Abstract
The goal of the present study was to determine the minimum concentration of systemic erythropoietin-R76E required for neuroprotection in the retina. Erythropoietin (EPO) exhibits neuroprotective effects in both in vitro and in vivo models of neuronal cell death although its classical function is the regulation of red blood cell production. It can cross the blood brain barrier and therefore can be delivered systemically to affect the retina. However, long-term treatment with exogenous erythropoietin causes polycythemia. To decrease this potentially lethal effect, we generated and tested a modified form that contains a single arginine to glutamate mutation at the 76th position (EPO-R76E). In previous studies, this mutant protected retinal neurons in mouse models of retinal degeneration and glaucoma with similar efficacy as wild-type EPO. However, EPO-R76E has attenuated erythropoietic activity, therefore, neuroprotection can be achieved without causing a significant rise in hematocrit. BALB/cByJ mice received a single intramuscular injection of recombinant adeno-associated virus carrying enhanced green fluorescent protein, Epo, or Epo-R76E. To result in continuous production of four different doses of EPO-R76E, two doses of two different serotypes (2/5 and 2/8) were used. Mice were subjected to optic nerve crush and analysis was performed thirty days later. EPO-R76E showed dose dependent protection of the retinal ganglion cell bodies, but was unable to prevent axonal degeneration. Furthermore, EPO-R76E induced a dose-dependent rise in the hematocrit that was still attenuated as compared to wild-type EPO.
Keywords: erythropoietin, neuroprotection, optic nerve crush, retinal ganglion cells, dose, gene therapy
1. INTRODUCTION
One relatively new and exciting addition to the field of neuroprotection is eythropoietin (EPO). The most notable role of EPO is the production of red blood cells. Since the pioneering work of Juul et al. (1998), many investigators have shown that endogenous EPO and its receptor are found in neural tissue. And, addition of exogenous EPO protects neurons from excitotoxic injury, ischemic insults, mechanical trauma and other neurodegenerative disease states (for review see Grimm et al., 2005). In the eye, intravitreal injection of EPO protects retinal ganglion cells (RGC) from death (Weishaupt et al., 2004; Kretz et al., 2005; Wang et al., 2009), and systemic or intraocular EPO protects the photoreceptors from inherited and light-induced cell death (Grimm et al., 2002, 2004; Rex et al., 2009). These results demonstrate that EPO can protect neurons from an immediate injury. We have taken a different approach to address neuronal diseases such as glaucoma that are slow and progressive. EPO is delivered into muscle using recombinant adeno-associated virus (rAAV)-mediated gene therapy. This method delivers neuroprotective levels of EPO continuously for the duration of the experiment, eliminating the need for repeated dosing (Rex et al., 2004; Sullivan et al., 2011a).
Our previous studies showed that intraocular delivery of rAAV2/2.Epo is not neuroprotective in multiple mouse models of retinal degeneration despite generating high levels of EPO in the eye (Rex et al., 2004). However, intramuscular delivery of rAAV2/2.Epo is effective at preventing retinal cell death (Rex et al., 2004). Direct injection of recombinant human EPO into the eye also prevents photoreceptor cell death without inducing a rise in hematocrit, implying that EPO itself is protective to the retina, and that the neuroprotective effect of systemic delivery of EPO is likely due to the EPO that reaches the retina and not due to an indirect effect of the increase in hematocrit (Rex et al., 2009). A direct effect of EPO on the retina is also supported by the studies of Weishaupt et al., 2004; Kretz et al., 2005; and Wang et al. 2009 showing that intraocular delivery of recombinant human EPO protects the retinal ganglion cells. These studies also imply that the therapeutic dose range for EPO is bell-shaped (Weishaupt et al., 2004; Rex et al., 2004, 2009). Thus, if intraocular gene delivery is pursued, there will be a need for precise regulation of gene expression.
