Abstract
This review briefly describes the changes in baroreflex function that occur during female reproductive life, specifically during the reproductive cycle and pregnancy. The sensitivity or gain of baroreflex control of heart rate and sympathetic activity fluctuates during the reproductive cycle, reaching a peak when gonadal hormones rise, during the follicular phase in women and proestrus in rats. The increase in baroreflex sensitivity (BRS) is likely mediated by estrogen, because ovariectomy in rats eliminates the BRS increase, the cyclic profile of changes in BRS mirror the changes in estrogen, and estrogen acts in the brainstem to increase BRS. Pregnancy, on the other hand, depresses both BRS and the maximal level of sympathetic activity and heart rate evoked by severe hypotension. The decrease in BRS may be mediated by a reduction in the actions of insulin in the arcuate nucleus to support the baroreflex. In addition, increased levels of the neurosteroid progesterone metabolite, 3α-OH-DHP, act downstream in the rostral ventrolateral medulla to suppress maximal baroreflex increases in sympathetic activity. Consequently, these changes in baroreflex function impair blood pressure regulation in the face of hypotensive challenges like orthostasis and hemorrhage, a common event during delivery. As a result, peripartum hemorrhage is a major cause of human maternal mortality.
The baroreceptor reflex is the major mechanism that maintains arterial pressure at set limits from moment to moment. Considerable data have documented that baroreflex function is not static, but changes under both physiologic and pathophysiological conditions. The purpose of this short review is to summarize the alterations in baroreflex function that occur in females during two physiological events: the reproductive cycle and pregnancy.
Baroreflex function during the reproductive cycle
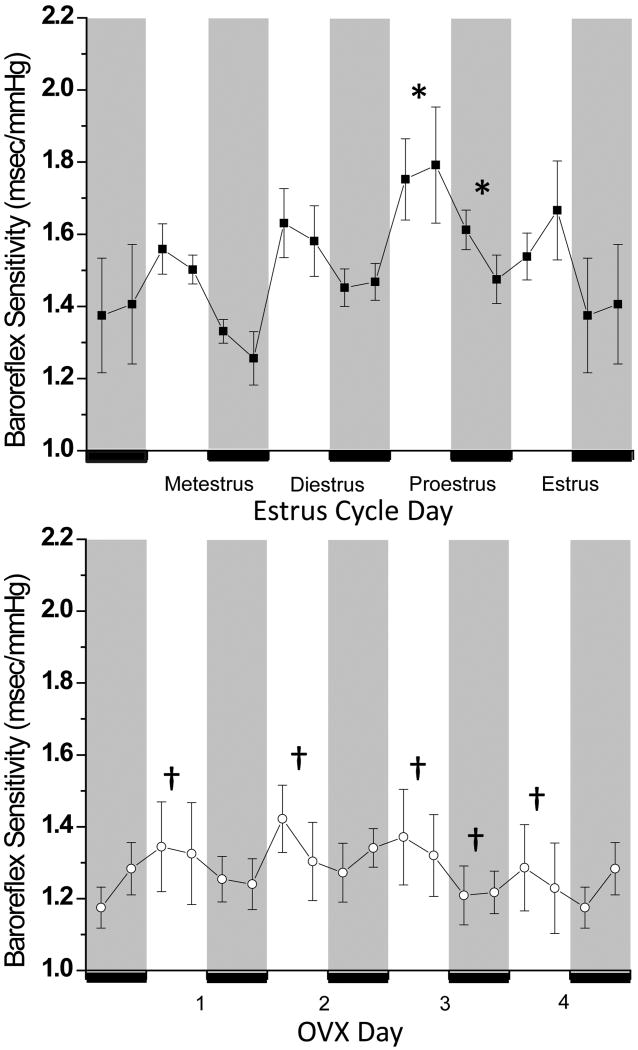
In women, the sensitivity or gain of baroreflex control of muscle sympathetic nerve activity (MSNA) increases during the mid-luteal phase of the menstrual cycle, when gonadal hormones (estrogen and progesterone) are elevated, compared to the early follicular phase, when these hormones are relatively low.1 Basal MSNA and plasma levels of norepinephrine increase in parallel during the luteal phase.1;2 Increases in cardiovagal baroreflex sensitivity (BRS) have also been observed in women at or near ovulation and the estrogen surge.3;4 Rats exhibit similar baroreflex and gonadal hormone fluctuations during the estrous cycle. Baroreflex control of renal sympathetic nerve activity (RSNA) is enhanced during proestrus compared to diestrus; more specifically, both baroreflex gain and the maximal level of RSNA achieved during severe hypotension are elevated.5 In addition, continuous measurements of spontaneous BRS (sBRS), which reflect largely cardiovagal reflex function, revealed increases in sBRS on the afternoon and evening of proestrus (Figure 1),5 when estrogen levels peak and progesterone is increasing.
