Abstract
Two antigenically distinct lineages of influenza B viruses have circulated globally since 1985. However, licensed trivalent seasonal influenza vaccines contain antigens from only a single influenza B virus and thus provide limited immunity against circulating influenza B strains of the lineage not present in the vaccine. In recent years, predictions about which B lineage will predominate in an upcoming influenza season have been no better than chance alone, correct in only 5 of the 10 seasons from 2001 to 2011. Consequently, seasonal influenza vaccines could be improved by inclusion of influenza B strains of both lineages. The resulting quadrivalent influenza vaccines would allow influenza vaccination campaigns to respond more effectively to current global influenza epidemiology. Manufacturing capacity for seasonal influenza vaccines has increased sufficiently to supply quadrivalent influenza vaccines, and methods to identify the influenza B strains to include in such vaccines are in place. Multiple manufacturers have initiated clinical studies of quadrivalent influenza vaccines. Data from those studies, taken together with epidemiologic data regarding the burden of disease caused by influenza B infections, will determine the safety, effectiveness, and benefit of utilizing quadrivalent vaccines for the prevention of seasonal influenza disease.
Keywords: influenza, public health, quadrivalent, surveillance, vaccine
Introduction
Influenza A/H1N1, A/H3N2 and B viruses have circulated and caused disease in humans on a global basis since 1977.1 Accordingly, licensed seasonal influenza vaccines have contained three strains, one from each A subtype and one type B virus. Because new variant strains of each type/subtype continually evolve, the specific strains to be included in seasonal influenza vaccines are chosen based on a prediction of the strains likely to circulate in the upcoming influenza season.1 Since 1985, two antigenically distinct lineages of influenza B viruses have circulated globally.2 However, as only one lineage can be selected for inclusion in current trivalent influenza vaccines, the vaccines have provided limited immunity against strains of the other lineage.3,4 Additionally, in 5 of the 10 influenza seasons between 2001–2002 and 2010–2011, the predominant circulating influenza B lineage was different from that chosen for the vaccine.5,6 Consequently, influenza vaccination campaigns have had limited effectiveness against influenza B epidemics during seasons in which a significant proportion of the disease was caused by opposite-lineage influenza B strains. This reduced effectiveness in such seasons could be avoided if seasonal influenza vaccines included four strains, one strain from each B lineage in addition to A/H1N1 and A/H3N2 strains. The current review describes the burden of disease caused by influenza B infection, evaluates the impact of two distinct circulating influenza B lineages, and provides data supporting the feasibility of the development and widespread adoption of quadrivalent influenza vaccines.
Influenza B Accounts for a Significant Proportion of the Overall Burden of Influenza
Both influenza A and B are orthomyxoviruses that cause annual epidemics in humans on a global scale. Influenza B predominantly circulates in human populations, in contrast to influenza A, which circulates in multiple animal species as well as in humans. Like influenza A, novel variant strains of influenza B continually emerge due to antigenic drift. In recent years, influenza B viruses have evolved more slowly than A/H3N2 viruses, as evidenced by a lower rate of nucleotide substitution, estimated at 0.14 × 10-3 to 3.32 × 10-3 substitutions/site/year for influenza B vs. 2.68 × 10-3 to 12.50 × 10-3 for influenza A/H3N2.7,8 The likelihood that substitutions result in amino acid changes is also lower for B viruses compared with A/H3N2 viruses.7 However, there is frequent segment reassortment between influenza B viruses, which is often the mechanism by which new dominant B viruses emerge.7