In contrast, an upper limit of a therapeutic dose for systemic administration of EPO has not been detected (possibly because the blood brain barrier limits the amount of EPO that reaches the neuronal tissue). But, one of the challenges with long-term systemic delivery of EPO is polycythemia (Rex et al., 2004, Sullivan et al., 2011a,b), which can lead to death. To overcome this lethal effect, a form of EPO that has attenuated eythropoietic activity (EPO-R76E) was generated and shown to be neuroprotective in the retinal degeneration slow mouse (Sullivan et al., 2011a). Our group has used rAAV.EpoR76E to protect neurons in a naturally occurring mouse model of glaucoma, the DBA/2J mouse. The continuous systemic delivery of 67mU/ml EPO-R76E preserved 70% of RGC somata and axons, and preserved visual function as measured by visual evoked potentials in the absence of a significant rise in hematocrit (Sullivan et al., 2011b). The goal of this study is to further define the levels of continuously produced EPO-R76E in the serum that are sufficient to protect RGC bodies from axonal injury. To induce different levels of EPO-R76E in the serum we utilized two concentrations of two rAAV serotypes known to be effective in transducing skeletal muscle, rAAV2/5 and rAAV2/8. Louboutin et al., 2005 performed direct comparisons of the transduction efficiency of rAAV2/5 and rAAV2/8 and showed that rAAV2/8 transduced about 30% more muscle fibers than rAAV2/5. They also found that both serotypes transduced slow and fast muscle fibers to a similar degree. The goal of the current study was not to compare these two serotypes and their efficiency, but rather, to use them as tools to generate different amounts of our therapeutic agent systemically, in vivo.
There are many proteins that have been identified as neuroprotective and a few have reached the level of clinical trials, however, to date, none have been successful either due to adverse effects or lack of efficacy (for review, see Meuth et al., 2010; www.ClinicalTrials.gov). One of the major challenges is delivery since most of these proteins do not cross the blood brain barrier, so repeat, invasive, technically challenging injections are required. In contrast, EPO is able to cross the blood brain barrier (Brines et al., 2000; Juul et al., 2004; Banks et al., 2004) and is already FDA approved and safely in use in the clinic for the treatment of anemia. In fact, induction of erythropoiesis is the major negative side-effect when considering systemic delivery of EPO for neuroprotective therapy. Therefore, we developed a mutant form of EPO that displayed the same level of neuroprotection as wild-type EPO (EPO-R76E; Sullivan et al., 2011a,b). In this study (Sullivan et al., 2011b), treatment with wild-type EPO elevated the hematocrit to lethal levels, requiring weekly phlebotomy. Treatment with similar doses of EPO-R76E did not require bleeding to maintain the hematocrit within the normal range, for this mutant form of EPO has attenuated erythropoietic activity.
Optic nerve crush is a commonly used model of glaucoma and acute RGC death (Bien et al., 1999; Levkovitch-Verbin, 2004). Like many models it does not perfectly emulate the disease but it does provide insight into the mechanisms involved in the death of the ganglion cells by providing a precise, short-term, synchronous insult to the axons resulting in secondary RGC apoptotic cell death as in glaucoma (Li et al., 1999; Levkovitch-Verbin et al., 2000). The mechanical injury to the optic nerve activates many of the same apoptotic pathways that are enabled during glaucoma (Quigley and Addicks, 1981; Quigley et al., 1981; Isenmann et al., 1997; Kermer et al., 1998; Li et al., 1999; Li et al., 2000; Libby et al., 2005; Zalewska et al., 2008). The present study uses this optic nerve crush model to define the therapeutic dose response curve for systemically delivered EPO-R76E.
2. MATERIALS and METHODS
2.1 Injections
BALB/cByJ mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Due to its genetic background, the BALB/cByJ mouse is the strain most susceptible to optic nerve crush (Brines and Cerami, 2008) and therefore serves as an ideal model to study the neuroprotective effects of EPO-R76E. The viral vectors were produced and purified by the University of Iowa or the University of Pennsylvania Vector Cores. A Hamilton syringe was used to deliver 10μl of: 1X1010 genome copies (gc) of rAAV2/5.CMV.eGFP; 1X1010 gc rAAV2/5.CMV.Epo; 3x1010 gc (dose 1) or 1 x1011 gc (dose 2) of rAAV2/5.EpoR76E; or 5 x109 gc (dose 3) or 1 x1012 gc (dose 4) of rAAV2/8.EpoR76E into the quadriceps of two month old mice. The Epo transgene was derived from rhesus macaque. All experimental procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee at the University Tennessee Health Science Center
2.2 Optic nerve crush
Thirty days after intramuscular vector delivery, mice were anesthetized with ketamine/xylazine/urethane (25/10/1000 μg/g body weight) and a small incision was made in the lateral aspect of the conjunctiva. With a pair of small forceps the edge of the conjunctiva next to the globe was retracted slightly and rotated laterally, allowing visualization of the posterior aspect of the globe and the optic nerve. Viewed under a binocular operating microscope, the surrounding connective tissue and muscle was gently separated from the nerve. The exposed optic nerve was crushed for 10 sec with a pair of Dumont cross-clamp #7 forceps (Roboz, cat. #RS = 5027, Gaithersburg, MD). This instrument was chosen because its spring action applied a moderate yet constant and consistent force to the optic nerve. The forceps were then removed and the eye was allowed to rotate back into place.
