Abstract
The presence of neighboring vegetation modifies the light environment experienced by plants, generating signals that are perceived by phytochromes and cryptochromes. These signals cause large changes in plant body form and function, including enhanced growth of the hypocotyl and petioles, a more erect position of the leaves and early flowering in Arabidopsis thaliana. Collectively, these so-called shade-avoidance responses tend to reduce the degree of current or future shade by neighbors. Shade light signals increase the abundance of PHYTOCHROME INTERACTING FACTOR 4 (PIF4) and PIF5 proteins, promote the synthesis and redirection of auxin, favor the degradation of DELLA proteins and increase the expression of auxin, gibberellins and brassinosteroid-promoted genes, among other events downstream the photoreceptors. Selectively disrupting these events by genetic or pharmacological approaches affects shade-avoidance responses with an intensity that depends on the developmental context and the environment. Shade-avoidance responses provide a model to investigate the signaling networks used by plants to take advantage of the cues provided by the environment to adjust to the challenges imposed by the environment itself.
INTRODUCTION
Shade-avoidance responses are the changes in plant body form and function that occur in response to the light signals provided by neighboring vegetation and that tend to reduce the degree of current or future shade. Plants rely on the availability of photo-synthetically-active radiation (PAR, 400–700 nm) to produce the carbohydrates used in their metabolism. The reduction of PAR below saturation levels lowers photosynthesis and can seriously compromise plant fitness. This has provided the evolutionary force to generate shade-avoidance responses.
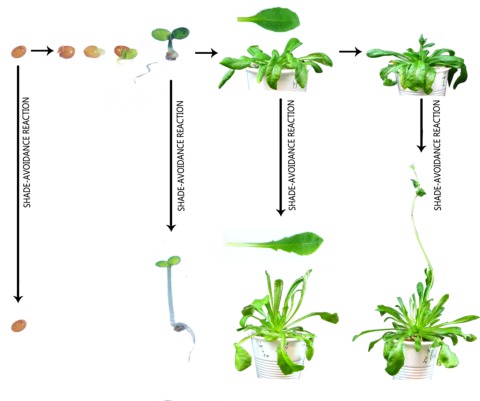
Figure 1 shows shade-avoidance responses at different stages of the life cycle in Arabidopsis thaliana. The germination of Arabidopsis seeds can be repressed by shade light and this is a shade-avoidance response because it prevents the generation of seedlings immediately exposed to the limiting PAR levels at the base of deep canopies. The seeds will then germinate when the canopy becomes disturbed and the seeds exposed to unfiltered sunlight. Arabidopsis seeds can germinate under dense canopies but this requires sensitization by incubation in full darkness, a condition experienced when the seeds are buried (Shinomura et al., 1996; Botto et al., 1996). For a more detailed treatment of seed-germination responses we refer to the chapter dedicated to this subject (Bentsink and Koornneef, 2008).
Figure 1.
Shade light signals cause shade-avoidance responses throughout the life cycle of plants of Arabidopsis thaliana.
The upper set of pictures shows the progression of Arabidopsis plants under open conditions. Shade light causes alternative growth and developmental patterns including the repression of seed germination, the promotion of hypocotyl growth, the promotion of petiole growth and more erect leaves and early flowering.
At the seedling stage, shade light signals promote hypocotyl extension growth (Figure 1), and a longer stem places the cotyledons and early true leaves of the rosette at a higher position within the canopy, reducing the degree of future or current shade. In addition, the gradients of light between sunflecks and shaded areas deviate the axis of hypocotyl growth towards the illuminated side. For a more detailed treatment of phototropic responses we refer to the chapter dedicated to this subject (Pedmale et al., 2010).
At the rosette stage, shade light signals cause upward bending of the cotyledons and true leaves, enhance petiole extension growth and can reduce leaf-lamina expansion (Figure 1). The upward bending of the leaves, caused by faster growth on their lower than their upper side, is called hyponasty. Hyponasty is a rapid response that places leaf lamina at a higher, better light position within the canopy. Longer petioles enhance this effect by increasing the amplitude of the change in leaf position. In addition, longer petioles can horizontally displace the position of the leaf lamina away from the shade of neighbors. If a plant is partially shaded by neighbors, reduced expansion of shaded leaves would reduce the proportion of shaded leaf area.
Shade light signals also accelerate flowering (Figure 1) and reduce branching. Since Arabidopsis typically grows as a rosette during the vegetative phase, the extension of the internodes that accompanies reproductive development places cauline leaves at higher strata within the canopy. Early flowering also reduces the chance of future shade by completing the cycle before the canopy becomes too closed. The advantage of extending the vegetative phase while the environment is favorable is that the more developed foliage can supply photoassimilates to a more developed reproductive structure, thus increasing fitness. However, delayed flowering would not be justified if the leaves become severely shaded. Reduced branching is a shade-avoidance reaction because new branches originate from the rosette, placed at the base of the plant and profuse branching would therefore increase the proportion of shaded tissues.
The adaptive benefits of the plasticity involved in shade-avoidance responses has been demonstrated by using mutants unable either to produce these responses in crowded vegetation stands or to restrain shade-avoidance reactions in open places (Ballaré et al., 1994; Casal et al., 1994; Schmitt et al., 1995; Keuskamp et al., 2010). The changes in plant body form that occur in response to shade light signals are beneficial in crowded environments and detrimental in open places where they, for instance, increase wind impact. Shade-avoidance responses are likely to have evolved with shade, as early as the Devonian (Mathews, 2006).
The first sections of this chapter describe the light signals able to initiate shade-avoidance responses and the photoreceptors that perceive these signals in Arabidopsis thaliana. Subsequent sections present the mechanisms involved in the control of stem growth, leaf growth and flowering by shade light signals in Arabidopsis thaliana. These responses are presented separately to highlight differences that are often not recognized. The final sections deal with the differences and connections between shade-avoidance and shade-acclimation responses; a brief description of shade-avoidance responses in other species (in particular species of agricultural importance) to aid the use of Arabidopsis in translational approaches to improve crops, and final remarks.
LIGHT SIGNALS PROVIDED BY NEIGHBORING VEGETATION
The differences between sunlight and shade light
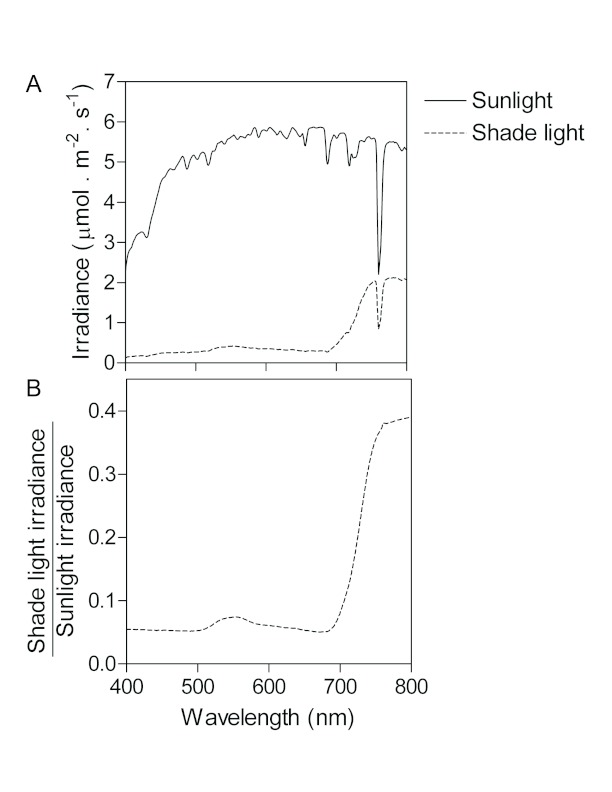
A light signal is a change in the light environment that can be perceived by plant photoreceptors. We will therefore define the changes of the light environment caused by the presence of neighbors and discern which of them are actually perceived by the plant and can therefore be considered as signals. Figure 2A shows the spectral distribution of incoming solar radiation between 400 and 800 nm compared to that observed under a dense canopy of green vegetation. The interference caused by the dense canopy reduces irradiance. Figure 2B shows the ratio between shade light and sunlight for the different wavelengths. Clearly, shade light shows a severe reduction of blue and red light, a slightly weaker reduction of green light and a relatively poor reduction of far-red light. This is caused by the optical properties of the green foliage, which absorbs more strongly in red and blue light than in green or far-red light.
Figure 2.
Differences between sunlight and shade light.
(A) Spectral distribution of sunlight reaching the top or the base of a dense vegetation canopy (respectively labeled sunlight and shade light).
(B) Spectrum of the ratio between the irradiance reaching the base of the canopy (shade light) and the irradiance of sunlight before making contact with the vegetation (calculated after A).
Shade light signals
Not all the changes caused by shade are shade-avoidance signals. Only those changes of the light environment perceived by photoreceptors and wired to the responses in body form that reduce the degree of current or future shade. Shade light signals include the reduction in red / far —red ratio (R:FR) perceived by phytochrome, the reduction in red plus far-red irradiance perceived by phytochrome, and the reduction in blue-UV-A irradiance perceived by cryptochromes. Plants are also able to respond to the reduced blue / green ratio in a cryptochrome-dependent manner. As light penetrates into the canopy these signals become more intense.
Dynamic changes in R:FR and irradiance signals
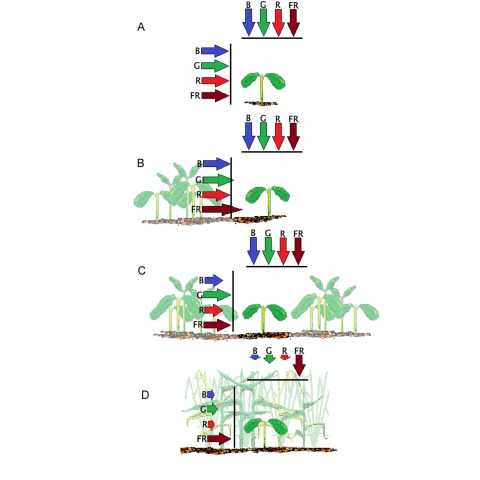
Full sunlight and deep shade light represent two extreme conditions. Figure 3 presents the different intensity stages of shade light signals in growing canopies. Compared to a fully isolated plant (Fig. 3A), a plant grown in a sparse canopy may experience increased levels of far-red light of the horizontally propagating light due to selective light reflection on the foliage of relatively distant, non-shading neighbors (Fig. 3B). This early warning signal of the presence of neighboring vegetation anticipates actual shade because the horizontally placed leaves are receiving full sunlight from above (Ballaré et al., 1987; Smith et al., 1990). With canopy growth there is a reduction of the irradiance of horizontally propagating radiation (Fig. 3C). Again, these changes impact mainly on vertical organs (typically the stem) and occur before the horizontal leaves become affected (Ballaré et al., 1989). Mutual shading of the most important photosynthetic organs begins only when the development of the foliage causes interference of direct light potentially reaching a given leaf by a neighbor leaf placed above it (Fig. 3D). This temporal pattern shows that neighbor signals able to initiate shade-avoidance reactions anticipate actual shade. For this reason shade avoidance can reduce the degree not only of current but also of future shade.
Figure 3.
The signals of the light environment change in response to the density of the surrounding vegetation.
(A) Plant isolated from nearby vegetation. The arrows represent the irradiance of vertically- and horizontally-propagating blue (B), green (G), red (R) and far-red (FR) light. The plant receives full incoming radiation in each waveband (represented by the size of each arrow touching the 100% black line).
(B) Plant surrounded by neighbors that do not project their shade on it but reflect far-red light (note that the horizontal arrow corresponding to far-red light crosses the black line, indicating more than 100% irradiance).
(C) Plant surrounded by neighbors that shade its stem (note arrows shorter than 100% for horizontally-propagated light).
(D) Plant shaded with horizontal foliage shaded by neighbors (note reduced size of vertical arrows).
Experimental simulation of shade light signals
Shade light signals are complex due to their spatial and temporal variation. One system often used to investigate shade-avoidance reactions is to reduce the R:FR while keeping PAR constant (Morgan and Smith, 1976). This is achieved by adding far-red light to a source of white light common to all the treatments. PAR can be provided by artificial sources or by sunlight. The advantage of this approach is that plants can be exposed to realistic R:FR without altering otherfeatures of the environment. Strictly speaking this does not reproduce any of the situations described in Figure 3 because although the second stage involves increasing far-red without large changes in other wavebands, the R:FR signal reaches the vertical stem and not the horizontal leaves (Fig. 3B), whereas the simulation affects the R:FR reaching the leaves. Far-red light can be provided from one side. Under sunlight this can be achieved by using artificial sources (Casal et al., 1987b), far-red light reflecting mirrors (Ballaré et al., 1987) or actual green neighbors that reflect far-red light (Ballaré et al., 1987). Fiber-optic probes can be used to direct far-red light to the stem (Morgan et al., 1980), and this approach reproduces the second stage in Figure 3.
Another approach is to use a pulse of far-red at the end of the photoperiod, i.e. end-of-day far-red (EODFR) (Downs et al., 1957). The principle of this treatment is that although the exposure to light is brief it causes changes in the status of the photoreceptors involved in the perception of the R:FR that persist during the subsequent night (see phytochromes below). To be effective this brief reduction in R:FR has to be severe (i.e. pure far-red light) as weak reductions of R:FR below sunlight values have no effects (contrary to similar reductions provided during daytime) (Casal et al., 1990a; Sellaro et al., 2011). This weaker sensitivity to reductions in R:FR at the end of the photoperiod prevents a response to the reduced R:FR caused by atmospheric factors at the extremes of the photoperiod. Clearly, a very severe reduction in R:FR restricted to the end of the photoperiod (i.e. EODFR) is not found in the natural situation.