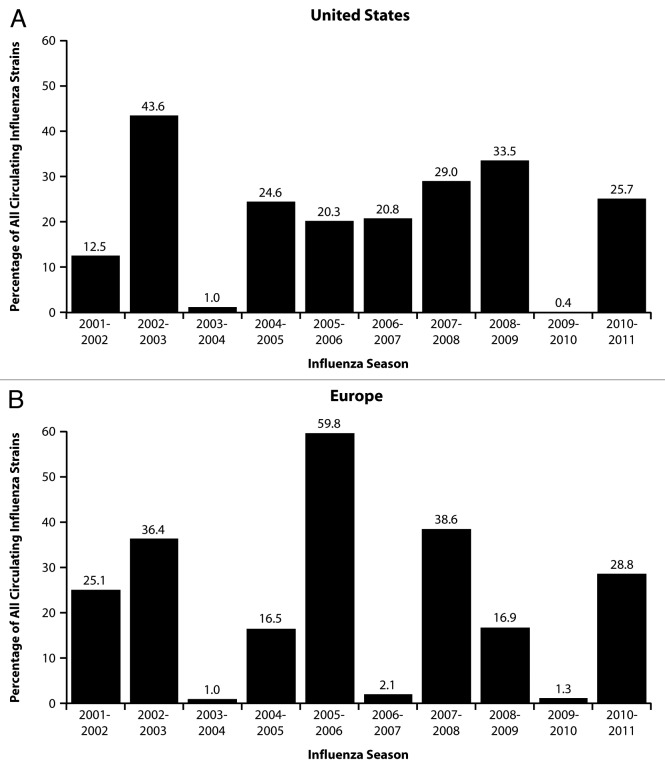
The incidence of influenza B can vary dramatically between influenza seasons. According to data from the US. Centers for Disease Control and Prevention (CDC), from 2001–2002 through 2010–2011 (excluding the 2009–2010 pandemic), influenza B was responsible for < 1% to 44% of influenza-positive samples submitted by participating laboratories (Fig. 1A). On average, 24% of influenza samples during this period were positive for influenza B. European surveillance data from the same seasons was similar, with influenza B being responsible for 1% to 60% of influenza-positive samples and a season average of 23% (Fig. 1B). These data are consistent with a Dutch study that systematically examined cases of influenza-like illness from 1992–1993 through 2006–2007 and found the proportion of influenza cases caused by influenza B to range from 0% to 82% by season, with a season average of 29%.9 Influenza B was responsible for more than 30% of cases in 7 of the 15 seasons. Similarly, in data from Finland from 1980 to 1999, influenza B accounted for 20% of influenza cases among children <17 y of age, and was the predominant cause of influenza illness in 5 of the 20 seasons.10
Figure 1. Influenza B circulation as a proportion of circulating influenza strains: US and European data for 2001 to 2011. US data (A) were extracted from cumulative data reported in the final CDC weekly influenza surveillance report for each season (available at www.cdc.gov/flu/weekly/pastreports.htm). Data for 2008–2009 represent cumulative data through April 18, 2009. EU data (B) were extracted from cumulative Euroflu sentinel site data reported in the final weekly influenza surveillance bulletin for each season beginning with the 2003–2004 season (available at www.euroflu.org/cgi-files/bulletin_v2.cgi). Because Euroflu bulletins issued before 2003–2004 do not provide cumulative season data, data presented for the 2001–2002 and 2002–2003 influenza seasons were extracted from the final cumulative European Influenza Surveillance Scheme report issued in 2007 (available at www.euroflu.org/documents/eiss_annual_report_2006-2007.pdf).
Studies of severe influenza disease in the US have shown that influenza B causes significant morbidity and mortality. For influenza-attributable respiratory- and circulatory-related hospitalizations in all ages from 1979–1980 through 2000–2001, the estimated hospitalization rate during seasons dominated by influenza B was 81.4 hospitalizations per 100,000, which approached the hospitalization rate observed in A/H3N2-predominant seasons (99.0 per 100,000) and was greater than that observed in A/H1N1-predominant seasons (55.9 per 100,000).11 Between 1990–1991 and 1998–1999, influenza B was estimated to account for 15% of all influenza-attributable respiratory- and circulatory-related deaths in the US. The estimated average annual deaths due to influenza B (5255) was lower than the number attributed to A/H3N2 (28,940), but higher than the number attributed to A/H1N1 (1960).12
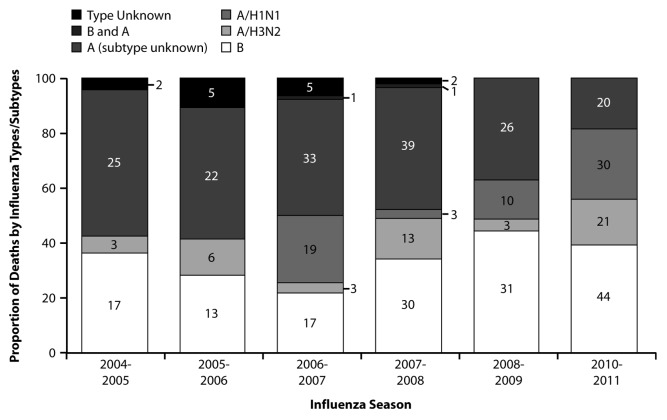
