of malnutrition (Fig. 1). ECW was 7.0–9.0% lower and TBW was 7.6–9.8% lower for underweight compared with not-underweight women at each visit. Similarly, ECW and TBW were each ~10% lower for short compared with normal-height women across pregnancy (data not shown).
FIGURE 1.
Extracellular water (A) and total body water (B) in women at 3 time points during gestation by early pregnancy (~10 wk of gestation) nutritional status characterized by height and BMI. Symbols are means and vertical lines represent 95% CI, n = 171 (black squares), 119 (black triangles), 60 (gray circles), and 27 (gray diamonds).
Differences in ECW and TBW gains were observed between nutritional status groups from 10–20 wk but not 20–32 wk of gestation (data not shown), but the gains did not follow a pattern across all groups. For both ECW and TBW, underweight women of normal height had a greater weight gain compared to normal-weight women who had short stature in early pregnancy. Also, among women with normal height, underweight women had a higher TBW gain than women of normal weight. No differences were observed in percentage change in plasma volume by nutritional status category.
In adjusted mixed-effects linear regression models, a higher BMI at ~10 wk was associated with a greater increment in ECW and TBW at all 3 visits (Table 3). Maternal height was also positively associated with ECW and TBW, independently of BMI. We then examined the relationship between initial nutritional status and body water changes. In adjusted linear regression models, early pregnancy BMI was negatively associated with ECW and TBW gain from 10 to 20 wk but not from 20 to 32 wk of gestation (Table 3), similar to the unadjusted findings based on nutritional status categories. A higher weight gain from 10 to 20 wk was associated with lower ECW and TBW changes from 20 to 32 wk of gestation. Finally, early pregnancy BMI, but neither height nor weight gain, was positively associated with plasma volume expansion from 20 to 32 wk, adjusted for confounders. When using impedance or height2/impedance alone (at 5 or 50 kHz) as the outcomes in place of ECW or TBW, respectively, the inference of associations did not change except that height was not associated with impedance alone in mixed-effects models (data not shown).
TABLE 3.
Associations of ECW, TBW, and percentage change in plasma volume with early pregnancy maternal nutritional status1
| ECW, kg |
TBW, kg |
Plasma volume, % Δ |
|||||||
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |
| ~10, ~20, and ~32 wk of gestation (n = 369)2 | |||||||||
| BMI, kg/m2 | 0.27 | (0.25, 0.29) | <0.001 | 0.66 | (0.62, 0.70) | <0.001 | |||
| Height, cm | 0.11 | (0.10, 0.12) | <0.001 | 0.25 | (0.23, 0.27) | <0.001 | |||
| Δ from ~10 to ~20 wk of gestation (n = 375)3 | |||||||||
| BMI, kg/m2 | −0.06 | (−0.08, −0.04) | <0.001 | −0.14 | (−0.18, −0.10) | <0.001 | 0.29 | (−0.37, 0.96) | 0.39 |
| Height, cm | 0.00 | (−0.01, 0.01) | 0.74 | 0.00 | (−0.02, 0.02) | 0.73 | −0.13 | (−0.42, 0.15) | 0.36 |
| Δ from ~20 to ~32 wk of gestation (n = 371)3 | |||||||||
| BMI, kg/m2 | −0.00 | (−0.03, 0.03) | 0.83 | −0.01 | (−0.07, 0.04) | 0.62 | 0.58 | (0.03, 1.14) | 0.040 |
| Weight Δ from 10–20 wk, kg | −0.04 | (−0.07, −0.01) | 0.009 | −0.08 | (−0.14, −0.02) | 0.009 | −0.11 | (−0.72, 0.51) | 0.73 |
| Height, cm | 0.01 | (−0.00, 0.02) | 0.15 | 0.02 | (−0.00, 0.04) | 0.07 | 0.14 | (−0.08, 0.35) | 0.20 |
ECW, extracellular water; TBW, total body water.
Outcomes at 3 visits (~10, ~20, and ~32 wk) regressed on maternal anthropometry at ~10 wk (fixed effect) with intercept for each participant (random effect) and random slope by gestational age with unstructured variance-covariance structure; adjusted for age (y), parity (0 vs. ≥1), infant sex, maternal education (y), living standard scale, and gestational age at assessment (wk).
Change between visits regressed on maternal anthropometry at ~10 wk with linear regression; adjusted for those in 2 plus days between measurements instead of gestational age.