Neutral filters can be used to simulate the reductions in irradiance and colored filters to reduce selected wavebands (Yanovsky et al., 1995a). For instance, yellow and orange filters cut blue light, cupper-sulphate filters reduce far-red. Green filters can simulate all aspects of shade light (Figure 3D) (Sánchez et al., 2011). The filters can be placed above the plants (then they will also affect PAR reaching the leaves) or surrounding the stem (Ballaré et al., 1991b), which is not a main photosynthetic organ.
While the aforementioned approaches simulate aspects of shade light and use a control exposed to unfiltered sunlight or an artificial white-light PAR source with high R:FR, it is also possible to use natural shade as a control and increase the R:FR by means of copper-sulphate filters placed either above the plants (Ballaré et al., 1991a) or surrounding the stem (Ballaré et al., 1990), or by using red-light emitting diodes selectively increasing the R:FR of photoreceptive tissues (Deregibus et al., 1985).
Finally, with the currently-available genetic tools it is also possible to compare real sunlight and shade light conditions and elucidate which of the environmental changes between these two conditions causes the observed physiological or molecular outputs by evaluating mutants disabled in the response to selected signals (Sellaro et al., 2010; Sellaro et al., 2011).
This brief overview demonstrates that there is a clear correlation between the accuracy of the simulation of the natural environment and the technical difficulties involved in this simulation. This should not discourage studies in this area because any of the above experimental conditions can be extremely useful to investigate shade-avoidance reactions provided that one is aware of the benefits and limitations (Fankhauser and Casal, 2004).
PHOTORECEPTORS INVOLVED IN THE PERCEPTION OF SHADE LIGHT SIGNALS
Phytochromes
Phytochromes are red and far-red light photoreceptors that bear a linear tetrapyrrole chromophore (Li et al., 2011). There are five phytochrome genes in Arabidopsis (PHYA through PHYE). The contribution of each phytochrome can vary with the different physiological outputs but phyA and phyB are clearly the most important. Phytochromes are synthesized in the Pr form that has the peak of absorption in red light. Upon light absorption excited Pr relaxes into the Pfr form, which has the peak of absorption in far-red light. Upon light absorption, excited Pfr relaxes to the Pr form. Pfr is biologically active and migrates from the cytosol to the nucleus. Under saturating light, a photoequilibrium is established between Pr and Pfr and the proportion of Pfr at photoequilibrium depends on the R:FR (Holmes and Smith, 1977; Smith et al., 1990). Phytochromes have a secondary peak of absorption in the blue-light region and can mediate responses to blue light compared to darkness (i.e. de-etiolation responses). However, in the context of shade-avoidance reactions where red and far-red light are present the blue-light component has no major influence on phytochrome status (Mancinelli, 1986).
The total amount (Pr+Pfr) of phyB is relatively stable. However, Arabidopsis phyB can undergo dark reversion from Pfr to Pr (Elich and Chory, 1997; Sweere et al., 2001). Under weak light, this dark reaction competes with photoconversion and therefore the level of Pfr of phyB will decrease not only with low R:FR but also with low irradiance. At high irradiance, photoconversion is much faster than dark reversion and the level of Pfr depends only on R:FR. The amount of phyB Pfr will depend on the R:FR but under low irradiances these levels will be below those corresponding to photoequilibrium. Therefore, phyB is a sensor of R:FR and of red irradiance. phyD, phyE and phyC are predicted to operate in a comparable manner (Franklin et al., 2003). The dark reversion of Pfr to Pr is variable in magnitude in different cellular contexts and biologically meaningful amounts of Pfr can persist for several hours even in full darkness. This is the principle of the EODFR treatment, which reduces Pfr levels to a minimum immediately prior to the beginning of the night. In the absence of EODFR, Pfr persists in darkness and a demonstration of this persistence is that plants can respond to a pulse of far-red (which acts by severely reducing Pfr levels) even if delayed several hours into the night (Downs et al., 1957; Casal, 1996).
The photoconversion properties of phyA are similar to those of phyB and the occurrence of phyA dark reversion shows natural variation in Arabidopsis (Eichhenberg et al., 2000). However, while total phyB levels are relatively stable, phyA Pfr is rapidly ubiquitinated and degraded in the 26S proteasome (Clough and Vierstra, 1997; Hennig et al., 1999). Therefore, the steady state level of phyA Pfr is not well related to R:FR because higher R:FR shifts photoconversion towards Pfr but exposes more Pfr to destruction. Furthermore, phyA Pfr migration from the cytosol to the nucleus requires binding FAR-RED ELONGATED HYPOCOTYL 1 (FHY1) and FHY1-LIKE (FHL) but once in the nucleus, FHY and FHL binding reduces Pfr activity. Therefore, Pfr must be photoconverted to Pr to release FHY/FHL and subsequently it must be photoconverted back to Pfr for biological activity (Rausenberger et al., 2011). As a result of phyA turnover (synthesis and degradation) and the photoconversions between Pr and Pfr and vice versa in the cytosol and the nucleus, the peak of phyA activity occurs at wavelengths closer to the peak of absorption of Pfr than of Pr absorption (Rausenberger et al., 2011), which corresponds to very low R:FR. phyA activity is strongly fluence-rate dependent, likely because higher fluence rates increase the rate of FH1/FHL-phyA complex assembly and disassembly and hence phyA transport capacity (Rausenberger et al., 2011). While the peak of phyA activity is in far-red light, phyA also operates under red light showing a weak contribution at very-low fluence rates (Mazzella et al., 1997) but a significant contribution at high irradiances (Franklin et al., 2007). Therefore phyA is a sensor of the reductions in red plus far-red irradiance caused by increasing shade. The activity of phyA is not significantly affected by R:FR in the range between unfiltered sunlight values (1.1) and 0.3 (Sellaro et al., 2010) but it increases with the very low R:FR observed in severely shaded environments (Smith et al., 1997). In summary, the activity of phyA decreases with increasing degrees of shade due to the reduced irradiance levels and under very dense canopies it increases again thanks to a very low (more favorable) R:FR.
Cryptochromes
Cryptochromes are photolyase-like blue light receptors that bind flavin adenine dinucleotide and could also bind a putative second chromophore (Yu et al., 2010). Arabidopsis has three cryptochrome genes but only CRYPTOCHROME 1 (CRY1) and secondarily CRY2 genes have been shown to participate in shade-avoidance reactions. The activity of cryptochromes increases with the levels of blue light and therefore cryptochromes are sensors of irradiance levels modified by the degree of shade. There are shade-avoidance responses to the blue / green ratio that require the action of cryptochromes (Sellaro et al., 2010) but the molecular basis of this dependency is a matter of debate (Banerjee et al., 2007; Bouly et al., 2007; Liu et al., 2010)
Other photoreceptors
In addition to the changes of the light environment described above, shade also reduces UV-B irradiance and this change perceived by UVR8 (Rizzini et al., 2011) could initiate shade-avoidance reactions (Ballaré et al., 1991c) but this possibility remains to be tested. Canopies are heterogeneous and create horizontal gradients that can be perceived by phototropins (Briggs and Christie, 2002). Some responses to green light are present in mutants of phytochromes and cryptochromes (Zhang et al., 2011). Therefore, while phyochromes, especially phyB, are the most important photoreceptors for shade-avoidance reactions followed by cryptochromes, especially cry1, other photoreceptors could enlarge the list in the future.
STEM GROWTH
Hypocotyl growth is promoted by different signals of neighboring vegetation. At the rosette stage, internode elongation is arrested but this is not the case in the cry1 phyB double mutant if grown at high temperatures (Mazzella et al., 2000), in the quadruple phyA phyB cry1 cry2 mutant (Mazzella et al., 2001), in the phyA phyB mutant exposed to EODFR (Devlin et al., 1996) and in the phyA phyB phyE mutant (Devlin et al., 1998). Although the photoreceptors are clearly important to maintain the rosette structure, internode elongation at this stage cannot be regarded as a physiological shade-avoidance response in Arabidopsis until the wild type is shown to extend these internodes under shade conditions. At a later stage, compared to the wild type grown under high R:FR, the height of the inflorescence stem is promoted by low R:FR and by the phyB mutation but these effects are relatively small (Finlayson et al., 2010). For this reason, most of the research on stem responses to shade light signals in Arabidopsis involves hypocotyl growth.
The hypocotyl is a transition organ that shows two temporally separable responses to light. When first exposed to light the hypocotyl shows de-etiolation (induced by light compared to darkness) and only then it becomes fully competent to respond to shade-light signals. The inhibition of hypocotyl-growth in response to light during de-etiolation is not simply the mirror image of the promotion of hypocotyl growth during shade avoidance. One important difference is that low R:FR are suitable for deetiolation and inhibit early hypocotyl growth almost as well as high R:FR, whereas at later stages low R:FR promote hypocotyl growth compared to high R:FR. A low R:FR can be achieved experimentally by adding far-red light to a PAR source with high R:FR. This treatment inhibits early hypocotyl growth when compared to the high R:FR condition because the higher level of red plus far-red irradiance perceived by phyA (Salter et al., 2003; Strasser et al., 2010) dominates over the low R:FR. Therefore, for hypocotyl-growth analysis under such experimental conditions it is advisable to allow a couple of days for de-etiolation under a high R:FR ratio source before applying the different R:FR treatments (Johnson et al., 1994).
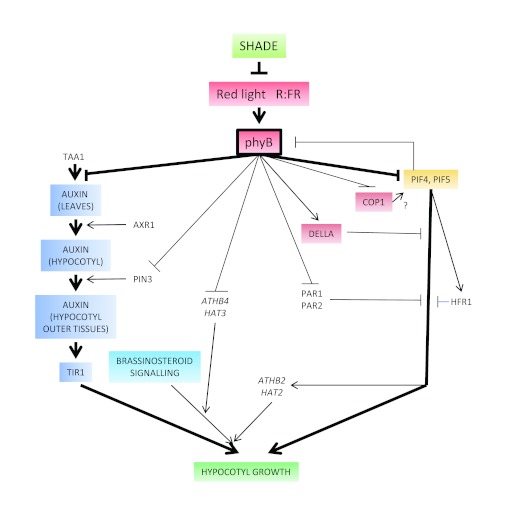
For the promotion of hypocotyl growth, the reduced red irradiance and R:FR caused by increasing shade are perceived by phyB (Figure 4), the reduced red plus far-red irradiance is perceived by phyA, the reduced blue irradiance is perceived by cry1 in the Landsberg erecta accession and by cry1 and cry2 in Columbia and the reduced blue / green ratio is perceived by or requires cry1 in Landsberg erecta (Sellaro et al., 2010). In addition to its direct role inhibiting hypocotyl growth in response to increasing irradiance, phyA has a more indirect role reducing the extent of phyB-mediated inhibition of hypocotyl growth (Cerdán et al., 1999). This regulation of phyB signaling by phyA is observed under natural radiation (Mazzella and Casal, 2001; Sellaro et al., 2010) and makes the seedlings more sensitive to shade-light signals as the phyA mutant does not show the promotion of hypocotyl growth caused by neighbors that reflect far-red light without shading (stage shown in Fig. 3B) (Casal, 1996).
Figure 4.
Simplified representation of the network involved in the promotion of hypocotyl growth by low R:FR and low red irradiance of shade light perceived by phyB.
When transferred from high to low R:FR, Arabidopsis seedlings show no hypocotyl-growth response during a lag of approximately 45 min, followed by a first phase of growth promotion between 45 and 150 min, a phase of reduced growth between 150 and 230 min and a second promotion of hypocotyl growth beyond 230 min (Cole et al., 2011). This kinetics of induction is similar (with quantitative differences) to that reported in classical experiments with Sinapis alba (Morgan et al., 1980; Child and Smith, 1987). The reversal of the promotion once the seedlings return to high R:FR depends on the duration of the exposure to low R:FR (Cole et al., 2011), and this could reflect an effect derived from leaf perception of low R:FR (Casal and Smith, 1988). Under free-running conditions the promotion of hypocotyl growth recorded 24 h after 2-h exposure to low R:FR is stronger if low R:FR are given during subjective afternoon (Salter et al., 2003). This promotion is attenuated and phase-shifted in timing of cab expression 1 (toc1) mutant affected in a central component of the clock (Strayer et al., 2000) indicating a circadian gating of hypocotyl shade-avoidance responses (Salter et al., 2003). However, a clock regulation is not apparent during the first 10 h of exposure to low R:FR when growth is monitored at high resolution (Cole et al., 2011). Under day-night cycles of natural radiation, shade events (2 h) are more effective to promote hypocotyl growth if they occur in the afternoon (Sellaro et al., 2012).
The strength of the hypocotyl-growth response to low R:FR shows substantial natural variation unrelated to the length of the hypocotyls under high R:FR, to the latitude of the location of original collection, or to the variability in the response of flowering to low R:FR (Botto and Smith, 2002). A few accessions, such as CT-1 and No-0, show long hypocotyls under white light and reduced response to EODFR (Coluccio et al., 2011).
Hypocotyl shade-avoidance responses require PHYTOCHROME-INTERACTING FACTOR 4 (PIF4), PIF5 and CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1).