Although influenza B causes disease in all age groups, its incidence relative to influenza A appears to be highest among older children and young adults. In studies that described influenza illness among children and adults, the proportion of illnesses caused by influenza B relative to influenza A was highest for individuals 5 to 29 y of age13 and 2 to 39 y of age.14 A study describing absenteeism among Japanese schoolchildren for the 24 y between 1984 and 2007 demonstrated that influenza B was responsible for the largest outbreaks, and outbreaks were observed even during seasons in which many children were vaccinated.15 Multiple studies of medically-attended influenza in children have demonstrated that children with influenza B illness are older than those with influenza A.10,16-20 Furthermore, while influenza B causes mortality in all age groups, it appears to be a disproportionate cause of pediatric influenza deaths. The CDC made US pediatric influenza deaths reportable beginning with the 2004–2005 season. From 2004–2005 through 2010–2011, with the exception of the 2009–2010 pandemic, influenza B was responsible for 22–44% of reported influenza deaths in children 0–18 y of age each season (Fig. 2). Overall, during this period, influenza B was responsible for 34% of reported pediatric influenza deaths. Thompson et al. also estimated that influenza B was a disproportionate cause of influenza deaths among children. Influenza B was responsible for 27% and 26% of influenza-attributable respiratory- and circulatory-related deaths among infants younger than 12 mo of age and children 1–4 y of age, respectively, as compared with 17%, 12%, and 15% among individuals 5–49 y of age, 50–64 y of age, and 65 y of age and older, respectively.12
Figure 2. Proportion of US pediatric influenza deaths by viral type (2004 to 2011, excluding 2009–2010 pandemic). Values in columns represent the number of deaths in each category for each season. Data for the 2004–2005 through 2008–2009 seasons were obtained via personal communication with the CDC. Pediatric influenza deaths became reportable in 2004–2005; as a result, comparable data are not available prior to 2004–2005. Data for the 2010–2011 season was obtained from the CDC 2010–2011 Influenza Season Summary (available at www.cdc.gov/flu/weekly/pastreports.htm).
Medically-attended illnesses in both children and adults due to influenza A and B are generally similar in regards to symptoms, severity, and rates of influenza-related complications.10,16-25 The principal differences observed across studies are that influenza B disease in children is more commonly associated with myalgia, myositis, and leukopenia.10,17,19,22,23 Three studies found that influenza B was less commonly associated with rhinorrhea in children.10,22,23 One study noted a shorter duration of illness in children with influenza B,16 but in other studies duration was similar.17,18 In adults, the duration of influenza A and B illness was similar.24,25
One Virus, Two Lineages
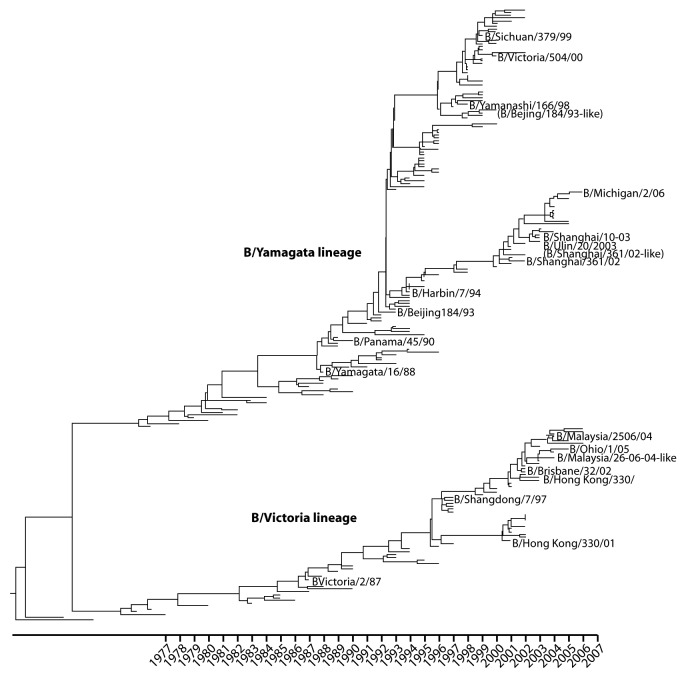
Although influenza B viruses are classified as a single influenza type, B viruses can be categorized into two antigenically distinct phylogenetic lineages, B/Victoria/02/87-like and B/Yamagata/16/88-like, based on divergence in the HA1 domain of the viral hemagglutinin gene (Fig. 3). Before 1985, there was a single lineage of influenza B in global circulation. This lineage was the precursor to the subsequent Yamagata lineage.2 The Victoria lineage appears to have emerged from a minor lineage of B viruses in China by 1975 but was not isolated outside of China until 1985.26 Following the global detection of Victoria-like strains in 1985, the Victoria lineage dominated global circulation from 1987 to 1989, followed by Yamagata dominance in the 1990s and subsequent re-emergence of the Victoria lineage in 2001–2002. From 2001–2002 to the present, both lineages have co-circulated each season at varying levels (Fig. 4).