2.3 Immunohistochemistry
Thirty days post-crush, mice were euthanized and eyes were enucleated and stored in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 at 4°C. Retinas were isolated and incubated in phosphate buffered saline (PBS) and blocked in 20% normal donkey serum in PBS containing 0.1% Triton-X-100 and 0.5% BSA for a minimum of 2 h at 4°C. The primary antibody, anti-NeuN, a marker for RGCs (Buckingham et al., 2008; Canola et al., 2007; Dijk et al., 2007; Zhong et al., 2007) and some displaced amacrine cells, (Chemicon, Temecula, CA) was used at 1:500 and secondary antibody was used at 1:200 (Alexa 488, Invitrogen, Carlsbad, CA). Retinas were placed RGC side up, mounted with Vectashield containing DAPI (Vector Labs, Burlingame, CA) and viewed using a Nikon Eclipse TE2000 laser scanning confocal microscope (Nikon, Japan). The retinal flatmounts were probed with anti-NeuN, a marker for RGCs and some displaced amacrine cells (Zhong et al., 2007; Canola et al., 2007; Dijk et al., 2007; Buckingham et al., 2008).
2.4 RGC imaging and counts
Retinas were first imaged at 4X magnification and a grid was placed over each retina to randomly select 8 locations to be imaged at 40X magnification. The NeuN-labeled cells in each region were counted manually using ImageJ software (available by ftp at http://rsbweb.nih.gov/ij/ developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) and Metamorph (Universal Imaging Corporation, Downingtown, PA).
2.5 EPO enzyme-linked immuno-sorbant analysis (ELISA) and hematocrit
Serum from blood samples was probed for EPO using the human Quantikine IVD EPO ELISA Kit according to manufacturer’s protocol (R&D Systems, Minneapolis, MN). It should be noted that the ELISA kit is calibrated against human EPO and has been shown to be 4-fold less sensitive for rhesus versus human EPO (Rivera et al., 2005). This was taken into account for calculations within the manuscript. The absorbance at 450 nm with a 600 nm reference was detected on a BioTek - μQuant plate reader (Winooski, VT). In some mice the serum samples were pooled in order to obtain sufficient material for the ELISA. Hematocrit was measured by capillary centrifugation.
2.6 Optic nerve histology
Optic nerves were isolated and placed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 for one week at 4°C. Next, samples were post-fixed in 1% osmium tetroxide in 0.1 M cacodylate buffer, dehydrated in a graded ethanol series, further dehydrated in propylene oxide and embedded in Embed-812 resin (EMS, Hatfield PA), cut into 1 μm-thick sections (Reichert-Jung Ultracut E, Austria) and stained with 1% p-phenylenediamine in 50% methanol (Sigma-Aldrich, St Louis, MO). Sections were viewed by light microscopy using an Olympus BX51 microscope (Olympus America inc, PA). Prior to beginning axon counts, the optic nerve was traced at 20X magnification and cross- sectional area automatically calculated using ImageJ software. The entire length from top to bottom of same cross section was then imaged using a 60X oil immersion lens. Approximately the same region of 0.010 mm2 was selected from each high magnification image; both live and dead axons were manually counted using ImageJ software. Measurements of the cross-sectional area of the optic nerve were used with axon density to estimate the total number of axons.
2.7 Statistical analysis
A one-way ANOVA followed by a pair-wise Bonferroni post hoc comparison test was used to determine statistical significance when comparing NeuN counts and axon counts. Statistical analysis was performed with Prism 4.0 software (GraphPad, San Diego, CA).
3. RESULTS
3.1 Dose dependent increase in hematocrit and serum EPO-R76E levels
The left quadriceps of BALB/cByJ mice were injected with rAAV vector carrying either enhanced green fluorescent protein (eGFP, negative control), Epo (positive control), or EpoR76E. The amount of circulating EPO (including EPO-R76E) in each animal and the hematocrit was quantified (Table 1). Negative control mice had no detectable circulating EPO and an average hematocrit of 46%. Positive control mice that received rAAV2/5.Epo had 83 ± 26mU/ml (±SD) of EPO in their serum and an expected increase in the hematocrit to 84%. Due to the short duration of the experiment no phlebotomy was required for the mice to survive to the experimental endpoint. Two different serotypes of rAAV.EpoR76E were used in the study. Serotype rAAV2/5 was injected at 3x1010 (dose 1) or 1 x1011 gc (dose 2), and serotype rAAV2/8 was injected at 5 x109 (dose 3) or 1 x1012 gc (dose 4). The resulting serum EPO-R76E concentrations are listed in Table 1 from lowest (dose 1) to highest (dose 4). Treatment with dose 1 yielded 9 ± 3.4mU/ml EPO-R76E in the serum, and the average hematocrit in these mice was 47%. Treatment with dose 2 produced 30 ± 4.6mU/ml EPO-R76E in the serum and an average hematocrit of 48%. Treatment with dose 3 generated 132 ± 13 mU/ml EPO-R76E in the serum and a hematocrit of 64%. Finally, treatment with dose 4 resulted in 264 ± 17mU/ml EPO-R76E in the serum, and a hematocrit of 67%. Thus, we were able to generate four different concentrations of EPO-R76E in the serum along with the positive and negative controls. The two highest doses of EPO-R76E induced a rise in hematocrit above normal levels. However, despite three times the level of the positive control (EPO-R76E) in the serum, the hematocrit was almost 20% lower in dose 4 mice than in the rAAV.Epo injected mice. These data demonstrate a significant attenuation of the erythropoietic activity in EPO-R76E.