Figure 1.
sBRS increases during proestrus in rats (n=5), and this daily estrous cycle variation is abolished following ovariectomy (OVX; n=6). * P<0.05 compared to at least one other day within group (comparisons made among either light or dark periods only); †: P<0.05 OVX compared to cycling at the same time. The dark phase of the day-night cycle is indicated by gray shading. This figure was reprinted from Reference 5 with kind permission of the American Physiological Society.
To test the role of gonadal hormones in these cyclical changes, sBRS was assessed in ovariectomized (OVX) rats.5 OVX not only lowered sBRS and abolished diurnal variations (sBRS is normally lower during the light phase), it also eliminated daily fluctuations in sBRS (Figure 1).5 These data indicate that gonadal steroids are required for both diurnal and for daily estrous cycle mediated changes in baroreflex function. Progesterone likely does not contribute to the increases in baroreflex function during the high gonadal hormone segment of the reproductive cycle, since a progesterone neurosteroid metabolite, 3α-hydroxy-dihydroprogesterone (3α-OH-DHP), decreases gain of baroreflex control of RSNA.6;7 On the other hand, considerable evidence implicates estrogen. First, estrogen changes in parallel with BRS in both rats5;8 and women.3 Second, estrogen enhances BRS via an action in the brainstem.9-14
Pregnancy impairs the baroreflex
During pregnancy, the cardiovascular system undergoes profound yet teleologically appropriate changes that serve to optimize fetal development and growth. Early in gestation, blood volume and cardiac output increase by 30-50%.6;15 However, arterial pressure falls due to even greater decreases in systemic vascular resistance.6;15 While most physiological changes induced by pregnancy are relatively benign to the mother, one deleterious consequence is a marked suppression of the function of the baroreceptor reflex.6;7;16;17 Baroreflex dysfunction has been documented in several species besides humans, including rabbits, rats, goats, sheep, and dogs.6 Because of an impaired ability to maintain arterial pressure, this change in autonomic regulation underlies frequent serious complications of pregnancy. Pregnant women are prone to orthostatic hypotension.18 In addition, since hemorrhage accompanies every delivery, when blood loss is significant, severe hypotension can result.19 Indeed, peripartum hemorrhage is a major cause of maternal mortality.6
A series of experiments in conscious rabbits, studied in both the nonpregnant and pregnant state, investigated the hemodynamic mechanisms by which pregnancy impairs blood pressure control during hemorrhage. Normally during blood loss, reflex-mediated increases in sympathetic activity help to maintain arterial pressure both by vasoconstriction, to increase systemic vascular resistance, and by venoconstriction, to maintain preload and cardiac output.20-22 During pregnancy, the more rapid development of hypotension during blood loss was largely attributed to a failure to maintain cardiac output.19 Therefore, pregnancy appears to attenuate reflex venoconstriction, due either to a blunted increase in sympathetic activity to veins or to reduced venous reactivity. In addition, in pregnant animals, insufficient increases in systemic vascular resistance also contribute to inadequate arterial pressure maintenance during hemorrhage.19 Studies of regional conductance changes revealed that the mesenteric and renal circulations are regulated normally.19;23 However, suboptimal reflex vasoconstriction of the terminal aortic or the femoral vascular bed was observed when pregnant rabbits were bled.19;23 Hindquarter vascular reactivity is preserved during pregnancy;24 therefore, it appears that reflex increases in sympathetic activity to the skin and muscle of the leg are also impaired. On the other hand, although the sympathetic innervation of the uterine circulation markedly regresses during pregnancy,25;26 it is unlikely that a failure to constrict this bed is a significant factor, since in many species uterine blood flow accounts for only a small fraction of the total cardiac output.23 Collectively, therefore, pregnant individuals may be more prone to hemorrhagic hypotension, because of impaired reflex sympathetically-mediated venoconstriction and femoral vascular vasoconstriction.