Figure 3. Evolution of two antigenically distinct lineages of influenza B (1970–2006). Phylogenetic tree of the influenza B virus HA1 domain based on 214 sequences sampled annually between 1970 and 2006. Data for the B/Victoria/02/87-like and B/Yamagata/16/88-like lineages are shown. Representative vaccine strains are also shown. Adapted with permission from Chen R. Holmes E.C. The evolutionary dynamics of human influenza B virus.7
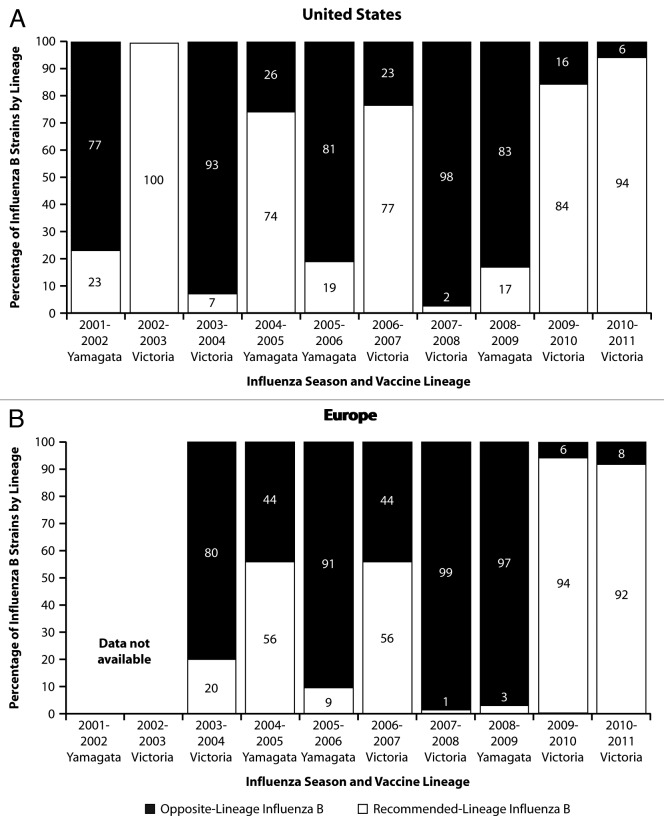
Figure 4. Influenza B circulation by lineage: US and European data for 2001 to 2011. Data were obtained as reported for Figure 1. US data are represented in panel A (A) and European data are represented in panel B (B). The influenza B lineage (Victoria or Yamagata) recommended for inclusion in the trivalent vaccine is shown on the x-axis for each season. EU data regarding the proportion of B viruses by lineage were not available for the 2001–2002 and 2002–2003 influenza seasons.
The pattern of varying dominance of the two influenza B lineages is likely driven by lineage-specific immunity in the population, with one lineage predominating until accumulated immunity to that lineage results in increased relative susceptibility to and spread of the other lineage.7 A serologic study of European children 0 to 7 y of age, almost none of whom had been previously vaccinated against influenza, demonstrated that children accumulated natural immunity to influenza B more slowly than to influenza A.27 This observation likely explains the increased incidence of influenza B illness relative to influenza A among older children and young adults. An important observation by Bodewes et al. was that antibody responses to influenza B infection in children were lineage-specific, with no cross-reactivity between lineages. A similar pattern of limited cross-reactivity occurs in response to influenza vaccination. For trivalent inactivated influenza vaccines (TIV), antibody responses to the lineage not contained in the vaccine are reduced in adults and negligible in children.4 In recent randomized, placebo-controlled efficacy studies of TIV in younger adults, efficacy against opposite-lineage B strains has been variable, ranging from 22% to 52%,28,29 which is lower than the observed efficacy against vaccine-matched strains of 78%.29 With trivalent live attenuated influenza vaccine in children, 31% efficacy has been observed against opposite-lineage influenza B strains, which is lower than the 86% efficacy against vaccine-matched B strains.3
Inability to Predict Which Influenza B Lineage Will Circulate
In recent years, predictions of the predominant influenza B lineage have been no better than chance alone. In 5 of the 10 influenza seasons in the US from 2001–2002 through 2010–2011, the predominant circulating influenza B lineage was different from that contained in the vaccine (Fig. 4A). Overall, an estimated 46% of influenza B samples during this period were influenza B strains of the lineage not included in the vaccine. Similarly, in Europe from 2003–2004 through 2010–2011 (the seasons for which data are available), the predominant lineage differed from that contained in the vaccine in 4 of 8 seasons (Fig. 4B), and an estimated 58% of influenza B samples were of the lineage not included in the vaccine. Because inclusion of the incorrect influenza B lineage in trivalent vaccines provides suboptimal protection against circulating opposite-lineage strains, inaccurate prediction of the predominant influenza B lineage leaves many vaccinated individuals with suboptimal protection from influenza B disease.