PHYTOCHROME-INTERACTING FACTOR (PIF) proteins are basic helix-loop-helix transcription factors that bind the active form of phyB (some also bind phyA) in the nucleus and as a result of this interaction become rapidly phosphorylated, apparently ubiquitylated and finally degraded in the proteasome (Leivar and Quail, 2011). The function of PIF proteins in shade-avoidance reactions is supported by genetic and molecular experiments. Under constant white light with high R:FR, compared to the wild type the hypocotyl is slightly shorter in the pif4, pif5 and pif4 pif5 mutants and longer in the PIF5 overexpressor. pif4, pif5 and pif4 pif5 mutants and the PIF5 overexpressor have reduced hypocotyl growth responses to low R:FR and are partially epistatic to the phyB mutation (Lorrain et al., 2008). These observations indicate that shade-avoidance responses require normal levels of PIF4 and PIF5. The pif4 pif5 mutant lacks key components of the signaling mechanisms leading to enhanced hypocotyl growth and the PIF5 overexpressor already has a long hypocotyl in the absence of shade signals. When Arabidopsis seedlings are transferred from white light with a high R:FR to a low R:FR PIF5 and PIF4 re-accumulate. Increased PIF5 levels are already observed 15 min after transfer to low R:FR and persists at least during the subsequent 2 h (Lorrain et al., 2008). While PIF4 and PIF5 levels increase rapidly, the pif4 pif5 double mutant shows only a weak reduction of the promotion of hypocotyl growth by low R:FR during the first 10 h of treatment (Cole et al., 2011), suggesting that the impact of PIF protein levels on growth is relatively slow. These results indicate that the long-term promotion of hypocotyl growth caused by the low levels of active phyB established by low R:FR is partially accounted for by the accumulation of growth promoting PIF4 and PIF5 transcription factors (Figure 4). In addition to the regulation of PIF protein levels by phyB there is a reciprocal regulation as PIF3, PIF4 and PIF7 also help to maintain low levels of phyB (Leivar et al., 2008a). Shade-avoidance reactions would be favored by both the re-accumulation of PIF proteins allowed by the reduced phyB Pfr levels and the further reduction of phyB Pfr by PIF proteins.
The hypocotyl-growth response to low R:FR or EODFR requires the E3 ligase CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1) (McNellis et al., 1994). However, the mechanism of COP1 action during shade-avoidance responses is not well established. In this regard, COP1 is required for the accumulation of PIF3 (and likely other PIF proteins) in the dark (Bauer et al., 2004; Leivar et al., 2008b) and it would be interesting to evaluate whether COP1 is also necessary for the low R:FR-induced reaccumulation of PIF (Figure 4). EARLY FLOWERING 3 (ELF3) may act at the biochemical level as an adaptor/scaffold protein facilitating COP1 activity (Yu et al., 2008) or by forming an evening complex required for the correct diurnal expression of PIF4 and PIF5 (Nusinow et al., 2011). ELF3 is a likely candidate gene to account for a quantitative trait locus for hypocotyl growth responses to low R:FR between the accessions Bayreuth-0 and Shahdara (Coluccio et al., 2011). This is also true for other shade-avoidance responses (see below) but the mechanisms of action of ELF3 during shade-avoidance responses remain to be elucidated.
Hypocotyl shade-avoidance responses require auxin, gibberellins and brassinosteroid signals
Auxin. Several mutations or pharmacological treatments that affect auxin synthesis, auxin transport or auxin perception impair the promotion of hypocotyl growth by low R:FR. The SHADE AVOIDANCE 3 (SAV3) /TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (TAA1) gene is expressed in the cotyledons (very poor expression in the hypocotyl) and SAV3/TAA1 catalyzes the formation of indole-3-pyruvic acid from L-tryptophan (L-Trp) in the auxin biosynthetic pathway (Tao et al., 2008). PIN-FORMED 3 (PIN3) (Friml et al., 2002) and PIN7 are auxin efflux transmembrane transporters, AUXIN RESISTANT 1 (AXR1) is an ubiquitin-activating enzyme that controls stability of auxin efflux carriers (Sieberer et al., 2000), and TRANSPORT INHIBITOR RESPONSE 1 is an auxin receptor (Dharmasih et al., 2005; Kepinski and Leyser, 2005). The promotion of hypocotyl growth by low R:FR is reduced in the sav3 (Tao et al., 2008), pin3, pin7, axr1 and tir1 mutants (Keuskamp et al., 2010) and in wild- type seedlings exposed either to naphthylphthalamic acid (NPA), which blocks polar auxin transport, or α-(phenylethyl-2-one)-IAA, which is an antagonist for the auxin receptor TIR1 and its homologs (Steindler, 1999; Keuskamp et al., 2010). The hypocotyl of the sav3-2 mutant does not respond to low R:FR during the first 4 h of treatment, and only then shows a mild promotion of extension growth (Tao et al., 2008; Cole et al., 2011).
Low R:FR not only require normal auxin synthesis, transport, perception and signaling; they also modify key aspects of these processes. At the whole-seedling level of resolution, low R:FR promote free auxin levels (Tao et al., 2008) and the expression of PIN3, PIN7 and the auxin-induced transcription factor genes IAA1, IAA3, IAA5, IAA11, and IAA19 among several other auxin-related genes (Devlin et al., 2003). Low R:FR increase auxin signaling in the cotyledons (Tao et al., 2008). Both free-auxin and cotyledon auxin signaling responses require SAV3, but the expression of SAV3/TAA1 is not increased by low R:FR (Tao et al., 2008). In the hypocotyls, low R:FR promote the expression of PIN3 and direct PIN3-GFP from the basal to the lateral side of the membrane of the endodermal cells, increase free IAA levels in a PIN3-dependent manner and increase the activity of the auxin-responsive IAA19 promoter fused to GUS, particularly in the outer tissues of the hypocotyl (Keuskamp et al., 2010). Therefore, low R:FR favors SAV3/TAA1-mediated auxin synthesis in the leaves (Tao et al., 2008) and PIN3-3 mediated lateral auxin redistribution towards epidermal and cortical cells of the hypocotyl (Keuskamp et al., 2010), which in turn would promote the elongation of these cells and of the whole organ (Morelli and Ruberti, 2000).
Low R:FR could also alter the responsivity to auxin. The expression of the homeodomain-leucine zipper transcription factor genes ARABIDOPSIS THALIANA HOMEOBOX PROTEIN 2 (ATHB2) / HAT4 and HAT2 is rapidly and reversibly promoted by low R:FR (Carabelli et al., 1993; Roig-Villanova et al., 2006). This promotion depends partially on PIF4 and PIF5 (Lorrain et al., 2008) (Figure 4). Transgenic seedlings with elevated levels of expression of ATHB2 or HAT2 have long hypocotyls under high R:FR and altered responses to auxin whereas transgenic seedlings with reduced levels of ATHB2 expression show short hypocotyls (Steindler, 1999; Morelli and Ruberti, 2002; Sawa et al., 2002).
The promotion of hypocotyl growth caused by lowering blue light is reduced in the sav3, pin3, pin7, tir1 and tir1afb1afb2afb3 mutants (where abf1, abf2 and abf3 are mutations at AUXIN SIGNALING F-BOX protein genes, homologs to TIR1 (Dharmasiri et al., 2005; Kepinski and Leyser, 2005)) compared to the wild type (Keuskamp et al., 2010). The response is also reduced in the wild type by the application of NPA or the inhibitior of auxin perception α-(phenylethyl-2-one)—indole-3-aceticacid (Keuskamp et al., 2010). These observations indicate that the promotion of hypocotyl growth by lowering blue light also requires auxin synthesis, auxin transport and auxin perception.
Gibberellins. The promotion of hypocotyl growth by the phyB mutation (Reed et al., 1996), low R:FR or low blue light (Djakovic-Petrovic et al., 2007) is impaired by the addition of the inhibitor of gibberellin biosynthesis paclobutrazol and by the use of mutant backgrounds deficient in gibberellin synthesis. Whether phyB affects total seedling levels of this hormone is not clear because some active gibberellins do not show differences between the wild type and the phyB mutant and others could not be detected (Reed et al., 1996). The expression of the GIBBERELLIN 20-OXI-DASE 3 (GA20OX3) gene, which encodes an enzyme involved in the synthesis of gibberellins, is strongly promoted by low R:FR (Devlin et al., 2003) but this response is difficult to interpret because GA20OX3 expression is down-regulated by elevated gibberellin signaling (Sun, 2008).
While phyB-mediated changes in active gibberellins have not been detected, phyB clearly reduces the responsivity to gibberellins (Reed et al., 1996), suggesting alterations downstream of hormone levels. The DELLA proteins REPRESSOR OF GA (RGA), GIBBERELLIC ACID INSENSITIVE (GAI), RGA-Like1 (RGL1), RGL2 and RGL3 become degraded after the activation of the receptor of gibberellins (Sun, 2008). DELLA proteins are negative regulators of stem extension growth that reduce hypocotyl growth in part by impeding PIF4 and PIF3 binding to DNA (De Lucas et al., 2008; Feng et al., 2008). Although low R:FR strongly promote the expression of the GAI gene (Devlin et al., 2003), both low R:FR and low blue light reduce the abundance of DELLA proteins in the hypocotyls and these responses are blocked by paclobutrazol (Djakovic-Petrovic et al., 2007). The gibberellin-insensitive gai gain-of-function mutant, which has a stable GAI protein, shows reduced responses to low R:FR or low blue light, indicating that the induction of DELLA degradation by these shade light signals is a requisite for the growth response. In addition, a quadruple gai rga rgl1 rgl2 loss-of-function mutant shows partially elongated hypocotyls, suggesting that degradation of DELLA could by itself account for part of the hypocotyl response to low R:FR (Djakovic-Petrovic et al., 2007). The quadruple gai rga rgl1 rgl2 mutant has a response to low blue light even larger than that observed in the WT (Djakovic-Petrovic et al., 2007) and this response is almost completely abolished by the addition of NPA (Pierik et al., 2009), suggesting that DELLA proteins could restrain auxin-mediated responses to low blue light.
Brassinosteroids. A full hypocotyl-growth response to shade light requires brassinosteroids. When grown under a plant canopy, the brassinosteroid biosynthesis mutant diminutol dwarf1 (Klahre et al., 1998) is unable to show the typical promotion of hypocotyl growth caused by shade light (Luccioni et al., 2002) (Figure 4). The promotion of hypocotyl growth observed in response to lowering blue light is reduced in the brassinosteroid synthesis mutant rotundifolia 3 (rot3) (Kim et al., 1998), in wild-type seedlings treated with the brassinosteroid synthesis inhibitor brasinazole and in the bri1 mutant (Keuskamp et al., 2011) affected in the BRASSINOSTEROID INSENSITIVE 1 (BRI1) brassinosteroid-receptor gene (Wang et al., 2001). However, none of these effects is complete and only the simultaneous blocking of both auxin and brassinosteroid pathways eliminates the response to low blue light (Keuskamp et al., 2011). XYLOGLUCAN ENDOTRANS-GLUCOSYLASE /HYDROLASE (XTH) enzymes represent a potential control point for cell elongation (Cosgrove, 2005) and the expression of several XTH genes is promoted by lowering blue light and the response of some of these genes requires either auxin perception or brassinosteroid synthesis (Keuskamp et al., 2011). Interestingly, the combined application of auxin and brassinosteroids has additive promotion effects on hypocotyl growth (Keuskamp et al., 2011). One mode of integration of auxin and brassinosteroid pathways involves the phosphorylation of AUXIN RESPONSE FACTOR 2 (ARF2) by brassinosteroid-regulated activity of the GSK3 kinase BRASSINOSTEROID-INSENSITIVE 2 (BIN2) (Vert et al., 2008). This reduces ARF2 DNA binding and repression activities, leading to synergistic increases in transcription of auxin-response genes (Vert et al., 2008).
The expression of BRI1 (Devlin et al., 2003) and of the homeodomain-leucine zipper transcription factors genes ARABIDOPSIS THALIANA HOMEOBOX PROTEIN 4 (ATHB4) and HAT3 (Roig-Villanova et al., 2006; Sorin et al., 2009) is promoted by low R:FR. The athb4 hat3 mutant has normal hypocoyl length under high R:FR but it fails to respond to low R:FR and responds poorly to the addition of brassinosteroids (Sorin et al., 2009), suggesting that low R:FR could increase the sensitivity to brassinosteroids (Figure 4).
Negative regulation of hypocotyl shade-avoidance responses
The previous paragraphs describe a largely positive action of shade signals on pathways that promote hypocotyl growth as those involving PIF proteins, auxin, gibberellins and brassinosteroid. This section describes the positive action of shade signals on negative regulators of hypocotyl shade-avoidance responses. While several genes show a transient promotion of expression in response to low R:FR, the bHLH family member LONG HYPOCOTYL IN FAR-RED LIGHT (HFR1) gene, is rapidly promoted by low R:FR and retains elevated levels of expression several days later (Sessa et al., 2005). This promotion depends on PIF4 and PIF5 (Lorrain et al., 2008). When the seedlings are grown for 3 d under high R:FR photopehods and then transferred for 4 d to low R:FR, the hfr1 mutant shows an exaggerated promotion of hypocotyl growth and a stronger and /or more persistent enhancement of the expression of the ATHB2, PHYTOCHROME INTERACTING FACTOR 3-LIKE 1 (PIL1), PIF6/PIL2, BIM1, PHYA and XTR1 genes among others typically promoted by low R:FR (Sessa et al., 2005; Roig-Villanova et al., 2007; Hornitschek et al., 2009). Similarly, PHYTOCHROME RAPIDLY REGULATED 1 (PAR1) and PAR2 are atypical bHLH genes whose expression is rapidly and reversibly promoted by low R:FR even in the presence of the protein synthesis inhibitor cycloheximide (Roig-Villanova et al., 2006). Overexpression of PAR1 or PAR2 reduces the hypocotyl-growth response to low R:FR, and the expression response to low R:FR of selected auxin-signaling genes, whereas reduced expression of PAR1 and/or PAR2 increases hypocotyl growth (Roig-Villanova et al., 2007). As noted by Roig-Villanova et al, (2007) the hfr1 mutant has a exaggerated response to low R:FR under low irradiances but the hfr1, par1 or par2 phenotypes are weak when low R:FR are provided at high irradiances. Therefore, HFR, PAR1 and PAR2 are negative regulators of shade-avoidance responses whose expression is promoted by shade light generating a negative feed-back (Figure 4).