The decrease in baroreflex control of the autonomic nervous system has been detected using several methodological approaches, including non-invasive techniques in women employing the analysis of heart rate and arterial pressure in the frequency domain.6;17 Similar conclusions have been derived from assessments of sBRS and infusion of vasoactive drugs to construct complete sigmoidal baroreflex relationships between arterial pressure and heart rate or sympathetic nerve activity (Figure 2).6;17 Three features of these curves are commonly attenuated: the maximum gain or slope of the most linear segment of the curves, the maximal level of sympathetic activity or heart rate achieved during severe hypotension, and the “setpoint” or the arterial pressure level associated with the midpoint of the curve. This latter change is likely mediated by resetting of baroreceptor afferents, which causes the baroreflex function curve to shift toward the lower arterial pressure level during pregnancy.6 However, the mechanisms that underlie the decreases in the baroreflex maximum and in baroreflex gain are complex and may involve distinct brain sites and hormonal mechanisms.6
Figure 2.
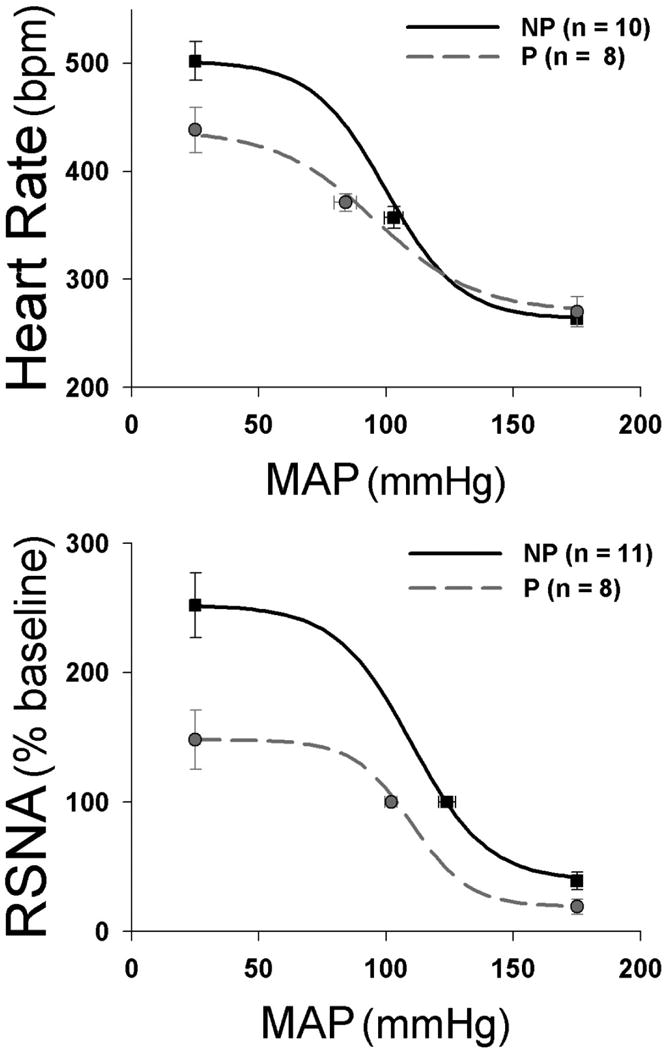
In conscious rats, pregnancy (P) impairs baroreflex control of heart rate (top) and renal sympathetic nerve activity (RSNA; bottom) compared to the nonpregnant (NP) state. This figure was reprinted from Reference 6 with kind permission of the American Physiological Society.
Within the brain, the core baroreflex pathway resides in the brainstem.27 Baroreceptor afferents terminate in the nucleus tractus solitarius (NTS) and in response to increases in pressure excite NTS second order neurons via a glutamatergic synapse. NTS neurons project to and excite (also via glutamate) interneurons in the caudal ventrolateral medulla that project to and release GABA to inhibit sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM). Thus, pregnancy-induced modulation of baroreflex function could occur at any or all of these central sites, although the RVLM appears pivotal.