Table 1.
Hematocrit and serum EPO levels in treated and control mice.
| Treatment | Hematocrit | EPO (mU/ml) | N |
|---|---|---|---|
| rAAV.CMV.eGFP | 46% ± 1.7 | 0 | 10 |
| rAAV.CMV.Epo | 84% ± 4.5 | 83 ± 26 | 8 |
| Dose 1 | 47% ± 3.0 | 9 ± 3.4 | 6 |
| Dose 2 | 48% ± 8.5 | 30 ± 4.6 | 18 |
| Dose 3 | 64% ± 11.0 | 132 ± 13 | 4 |
| Dose 4 | 67% ± 7.8 | 264 ± 17 | 11 |
The values for hematocrit are ± SD and the values for EPO concentration are ± SEM. N= number of mice.
3.2 Systemic delivery of EPO-R76E protects NeuN positive cells in the ganglion cell layer in a dose dependent manner
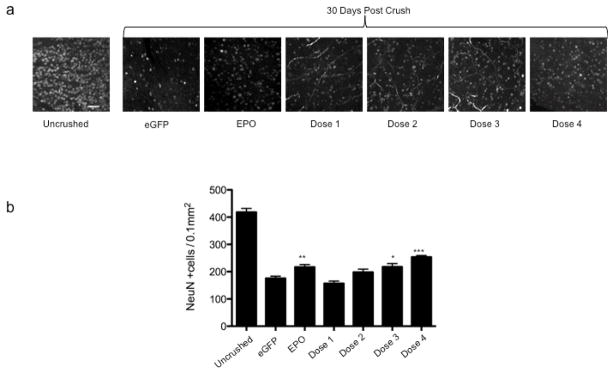
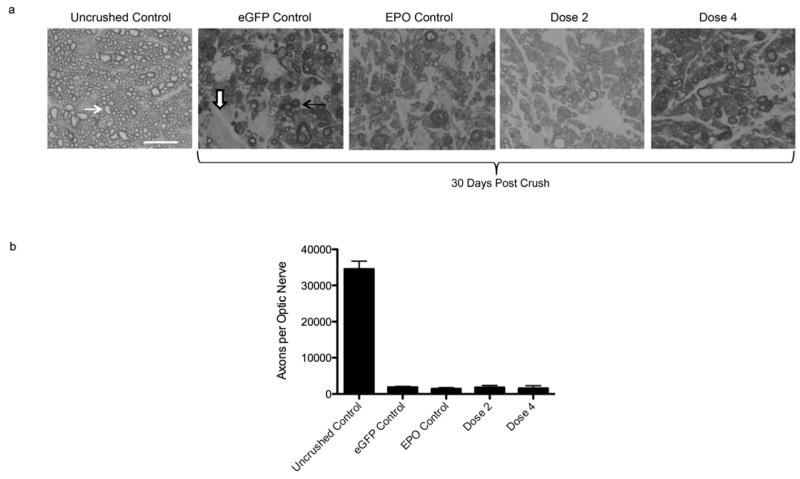
Retinas from uninjected, normal mice had a high density of anti-Neuronal Nuclei (NeuN)- labeled cells in the ganglion cell layer (GCL) (Fig. 1a). As expected, after optic nerve crush there was a large decrease in the number of NeuN-labeled cells in negative control mice. Even in treated mice (EPO or Doses 1–4 of EPO-R76E) there were many fewer NeuN-positive cells in the GCL as compared to the no crush control. However, the retinas from mice treated with rAAV.Epo did appear to have more NeuN-labeled cells in the GCL after optic nerve crush than the retinas from mice treated with rAAV.eGFP. The number of NeuN-labeled cells also appeared to be higher in mice treated with either doses 3 or 4 of EPO-R76E as compared to the negative control group. In fact, retinas from mice treated with either dose 3 or 4 appeared to have a similar density of NeuN-labeled cells as was observed in positive control animals. To confirm these observations, NeuN-labeled cells in the GCL layer in each group were manually counted (Fig. 1b). The average cell density of negative control mice 30 days after optic nerve crush was 175 ± 34 cells/0.1mm2, a 58% reduction from normal retina (418 ± 82 cells/0.1mm2). Treatment with rAAV.Epo (84mU/ml EPO) prevented 10% of the neuronal loss (217 ± 40 cells/0.1mm2, p ≤ 0.01). The protection provided by EPO-R76E was concentration dependent. At the lowest concentration, dose 1, EPO-R76E was unable to prevent neuronal cell death (157 ± 31 cells/0.1mm2). Treatment with dose 2 of EPO-R76E increased the NeuN-positive cell density to 198 ± 45 cells/ 0.1mm2 (5% greater than the negative control). This level trended towards neuroprotection, but was not statistically significant. The two highest concentrations of EPO-R76E tested provided statistically significant protection using the stringent Bonferroni post-hoc test. The NeuN-labeled cell density was 218 ± 37 cells/0.1mm2 (p ≤ 0.05) and 253 ± 25 cells/0.1mm2 (p ≤ 0.001), for doses 3 and 4, respectively. For Dose 3, this correlated to a 52% of no crush control levels (10% more than the negative control, and identical to treatment with rAAV2/5.Epo; Fig. 1b). Dose 4 of EPO-R76E resulted in 14% more NeuN-labeled cells than mice treated with the Epo vector (p ≤ 0.05), a preservation of 60% of retinal ganglion cells (60% of no-crush control levels). These results demonstrate that neuroprotection by systemic EPO-R76E is dose-dependent, and the two lowest doses were below the effective therapeutic concentration.