PAR1, PAR2 and HFR1 proteins lack a typical basic domain necessary for binding to E-box and G-box motifs in the promoter of target genes. HFR1 forms non-DNA-binding heterodimers with PIF4 and PIF5, preventing their binding to DNA and biological activity (Hornitschek et al., 2009). The mechanism of action of PAR1 is apparently similar to that of HFR1 (Galstyan et al., 2011).
PIL1 expression shows a strong and rapid promotion by low R:FR (Salter et al., 2003), which depends on PIF4 and PIF5 (Lorrain et al., 2008). The pil1 mutants show phase-shifted and attenuated promotion of hypocotyl growth caused by 2 h exposure to low R:FR measured 24 h later (Salter et al., 2003) and an enhanced promotion when the seedlings are grown for 5 d under low R:FR (Roig-Villanova et al., 2006). Therefore, PIL1 can be a positive or negative regulator of shade-avoidance responses apparently depending on the kinetics of the shade light signal.
Several B-box-containing zinc finger transcription factors (B-BOX DOMAIN PROTEIN, BBX) are involved in hypocotyl-growth responses to shade light. The bbx19, bbx21 and bbx22 mutant seedlings show long hypocotyls whereas bbx18 and bbx24 mutant seedlings show short hypocotyls under natural or simulated shade (Crocco et al., 2010). The bbx21 bbx22 double mutation restores the hypocotyl-growth response to shade light in the cop1 mutant background, suggesting that BBX proteins act down-stream COP1 (Crocco et al., 2010). Compared to sunlight, natural shade promotes the expression of BBX21, BBX19 and BBX22 and reduces the expression of BBX18 and BBX24, indicating that these genes are also part of the feed-back inhibition of shade-avoidance responses (Crocco et al., 2010).
Sunfleck repression of hypocotyl shade-avoidance response
Plant canopies are heterogeneous and have gaps that allow the penetration of sunflecks. These sunflecks depend on the position of the gap and on solar elevation and therefore, their occurrence is repeated every clear day at approximately the same time. The activation primarily of phyB and secondarily of phyA by sunflecks of 2 h duration causes a strong inhibition of hypocotyl growth compared to continuous shade light, particularly when the sunflecks take place late in the photoperiod (Sellaro et al., 2011). Sunflecks cause large changes in the transcriptome, including the enhanced expression of the ELONGATED HYPOCOTYL 5 (HY5) bZip transcription-factor gene (Oyama et al., 1997). The levels of HY5 are low both in seedlings grown under sunlight as well as in seedlings grown under uninterrupted shade light. However, after several hours of shade light the expression of HY5 is promoted by the transition to sunlight, i.e. the expression of HY5 is selectively high under sunfleck conditions (Sellaro et al., 2011). The hy5 mutant shows impaired hypocotyl-growth inhibition and transcriptome responses when exposed to sunflecks. In particular, sunflecks reduce the expression of auxin-related genes and PHYTOCHROME KINASE 4 (PKS4) (Schepens et al., 2008) in an HY5-dependent manner (Sellaro et al., 2011). Mutants with severely impaired rhythms fail to respond to sunflecks even in the afternoon while the late elongated hypocotyl (lhy) circadian clock associated 1 (cca1) double mutant (Mizoguchi et al., 2002) shows a slightly higher response to morning sunflecks but it retains the higher effectiveness of afternoon sunflecks. In summary, the activation of phyB and phyA after several hours of shade light in the day promotes the expression of HY5 which represses the expression of auxin-related and PKS4 genes causing reduced hypocotyl growth. A permissive action of the clock helps to reinforce the action afternoon compared to morning sunflecks (Sellaro et al., 2011).
LEAF GROWTH
Petiole elongation
Compared to Arabidopsis plants grown isolated from nearby vegetation, plants grown in dense canopies show longer petioles (Ballaré and Scopel, 1997; Djakovic-Petrovic et al., 2007). Lowering the R:FR causes a promotion of petiole growth already detectable 2 h after the beginning of the treatment (Djakovic-Petrovic et al., 2007; Sasidharan et al., 2010). EODFR also promotes petiole growth compared to the controls directly transferred from white light to darkness (Kozuka et al., 2010). The phyB mutant has longer petioles than the wild-type (Nagatani et al., 1991; Reed et al., 1993) and the phyA phyB phyD and phyA phyB phyE mutants have longer petioles than the phyA phyB mutant, (Devlin et al., 1998; Devlin et al., 1999). The promotion of petiole growth in response to either low daytime R:FR or EODFR is absent or even inverted (inhibition) in the phyB mutant (Nagatani et al., 1991; Devlin et al., 1996; Pierik et al., 2009; Kozuka et al., 2010), which retains some response to increasing canopy shade (Ballaré and Scopel, 1997). Lowering blue light caused little promotion of petiole growth in some experiments (Djakovic-Petrovic et al., 2007; Pierik et al., 2009) and a robust promotion of petiole elongation in others, where the cry1 mutant showed constitutively long petioles and no response to low blue light (Keller et al., 2011). Therefore, the promotion of petiole growth by shade light signals is mediated primarily by phyB and secondarily by phyD, phyE and cry1.
Under constant white light pif5 but not the pif4 mutant shows reduced petiole response to low R:FR. The pif4 pif5 double mutant has a shorter petiole under high R:FR but apparently normal responses to low R:FR. The PIF5 overexpressor shows long petioles under high R:FR that do not respond to low R:FR but do not reach the length observed in wild-type plants exposed to low R:FR (Lorrain et al., 2008). The pif4, pif5 and pif4 pif5 mutants show reduced petiole-growth responses to low blue light, (Keller et al., 2011). These observations indicate that while altered levels of PIF4 and/or PIF5 can distort petiole growth responses, the function of these proteins is probably less central for petiole than for hypocotyl growth.
The promotion of petiole growth by shade light signals is impaired in several auxin-or brassinosteroid-related mutants. The doc1/big mutant, affecting a calossin-like protein gene involved in auxin transport (Gil et al., 2001) and the rot3 mutant, deficient in brassinosteroid synthesis have short petioles with reduced responses to EODFR and are epistatic to the phyB mutation (Kozuka et al., 2010). The sav3 auxin synthesis mutant (Tao et al., 2008) and the axr1 mutant and the axr2-1/iaa7 gain-of-function mutant (Nagpal et al., 2000) also impair petiole-growth responses to daytime low R:FR (Sasidharan et al., 2010). The sav3 mutant and a partial loss of function bri1 mutant show severely reduced petiole-growth responses to low blue light, while these responses are normal in pin3 and the quintuple yuc3 yuc5 yuc7 yuc8 yuc9 mutant (Keller et al., 2011). YUCCA (YUC) enzymes catalyse a rate-limiting step in tryptophan-dependent auxin biosynthesis (Zhao et al., 2001). Clearly, petiole-growth responses to shade light signals require auxin and brassinosteroid signaling. However, there is only partial information concerning the effects of light signals on these hormone pathways controlling petiole growth. It is known that auxin-responsive and brassinosteroid-responsive genes are overrepresented among the EODFR-induced genes in the petiole 2 h after the transition to darkness and that EODFR does not cause a detectable increase in auxin levels in the petiole or leaf blade (Kozuka et al., 2010).
EODFR given only to the blade is more effective to promote petiole growth and petiole changes in gene expression than EODFR given to the petiole itself (Kozuka et al., 2010). The aux-in-transport inhibitor NPA reduces the petiole growth response to EODFR when added to the agar in experiments with excised leaves and when sprayed to intact plants (Kozuka et al., 2010) and the response to low daytime R:FR when brushed onto the leaves (Pierik et al., 2009). The pin3 mutant shows a slightly delayed promotion of petiole extension in growing canopies (Keuskamp et al., 2010). Therefore, auxin transport might play a role in blade-petiole communication (Kozuka et al., 2010).
EODFR up-regulates the expression of the GA20OX2 gene involved in gibberellin biosynthesis, and transgenic Arabidopsis plants with reduced GA20OX2 expression show reduced petiole elongation response to EODFR (Hisamatsu et al., 2005). This observation suggests that the EODFR promotion of petiole growth could involve increased synthesis of gibberellins in response to EODFR, but the latter interpretation remains to be tested. Low R:FR reduce the abundance of DELLA in the petiole with a kinetics that matches that of petiole growth (Djakovic-Petrovic et al., 2007). Wild-type plants treated with paclobutrazol, the gibberellins-deficient ga1 mutant and the gai mutant that bears a stable GAI protein respond deficiently to low R:FR, indicating that the promotion of petiole growth requires gibberellins and degradation of DELLA. However, quadruple mutants of DELLA proteins do not show elongated petioles and retain apparently normal responses to low R:FR. Therefore, the low R:FR-induced degradation of DELLA is a requisite for normal petiole responses to R:FR but in contrast to the case of hypocotyl growth, lowering DELLA levels genetically is not enough to phenocopy petiole-growth promotion by low R:FR (Djakovic-Petrovic et al., 2007). Although auxin facilitates the degradation of DELLA proteins in the petiole, they do not exert their effect via this pathway (Pierik et al., 2009). The quintuple delta mutant conserves normal responses to reduced blue light. Lowering blue light does not trigger DELLA degradation in Arabidopsis petioles, but the gain of function gai1 mutant fails to respond, implying that elevated levels of DELLA impair the response (Keller et al., 2011).
Low R:FR stimulates ethylene production by the shoot (Pierik et al., 2009). The ethylene-insensitive 2 (ein2) mutant, the ein3 ein-3 like 1 (eil1) double mutant (Schaller and Kieber, 2002) and wild-type plants treated with the ethylene action inhibitor 1-methylcyclopropane do not show the promotion of petiole growth by low R:FR indicating that ethylene is required for this shade-avoidance response. However, supplementary ethylene has only minor effects on petiole growth (Pierik et al., 2009).
In the petiole, enhanced xyloglucan degrading activity and enhanced expression of XTH5 and XTH17 (among other XTH genes) accompany the growth promotion induced by low R:FR or simulated shade light and this growth promotion is absent or reduced in xth15 and xth17 mutants (Sasidharan et al., 2010). Conversely, expansin activity (Cosgrove, 2005) does not obviously correlate with the petiole growth responses (Sasidharan et al., 2010). The hfr1 mutant has exaggerated petiole elongation, indicating that the negative regulation of shade-avoidance responses operates beyond the hypocotyl stage (Sessa et al., 2005).
Leaf expansion
Compared to the wild type under high R:FR, leaf area is reduced by low R:FR and by the phyB mutation (Nagatani et al., 1991; Reed et al., 1993; Devlin et al., 1999). Leaf area is also reduced in phyB phyD compared to phyB, indicating a role of phyD (Devlin et al., 1999). However, the phyB mutation can increase leaf area (Robson et al., 1993) suggesting that the final output depends on growth conditions (Devlin et al., 1999). The leaf area response to low R:FR is also strongly context dependent and plants grown at 16 rather than 22 °C show a promotion and not an not a reduction in leaf area compared to high R:FR controls (Franklin et al., 2003). The hfr1 mutant has reduced leaf area expansion, indicating that HFR1 negatively regulates diverse shade-avoidance responses (Sessa et al., 2005).
The reduction in leaf lamina area can be the result of both increased demand of resources by the petioles and direct mechanisms of shade light action on the lamina. In experiments conducted in Sinapis alba where one leaf of the first pair was covered while the other leaf (and the rest of the shoot, including the stem) was exposed to EODFR, the leaf exposed to EODFR showed reduced extension growth and accumulation of dry matter and structural carbohydrates, and higher activities of sucrose-phosphate synthase (an enzyme positively linked to carbon export from the leaves), than the leaf not exposed to EODFR (Yanovsky et al., 1995b). Since both leaves were attached to the same internode these effects cannot be assigned to increased stem demand and demonstrate the occurrence of direct effects of shade light on the leaves. However, the leaf covered during the exposure to EODFR showed some growth reduction compared to the leaves of control plants not exposed to EODFR. This suggests that increased growth of the stem could reduce the growth of the leaf covered during the EODFR treatment (Yanovsky et al., 1995b).
Among the direct effects, in Arabidopsis the reduced leaf area under low R:FR is caused by decreased cell proliferation (Carabelli et al., 2007). When seedlings are transferred to low R:FR after 7 d under high R:FR, a significant reduction in the activity of a cell division marker is observed 8 h after transfer to low R:FR. In seedlings grown for only 4–5 d under high R:FR, transfer to low R:FR causes a rapid promotion of the synthetic DR5 promoter activity (used to estimate auxin signaling intensity) and the CYTOKININ OXIDASE 6 (CKX6) (Werner and Schmülling, 2009) promoter activity in leaf phmordia (already detectable 4 h after transfer) and a severe arrest of leaf primordia growth that is not observed in the tir1 or ckx6 mutants (Carabelli et al., 2007). These observations support a rapid promotion in auxin signaling by low R:FR inducing cytokinin degradation via the promotion of CKX6, which would reduce cell proliferation (Carabelli et al., 2007). Despite the differential effect of EODFR on petiole and leaf-lamina growth, EODFR increases the expression of auxin-responsive and brassinosteroid-responsive genes in both organs (Kozuka et al., 2010). However, the doc1/big or rot3 mutants that affect the petiole response, retain normal leaf-blade responses to EODFR (Kozuka et al., 2010).