The magnitude of the problem created by mismatch between circulating influenza B strains and the influenza B lineage contained in the vaccine varies by season. The most significant recent examples occurred during the 2005–2006 and 2007–2008 seasons. In 2005–2006, the influenza B component of the northern hemisphere influenza vaccine was of the B/Yamagata lineage, but 81–91% of the circulating influenza B viruses antigenically characterized in the US and Europe were of the B/Victoria lineage, and influenza B was found in 34–60% of all samples (Figs. 1 and 4). Similarly, in 2007–2008, the influenza B component of the vaccine was of the B/Victoria lineage, but 98–99% of the circulating influenza B viruses characterized in the US and Europe belonged to the B/Yamagata lineage, and influenza B was found in 29–39% of all samples (Fig. 1 and 4). In other seasons, the magnitude of the problem was less, such as when the vaccine component was a good match to circulating strains (e.g., 2002–2003 and 2010–2011) or when influenza B illness was less common (e.g., 2003–2004 and 2009–2010).
Potential Benefits of Quadrivalent Influenza Vaccines
Based on the demonstrated burden of influenza B, the limited cross-protection between the two influenza B lineages, and the inability to accurately predict which influenza B lineage will circulate, it is clear that seasonal influenza vaccines could be improved by the inclusion of influenza B strains from both lineages. The rationale for the shift from trivalent to quadrivalent influenza vaccines is similar to that for the 1977 transition from a bivalent (A/H3N2 and B) vaccine to a trivalent (A/H1N1, A/H3N2, B) vaccine, which was based on the lack of cross-protection between A/H3N2 and A/H1N1. The inclusion of both influenza B lineages would provide a direct benefit to vaccine recipients whenever a large number of circulating influenza B viruses does not match the lineage chosen for trivalent vaccines, either because the lineage prediction was incorrect or because both lineages co-circulated to a significant degree. Moreover, in seasons in which influenza B circulation is minimal or B viruses are well matched to the trivalent vaccine strain, vaccination with a quadrivalent influenza vaccine would still provide benefit to the individual by increasing immunity to both lineages of influenza B, with potential clinical benefit in subsequent seasons.30 Accumulated immunity may be more relevant for influenza B than for influenza A because antigenic drift is more limited with influenza B viruses. From a public health perspective, if quadrivalent vaccines led to fewer mismatched seasonal vaccine campaigns, the public’s confidence and acceptance of influenza vaccination might also be enhanced.31
The CDC recently conducted an analysis to quantify the potential public health impact of a quadrivalent influenza vaccine strategy relative to dependence on trivalent vaccines.32 Based on the available input data and the assumption that quadrivalent vaccine production would not decrease influenza vaccine supply, the analysis suggested that use of quadrivalent vaccines in the US during the 2001–2008 seasons would have been beneficial in each season, cumulatively resulting in approximately 2.1 million fewer cases of influenza, 20,000 fewer hospitalizations, and 1,200 fewer deaths.
Optimal target populations for quadrivalent influenza vaccines are a subject of ongoing discussion (FDA VRBPAC 2007, 2009, 2011).33-35 The primary benefit of a quadrivalent vaccine is in eliminating the risk that the incorrect B lineage is selected for inclusion in the vaccine. The clinical benefit is likely to be highest among children and young adults because of the increased incidence of influenza B disease in these age groups, and studies in children with live attenuated and inactivated influenza vaccines have shown little to no cross-protection against opposite-lineage strains following vaccination. However, cross-lineage protection from vaccination is also reduced in adults, and influenza B accounts for a significant proportion of influenza-related morbidity and mortality in all age groups, even among older adults. Because individuals of all ages would be expected to benefit from quadrivalent influenza vaccines, there is a strong rationale for providing quadrivalent influenza vaccines to all indicated age groups, as long as the incremental cost relative to trivalent vaccines is not excessive.