One hormonal mechanism that underlies decreases in the baroreflex maximum is the action of 3α-OH-DHP to enhance GABAergic suppression of RVLM premotor neurons6 (Figure 3). 3α-OH-DHP levels, as well as the enzymes responsible for the synthesis of 3α-OH-DHP from progesterone, are increased in the brain at end-gestation, the time at which baroreflex function reaches its nadir. In addition, acute systemic or RVLM administration of 3α-OH-DHP in virgin rats reduces the maximal reflex levels of RSNA similarly to pregnancy. The link between 3α-OH-DHP and increased GABAergic tone in the RVLM is supported by the well-documented ability of this neurosteroid, by binding to the GABAA receptor, to enhance its function. More importantly, evidence indicates that RVLM premotor neurons receive greater tonic GABAergic suppression during pregnancy.6
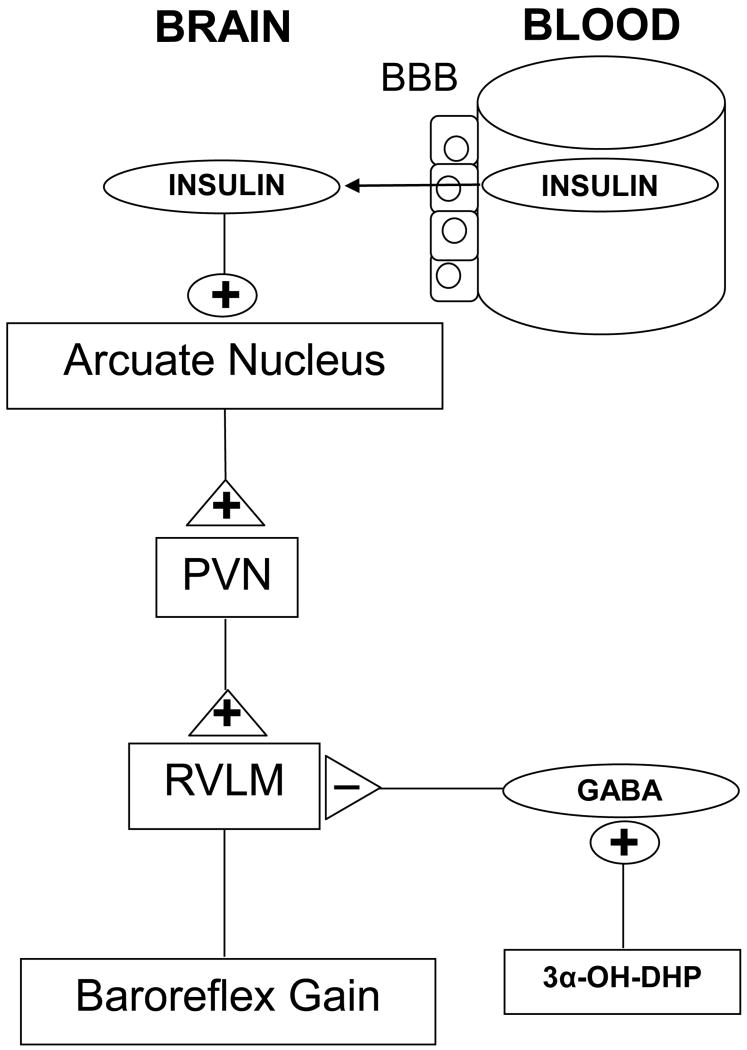
Figure 3.
Mechanisms that contribute to impaired baroreflex function during pregnancy. Insulin in blood is transported across the blood-brain barrier (BBB) into brain, where, via actions in the hypothalamic arcuate nucleus, it enhances baroreflex function. The excitation initiated by insulin in the arcuate nucleus is conveyed via the paraventricular nucleus (PVN) to a brainstem baroreflex relay, the rostral ventrolateral medulla (RVLM). During pregnancy, falls in brain insulin (which supports baroreflex function) decreases baroreflex gain. In addition, GABAergic suppression of RVLM premotor neurons mediated by the neurosteroid 3α-OH-DHP decreases maximum levels of sympathetic activity elicited by severe hypotension.