Hyponastic leaf movement
When Arabidopsis plants are grown in dense canopies, the leaves adopt a more erect position (Ballaré and Scopel, 1997; Djakovic-Petrovic et al., 2007). This response is rapid and can be readily appreciated in a few hours (Faigón-Soverna et al., 2006). Both, lowering the R:FR (Vandenbussche et al., 2003; Tao et al., 2008; Keuskamp et al., 2010) or irradiance (Vandenbussche et al., 2003; Mullen et al., 2006) cause leaf hyponasty and lowering both has a stronger effect (Sasidharan et al., 2010). The response to irradiance is gradual at least between 5 and 200 µmol m2 s-1, can easily be detected 2 h after the reduction in irradiance and reaches a maximum after 16 h (Millenaar et al., 2009). The phyB mutant has hyponastic leaves in the absence of shade and retains only a minor response to dense canopies (Ballaré and Scopel, 1997) and a delayed response to reduced irradiance (Millenaar et al., 2009). Lowering specifically blue light causes leaf hyponasty. The cry1 mutant may present slightly hyponastic leaves that do not respond to low blue light (Keller et al., 2011) but this hyponastic phenotype is not always observed (Ballaré and Scopel, 1997; Mullen et al., 2006).
The hyponastic response to low R:FR is severely reduced in sav3 (Tao et al., 2008; Moreno et al., 2009) and pin3 mutants (Keuskamp et al., 2010). The hyponastic response to lowering irradiance is strongly reduced in wild-type plants treated with the polar auxin transport inhibitor, TIB, and in the tir1, tir1 afb1 afb2 afb3, pin3, pin7, tir3, axr2 or axr3 mutants (Vandenbussche et al., 2003; Millenaar et al., 2009), indicating that the response to low R:FR or low irradiance requires intact auxin signaling. Conversely, the response to lowering blue light is conserved in the sav3, pin3, quintuple yucca, quintuple della, gai1 and bri1 mutants and partially attenuated in the pif4 and pif5 and pif4 pif5 mutants (Keller et al., 2011). There are conflicting results regarding the role of ethylene (Vandenbussche et al., 2003; Millenaar et al., 2009).
BRANCHING
Plants of Arabidopsis either of the phyB mutant or of the wild type grown under low R:FR produce less branches than plants of the wild type grown under high R:FR (Reed et al., 1993; Finlayson et al., 2010). This indicates that high R:FR perceived by phyB promote branching compared to low R:FR. Branching involves a series of steps that begin with the generation of the leaves and the generation of the buds in the leaf axils. Low R:FR-treated plants and phyB mutants grown under high R:FR cause accelerated flowering and therefore reduce the number of leaves. However, these plants reduce branching more than expected based on their reduction in leaf number as indicated by the number of branches standardized to a leaf number basis (Finlayson et al., 2010). Similarly, low R:FR and the phyB mutation cause some reduction in the number of buds per leaf but this effect does not account for the effects on branch numbers, because buds are always in excess of branches. Therefore, phytochrome affects branching mainly by modulating the bud outgrowth process (Finlayson et al., 2010). In turn, the effect of phyB on bud outgrowth has two opposite components. On the one hand phyB opposes the correlative inhibition (i.e. the inhibition imposed by one organ on the growth of another organ) resulting from the influence of the main shoot and other branches. On the other, phyB represses stem growth (see previous sections of this chapter) and therefore, low R:FR can promote the growth of some branches (Finlayson et al., 2010).
There is a correlative control of branching by signals received from more or less remote parts of the plant. Auxin produced in young expanding leaves at the shoot apex is transported basipetally down the stem and indirectly inhibits shoot branching, establishing apical dominance (Leyser, 2005). Strigolactones are synthesized in both the roots and the shoots and are transported acropetally, presumably in the xylem, to repress bud activity (Domagalska and Leyser, 2011). The control of bud outgrowth by phyB perception of low R:FR is impaired in axr1, more axillary branches 2 (max2) and max4 (Domagalska and Leyser, 2011) mutants, indicating that this control requires intact auxin and strigolactone signaling (Finlayson et al., 2010). Domagalska and Leyser (2011) have proposed that phyB could theoretically exert part of its action on branching by altering strigolactone signaling and polar auxin transport.
In addition to the correlative or systemic regulation of branching there is a local control. The TCP-domain transcription factor genes BRANCHED 1 (BRC1) and BRC2 are expressed in the buds and suppress bud outgrowth (Aguilar-Martínez et al., 2007; Finlayson, 2007). The control of branching by phyB and R:FR is impaired in the brc1 and brc2 mutants (Finlayson et al., 2010). When mRNA levels are analyzed in unelongated primary rosette buds at two different positions of plants of the wild type grown under high or low R:FR and plants of the phyB mutant grown under high R:FR, the expression of BRC1 and BRC2 correlate with different genes, indicating that they are part of different gene networks (Finlayson et al., 2010).
In Arabidopsis the effects of the white light irradiance are relatively small and increasing irradiance levels can either increase or decrease branching (Buchovsky et al., 2008; Su et al., 2011). High levels of PAR reduce the impact of the phyB mutation on branching (Su et al., 2011).
FLOWERING
Arabidopsis plants exposed to natural (Pigliucci and Schmitt, 1999) or simulated (Sánchez et al., 2011) shade light conditions (low irradiance, low R:FR) flower after producing less leaves than sunlight controls. Lowering the R:FR accelerates flowering and under high R:FR the phyB mutant flowers earlier than the wild type, while the phyB phyD and phyB phyE mutants flower earlier than the phyB mutant (Devlin et al., 1998; Devlin et al., 1999). The effects of the phyB and phyB phyD mutations involve increased expression of FLOWERING LOCUS T (FT) (Cerdán and Chory, 2003; Halliday et al., 2003). The FT protein is produced in the leaves in response to diverse flowering stimuli and migrates to the apex where it promotes the transition from the vegetative to the reproductive stage (Corbesier et al., 2007). In contrast to R:FR, irradiance levels have week effects on the timing of flowering measured on a biological scale (i.e., leaf number) (Buchovsky et al., 2008; Zhao et al., 2011). Therefore, under shade, the low R:FR perceived primarily by phyB and secondarily by phyD and phyE induce Arabidopsis flowering at an earlier developmental stage.
The expression of FT depends on the balance between the repression mediated by FLOWERING LOCUS C (FLC) and that promotion mediated by CONSTANS (CO), both of which act at the FT promoter itself (Li et al., 2008; Adrian et al., 2010). Extended periods of low temperatures (vernalization) reduce the expression of FLC (Michaels and Amasino, 1999) and allow the induction of flowering by long days. Low R:FR or phyB phyD phyE mutations accelerate flowering even in lines with constitutive high expression of FLC indicating that shade light signals can bypass the requirement of vernalization (Wollenberg et al., 2008).
In the photoperiodic pathway of flowering induction, the expression of FT in the leaves is induced by the coincidence between the presence of light and the expression of CONSTANS, which is controlled by the clock and peaks at night (Suarez-Lopez et al., 2001; Yanovsky and Kay, 2002). This coincidence occurs close to the end of the photoperiod under long days and is accounted for by the stabilization of the CO protein by light perceived by phyA, cry2 and cry1 (Valverde et al., 2004). Low R:FR increase the expression and activity of CO at dawn and dusk (Wollenberg et al., 2008). Active phyB reduces the stability of CO (Valverde et al., 2004) and this could account for the enhanced activity of CO under low R:FR. Therefore, shade light signals could partially act by enhancing the photoperiodic pathway and in accordance with this idea low R:FR has little effect under short days (Wollenberg et al., 2008). However, it is clear that not all the effects of lowering active phytochrome levels can be accounted for by enhancing the photoperiodic pathway. For instance, the phyA phyB phyE co mutant flowers earlier than the phyA phyB co (Halliday et al., 2003). Simulated shade (low irradiance, low R:FR) shows a stronger acceleration of flowering under short than under long days (Sánchez et al., 2011) and the phyB (Reed et al., 1993; Halliday et al., 1994) or phyB phyD phyE (Wollenberg et al., 2008) mutants flower early under short days. Early flowering of the phyB mutant or in response to EODFR requires PHYTOCHROME AND FLOWERING TIME 1 (PFT1), which is not required for the photoperiodic response (Cerdán and Chory, 2003). PTF1 is the MED25 subunit of the plant Mediator complex and promotes flowering through CO-dependent and independent mechanisms (Iñigo et al., 2011). The relative importance of each way of action could depend on the context. As a matter of fact, the acceleration of flowering by low R:FR or by the phyB, phyD and phyE mutations is reduced when the plants are grown at 16 °C instead of 22 °C (Halliday et al., 2003).
Some of the pathways involved in the vegetative shade-avoidance responses also affect the early-flowering response to low R:FR but others do not. For instance, the hfr1 mutant flowers earlier and has higher FT expression levels than the wild type under low R:FR (Sessa et al., 2005). Similarly, natural alleles of ELF3 regulate the flowering time response to shade (Jiménez-Gómez et al., 2010). A role of natural variation at the PIF4 locus in the control of flowering time has also been proposed (Brock et al., 2010) but its significance has been questioned (Shin et al., 2009; Leivar and Quail, 2011). However, the sav3 mutant is affected in vegetative shade-avoidance responses but it flowers at the same time as the wild type (Tao et al., 2008). Conversely, the doc1/big mutant suppresses the accelerated flowering of the phyB mutant but retains normal hypocotyl EOD responses (Kanyuka et al., 2003).
ACCLIMATION TO SHADE AND SHADE-AVOIDANCE REACTIONS
The aforementioned processes are shade-avoidance reactions because they reduce the degree of current or future shade. Other responses to shade light do not reduce per se the degree of shade; rather, they help to optimize the use of resources under shade. Therefore, they could indirectly contribute to shade-avoidance when the plant or its lower leaves are already shaded.
The photosynthetic apparatus acclimates both in terms of morphology and stoichiometry of its components in response to the changes in irradiance and spectral composition associated to the degree of shade by neighbors. These changes in the light environment are perceived at least in part by the photosynthetic apparatus itself but phytochromes and cryptochromes are also involved (Walters, 2005). EODFR (Casal et al., 1990b), low R:FR (McLaren and Smith, 1978) and the phyB mutation (Reed et al., 1996) reduce leaf chlorophyll levels. Low irradiance (Casson et al., 2009) and low R:FR (Boccalandro et al., 2009) perceived by phyB reduce stomata density. This in turn reduces transpiration and maximum photosynthesis but increases water-use efficiency (Boccalandro et al., 2009). Cryptochromes are also involved in the control of stomata development (Kang et al., 2009) and indirectly regulate leaf conductance by reducing the levels of abscisic acid (Boccalandro et al., 2011).
Exposure to low R:FR and the phyB mutation increase the vulnerability of Arabidopsis plants to insect herbivores at least in part by reducing the sensitivity to jasmonic acid (Moreno et al., 2009; Ballaré, 2011). The expression of many plant disease resistance genes is modulated by the activity of phytochromes (Devlin et al., 2003). The growth of an incompatible strain of Pseudomonas syringae is enhanced in the phyA phyB double mutant (Genoud et al., 2002) and P. syringae pv. tomato (Pst.) DC3000 proliferates more abundantly in the cry1 mutant of Arabidopsis than in the wild type (Wu and Yang, 2010). The reduced investment in defense would release resources for shade-avoidance reactions. The constitutive shade-avoidance1 (csa1) mutant shows shade-avoidance responses in the absence of shade signals (including elongated hypocotyls and petioles, hyponastic leaves, early flowering) and enhanced expression of shade-response marker genes like HAT4 and HFR1. The csa1 phenotype is caused by the expression of a truncated version of a TOLL/INTERLEUKIN1 RECEPTOR—NUCLEOTIDE BINDING SITE-LEUCINE-RICH REPEAT (TIR-NBS-LRR) gene (Faigón-Soverna et al., 2006). TIR-NBS-LRR proteins have been implicated in defense responses in plants and csa1 shows enhanced growth of a bacterial pathogen (Faigón-Soverna et al., 2006). Therefore, CSA1 provides a molecular link between shade-avoidance and defense responses.
SHADE-AVOIDANCE RESPONSES IN CROPS AND WEEDS
The promotion of stem growth by low R:FR and/or EODFR has been demonstrated in many crop species including beans (Downs et al., 1957), mustard (Sinapis alba) (Morgan et al., 1980), tobacco (Nicotiana tabacum) (Kasperbauer, 1971), sunflower (Helianthus annus) (Libenson et al., 2002), tomato (Solanum lycopersicon) (Selman and Ahmed, 1962) and cucumber (Cucumis sativus) (Ballaré et al., 1991c), and weeds such as Chenopodium album (Morgan and Smith, 1976) and Datura ferox (Ballaré et al., 1987). The response to reduced blue light or reduced red plus far-red light has also been demonstrated for instance in tobacco (Casal and Sánchez, 1994), tomato (Casal, 1994), Sinapis alba, and Datura ferox (Ballaré et al., 1991b). There are large quantitative differences among species in the extent of response to low R:FR, which are highly significant in species from open habitats such as Senecio vulgaris and Chenopodium album and hardly detectable in species native of shaded woodland habitats such as Mercurialis perennis (Morgan and Smith, 1979). Despite the fact that the promotion of stem extension growth by shade light signals is arguably the most conspicuous shade-avoidance response, there are clear exceptions to this rule. In wheat, for instance, the lowermost internodes are very short and show some promotion of extension under low R:FR but the uppermost internode or peduncle, which bears the ear, shows reduced rather than enhanced growth in response to low R:FR (Casal, 1993; Ugarte et al., 2010). It is interesting to note that grain yield in sunflower can be reduced by promoting stem growth by selectively lowering the R:FR reaching the stem, suggesting an indirect effect of shade-avoidance responses on yield (Libenson et al., 2002), while wheat plants show a direct effect of low R:FR on grain yield in the absence of changes in plant stature (Casal, 1993; Ugarte et al., 2010).