Discussion
This prospective, longitudinal study examined the associations between early pregnancy maternal nutritional status and changes in ECW, TBW, and plasma volume during pregnancy. Uniquely, this study was conducted in rural Bangladesh where ~25–30% of women exhibit wasting malnutrition (mid-upper arm circumference <22 cm) and over one-half of babies are born low birth weight (27, 28), an area with a great public health need for understanding the connections between maternal nutritional status and fetal growth. Early pregnancy BMI and height, as proxies for prepregnancy measures, were positively associated with ECW and TBW during pregnancy (at ~10, 20, and 32 wk of gestation). Early pregnancy BMI, but not height, was negatively associated with ECW and TBW gains from 10 to 20 wk. BMI was positively associated with estimated percentage change in plasma volume from 20 to 32 wk, but maternal height and weight gain during pregnancy were not.
In this study, we were most interested in the plasma volume expansion of pregnancy due to its importance in nutrient transfer and association with fetal growth restriction (21, 22, 39). We were also interested in patterns of change, because plasma volume expansion is not constant during pregnancy, and therefore estimated changes across early and late gestational periods rather than examining 10- to 32-wk changes. Because we estimated plasma volume change with an equation in lieu of direct measurement, we also estimated ECW and TBW with BIA as gross proxies of plasma volume. In adult nonpregnant women, TBW is ~50–55% of body weight, ECW volume is ~40–45% of TBW, and plasma volume is ~20% of ECW (8–10, 40). Thus, it is possible that plasma volume changes can be inferred by capturing ECW and TBW and other authors have also considered BIA-based measures as proxies for plasma volume measurement (17, 18). Importantly, though, body water is representative of body composition. Increases in ECW and TBW are partially due to increases in maternal lean tissue and the products of conception, in addition to plasma volume expansion (8), with the products of conception contributing 47% of TBW gain from ~14 to 37 wk (18). In 2 unique studies examining both plasma volume and TBW longitudinally in human pregnancies, the mean increase in plasma volume was 9–10% of the mean increase in TBW (41, 42).
Low plasma volume, ECW, and TBW during pregnancy have all been associated with fetal growth restriction (2, 19, 21, 22, 43), directing research toward risk factors for low plasma volume and body water. Over 40 y ago, Hytten and Paintin (4) found that nonpregnant plasma volume was positively related to the weight and height of the mother but that the total increase in plasma volume during pregnancy was similar among all women. A small study in Chile showed that plasma volume at 35–37 wk of gestation was 15% lower in underweight compared with normal-weight women, but changes in plasma volume were not assessed (3). Butte et al. (16, 26) found that TBW in each trimester was 6–12% lower in women with low BMI (<19.8 kg/m2) than in normal BMI (19.8–26.0 kg/m2) and along with another U.S. study found that TBW gain did not differ by prepregnancy BMI status.
In this study, similar to studies of body water and plasma volume, body water was 7–10% lower in each trimester for underweight compared with not-underweight women (in unadjusted comparisons), and in adjusted analysis, lower BMI and height were independently associated with lower ECW and TBW in each trimester of pregnancy. Unlike other studies, we also found differences in gains of body water and plasma volume by early pregnancy BMI. Gains in ECW and TBW from 10 to 20 wk were negatively associated with BMI, i.e., women with a higher BMI had significantly lower increases in these body water compartments. The association with BMI was positive for plasma volume; women with a higher BMI had a higher expansion of plasma volume from 20 to 32 wk. We expect that the differences in our findings from other studies may be partially due to the poorer nutritional status in our population in addition to differences in measurement methods.
Although plasma volume and body water sometimes yield similar associations with maternal nutritional status or fetal growth, they have not been well studied jointly nor across maternal populations with differing nutritional status. As described, we expected body water to serve as a proxy for plasma volume and yield similar associations with early pregnancy nutritional status; however, this was not the case. Perhaps ECW and TBW are more representative of maternal weight gain, including that of the conceptus, rather than change in plasma volume, because it is well established that women with lower prepregnancy BMI gain more than women starting with a higher BMI. However, using the Strauss equation, we were able to establish that low early pregnancy BMI (although not height) is associated with reduced plasma volume expansion in this malnourished population, a pathway leading to fetal growth restriction (44). Because we found that BMI was negatively associated with early changes in ECW and TBW yet positively associated with later percentage change in plasma volume, our results may imply that women with a lower BMI gain more body water by gaining more weight while still experiencing lower expansion of plasma volume, possibly due to beginning the pregnancy undernourished.