Another hormone that is involved in the pregnancy-induced baroreflex impairment is insulin. Insulin resistance is a normal adaption of pregnancy that, by increasing circulating glucose levels, serves to enhance placental glucose transfer to the fetus. However, several lines of evidence support the hypothesis that insulin resistance also contributes to the decrease in baroreflex gain, by decreasing brain insulin levels.6 First, decreases in insulin sensitivity and baroreflex gain are temporally correlated in pregnant rabbits, rats and humans.6;28;29 Second, treatment of pregnant rabbits with the insulin sensitizing drug, rosiglitazone, improves baroreflex function.29 Third, insulin enters the brain via transport from plasma across the blood brain barrier, and insulin resistant states are associated with decreases in insulin transport.30 Indeed, during pregnancy, brain insulin levels fall.29;31 Fourth, in conscious pregnant rats, intracerebroventricular (icv) infusion of insulin normalizes baroreflex gain, while in virgin rats insulin infusion is ineffective.31 Thus, during pregnancy, it appears that a factor that contributes to insulin resistance may also reduce transport of insulin into the brain. Since insulin acts centrally to enhance baroreflex function,32;33 the consequent fall in brain insulin results in a parallel decrease in BRS.
Additional recent data suggest that low brain insulin may underlie in part impaired baroreflex function in other insulin resistant states. Like pregnancy, obesity impairs cardiac baroreflex gain34-37 and decreases brain insulin.38-40 Also like pregnancy, rosiglitazone treatment of obese animals improves cardiac baroreflex gain,41 and icv insulin infusion normalizes baroreflex function, both by increasing baroreflex gain and by increasing the baroreflex maximum.42 Thus, normal brain insulin levels may be required for maintenance of optimal baroreflex control of heart rate. Furthermore, low brain insulin may contribute to the baroreflex impairment noted in other insulin resistant states, such as aging, congestive heart failure, hypertension and Alzheimer's Disease. However, future study is required to test this hypothesis.
The brain sites and circuitry by which insulin supports baroreflex function have recently been identified (Figure 3). Unlike 3α-OH-DHP, which acts in the RVLM, initial studies revealed that insulin initiates its effects in the forebrain, since lateral icv, but not fourth ventricular, insulin infusion increased baroreflex gain.32 Further work identified the arcuate nucleus as the site at which insulin acts to activate the sympathetic nervous system and increase baroreflex gain, via a neural pathway that includes the paraventricular nucleus of the hypothalamus (PVN).43 Two major sets of neurons in the arcuate nucleus project to the PVN: pro-opiomelanocortin (POMC) neurons, which release alpha-melanocyte-stimulating hormone, and neuropeptide Y neurons.44 Recently, PVN melanocortin receptors were shown to contribute to the sympathoexcitatory response to insulin, suggesting that POMC neurons convey, at least in part, the signal from the arcuate nucleus to PVN.45 The role of neuropeptide Y neurons has not been investigated. From the PVN, the neuronal pathway appears to converge with brainstem baroreflex circuitry in the RVLM, since the sympathoexcitatory effect of insulin is reversed by blockade of RVLM ionotropic glutamate receptors.45
This circuitry, specifically the potential for integration of insulin and 3α-OH-DHP signaling in the RVLM, provides a mechanistic explanation for the findings that in pregnant animals, rosiglitazone treatment to increase insulin sensitivity29 and icv insulin infusion31 each normalized baroreflex gain, yet failed to improve the attenuated baroreflex maximum levels of heart rate normally observed. Because GABAergic inhibition of RVLM premotor neurons is increased during pregnancy due to increases in 3α-OH-DHP, this suppression could prevent insulin's usual effect to increase baroreflex maximum levels32 as well (Figure 3).
Summary
Normal reproductive events in females evoke significant changes in baroreflex function. During the reproductive cycle, the sensitivity or gain of baroreflex control of sympathetic nerve activity and heart rate increases with the surge of gonadal hormonal levels, in particular of estrogen. In contrast, pregnancy depresses both baroreflex gain and the maximal level of sympathetic activity and heart rate induced by severe hypotension. The decrease in baroreflex gain may be mediated by a reduction in the actions of insulin in the arcuate nucleus to support the baroreflex. In addition, increased levels of the neurosteroid 3α-OH-DHP act downstream in the RVLM to suppress maximal reflex-induced increases in sympathetic activity.