Reduced branching in response to low R:FR and/or EODFR is another shade-avoidance response that has been observed in many crop species including tobacco (Kasperbauer, 1971) tomato, where EODFR has been suggested as a replacement for manual pruning in commercial crops (Tucker, 1975), wheat (Triticum aestivum) (Casal, 1988), barley (Skinner and Simmons, 1993), and forage grasses such as Lolium multiflorum (Deregibus et al., 1983). The enrichment of red light beneath the canopy by means of red-light emitting diodes directed towards the base of the plant has been shown to promote tillering of Paspalum dilatatum and Sporobolus indicus in natural grasslands (Deregibus et al., 1985). The phyB mutant of sorghum (Sorghum bicolor) and wild-type plants exposed to low R:FR show reduced bud outgrowth, and enhanced bud expression of the SbTEOSINTE BRANCHED and DORMANCY-ASSOCIATED 1 SbDRM1 genes (Kebrom et al., 2006).
Leaf growth responses to low R:FR or EODFR show significant variation among dicotyledonous species ranging from inhibition to promotion (for references see (Casal and Smith, 1989). In grasses like Lolium multiflorum, Paspalum dilatatum and barley (Hordeum vulgare L.), low R:FR or EOD FR (Casal et al., 1987a; Skinner and Simmons, 1993) and low blue light (Casal and Alvarez, 1988) promote leaf sheath growth, placing leaf lamina at a higher stature In some maize cultivars, the leaves grow away from the low R:FR signals of neighbors, reducing mutual plant shading in crops (Maddonni et al., 2002). The increased leaf senescence under reduced PAR and R:FR is a response to shade light observed in crop species like sunflower (Rousseaux et al., 1996) that has received little attention in Arabidopsis thaliana.
Accelerated flowering in response to shade light signals is not observed in many crop species. Low R:FR accelerate flowering in barley (Deitzer et al., 1979) and Lolium multiflorum (Casal et al., 1985). In wheat, EODFR can affect the timing of anthesis as a result of changes in the rate of development of the reproductive structures without apparent changes in the time of apex transition to the reproductive stage revealed by differences in leaf number (Casal, 1993; Ugarte et al., 2010).
Defense against biotic agents is also reduced by shade light signals in crop species. The phyB mutation in tomato increases susceptibility to insect herbivores (Izaguirre et al., 2006). The rice phyA phyB phyC triple mutant shows reduced expression of pathogenesis-related class 1 (PR1) proteins and enhanced susceptibility to blast fungus (Magnaporthe grisea) (Xie et al. 2011). The symbiosis between legumes and rhizobia provides a different pattern of light effects on biotic relationships. The phyB mutant of Lotus japonicus and wild-type plants exposed to low R:FR show reduced nodule development after Mesorhizobium loti inoculation (Suzuki et al., 2011). This is caused by a shoot-derived signal which involves jasmonic acid.
CONCLUDING REMARKS
As sessile organisms, plants have evolved mechanisms to evade or alleviate the detrimental consequences of the prevailing environmental conditions, including the limitations imposed by shade and the consequent reduced availability of PAR. This involves wiring environmental signals perceived by photoreceptors to the endogenous signals and the structural components controlling growth and development (e.g. the enzymes that modulate cellwall extensibility). Therefore, it is not unexpected to see changes in selected hormone signaling components in response to shade light signals. Auxin plays a dominant role but at least gibberellins, brassinosteroids, cytokinins and ethylene are also important. PIF proteins, discovered in the phytochrome field, are now considered hubs controlling different growth and developmental responses, and play a key role in shade-avoidance responses. This chapter presents a separate analysis of the various shade-avoidance responses to demonstrate that the significance of the different molecular and cellular events is dependent on the developmental context and on the environment (temperature, for instance, can change even the direction of a growth or developmental response). Fuller understanding of this complexity will lead us to learn how plants optimize their function dealing with the fluctuating environment they have to face.
ACKNOWLEDGMENTS
I thank Dr Santiago Trupkin and Mr Tomás E. Casal for their help with the figures. Work in my lab is supported by Agenda Nacional de Promoción Científica y Ténica (ANPCYT), Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and International Centre for Genetic Engineering and Technology (ICGEB).
Footnotes
Citation: Jorge J. Casal (2012) Shade Avoidance. The Arabidopsis Book 10:e0157. doi:10.1199/tab.0157
elocation-id: e0157
First published on January 19, 2012: e0157. doi: 10.1199/tab.0157
REFERENCES
- Adrian J., Farrona S., Reimer J.J., Albani M.C., Coupland G., Turck F. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell. 2010;22:1425–1440. doi: 10.1105/tpc.110.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Martínez J.A., Poza-Carrión C., Cubas P. Arabidopsis Branched1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19:458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré C.L. Jasmonate-induced defenses: A tale of intelligence, collaborators and rascals. Trends Plant Sci. 2011;16:249–257. doi: 10.1016/j.tplants.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Ballaré C.L., Scopel A.L. Phytochrome signalling in plant canopies: Testing its population-level implications with photoreceptor mutants of Arabidopsis. Funct. Ecol. 1997;11:441–450. [Google Scholar]
- Ballaré C.L., Scopel A.L., Sáanchez R.A. Photomodulation of axis extension in sparse canopies. Plant Physiol. 1989;89:1324–1330. doi: 10.1104/pp.89.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré C.L., Scopel A.L., Sánchez R.A. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science. 1990;247:329–332. doi: 10.1126/science.247.4940.329. [DOI] [PubMed] [Google Scholar]
- Ballaré C.L., Scopel A.L., Sanchez R.A. On the opportunity cost of the photosynthate invested in stem elongation reactions mediated by phytochrome. Oecologia. 1991a;86:561–567. doi: 10.1007/BF00318323. [DOI] [PubMed] [Google Scholar]
- Ballaré C.L., Scopel A.L., Sánchez R.A. Photocontrol of stem elongation in plant neighbourhoods: effects of photon fluence rate under natural conditions of radiation. Plant Cell Environ. 1991b;14:57–65. [Google Scholar]
- Ballaré C.L., Casal J.J., Kendrick R.E. Responses of light-grown wild-type and long-hypocotyl mutant cucumber seedlings to natural and simulated shade light. Photochem Photobiol. 1991c;54:819–826. [Google Scholar]
- Ballaré C.L., Scopel A.L., Jordan E.T., Vierstra R.D. Signaling among neighboring plants and the development of size inequalities in plant populations. Proc. Natl. Acad. Sci. USA. 1994;91:10094–10098. doi: 10.1073/pnas.91.21.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré C.L., Sánchez R.A., Scopel A.L., Casal J.J., Ghersa C.M. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ. 1987;10:551–557. [Google Scholar]
- Banerjee R., Schleicher E., Meier S., Viana R.M., Pokorny R., Ahmad M., Bittl R., Batschauer A. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem. 2007;282:14916–14922. doi: 10.1074/jbc.M700616200. [DOI] [PubMed] [Google Scholar]
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C.S., Ádám E., Fejes E., Schüfer E., Nagy F. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting Factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentsink L., Koornneef M. Seed dormancy and germination. The Arabidopsis Book. 2008;6:e0119. doi: 10.1199/tab.0119. doi:10.1199/tab.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccalandro H.E., Giordano C.V., Ploschuk E.L., Piccoli P.N., Bottini R., Casal J.J. Phototropins but not cryptochromes mediate the blue light-specific promotion of stomatal conductance, while both enhance photosynthesis and transpiration under full sunlight. Plant Physiol. (in press). 2011. [DOI] [PMC free article] [PubMed]
- Boccalandro H.E., Rugnone M.L., Moreno J.E., Ploschuk E.L., Serna L., Yanovsky M.J., Casal J.J. Phytochrome B enhances photosynthesis at the expense of water-use efficiency in arabidopsis. Plant Physiol. 2009;150:1083–1092. doi: 10.1104/pp.109.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto J.F., Smith H. Differential genetic variation in adaptive strategies to a common environmental signal in Arabidopsis accessions: phytochrome-mediated shade avoidance. Plant Cell Environ. 2002;25:53–63. [Google Scholar]
- Botto J.F., Sánchez R.A., Whitelam G.C., Casal J.J. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouly J.-P., Schleicher E., Dionisio-Sese M., Vandenbussche F., van der Straeten D., Bakrim N., Meier S., Batschauer A., Galland P., Bittl R., Ahmad M. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states J. Biol. Chem. 2007;282:9383–9391. doi: 10.1074/jbc.M609842200. [DOI] [PubMed] [Google Scholar]
- Briggs W., Christie J. Phototropins 1 and 2: Versatile plant blue-light receptors. Trend Plant Sci. 2002;7:204–210. doi: 10.1016/s1360-1385(02)02245-8. [DOI] [PubMed] [Google Scholar]
- Brock M.T., Maloof J.N., Weinig C. Genes underlying quantitative variation in ecologically important traits: PIF4 (PHYTOCHROME INTERACTING FACTOR 4) is associated with variation in internode length, flowering time, and fruit set in Arabidopsis thaliana. Mol. Ecol. 2010;19:1187–1199. doi: 10.1111/j.1365-294X.2010.04538.x. [DOI] [PubMed] [Google Scholar]
- Buchovsky A.S., Strasser B., Cerdán P.D., Casal J.J. Suppression of pleiotropic effects of functional CRYPTOCHROME genes by TERMINAL FLOWER. Genetics. 2008;180:1467–1474. doi: 10.1534/genetics.108.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli M., Sessa G., Baima S., Morelli G., Ruberti I. The Arabidopsis Athb-2 and -4 genes are strongly induced by far-red-rich light. Plant J. 1993;4:469–479. doi: 10.1046/j.1365-313x.1993.04030469.x. [DOI] [PubMed] [Google Scholar]
- Carabelli M., Possenti M., Sessa G., Ciolfi A., Sassi M., Morelli G., Ruberti I. Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Gene Dev. 2007;21:1863–1868. doi: 10.1101/gad.432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J.J. Light quality effects on the appearance of tillers of different order in wheat (Triticum aestivum). Ann. Appl. Biol. 1988;112:167–173. [Google Scholar]
- Casal J.J. Novel effects of phytochrome status on reproductive shoot growth in Triticum aestivum L. New Phytol. 1993;123:45–51. [Google Scholar]
- Casal J.J. Stem extension-growth responses to blue-light require Pfr in tomato seedlings but are not reduced by the low phytochrome levels of the aurea mutant. Physiol Plant. 1994;91:263–267. [Google Scholar]
- Casal J.J. Phytochrome A enhances the promotion of hypocotyl growth caused by reductions of phytochrome B Pfr levels in light-grown Arabidopsis thaliana. Plant Physiol. 1996;112:965–973. doi: 10.1104/pp.112.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J.J., Smith H. Persistent effects of changes in phytochrome status on internode growth in light-grown mustard: Occurrence, kinetics and locus of perception. Planta. 1988;175:214–220. doi: 10.1007/BF00392430. [DOI] [PubMed] [Google Scholar]
- Casal J.J., Alvarez M.A. Blue light effects on the growth of Lolium multiflorum Lam leaves under natural radiation. New Phytol. 1988;109:41–45. [Google Scholar]
- Casal J.J., Smith H. The function, action and adaptive significance of phytochrome in light-grown plants. Plant Cell Environ. 1989;12:855–862. [Google Scholar]
- Casal J.J., Sánchez R.A. Impaired stem-growth responses to blue light irradiance in light-grown transgenic tobacco seedlings overexpressing Avena phytochrome A. Physiol. Plant. 1994;91:268–272. [Google Scholar]
- Casal J.J., Deregibus V.A., Sánchez R.A. Variations in tiller dynamics and morphology in Lolium multiflorum Lam vegetative and reproductive plants as affected by differences in red/far-red irradiation. Ann Bot. 1985;56:553–559. [Google Scholar]
- Casal J.J., Sánchez R.A., Deregibus V.A. The effect of light quality on shoot extension growth in three species of grasses. Ann Bot. 1987a;59:1–7. [Google Scholar]
- Casal J.J., Sánchez R.A., Deregibus V.A. Tillering responses of Lolium multiflorum plants to changes of red/far-red ratio typical of sparse canopies. J Exp Bot. 1987b;38:1432–1439. [Google Scholar]
- Casal J.J., Sánchez R.A., Gibson D. The significance of changes in the red/far-red ratio, associated with either neighbour plants or twilight, for tillering in Lolium multiflorum Lam. New Phytol. 1990a;116:565–572. [Google Scholar]
- Casal J.J., Whitelam G.C., Smith H. Phytochrome effects on the relationship between chlorophyll and steady-state levels of thylakoid polypeptides in light-grown tobacco. Plant Physiol. 1990b;94:370–374. doi: 10.1104/pp.94.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J.J., Ballaré C.L., Tourn M., Sánchez R.A. Anatomy, growth and survival of a long-hypocotyl mutant of Cucumis sativus deficient in phytochrome B. Ann Bot. 1994;73:569–575. [Google Scholar]
- Casson S.A., Franklin K.A., Gray J.E., Grierson C.S., Whitelam G.C., Hetherington A.M. Phytochrome B and PIF4 regulate stomatal development in response to light quantity Comp Biochem Phys A. 2009;19:229–234. doi: 10.1016/j.cub.2008.12.046. [DOI] [PubMed] [Google Scholar]
- Cerdán P., Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- Cerdän P.D., Yanovsky M.J., Reymundo F.C., Nagatani A., Staneloni R.J., Whitelam G.C., Casal J.J. Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J. 1999;18:499–507. doi: 10.1046/j.1365-313x.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- Clough R.C., Vierstra R.D. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721. [Google Scholar]