Studies using gold standard measurements for ECW and plasma volume will be useful to investigate the distinction between changes in these water compartments in malnourished populations, especially as the public health goal is to optimize plasma volume expansion to support appropriate fetal growth. Our TBW equation was validated by 2H2O dilution, with a predicted residual sums of squares statistic that compares favorably to that observed by Sun et al. (45) in a large study in an American population. Using the BIA-based body water prediction equations could be considered problematic, because height and weight are components of the prediction of TBW and ECW. However, in a sensitivity analysis, we found consistent strengths of association with BMI and weight gain when impedance alone was used as a proxy for TBW and ECW, suggesting that bioelectrical properties rather than height and weight were driving the associations. We did not find an association between height and impedance alone, but because impedance is inversely related to the distance the current travels, height is a necessary component of expression for its interpretation. Just as the inclusion of height-squared in calculating BMI renders the association of body mass independent of height, its use with impedance does not drive the association with height. So height being associated with height2/Z (i.e., without weight in the equation) supports our positive finding of the height and body water association using the respective prediction equations.
A limitation of this study was the absence of a validated measure of plasma volume in pregnant women, especially because this is our main outcome of interest. However, using the Strauss equation is a reasonable approach, as discussed by Greenleaf et al. (33), because it includes Hb to account for possible changes in mean corpuscular volume that would affect plasma volume change estimation using Hct alone. Ideally, equations will be developed and validated during pregnancy for ECW and plasma volume in a similar setting of undernourishment, because gold standard methods are costly, invasive, and impractical in large field studies.
In addition to using estimates of body water and plasma volume, another limitation of this study is the absence of plasma volume changes starting from conception. Although maternal weight and TBW may not change for most in the first trimester (46), the expansion of plasma volume can occur and be detected as early as 6–10 wk gestation (39, 47) and it may have already expanded by ~14% at 12 wk (7). So our ~10-wk pregnancy estimates of BMI and TBW may be similar to preconception values, but we may not be capturing the earliest changes in plasma volume. Plasma volume plateaus at around 30–34 wk (21); thus, we did capture the majority of body water increases from plasma volume by taking the last measurement at 32 wk.
There is a public health need for examining how maternal nutritional status is connected to fetal growth, especially in regions of the world where maternal malnutrition and fetal growth restriction are common. Both plasma volume and body water have been linked to fetal growth restriction, but little is known about how a woman’s initial nutritional status influences plasma volume and body water across pregnancy. In this study, early pregnancy BMI and height were both positively associated with ECW and TBW at individual time points, and BMI was negatively associated with the change in body water in the first half of pregnancy. BMI was also positively associated with plasma volume expansion in the second half of pregnancy. Thus, in an undernourished population, women tend to follow a positive trajectory of body water increases based on initial BMI and height, whereas a lower BMI predicts higher body water gains and lower plasma volume expansion. Investigations of risk factors for poor plasma volume and methods for new, less invasive estimates of plasma volume in pregnancy are needed.
Acknowledgments
The authors thank Dr. Rolf Klemm and Dr. Hasmot Ali for their contributions to study planning and implementation and Maithilee Mitra for data oversight. A.D.G. designed the research, oversaw the field work, analyzed data, wrote the paper, and had primary responsibility for final content; P.C. designed the research and made critical revisions; K.J.S. designed the research and made critical revisions; A.B.L. designed the research; K.P.W. designed the research and made critical revisions; and S.S. and A.A.S. oversaw the field work. All authors read and approved the final manuscript.
Footnotes
Supported by the Bill and Melinda Gates Foundation and NIH grant T32HD046405 (doctoral support to A.D.G.).
Abbreviations used: BIA, bioelectrical impedance analysis; ECW, extracellular water; Hb, hemoglobin; Hct, hematocrit; LMP, last menstrual period; TBW, total body water; Z, impedance.