Path Forward for Quadrivalent Vaccines
Presumably, quadrivalent influenza vaccines would be very similar to currently licensed trivalent vaccines in regards to manufacturing processes, as well as vaccine excipients, dose, and administration. The only expected difference would be the inclusion of four influenza vaccine strains (A/H1N1, A/H3N2, and B strains of both lineages). Influenza B strains to be included in the vaccines could be chosen through the current processes for strain selection. In fact, the World Health Organization (WHO) recommendations for the 2011–2012 northern hemisphere seasonal influenza vaccine formulation already identify candidate vaccine strains from both influenza B lineages.36
Multiple manufacturers have initiated clinical studies to evaluate the immunogenicity and safety of quadrivalent influenza vaccines relative to trivalent vaccines in children and adults.33,37-44 In the past, efforts to advance quadrivalent influenza vaccines were complicated by concerns about influenza vaccine manufacturing capacity, but now capacity has increased to the point that introduction of quadrivalent vaccines should not impact the overall supply of influenza vaccines.32 In 2006, the WHO estimated that global manufacturing capacity for seasonal trivalent influenza vaccine was 347 million doses.45 In 2009, capacity increased to 876 million doses due to investments by manufacturers and governments. However, only 66% of the available global capacity in 2009 was utilized for production of 2008–2009 seasonal influenza vaccines.46 Production of quadrivalent vaccines could help maintain the current global manufacturing capacity, which would also enhance pandemic preparedness since the capacity could be redirected to manufacture monovalent pandemic doses.
Widespread utilization of quadrivalent influenza vaccines will require confirmation that quadrivalent influenza vaccines produce immune responses in children and adults comparable to those observed with trivalent seasonal influenza vaccines. Additionally, clinical studies must confirm that quadrivalent influenza vaccines have an acceptable safety profile in children and adults. Cost-effectiveness analyses comparing quadrivalent and trivalent vaccines will also be required if there is a significant incremental cost associated with quadrivalent vaccines. Lastly, acceptance of quadrivalent influenza vaccines by providers, payers, and the general public will require education regarding the benefits and safety of vaccines containing the additional strain.
Conclusion
Quadrivalent formulations represent a next logical step for seasonal influenza vaccines. Because 2 antigenically distinct influenza B lineages have been circulating since 1985 and the predominant influenza B lineage has been unpredictable in recent years, quadrivalent vaccines would more accurately reflect the current epidemiology of influenza and would allow vaccination campaigns to more effectively protect their target populations. Multiple manufacturers have initiated clinical studies of quadrivalent influenza vaccines. Data from those studies, as well as epidemiologic data regarding the burden of influenza B infections, will determine the safety, effectiveness, and benefit of utilizing quadrivalent vaccines for the prevention of seasonal influenza disease.
Acknowledgments
C.S.A. is an employee of MedImmune and was invited by Susanna Esposito, Guest Editor, to author this review. M.J.L. has served as a consultant for MedImmune. Formatting of the manuscript was provided by Complete Healthcare Communications, Inc. (Chadds Ford, PA) and funded by MedImmune.