- Cole B., Kay S.A., Chory J. Automated analysis of hypocotyl growth dynamics during shade avoidance in Arabidopsis. Plant J. 2011;65:991–1000. doi: 10.1111/j.1365-313X.2010.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccio M.P., Sánchez S.E., Kasulin L., Yanovsky M.J., Botto J.F. Genetic mapping of natural variation in a shade avoidance response: ELF3 is the candidate gene for a QTL in hypocotyl growth regulation. J Exp Bot. 2011;62:167–176. doi: 10.1093/jxb/erq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L., Vincent C., Jang S., Fornara F., Fan Q., Searle I., Giakountis A., Farrona S., Gissot L., Turnbull C., Coupland G. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- Crocco C.D., Holm M., Yanovsky M.J., Botto J.F. AtB-BX21 and COP1 genetically interact in the regulation of shade avoidance. Plant J. 2010;64:551–562. doi: 10.1111/j.1365-313X.2010.04360.x. [DOI] [PubMed] [Google Scholar]
- Child R., Smith H. Phytochrome action in light-grown mustard: Kinetics, fluence-rate compensation and ecological significance. Planta. 1987;172:219–229. doi: 10.1007/BF00394591. [DOI] [PubMed] [Google Scholar]
- De Lucas M., Daviere J.M., Rodríguez-Falcón M., Pontin M., Iglesias-Pedraz J.M., Lorrain S., Fankhauser C., Blázquez M.A., Titarenko E., Prat S. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- Deitzer G.F., Hayes R., Jabben M. Kinetics and time dependence of the effect of far-red light on the photoperiodic induction of flowering in wintex Barley. Plant Physiol. 1979;64:1015–1021. doi: 10.1104/pp.64.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregibus V.A., Sánchez R.A., Casal J.J. Effects of light quality on tiller production in Lolium spp. . Plant Physiol. 1983;72:900–902. doi: 10.1104/pp.72.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregibus V.A., Sánchez R.A., Casal J.J., Trlica M.J. Tillering responses to enrichment of red light beneath the canopy in a humid natural grassland. J. App. Ecol. 1985;22:199–206. [Google Scholar]
- Devlin P., Patel S.R., Whitelam G.C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1488. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin P.F., Yanovsky M.J., Kay S.A. A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol. 2003;133:1617–1629. doi: 10.1104/pp.103.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin P.F., Halliday K.J., Harberd N.P., Whitelam G.C. The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: novel phytochromes control internode elongation and flowering time. Plant J. 1996;10:1127–1134. doi: 10.1046/j.1365-313x.1996.10061127.x. [DOI] [PubMed] [Google Scholar]
- Devlin P.F., Robson P.R.H., Patel S.R., Goosey L., Sharrock R.A., Whitelam G.C. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Djakovic-Petrovic T., Wit M.D., Voesenek L.A.C.J., Pierik R. DELLA protein function in growth responses to canopy signals. Plant J. 2007;51:117–126. doi: 10.1111/j.1365-313X.2007.03122.x. [DOI] [PubMed] [Google Scholar]
- Domagalska M.A., Leyser O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011;12:211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- Downs R.J., Hendricks S.B., Borthwick H.A. Photoreversible control of elongation of pinto beans and other plants under normal conditions of growth. Bot. Gaz. 1957;118:199–208. [Google Scholar]
- Eichhenberg K., Hennig L., Martin A., Schäfer E. Variation in dynamics of phytochrome A in Arabidopsis ecotypes and mutants. Plant Cell Environ. 2000;23:311–319. [Google Scholar]
- Elich T.D., Chory J. Biochemical characterization of Arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell. 1997;9:2271–2280. doi: 10.1105/tpc.9.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigón-Soverna A., Harmon F.G., Storani L., Karayekov E., Staneloni R.J., Gassmann W., Mas P., Casal J.J., Kay S.A., Yanovsky M.J. A constitutive shade-avoidance mutant implicates TIR-NBS-LRR proteins in Arabidopsis photomorphogenic development. Plant Cell. 2006;18:2919–2928. doi: 10.1105/tpc.105.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Casal J. Phenotypic characterization of a photomorphogenic mutant. Plant J. 2004;39:747–760. doi: 10.1111/j.1365-313X.2004.02148.x. [DOI] [PubMed] [Google Scholar]
- Feng S., Martinez C., Gusmaroli G., Wang Y., Zhou J., Wang F., Chen L., Yu L., Iglesias-Pedraz J.M., Kircher S., Schäfer E., Fu X., Fan L.M., Deng X.W. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson S.A. Arabidopsis TEOSINTE BRANCHED1-LIKE 1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiol. 2007;48:667–677. doi: 10.1093/pcp/pcm044. [DOI] [PubMed] [Google Scholar]
- Finlayson S.A., Krishnareddy S.R., Kebrom T.H., Casal J.J. Phytochrome regulation of branching in arabidopsis. Plant Physiol. 2010;152:1914–1927. doi: 10.1104/pp.109.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K.A., Allen T., Whitelam G.C. Phytochrome A is an irradiance-dependent red light sensor. Plant J. 2007;50:108–117. doi: 10.1111/j.1365-313X.2007.03036.x. [DOI] [PubMed] [Google Scholar]
- Franklin K.A., Praekelt U., Stoddart W.M., Billingham O.E., Halliday K.J., Whitelam G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003;131:1340–1346. doi: 10.1104/pp.102.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J., Wisniewska J., Benková E., Mendgen K., Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Galstyan A., Cifuentes-Esquivel N., Bou-Torrent J., Martinez-Garcia J.F. The shade avoidance syndrome in Arabidopsis: A fundamental role for atypical basic helix-loop-helix proteins as transcriptional cofactors. Plant J. 2011;66:258–267. doi: 10.1111/j.1365-313X.2011.04485.x. [DOI] [PubMed] [Google Scholar]
- Genoud T., Buchala A., Chua N.-H., Métraux J.-P. Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J. 2002;31:87–95. doi: 10.1046/j.1365-313x.2002.01338.x. [DOI] [PubMed] [Google Scholar]
- Gil P., Dewey E., Friml J., Zhao Y., Snowden K.C., Putterill J., Palme K., Estelle M., Chory J. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Gene Dev. 2001;15:1985–1997. doi: 10.1101/gad.905201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday K.J., Koornneef M., Whitelam G.C. Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana L. to low red/far-red ratio. Plant Physiol. 1994;104:1311–1315. doi: 10.1104/pp.104.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday K.J., Salter M.G., Thingnaes E., Whitelam G.C. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J. 2003;33:875–885. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- Hennig L., Büche C., Eichenberg K., Schäfer E. Dynamic properties of endogenous phytochrome A in Arabidopsis seedlings. Plant Physiol. 1999;121:571–577. doi: 10.1104/pp.121.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamatsu T., King R.W., Helliwell C.A., Koshioka M. The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol. 2005;138:1106–1116. doi: 10.1104/pp.104.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M.G., Smith H. The function of phytochrome in the natural environment--III. Measurement and calculation of phytochrome photoequilibria. Photochem Photobiol. 1977;25:547–550. [Google Scholar]
- Hornitschek P., Lorrain S., Zoete V., Michielin O., Fankhauser C. Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 2009;28:3893–3902. doi: 10.1038/emboj.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñigo S., Alvarez M.J., Strasser B., Califano A., Cerdán P.D. PFT1, the MED25 subunit of the plant Mediator complex, promotes flowering through CONSTANS dependent and independent mechanisms in Arabidopsis. Plant J., in press. 2011. [DOI] [PubMed]
- Izaguirre M.M., Mazza C.A., Biondini M., Baldwin I.T., Ballararé C.L. Remote sensing of future competitors: Impacts on plants defenses. Proc. Natl. Acad. Sci. USA. 2006;103:7170–7174. doi: 10.1073/pnas.0509805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Gómez J.M., Wallace A.D., Maloof J.N. Network analysis identifies ELF3 as a QTL for the shade avoidance response in arabidopsis. PLOS Genet. 2010;6:e1001100. doi: 10.1371/journal.pgen.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E., Bradley M., Harberd P., Whitelam G.C. Photoresponses of light-grown phyA mutants of Arabidopsis. Phytochrome A is required for the perception of daylength extensions. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C.Y., Lian H.L., Wang F.F., Huang J.R., Yang H.Q. Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in arabidopsis. Plant Cell. 2009;21:2624–2641. doi: 10.1105/tpc.109.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanyuka K., Praekelt U., Franklin K.A., Billingham O.E., Hooley R., Whitelam G.C., Halliday K.J. Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J. 2003;35:57–70. doi: 10.1046/j.1365-313x.2003.01779.x. [DOI] [PubMed] [Google Scholar]
- Kasperbauer M.J. Spectral distribution of light in a tobacco canopy and effects of end-of-day light quality on growth and development. Plant Physiol. 1971;47:775–778. doi: 10.1104/pp.47.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom T.H., Burson B.L., Finlayson S.A. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol. 2006;140:1109–1117. doi: 10.1104/pp.105.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.M., Jaillais Y., Pedmale U.V., Moreno J.E., Chory J., Ballaré C.L. Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 2011;67:195–207. doi: 10.1111/j.1365-313X.2011.04598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S., Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Keuskamp D.H., Pollmann S., Voesenek L.A.C.J., Peeters A.J.M., Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci. USA. 2010;107:22740–22744. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp D.H., Sasidharan R., Vos I., Peeters A.J.M., Voesenek L.A.C.J., Pierik R. Blue-light-mediated shade avoidance requires combined auxin and brassinosteroid action in Arabidopsis seedlings. Plant J. 2011;67:208–217. doi: 10.1111/j.1365-313X.2011.04597.x. [DOI] [PubMed] [Google Scholar]
- Kim G.T., Tsukaya H., Uchimiya H. The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells. Gene Dev. 1998;12:2381–2391. doi: 10.1101/gad.12.15.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahre U., Noguchi T., Fujioka S., Takatsuto S., Yokota T., Nomura T., Yoshida S., Chua N.H. The arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;10:1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka T., Kobayashi J., Horiguchi G., Demura T., Sakakibara H., Tsukaya H., Nagatani A. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 2010;153:1608–1618. doi: 10.1104/pp.110.156802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Quail P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Al-Sady B., Carle C., Storer A., Alonso J.M., Ecker J.R., Quaila P.H. The Arabidopsis phytochrome-interacting factor PIF7, together with PIF3 and PIF4, regulates responses to prolonged red light by modulating phyB levels. Plant Cell. 2008a;20:337–352. doi: 10.1105/tpc.107.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E., Quail P.H. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 2008b;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. The fall and rise of apical dominance. Curr. Opin. Genet. Dev. 2005;15:468–471. doi: 10.1016/j.gde.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Li D., Liu C., Shen L., Wu Y., Chen H., Robertson M., Helliwell C.A., Ito T., Meyerowitz E., Yu H. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev. Cell. 2008;15:110–120. doi: 10.1016/j.devcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Li J., Lib G., Wang H., Deng X.-W. Phytochrome signaling mechanisms. The Arabidopsis Book. 2011;9:e0148. doi: 10.1199/tab.0148. doi: 0110.1199/tab.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libenson S., Rodriguez V., Sánchez R.A., Casal J.J. Low red to far-red ratios reaching the stem reduce grain yield in sunflower. Crop Sci. 2002;42:1180–1185. [Google Scholar]
- Liu B., Liu H., Zhong D., Lin C. Searching for a photo-cycle of the cryptochrome photoreceptors. Curr. Opin. Plant Biol. 2010;13:578–586. doi: 10.1016/j.pbi.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- Luccioni L.G., Oliverio K.A., Yanovsky M.J., Boccalandro H., Casal J.J. Brassinosteroid mutants uncover fine tunning of phytochrome signaling. Plant Physiol. 2002;178:173–181. [PMC free article] [PubMed] [Google Scholar]
- Maddonni G.A., Otegui M.E., Andrieu B., Chelle M., Casal J.J. Maize leaves turn away from neighbors. Plant Physiol. 2002;130:1181–1189. doi: 10.1104/pp.009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A.L. Comparison of spectral properties of phytochromes from different preparations. Plant Physiol. 1986;82:956–961. doi: 10.1104/pp.82.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S. Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol Ecol. 2006;15:3483–3503. doi: 10.1111/j.1365-294X.2006.03051.x. [DOI] [PubMed] [Google Scholar]
- Mazzella M.A., Casal J.J. Interactive signalling by phytochromes and cryptochromes generates de-etiolation homeostasis in Arabidopsis thaliana. Plant Cell Environ. 2001;24:155–162. [Google Scholar]
- Mazzella M.A., Alconada Magliano T.M., Casal J.J. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997;20:261–267. [Google Scholar]
- Mazzella M.A., Bertero D., Casal J.J. Temperature-dependent internode elongation in vegetative plants of Arabidopsis thaliana lacking phytochrome B and cryptochrome 1. Planta. 2000;210:497–501. doi: 10.1007/PL00008157. [DOI] [PubMed] [Google Scholar]
- Mazzella M.A., Cerdán P.D., Staneloni R., Casal J.J. Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsis development. Development. 2001;128:2291–2299. doi: 10.1242/dev.128.12.2291. [DOI] [PubMed] [Google Scholar]
- McLaren J.S., Smith H. Phytochrome control of the growth and development of Rumex obtusifolius under simulated canopy light environments. . Plant Cell Environ. 1978;1:61–67. [Google Scholar]
- McNellis T.W., von Arnim A.G., Araki T., Komeda Y., Misera S., Deng X.-W. Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell. 1994;6:487–500. doi: 10.1105/tpc.6.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels S.D., Amasino R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar F.F., Van Zanten M., Cox M.C.H., Pierik R., Voesenek L.A.C.J., Peeters A.J.M. Differential petiole growth in Arabidopsis thaliana: Photocontrol and hormonal regulation. New Phytol. 2009;184:141–152. doi: 10.1111/j.1469-8137.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T., Wheatley K., Hanzawa Y., Wright L., Mizoguchi M., Song H.R., Carré I.A., Coupland G. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev Cell. 2002;2:629–641. doi: 10.1016/s1534-5807(02)00170-3. [DOI] [PubMed] [Google Scholar]