Literature Cited
- 1.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–12 [PubMed] [Google Scholar]
- 2.Ghezzi F, Franchi M, Balestreri D, Lischetti B, Mele MC, Alberico S, Bolis P. Bioelectrical impedance analysis during pregnancy and neonatal birth weight. Eur J Obstet Gynecol Reprod Biol. 2001;98:171–6 [DOI] [PubMed] [Google Scholar]
- 3.Rosso P, Donoso E, Braun S, Espinoza R, Salas SP. Hemodynamic changes in underweight pregnant women. Obstet Gynecol. 1992;79:908–12 [PubMed] [Google Scholar]
- 4.Hytten FE, Paintin DB. Increase in plasma volume during normal pregnancy. J Obstet Gynaecol Br Emp. 1963;70:402–7 [DOI] [PubMed] [Google Scholar]
- 5.Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw. 1973;80:884–7 [DOI] [PubMed] [Google Scholar]
- 6.Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393–9 [DOI] [PubMed] [Google Scholar]
- 7.Bernstein IM, Ziegler W, Badger GJ. Plasma volume expansion in early pregnancy. Obstet Gynecol. 2001;97:669–72 [DOI] [PubMed] [Google Scholar]
- 8.Pipe NG, Smith T, Halliday D, Edmonds CJ, Williams C, Coltart TM. Changes in fat, fat-free mass and body water in human normal pregnancy. Br J Obstet Gynaecol. 1979;86:929–40 [DOI] [PubMed] [Google Scholar]
- 9.Van Loan MD, Kopp LE, King JC, Wong WW, Mayclin PL. Fluid changes during pregnancy: use of bioimpedance spectroscopy. J Appl Physiol. 1995;78:1037–42 [DOI] [PubMed] [Google Scholar]
- 10.Lof M, Forsum E. Evaluation of bioimpedance spectroscopy for measurements of body water distribution in healthy women before, during, and after pregnancy. J Appl Physiol. 2004;96:967–73 [DOI] [PubMed] [Google Scholar]
- 11.Deurenberg P, Tagliabue A, Schouten FJ. Multi-frequency impedance for the prediction of extracellular water and total body water. Br J Nutr. 1995;73:349–58 [DOI] [PubMed] [Google Scholar]
- 12.Yasuda R, Takeuchi K, Funakoshi T, Maruo T. Bioelectrical impedance analysis in the clinical management of preeclamptic women with edema. J Perinat Med. 2003;31:275–80 [DOI] [PubMed] [Google Scholar]
- 13.Lukaski HC, Hall CB, Siders WA. Assessment of change in hydration in women during pregnancy and postpartum with bioelectrical impedance vectors. Nutrition. 2007;23:543–50 [DOI] [PubMed] [Google Scholar]
- 14.Lukaski HC, Siders WA, Nielsen EJ, Hall CB. Total body water in pregnancy: assessment by using bioelectrical impedance. Am J Clin Nutr. 1994;59:578–85 [DOI] [PubMed] [Google Scholar]
- 15.Shaikh S, Schulze KJ, Kurpad A, Ali H, Shamim AA, Mehra S, Wu LS-F, Rashid M, Labrique AB, Christian P, et al. Development of bioelectrical impedance analysis based equations for estimation of body composition in postpartum rural Bangladeshi women. Br J Nutr. (In press) [DOI] [PubMed] [Google Scholar]
- 16.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. 2003;189:1423–32 [DOI] [PubMed] [Google Scholar]
- 17.Mardones-Santander F, Salazar G, Rosso P, Villarroel L. Maternal body composition near term and birth weight. Obstet Gynecol. 1998;91:873–7 [DOI] [PubMed] [Google Scholar]
- 18.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Maternal body fat and water during pregnancy: do they raise infant birth weight? Am J Obstet Gynecol. 1999;180:235–40 [DOI] [PubMed] [Google Scholar]
- 19.Sanin Aguirre LH, Reza-Lopez S, Levario-Carrillo M. Relation between maternal body composition and birth weight. Biol Neonate. 2004;86:55–62 [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria Siega-Riz A. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol. 2010;116:1191–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–8 [DOI] [PubMed] [Google Scholar]
- 22.Salas SP, Rosso P, Espinoza R, Robert JA, Valdes G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol. 1993;81:1029–33 [PubMed] [Google Scholar]
- 23.Salas SP, Rosso P. Plasma volume, renal function, and hormonal levels in pregnant women with idiopathic fetal growth restriction or preeclampsia. Hypertens Pregnancy. 1998;17:69–79 [Google Scholar]
- 24.Larciprete G, Valensise H, Vasapollo B, Di Pierro G, Menghini S, Magnani F, De Lorenzo A, Arduini D. Maternal body composition at term gestation and birth weight: is there a link? Acta Diabetol. 2003;40 Suppl 1:S222–4 [DOI] [PubMed] [Google Scholar]