Glossary
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- WHO
World Health Organization
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/17623
References
- 1.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 2.Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175:59–68. doi: 10.1016/0042-6822(90)90186-U. [DOI] [PubMed] [Google Scholar]
- 3.Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28:2149–56. doi: 10.1016/j.vaccine.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 4.Couch RB. Background and presentation of possible vaccine options. Presented at: Food and Drug Administration, Center for Biologics Evaluation and Research, Vaccines and Related Biological Products Advisory Committee Meeting; February 27-28, 2007; Washington, DC. [Google Scholar]
- 5.US Centers for Disease Control and Prevention. Seasonal influenza activity surveillance reports: 1999-2000 to 2010-2011 seasons. Available at: http://www.cdc.gov/flu/weekly/pastreports.htm. Accessed November 17, 2011
- 6.World Health Organization. WHO/Europe influenza surveillance. Available at: http://www.euroflu.org/index.php. Accessed June 20, 2011
- 7.Chen R, Holmes EC. The evolutionary dynamics of human influenza B virus. J Mol Evol. 2008;66:655–63. doi: 10.1007/s00239-008-9119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindstrom SE, Hiromoto Y, Nishimura H, Saito T, Nerome R, Nerome K. Comparative analysis of evolutionary mechanisms of the hemagglutinin and three internal protein genes of influenza B virus: multiple cocirculating lineages and frequent reassortment of the NP, M, and NS genes. J Virol. 1999;73:4413–26. doi: 10.1128/jvi.73.5.4413-4426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkstra F, Donker GA, Wilbrink B, Van Gageldonk-Lafeber AB, Van Der Sande MA. Long time trends in influenza-like illness and associated determinants in The Netherlands. Epidemiol Infect. 2009;137:473–9. doi: 10.1017/S095026880800126X. [DOI] [PubMed] [Google Scholar]
- 10.Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis. 2003;36:299–305. doi: 10.1086/345909. [DOI] [PubMed] [Google Scholar]
- 11.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 12.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 13.Grant KA, Carville K, Fielding JE, Barr IG, Riddell MA, Tran T, et al. High proportion of influenza B characterises the 2008 influenza season in Victoria. Commun Dis Intell. 2009;33:328–36. doi: 10.33321/cdi.2009.33.36. [DOI] [PubMed] [Google Scholar]
- 14.Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4:e247. doi: 10.1371/journal.pmed.0040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai S, Nanri S, Ban E, Inokuchi M, Tanaka T, Tokumura M, et al. Influenza vaccination of schoolchildren and influenza outbreaks in a school. Clin Infect Dis. 2011;53:130–6. doi: 10.1093/cid/cir336. [DOI] [PubMed] [Google Scholar]
- 16.Esposito S, Cantarutti L, Molteni CG, Daleno C, Scala A, Tagliabue C, et al. Clinical manifestations and socio-economic impact of influenza among healthy children in the community. J Infect. 2011;62:379–87. doi: 10.1016/j.jinf.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Hite LK, Glezen WP, Demmler GJ, Munoz FM. Medically attended pediatric influenza during the resurgence of the Victoria lineage of influenza B virus. Int J Infect Dis. 2007;11:40–7. doi: 10.1016/j.ijid.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Principi N, Esposito S, Gasparini R, Marchisio P, Crovari P. Burden of influenza in healthy children and their households. Arch Dis Child. 2004;89:1002–7. doi: 10.1136/adc.2003.045401. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Shen CF, Huang SC, Wang SM, Wang JR, Liu CC. Decreased leukocytes and other characteristics of laboratory findings of influenza virus infections in children. J Microbiol Immunol Infect. 2008;41:294–300. [PubMed] [Google Scholar]
- 20.Daley AJ, Nallusamy R, Isaacs D. Comparison of influenza A and influenza B virus infection in hospitalized children. J Paediatr Child Health. 2000;36:332–5. doi: 10.1046/j.1440-1754.2000.00533.x. [DOI] [PubMed] [Google Scholar]
- 21.Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, et al. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004-2005 through 2007-2008. Influenza Other Respi Viruses 2011; [Epub ahead of print]; PMID: 21668663; DOI: 10.1111/j.1750-2659.2011.00263.x. [DOI] [PMC free article] [PubMed]
- 22.Chi CY, Wang SM, Lin CC, Wang HC, Wang JR, Su IJ, et al. Clinical features of children infected with different strains of influenza B in southern Taiwan. Pediatr Infect Dis J. 2008;27:640–5. doi: 10.1097/INF.0b013e31816be008. [DOI] [PubMed] [Google Scholar]
- 23.Hu JJ, Kao CL, Lee PI, Chen CM, Lee CY, Lu CY, et al. Clinical features of influenza A and B in children and association with myositis. J Microbiol Immunol Infect. 2004;37:95–8. [PubMed] [Google Scholar]