Figure 1. Treatment with rAAV.CMV.Epo or rAAV.CMV.EpoR76E protects NeuN positive cells in the GCL layer against optic nerve crush.
(a) Confocal micrographs of retinal flat mounts labeled with anti NeuN. Images are from the ganglion cell layer of the retina. (b) Bar graph showing the average density of NeuN positive cells. Data are means ± S.E.M., and statistical analysis was done by one-way ANOVA with pair-wise Bonferroni post hoc test. eGFP = enhanced green fluorescent protein, * p ≤ 0.05 , ** p ≤ 0.01, *** p ≤ 0.001 vs. eGFP. Scale bar = 50 μm.
3.3 EPO is unable to protect axons in the optic nerve crush model
p-Phenylenediamine was used to label myelin surrounding axons in the optic nerve. Stained cross sections of the nerves were examined by high magnification bright-field microscopy to determine the percentage of surviving axons in treated animals (Fig. 2a). The optic nerves from all mice that received an optic nerve crush, regardless if treated or controls, looked similar. They all had large areas of gliosis and many darkly labeled axons indicative of axonal degeneration. To confirm this observation, the myelinated axons with clear axoplasms were manually counted using a grid system (Fig. 2b). Cross sections from uncrushed optic nerves contained an average of 34,562 healthy axons with few if any dead axons. Following optic nerve crush this number decreased to 1,877 normal appearing axons per optic nerve in negative control mice, 1,452 normal appearing axons per optic nerve in positive control mice, and 1,808 normal appearing axons per optic nerve in mice that received Dose 4 of EPO-R76E. There was no statistically significant difference in the number of surviving axons in any of the optic nerve crush groups between any treatment or control conditions. All groups had a statistically significant decrease in the number of axons as compared to the no crush control.
Figure 2. Treatment with rAAV.CMV.Epo or rAAV.CMV.EpoR76E does not protect axons from optic nerve crush.
(a) Brightfield micrographs of optic nerve cross sections stained with PPD. (b) Bar graph of average number of axons per optic nerve. There was no statistically significant difference between groups that received vector treatment. Data are means ± S.E.M., and statistical analysis was performed by one-way ANOVA with pair-wise Bonferroni post hoc test. Healthy axons (white arrow), degenerating/dying axons (
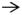 ), and gliosis (
), and gliosis (
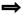 ) are indicated. Scale bar = 10 μm.
) are indicated. Scale bar = 10 μm.
4. DISCUSSION
The mouse GCL is composed of nearly 60% displaced amacrine cells and 40% RGCs (Jeon et al., 1998). In addition to labeling RGCs, NeuN also labels a small population of displaced amacrine cells (5–15%) in the GCL (Buckingham et al., 2008). Therefore, it is possible that the population of neurons being protected by EPO is composed of RGCs, displaced amacrine cells, or both. However, in rat, displaced amacrine cells are not affected one month after optic nerve lesion (Kielczewski et al., 2005), making it likely that EPO and EPO-R76E are protecting RGCs only and not displaced amacrine cells.