Acknowledgments
This work was supported in part by NIH grants HL088552 and AHA Grant-in-Aid 09GRNT2060630.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS, Levinson P, Keiser HR. Plasma and urinary catecholamines during the human ovulatory cycle. Am J Obstet Gynecol. 1983;146:824–829. doi: 10.1016/0002-9378(83)91086-4. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1091–R1097. doi: 10.1152/ajpregu.00162.2003. [DOI] [PubMed] [Google Scholar]
- 4.Saeki Y, Atogami F, Takahashi K, Yoshizawa T. Reflex control of autonomic function induced by posture change during the menstrual cycle. J Auton Nerv Syst. 1997;66:69–74. doi: 10.1016/s0165-1838(97)00067-2. [DOI] [PubMed] [Google Scholar]
- 5.Goldman RK, Azar AS, Mulvaney JM, Hinojosa-Laborde C, Haywood JR, Brooks VL. Baroreflex sensitivity varies during the rat estrous cycle: role of gonadal steroids. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1419–R1426. doi: 10.1152/ajpregu.91030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2010;299:R439–R451. doi: 10.1152/ajpregu.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heesch CM, Foley CM. CNS effects of ovarian hormones and metabolites on neural control of circulation. Ann N Y Acad Sci. 2001;940:348–360. doi: 10.1111/j.1749-6632.2001.tb03690.x. [DOI] [PubMed] [Google Scholar]
- 8.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 9.De Meersman RE, Zion AS, Giardina EG, Weir JP, Lieberman JS, Downey JA. Estrogen replacement, vascular distensibility, and blood pressures in postmenopausal women. Am J Physiol. 1998;274:H1539–H1544. doi: 10.1152/ajpheart.1998.274.5.H1539. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed MK, El Mas MM, Abdel-Rahman AA. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol. 1999;276:R1030–R1037. doi: 10.1152/ajpregu.1999.276.4.R1030. [DOI] [PubMed] [Google Scholar]
- 11.Pamidimukkala J, Taylor JA, Welshons WV, Lubahn DB, Hay M. Estrogen modulation of baroreflex function in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R983–R989. doi: 10.1152/ajpregu.00761.2001. [DOI] [PubMed] [Google Scholar]
- 12.Pamidimukkala J, Hay M. 17 beta-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol. 2003;285:H1515–H1520. doi: 10.1152/ajpheart.00174.2003. [DOI] [PubMed] [Google Scholar]
- 13.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res. 2000;879:105–114. doi: 10.1016/s0006-8993(00)02757-8. [DOI] [PubMed] [Google Scholar]
- 14.Saleh TM, Connell BJ. Centrally mediated effect of 17beta-estradiol on parasympathetic tone in male rats. Am J Physiol. 1999;276:R474–R481. doi: 10.1152/ajpregu.1999.276.2.R474. [DOI] [PubMed] [Google Scholar]
- 15.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24:11–14. doi: 10.1016/s0146-0005(00)80047-6. [DOI] [PubMed] [Google Scholar]
- 16.Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Semin Reprod Med. 2009;27:330–337. doi: 10.1055/s-0029-1225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rang S, Wolf H, Montfrans GA, Karemaker JM. Non-invasive assessment of autonomic cardiovascular control in normal human pregnancy and pregnancy- associated hypertensive disorders: a review. J Hypertens. 2002;20:2111–2119. doi: 10.1097/00004872-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Easterling TR, Schmucker BC, Benedetti TJ. The hemodynamic effects of orthostatic stress during pregnancy. Obstet Gynecol. 1988;72:550–552. [PubMed] [Google Scholar]
- 19.Clow KA, Giraud GD, Ogden BE, Brooks VL. Pregnancy alters hemodynamic responses to hemorrhage in conscious rabbits. Am J Physiol Heart Circ Physiol. 2003;284:H1110–H1118. doi: 10.1152/ajpheart.00626.2002. [DOI] [PubMed] [Google Scholar]