- 24.Ng S, Cowling BJ, Fang VJ, Chan KH, Ip DK, Cheng CK, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010;50:707–14. doi: 10.1086/650458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bettis R, Iacuzio D, Jung T, Fuchs R, Aultman R, Gyldmark M. Impact of influenza treatment with oseltamivir on health, sleep and daily activities of otherwise healthy adults and adolescents. Clin Drug Investig. 2006;26:329–40. doi: 10.2165/00044011-200626060-00004. [DOI] [PubMed] [Google Scholar]
- 26.Chen JM, Guo YJ, Wu KY, Guo JF, Wang M, Dong J, et al. Exploration of the emergence of the Victoria lineage of influenza B virus. Arch Virol. 2007;152:415–22. doi: 10.1007/s00705-006-0852-6. [DOI] [PubMed] [Google Scholar]
- 27.Bodewes R, de Mutsert G, van der Klis FR, Ventresca M, Wilks S, Smith DJ, et al. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol. 2011;18:469–76. doi: 10.1128/CVI.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beran J, Wertzova V, Honegr K, Kaliskova E, Havlickova M, Havlik J, et al. Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis. 2009;9:2. doi: 10.1186/1471-2334-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, et al. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51:997–1004. doi: 10.1086/656578. [DOI] [PubMed] [Google Scholar]
- 30.Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008;27:744–8. doi: 10.1097/INF.0b013e318174e0f8. [DOI] [PubMed] [Google Scholar]
- 31.Reed C, Meltzer M, Finelli L, Fiore A. Public health impact of including two influenza B strains in seasonal influenza vaccines. Available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm176375.htm. Accessed March 29, 2011 [DOI] [PubMed]
- 32.Reed C, Meltzer M, Finelli L, Fiore A. Public health impact of including two influenza B strains in seasonal influenza vaccines. Presented at: Options for the Control of Influenza VII; September 3-7, 2010; Hong Kong SAR, China. [Google Scholar]
- 33.US Food and Drug Administration. February 25, 2011: Vaccines and Related Biological Products Advisory Committee Meeting Transcript. Strain selection for the influenza virus vaccine for the 2011-2012 season. Available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm249303.htm. Accessed June 28, 2011.
- 34.US Food and Drug Administration. February 18, 2009: Vaccines and Related Biological Products Advisory Committee Meeting Transcript. Strain selection for the influenza virus vaccine for the 2009-2010 season. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM167159.pdf. Accessed June 28, 2011
- 35.US Food and Drug Administration. February 28, 2007: Vaccines and Related Biological Products Advisory Committee Meeting Transcript. Strain selection for the influenza virus vaccine for the 2007-2008 season. Available at: http://www.fda.gov/ohrms/dockets/ac/07/transcripts/2007-4282t2.htm. Accessed June 28, 2011
- 36.World Health Organization. Summary of status of development and availability of influenza B (Yamagata and Victoria lineage) candidate vaccine viruses* and potency testing reagents. Available at: http://www.who.int/csr/disease/influenza/summary_b_cvv_reagents_4_may_2011.pdf. Accessed June 20, 2011
- 37.ClinicalTrials.gov. A study to evaluate the immunogenicity of quadrivalent live attenuated influenza vaccine in children (NCT01091246). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01091246. Accessed June 28, 2011
- 38.ClinicalTrials.gov. A study to evaluate the safety and immunogenicity of GSK Biologicals' seasonal influenza vaccine in adults (NCT01196975). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01196975. Accessed June 28, 2011
- 39.ClinicalTrials.gov. Trial to evaluate safety and immunogenicity of GSK Biologicals' influenza vaccine GSK2584786A in healthy children (NCT01195779). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01195779. Accessed June 28, 2011
- 40.ClinicalTrials.gov. A study to evaluate the efficacy of GSK Biologicals' seasonal influenza vaccine in children (NCT01218308). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01218308. Accessed June 28, 2011
- 41.ClinicalTrials.gov. A study to evaluate the safety and immunogenicity of GSK Biologicals' seasonal influenza vaccine in children (NCT01198756). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01198756. Accessed June 28, 2011
- 42.ClinicalTrials.gov. Study of quadrivalent influenza vaccine among adults (NCT01218646). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01218646. Accessed June 28, 2011
- 43.ClinicalTrials.gov. A study of influenza virus vaccines in children and adults (NCT00988143). Available at: http://www.clinicaltrials.gov/ct2/show/NCT00988143. Accessed June 28, 2011
- 44.ClinicalTrials.gov. Study of quadrivalent influenza vaccine among children (NCT01240746). Available at: http://www.clinicaltrials.gov/ct2/show/NCT01240746. Accessed June 28, 2011
- 45.Kieny MP, Costa A, Hombach J, Carrasco P, Pervikov Y, Salisbury D, et al. A global pandemic influenza vaccine action plan. Vaccine. 2006;24:6367–70. doi: 10.1016/j.vaccine.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Collin N, de Radigues X. World Health Organization H1N1 Vaccine Task Force. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine. 2009;27:5184–6. doi: 10.1016/j.vaccine.2009.06.034. [DOI] [PubMed] [Google Scholar]