Systemic EPO-R76E protects RGCs in a dose-dependent manner. At the lowest dose of EPO-R76E (9mU/ml) there was no neuroprotection, at the second lowest dose (30mU/ml), there appeared to be a trend towards neuroprotection but it was not significant using stringent statistical criterion. We have detected significant protection of RGCs in the DBA/2J mouse model of glaucoma with 67mU/ml EPO-R76E (Sullivan et al., 2011b). In the current study, the third dose (132mU/ml) caused a significant increase in the numbers of RGC surviving after ONC, and the treatment effect increased with the highest dose tested (264mU/ml). But, even the highest dose of EPO-R76E only prevented 18% of the RGC death, indicating that there may be a more effective dose than those tested in this study. These results indicate that the low end of the therapeutic dose curve for EPO-R76E lies between 30 and 67mU/ml. The correlative increase in hematocrit levels precluded assessment of higher doses of systemic EPO-R76E.
The neuroprotective effect of EPO is well characterized in RGCs and is similar to that observed in other neurons (Kretz et al., 2005; Weishaupt et al., 2004; Zhong et al., 2007; King et al., 2007). Following injury of the optic nerve RGC cell bodies die by apoptosis, characterized by activation of caspases 3 and 9 (Kermer et al., 1998; Kermer et al., 2000), downregulation of PI-3K/Akt kinases (Klocker et al., 2000), degradation of nuclear DNA and condensation of the nuclei (Bien et al., 1999). EPO blocks apoptosis preventing death by blocking the activation of caspase 3 through the PI-3-Kinase, Akt pathway (Weishaupt et al., 2004), and by upregulating Bcl-XL (Kretz et al., 2005). The findings of the present study do not contradict these findings and in fact support them as a potential mechanism.
We did not observe any axonal protection at the doses used; however this does not preclude the possibility that higher doses of EPO-R76E may protect axons from Wallerian degeneration. The axons undergo Wallerian degeneration (Wang et al., 2006; Howell et al., 2007; Beirowski et al., 2008) a process that is independent of the apoptotic pathway (Raff et al., 2002; Ehlers, 2004). Either no axonal protection was achieved in our study due to the severity of the crush, or we may have missed preserved axons because of the way we analyzed the optic nerves and/or the small number of potentially surviving axons based on the number of surviving RGCs. King et al., 2007 imaged the optic nerve in longitudinal sections and reported axonal re-growth at two regions of the optic nerve, both proximal to the transection site. Here we only examined a 1 μm cross section of the optic nerve and the spatial relationship to the injury site was unknown. Therefore it is possible that neuroprotection may be provided in other regions of the optic nerve. Although we did not observe axonal protection in the current study, King et al., 2007 observed stimulation of axonal growth into peripheral nerve grafts after intraocular injections of EPO in a nerve transection model. Furthermore, EPO stimulates axonal growth in cultured retinal explants (Kretz et al., 2005). Nonetheless, previous studies from our laboratory have demonstrated axonal sparing in the DBA/2J mouse model of glaucoma when the animals were treated with systemic EPO or EPO-R76E using gene therapy (Sullivan et al., 2011b). In this earlier study, we detected a 70% axonal preservation 9 months after EPO or EPO-R76E treatment (4 months after onset axonal damage in untreated mice). The data from the DBA/2J mouse reveal that EPO and EPO-R76E can promote axon survival, and suggests that the lack of protection in the ONC model was due to the severity of the crush injury.
5. CONCLUSION
Obtaining the optimal therapeutic dose is critical for any drug delivery, maximizing the wanted effect of the agent and minimizing negative side effects. To be effective as a neuroprotectant EPO must be administered at levels higher than is approved for treatment of anemia in humans (for review see, Ehlers, 2004). EPO, like many other cytokines, may have a bell shaped dose curve, making it difficult to define the optimal therapeutic dose (Weishaupt et al., 2004; Zhong et al., 2007). We identify the lower limit of circulating EPO required for neuroprotection of RGCs, with the threshold of statistically significant neuroprotection being greater than 30mU/ml EPO-R76E in the serum. Further protection of the RGCs was achieved by treatment with higher doses and it is possible that we have not detected the peak effect since the highest dose tested still showed improved protection of RGCs. Unfortunately, the two highest doses of continuous, systemic EPO-R76E (132 and 264mU/ml), raised the hematocrit to levels that would not be acceptable in humans (65–67%). While this level of erythropoietic activity is still much attenuated from wild-type EPO, which, at lower doses, would require regular phlebotomy to maintain a hematocrit of approximately 80% (Sullivan et al., 2011b), it does indicate that achieving the maximal level of neuroprotection possible by systemic EPO-R76E may require further attenuation of the erythropoietic effects of EPO. One possibility is to generate compound mutants of EPO in which the amino acids at positions 100 and/or 103 are converted to glutamate. These mutations have also been shown to be neuroprotective, but have decreased ability to bind the erythropoietin receptor (Leist et al., 2004). The promise of EPO-R76E for neuroprotection warrants its examination as a treatment for many neurodegenerative diseases, such as: glaucoma, Alzheimer’s disease, Parkinson’s Disease, spinal cord injury, and even neurotrauma.