- 20.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991;260:H305–H318. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 21.Chalmers JP, Korner PI, White SW. The effects of haemorrhage in the unanaesthetized rabbit. J Physiol. 1967;189:367–391. doi: 10.1113/jphysiol.1967.sp008174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korner PI, Oliver JR, Zhu JL, Gipps J, Hanneman F. Autonomic, hormonal, and local circulatory effects of hemorrhage in conscious rabbits. Am J Physiol. 1990;258:H229–H239. doi: 10.1152/ajpheart.1990.258.1.H229. [DOI] [PubMed] [Google Scholar]
- 23.Brooks VL, Kane CM, Welch LS. Regional conductance changes during hemorrhage in pregnant and nonpregnant conscious rabbits. Am J Physiol. 1999;277:R675–R681. doi: 10.1152/ajpregu.1999.277.3.R675. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin MK, Quinn P, Farnham MS. Vascular reactivity in the hind limb of the pregnant ewe. Am J Obstet Gynecol. 1985;152:593–598. doi: 10.1016/0002-9378(85)90634-9. [DOI] [PubMed] [Google Scholar]
- 25.Latini C, Frontini A, Morroni M, Marzioni D, Castellucci M, Smith PG. Remodeling of uterine innervation. Cell Tissue Res. 2008;334:1–6. doi: 10.1007/s00441-008-0657-x. [DOI] [PubMed] [Google Scholar]
- 26.Brauer MM. Cellular and molecular mechanisms underlying plasticity in uterine sympathetic nerves. Auton Neurosci. 2008;140:1–16. doi: 10.1016/j.autneu.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 28.Brooks VL, Mulvaney JM, Azar AS, Zhao D, Goldman RK. Pregnancy impairs baroreflex control of heart rate in rats: role of insulin sensitivity. Am J Physiol Regul Integr Comp Physiol. 2010;298:R419–R426. doi: 10.1152/ajpregu.00441.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2188–R2195. doi: 10.1152/ajpregu.00614.2006. [DOI] [PubMed] [Google Scholar]
- 30.Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 31.Azar AS, Brooks VL. Impaired baroreflex gain during pregnancy in conscious rats: role of brain insulin. Hypertension. 2011;00 doi: 10.1161/HYPERTENSIONAHA.110.162354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- 33.Okada M, Bunag RD. Insulin acts centrally to enhance reflex tachycardia in conscious rats. Am J Physiol. 1994;266:R481–R486. doi: 10.1152/ajpregu.1994.266.2.R481. [DOI] [PubMed] [Google Scholar]
- 34.Straznicky NE, Lambert GW, Lambert EA. Neuroadrenergic dysfunction in obesity: an overview of the effects of weight loss. Curr Opin Lipidol. 2010;21:21–30. doi: 10.1097/MOL.0b013e3283329c62. [DOI] [PubMed] [Google Scholar]
- 35.Beske SD, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2002;282:H630–H635. doi: 10.1152/ajpheart.00642.2001. [DOI] [PubMed] [Google Scholar]
- 36.Barringer DL, Bunag RD. Uneven blunting of chronotropic baroreflexes in obese Zucker rats. Am J Physiol. 1989;256:H417–H421. doi: 10.1152/ajpheart.1989.256.2.H417. [DOI] [PubMed] [Google Scholar]
- 37.Bunag RD, Barringer DL. Obese Zucker rats, though still normotensive, already have impaired chronotropic baroreflexes. Clin Exp Hypertens A. 1988;10(Suppl 1):257–262. doi: 10.3109/10641968809075977. [DOI] [PubMed] [Google Scholar]
- 38.Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D, Jr, Greenwood MR, DORSA DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985;36:627–633. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- 39.Gerozissis K. Brain insulin: regulation, mechanisms of action and functions. Cell Mol Neurobiol. 2003;23:1–25. doi: 10.1023/A:1022598900246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- 41.Zhao D, Brooks VL. Rosiglitazone improves insulin sensitivity and baroreflex gain in rats with diet-induced obesity. FASEB J. 2010;24:1051.3. doi: 10.1124/jpet.112.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao D, Brooks VL. Diet-induced obesity impairs baroreflex gain: role of brain insulin. Hypertension. 2010;56:e102. [Google Scholar]
- 43.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic Response to Insulin Is Mediated by Melanocortin 3/4 Receptors in the Hypothalamic Paraventricular Nucleus. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]