- 25.Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987;80:502–11 [PubMed] [Google Scholar]
- 26.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90:483–8 [DOI] [PubMed] [Google Scholar]
- 27.Klemm RD, Labrique AB, Christian P, Rashid M, Shamim AA, Katz J, Sommer A, West KP., Jr Newborn vitamin A supplementation reduced infant mortality in rural Bangladesh. Pediatrics. 2008;122:e242–50 [DOI] [PubMed] [Google Scholar]
- 28.West KP, Jr, Christian P, Labrique AB, Rashid M, Shamim AA, Klemm RD, Massie AB, Mehra S, Schulze KJ, Ali H, et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: a cluster randomized trial. JAMA. 2011;305:1986–95 [DOI] [PubMed] [Google Scholar]
- 29.UNICEF/WHO/UNU Composition of a multi-micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. New York: UNICEF; 1999 [Google Scholar]
- 30.Labrique AB, Christian P, Klemm RD, Rashid M, Shamim AA, Massie A, Schulze K, Hackman A, West KP., Jr A cluster-randomized, placebo-controlled, maternal vitamin A or beta-carotene supplementation trial in Bangladesh: design and methods. Trials. 2011;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson RS. Principles of nutritional assessment. 2nd ed New York: Oxford University Press; 2005 [Google Scholar]
- 32.Strauss MB, Davis RK, Rosenbaum JD, Rossmeisl EC. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest. 1951;30:862–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenleaf JE, Convertino VA, Mangseth GR. Plasma volume during stress in man: osmolality and red cell volume. J Appl Physiol. 1979;47:1031–8 [DOI] [PubMed] [Google Scholar]
- 34.Lagi A, Rossi A, Sorelli P, Cartei A, Cencetti S. Plasma volume and hematocrit changes in recurrent fainters. Clin Auton Res. 2003;13:439–42 [DOI] [PubMed] [Google Scholar]
- 35.Knechtle B, Knechtle P, Rosemann T. Do male 100-km ultra-marathoners overdrink? Int J Sports Physiol Perform. 2011;6:195–207 [DOI] [PubMed] [Google Scholar]
- 36.Buchheit M, Laursen PB, Al Haddad H, Ahmaidi S. Exercise-induced plasma volume expansion and post-exercise parasympathetic reactivation. Eur J Appl Physiol. 2009;105:471–81 [DOI] [PubMed] [Google Scholar]
- 37.Gunnsteinsson S, Labrique AB, West KP, Jr, Christian P, Mehra S, Shamim AA, Rashid M, Katz J, Klemm RD. Constructing indices of rural living standards in Northwestern Bangladesh. J Health Popul Nutr. 2010;28:509–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. 2nd ed College Station (TX): Stata Press; . 2008 [Google Scholar]
- 39.Peck TM, Arias F. Hematologic changes associated with pregnancy. Clin Obstet Gynecol. 1979;22:785–98 [DOI] [PubMed] [Google Scholar]
- 40.Aronson PS, Boron WF, Boulpaep EL. Transport of solutes and water. : Boron WF, Boulpaep EL, Medical physiology: a cellular and molecular approach. Philadelphia: Saunders Elsevier; 2008 [Google Scholar]
- 41.Pirani BB, MacGillivray I. Smoking during pregnancy. Its effect on maternal metabolism and fetoplacental function. Obstet Gynecol. 1978;52:257–63 [PubMed] [Google Scholar]
- 42.Campbell DM, MacGillivray I. Comparison of maternal response in first and second pregnancies in relation to baby weight. J Obstet Gynaecol Br Commonw. 1972;79:684–93 [DOI] [PubMed] [Google Scholar]
- 43.Levario-Carrillo M, Rodriguez N, Tufino-Olivares E, Jimenez Mdel R, Delgado-Monge MC, Reza-Lopez S. Body composition of women with newborns who are small for gestational age. Neonatology. 2009;95:15–22 [DOI] [PubMed] [Google Scholar]
- 44.Rosso P, Donoso E, Braun S, Espinoza R, Fernandez C, Salas SP. Maternal hemodynamic adjustments in idiopathic fetal growth retardation. Gynecol Obstet Invest. 1993;35:162–5 [DOI] [PubMed] [Google Scholar]
- 45.Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, Kuczmarski RJ, Flegal KM, Johnson CL, Hubbard VS. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–40 [DOI] [PubMed] [Google Scholar]
- 46.Fattah C, Farah N, Barry SC, O'Connor N, Stuart B, Turner MJ. Maternal weight and body composition in the first trimester of pregnancy. Acta Obstet Gynecol Scand. 2010;89:952–5 [DOI] [PubMed] [Google Scholar]
- 47.Blackburn ST. Maternal, fetal, and neonatal physiology: a clinical perspective. 3rd ed St. Louis: Saunders Elsevier; 2007 [Google Scholar]