Systemic gene delivery of EPO-R76E protects RGC somata from optic nerve crush.
This protection is dose-dependent.
No protection of the RGC axons was detected in the optic nerve.
Acknowledgments
The authors thank Kathy Troughton and Yuming Hu in the UTHSC Neuroscience Imaging Core for resin embedding and sectioning the optic nerves.
This work was supported by NEI Core Grant P50EY13080, unrestricted grant from Research to Prevent Blindness, PHS Grant RO1EY017841 (EEG), U.S. Army Medical Research and Materiel Command and the Telemedicine & Advanced Technology Research Center W81XWH-10-1-0528 (TSR); Research to Prevent Blindness Career Development Award (TSR); Glaucoma Research Foundation (TSR); UTHSC Neuroscience Institute (TSR); and Roche Foundation for Anemia Research (TSR).
Abbreviations
- RGC
retinal ganglion cells
- EPO
erythropoietin
- rAAV
recombinant adeno-associated virus
- NeuN
Neuronal Nuclei
Footnotes
Conflicts of Interest: Timothy A. Sullivan and Tonia S. Rex are co-authors on a patent application for the use of EPO-R76E in the treatment of neurodegenerative diseases. No commercialization or marketing has been performed.
Department of Defense Non-endorsement Disclaimer: The views, opinions and/or findings contained in this research presentation are those of the authors and do not necessarily reflect the views of the Department of Defense and should not be construed as an official DoD/Army position, policy or decision unless so designated by other documentation. No official endorsement should be made.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Timothy A. Sullivan, Email: timothy.sullivan@armstrong.edu.
Eldon E. Geisert, Email: egeisert@uthsc.edu.
Justin P. Templeton, Email: jtemplet@uthsc.edu.
Tonia S. Rex, Email: trex@uthsc.edu.
References
- Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC. Passage of erythropoietic agents across the blood-brain barrier: A comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol. 2004;505:93–101. doi: 10.1016/j.ejphar.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Coleman MP, Martin KR. The wlds gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur J Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- Bien A, Seidenbecher CI, Bockers TM, Sabel BA, Kreutz MR. Apoptotic versus necrotic characteristics of retinal ganglion cell death after partial optic nerve injury. J Neurotrauma. 1999;16:153–163. doi: 10.1089/neu.1999.16.153. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2008;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canola K, Angenieux B, Tekaya M, Quiambao A, Naash MI, Munier FL, Schorderet DF, Arsenijevic Y. Retinal stem cells transplanted into models of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate. Invest Ophthalmol Vis Sci. 2007;48:446–454. doi: 10.1167/iovs.06-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk F, Bergen AA, Kamphuis W. Gap-43 expression is upregulated in retinal ganglion cells after ischemia/reperfusion-induced damage. Exp Eye Res. 2007;84:858–867. doi: 10.1016/j.exer.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Deconstructing the axon: Wallerian degeneration and the ubiquitin-proteasome system. Trends Neurosci. 2004;27:3–6. doi: 10.1016/j.tins.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Remé CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, Naash M, Gassmann M, Remé C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Hermann DM, Bogdanova A, Hoptop S, Kilic U, Wenzel A, Kilic E, Gassmann M. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005;16:531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, Whitmore AV, Masland RH, John SW. Axons of retinal ganglion cells are insulted in the optic nerve early in dba/2j glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenmann S, Wahl C, Krajewski S, Reed JC, Bahr M. Up-regulation of bax protein in degenerating retinal ganglion cells precedes apoptotic cell death after optic nerve lesion in the rat. Eur J Neurosci. 1997;9:1763–1772. doi: 10.1111/j.1460-9568.1997.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erythropoeitin concentrations in cerebrospinal fluid on nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Bahr M. Inhibition of cpp32–like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci. 1998;18:4656–4662. doi: 10.1523/JNEUROSCI.18-12-04656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermer P, Ankerhold R, Klocker N, Krajewski S, Reed JC, Bahr M. Caspase-9: Involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res. 2000;85:144–150. doi: 10.1016/s0169-328x(00)00256-4. [DOI] [PubMed] [Google Scholar]
- Kielczewski JL, Pease ME, Quigley HA. The effect of experimental glaucoma and optic nerve transection on amacrine cells in the rat retina. Invest Ophthalmol Vis Sci. 2005;46:3188–3196. doi: 10.1167/iovs.05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Rodger J, Bartlett C, Esmaili T, Dunlop SA, Beazley LD. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp Neurol. 2007;205:48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Klocker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3'-kinase/protein kinase b signaling. J Neurosci. 2000;20:6962–6967. doi: 10.1523/JNEUROSCI.20-18-06962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz A, Happold CJ, Marticke JK, Isenmann S. Erythropoietin promotes regeneration of adult cns neurons via JAK2/STAT3 and PI3K/Akt pathway activation. Mol Cell Neurosci. 2005;29:569–579. doi: 10.1016/j.mcn.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Billa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H. Animal models of optic nerve diseases. Eye (Lond) 2004;18:1066–1074. doi: 10.1038/sj.eye.6701576. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Harris-Cerruti C, Groner Y, Wheeler LA, Schwartz M, Yoles E. Rgc death in mice after optic nerve crush injury: Oxidative stress and neuroprotection. Invest Ophthalmol Vis Sci. 2000;41:4169–4174. [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Nickells RW. Experimental induction of retinal ganglion cell death in adult mice. Invest Ophthalmol Vis Sci. 1999;40:1004–1008. [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Poulsen KP, Nickells RW. Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Exp Eye Res. 2000;71:209–213. doi: 10.1006/exer.2000.0873. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Semaan SJ, Schlamp CL, Nickells RW. Dominant inheritance of retinal ganglion cell resistance to optic nerve crush in mice. BMC Neurosci. 2007;8:19. doi: 10.1186/1471-2202-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louboutin JP, Wang L, Wilson JM. Gene transfer into skeletal muscle using novel AAV serotypes. J Gene Med. 2005;7:442–451. doi: 10.1002/jgm.686. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Bittner S, Ulzheimer JC, Kleinschnitz C, Kieseier BC, Wiendl H. Therapeutic approaches to multiple sclerosis: an update on failed, interrupted, or inconclusive trials of neuroprotective and alterative treatment strategies. BioDrugs. 2010;24:317–330. doi: 10.2165/11537190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–143. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–649. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- Rex TS, Allocca M, Domenici L, Surace EM, Maguire AM, Lyubarsky A, Cellerino A, Bennett J, Auricchio A. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol Ther. 2004;10:855–861. doi: 10.1016/j.ymthe.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Rex TS, Wong Y, Kodali K, Merry S. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp Eye Res. 2009;89:735–740. doi: 10.1016/j.exer.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, Clackson T, Wilson JM. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Kodali K, Rex TS. Systemic gene delivery protects the photoreceptors in the retinal degeneration slow mouse. Neurochem Res. 2011a;36:613–618. doi: 10.1007/s11064-010-0272-6. [DOI] [PubMed] [Google Scholar]
- Sullivan TA, Geisert EE, Hines-Beard J, Rex TS. Systemic AAV-mediated gene therapy preserves retinal ganglion cells and visual function in DBA/2J glaucomatous mice. J Hum Gene Ther. 2011b;22:1191–1200. doi: 10.1089/hum.2011.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Yuan M, Neufeld AH. Degeneration of neuronal cell bodies following axonal injury in wld(s) mice. J Neurosci Res. 2006;84:1799–1807. doi: 10.1002/jnr.21075. [DOI] [PubMed] [Google Scholar]
- Weishaupt JH, Rohde G, Polking E, Siren AL, Ehrenreich H, Bahr M. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu ZL, Zhuang XT, Wang MF, Xu L. Neuroprotective effect of recombinant human erythropoietin on optic nerve injury in rats. Chin Med J (Engl) 2009;122:2008–2012. [PubMed] [Google Scholar]
- Zalewska R, Zalewski B, Reszec J, Mariak Z, Zimnoch L, Proniewska-Skretek E. The expressions of fas and caspase-3 in human glaucomatous optic nerve axons. Med Sci Monit. 2008;14:BR274–278. [PubMed] [Google Scholar]
- Zhong L, Bradley J, Schubert W, Ahmed E, Adamis AP, Shima DT, Robinson GS, Ng YS. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest Ophthalmol Vis Sci. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]