- Morelli G., Ruberti I. Shade avoidance responses. Driving auxin along lateral routes. Plant Physiol. 2000;122:621–626. doi: 10.1104/pp.122.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli G., Ruberti I. Light and shade in photocontrol of Arabidopsis growth. Trends Plant Sci. 2002;7:399–404. doi: 10.1016/s1360-1385(02)02314-2. [DOI] [PubMed] [Google Scholar]
- Moreno J.E., Tao Y., Chory J., Ballaré C.L. Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA. 2009;106:4935–4940. doi: 10.1073/pnas.0900701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.C., Smith H. Linear relationship between phytochrome photoequilibrium and growth in plants under natural radiation. Nature. 1976;262:210–212. [Google Scholar]
- Morgan D.C., Smith H. A systematic relationship between phytochrome-controlled development and species habitat, for plants grown in simulated natural radiation. Planta. 1979;145:253–258. doi: 10.1007/BF00454449. [DOI] [PubMed] [Google Scholar]
- Morgan D.C., O'Brien T., Smith H. Rapid photomodulation of stem extension in light-grown Sinapis alba L. Studies on kinetics, site of perception and photoreceptor. Planta. 1980;150:95–101. doi: 10.1007/BF00582351. [DOI] [PubMed] [Google Scholar]
- Mullen J.L., Weinig C., Hangarter R.P. Shade avoidance and the regulation of leaf inclination in Arabidopsis. Plant Cell Environ. 2006;29:1099–1106. doi: 10.1111/j.1365-3040.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- Nagatani A., Chory J., Furuya M. Phytochrome B is not detectable in the hy3 mutant of Arabidopsis, which is deficient in responding to end-of-day far- red light treatments. Plant Cell Physiol. 1991;32:1119–1122. [Google Scholar]
- Nagpal P., Walker L.M., Young J.C., Sonawala A., Timpte C., Estelle M., Reed J.W. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000;123:563–573. doi: 10.1104/pp.123.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinow D.A., Helfer A., Hamilton E.E., King J.J., Imaizumi T., Schultz T.F., Farré E.M., Kay S.A. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature. 2011;475:398–404. doi: 10.1038/nature10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T., Shimura Y., Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Gene Dev. 1997;11:2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedmale U.V., Celaya R.B., Liscum E. Phototropism: mechanism and outcomes. The Arabidopsis Book. 2010;8:e0125. doi: 10.1199/tab.0125. doi:10.1199/tab.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R., Djakovic-Petrovic T., Keuskamp D.H., De Wit M., Voesenek L.A.C.J. Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and DELLA proteins in Arabidopsis. Plant Physiol. 2009;149:1701–1712. doi: 10.1104/pp.108.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M., Schmitt J. Genes affecting phenotypic plasticity in Arabidopsis: Pleiotropic effects and reproductive fitness of photo-morphogenic mutants. J. Evol. Biol. 1999;12:551–562. [Google Scholar]
- Rausenberger J., Tscheuschler A., Nordmeier W., Wüst F., Timmer J., Schäfer E., Fleck C., Hiltbrunner A. Photo-conversion and nuclear trafficking cycles determine phytochrome As response profile to far-red light. Cell. 2011;146:813–825. doi: 10.1016/j.cell.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Reed J.W., Foster K.R., Morgan P.W., Chory J. Phytochrome B affects responsiveness to giberellins in Arabidopsis. Plant Physiol. 1996;112:337–342. doi: 10.1104/pp.112.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagpal P., Poole D.S., Furuya M., Chory J. Mutations in the gene for the Red/Far-Red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L., Favory J.J., Cloix C., Faggionato D., O'Hara A., Kaiserli E., Baumeister R., Schäfer E., Nagy F., Jenkins G.I., Ulm R. Perception of UV-B by the arabidopsis UVR8 protein. Science. 2011;332:103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- Robson P.R.H., Whitelam G.C., Smith H. Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol. 1993;102:1179–1184. doi: 10.1104/pp.102.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig-Villanova I., Bou J., Sorin C., Devlin P.F., Martinez-García J.F. Identification of primary target genes of phytochrome signaling. Early transcriptional control during shade avoidance responses in arabidopsis. Plant Physiol. 2006;141:85–96. doi: 10.1104/pp.105.076331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig-Villanova I., Bou-Torrent J., Galstyan A., Carretero-Paulet L., Portolés S., Rodríguez-Concepción M., Martínez-García J.F. Interaction of shade avoidance and auxin responses: A role for two novel atypical bHLH proteins. EMBO J. 2007;26:4756–4767. doi: 10.1038/sj.emboj.7601890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux M., Hall A., Sanchez R. Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiol Plant. 1996;96:217–224. [Google Scholar]
- Salter M.G., Franklin K.A., Whitelam G.C. Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature. 2003;426:680–683. doi: 10.1038/nature02174. [DOI] [PubMed] [Google Scholar]
- Sánchez S.E., Cagnola J.I., Crepy M., Yanovsky M.J., Casal J.J. Balancing forces in the photoperiodic control of flowering. Photochem. Photobiol. 2011;10:451–460. doi: 10.1039/c0pp00252f. [DOI] [PubMed] [Google Scholar]
- Sasidharan R., Chinnappa C.C., Staal M., Elzenga J.T.M., Yokoyama R., Nishitani K., Voesenek L.A.C.J., Pierik R. Light quality-mediated petiole elongation in arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosyl ase/hydrolases. Plant Physiol. 2010;154:978–990. doi: 10.1104/pp.110.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S., Ohgishi M., Goda H., Higuchi K., Shimada Y., Yoshida S., Koshiba T. The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J. 2002;32:1011–1022. doi: 10.1046/j.1365-313x.2002.01488.x. [DOI] [PubMed] [Google Scholar]
- Schaller G.E., Kieber J.J. Ethylene. The Arabidopsis Book. 2002;1:e0071. doi: 10.1199/tab.0071. doi:10.1199/tab.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens I., Boccalandro H.E., Kami C., Casal J.J., Fankhauser C. Phytochrome Kinase Substrate 4 modulates phytochrome-mediated control of hypocotyl growth orientation. Plant Physiol. 2008;147:661–671. doi: 10.1104/pp.108.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J., McCormac A.C., Smith H. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome- mediated elongation responses to neighbors. Am. Nat. 1995;146:937–953. [Google Scholar]
- Selman I.W., Ahmed E.O.S. Some effects of far-red irradiation and gibberellic acid on the growth of tomato plants. Ann. Appl. Biol. 1962;50:479–485. [Google Scholar]
- Sellaro R., Yanovsky M.J., Casal J.J. Repression of shade-avoidance reactions by sunfleck induction of HY5 in Arabidopsis. Plant J. 2011;68:919–928. doi: 10.1111/j.1365-313X.2011.04745.x. [DOI] [PubMed] [Google Scholar]
- Sellaro R., Pacín M., Casal J.J. Diurnal dependence of growth responses to shade in Arabidopsis. Role of hormone, clock and light signalling. Mol. Plant, accepted pending minor revision. 2012. [DOI] [PubMed]
- Sellaro R., Crepy M., Trupkin S.A., Karayekov E., Buchovsky A.S., Rossi C., Casal J.J. Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol. 2010;154:401–409. doi: 10.1104/pp.110.160820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G., Carabelli M., Sassi M., Ciolfi A., Possenti M., Mittempergher F., Becker J., Morelli G., Ruberti I. A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Gene Dev. 2005;19:2811–2815. doi: 10.1101/gad.364005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Kim K., Kang H., Zulfugarov I.S., Bae G., Lee C.H., Lee D., Choi G. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA. 2009;106:7660–7665. doi: 10.1073/pnas.0812219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T., Nagatani A., Hanzawa H., Kubota M., Watanabe M., Furuya M. Action spectra for phytochrome A-and phytochrome B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Nat. Acad. Sci. USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer T., Seifert G.J., Hauser M.T., Grisafi P., Fink G.R., Luschnig C. Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Curr. Biol. 2000;10:1595–1598. doi: 10.1016/s0960-9822(00)00861-7. [DOI] [PubMed] [Google Scholar]
- Skinner R.H., Simmons S.R. Modulation of leaf elongation, tiller appearance and tiller senescence in spring barley by far-red light. Plant Cell Environ. 1993;16:555–562. [Google Scholar]
- Smith H., Casal J.J., Jackson G.M. Reflection signals and the perception by phytochrome of the proximity of neighbouring vegetation. . Plant Cell Environ. 1990;13:73–78. [Google Scholar]
- Smith H., Xu Y., Quail P.H. Antagonistic but complementary actions of phytochromes A and B allow optimum seedling de-etiolation. Plant Physiol. 1997;114:637–641. doi: 10.1104/pp.114.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorin C., Salla-Martret M., Bou-Torrent J., Roig-Villanova I., Martínez-García J.F. ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. Plant J. 2009;59:266–277. doi: 10.1111/j.1365-313X.2009.03866.x. [DOI] [PubMed] [Google Scholar]
- Steindler C., Matteucci A., Sessa G., Weimar T., Ohgishi M., Aoyama T., Morelli G., Ruberti I. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development. 1999;126:4235–4245. doi: 10.1242/dev.126.19.4235. [DOI] [PubMed] [Google Scholar]
- Strasser B., Sánchez-Lamas M., Yanovsky M.J., Casal J.J., Cerdán P.D. Arabidopsis thaliana life without phytochromes. Proc. Natl. Acad. Sci. USA. 2010;107:4776–4781. doi: 10.1073/pnas.0910446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer C., Oyama T., Schultz T.F., Raman R., Somers D.E., Más P., Panda S., Kreps J.A., Kay S.A. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science. 2000;289:768–771. doi: 10.1126/science.289.5480.768. [DOI] [PubMed] [Google Scholar]
- Su H., Abernathy S.D., White R.H., Finlayson S.A. Photosynthetic photon flux density and phytochrome B interact to regulate branching in Arabidopsis. Plant Cell Environ. 2011;34:1986–1998. doi: 10.1111/j.1365-3040.2011.02393.x. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez P., Wheatley K., Robson F., Onouchi H., Valverde F., G. C. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;26:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Sun T. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The Arabidopsis Book. 2008;6:e0103. doi: 10.1199/tab.0103. doi:0110.1199/tab.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Suriyagoda L., Shigeyama T., Tominaga A., Sasaki M., Hiratsuka Y., Yoshinaga A., Arima S., Agarie S., Sakai T., Inada S., Jikumaru Y., Kamiya Y., Uchiumi T., Abe M., Hashiguchi M., Akashi R., Sato S., Kaneko T., Tabata S., Hirsch A.M. Lotus japonicus nodulation is photomorphogenetically controlled by sensing the red/far red (R/FR) ratio through jasmonic acid (JA) signaling Proc. Natl. Acad. Sci. USA. 2011;108:16837–16842. doi: 10.1073/pnas.1105892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere U., Eichhenberg K., Lohrmann J., Mira-Rodado V., Baurle I., Kudla J., Nagy F., Schafer E., Harter K. Interaction of the Response Regulator ARR4 with Phytochrome B in modulating red light signalling. Science. 2001;294:1108–1111. doi: 10.1126/science.1065022. [DOI] [PubMed] [Google Scholar]
- Tao Y., Ferrer J.L., Ljung K., Pojer F., Hong F., Long J.A., Li L., Moreno J.E., Bowman M.E., Ivans L.J., Cheng Y., Lim J., Zhao Y., Ballaré C.L., Sandberg G., Noel J.P., Chory J. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker D.J. Far-red light as a suppressor of side shoot growth in the tomato. Plant Sci. Let. 1975;5:127–130. [Google Scholar]
- Ugarte C.C., Trupkin S.A., Ghiglione H., Slafer G., Casal J.J. Low red/far-red ratios delay spike and stem growth in wheat. J. Exp. Bot. 2010;61:3151–3162. doi: 10.1093/jxb/erq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F., Mouradov A., Soppe W., Ravenscroft D., Samach A., Coupland G. Photoreceptor regulation of CONSTANS protein and the mechanism of photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- Vandenbussche F., Vriezen W.H., Smalle J., Laarhoven L.J.J., Harren F.J.M., van der Straeten D. Ethylene and Auxin Control the Arabidopsis Response to Decreased Light Intensity. Plant Physiol. 2003;133:517–527. doi: 10.1104/pp.103.022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G., Walcher C.L., Chory J., Nemhauser J.L. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA. 2008;105:9829–9834. doi: 10.1073/pnas.0803996105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R.G. Towards an understanding of photosynthetic acclimation J. Exp. Bot. 2005;56:435–447. doi: 10.1093/jxb/eri060. [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Seto H., Fujioka S., Yoshida S., Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- Werner T., Schmülling T. Cytokinin action in plant development. Cur. Opin. Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Wollenberg A.C., Strasser B., Cerdán P.D., Amasino R.M. Acceleration of flowering during shade avoidance in Arabidopsis alters the balance between Flowering Locus C-mediated repression and photoperiodic induction of flowering. Plant Physiol. 2008;148:1681–1694. doi: 10.1104/pp.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Yang H.Q. CRYPTOCHROME 1 is implicated in promoting R protein-mediated plant resistance to Pseudomonas syringae in arabidopsis. Mol. Plant. 2010;3:539–548. doi: 10.1093/mp/ssp107. [DOI] [PubMed] [Google Scholar]
- Xie X.Z., Xue Y.J., Zhou J.J., Zhang B., Chang H., Takano M. Phytochromes regulate SA and JA signaling pathways in rice and are required for developmentally controlled resistance to Magnaporthe grisea. Mol. Plant. 2011;4:688–696. doi: 10.1093/mp/ssr005. [DOI] [PubMed] [Google Scholar]
- Yanovsky M.J., Kay S.A. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- Yanovsky M.J., Casal J.J., Whitelam G.C. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 1995a;18:788–794. [Google Scholar]
- Yanovsky M.J., Casal J.J., Salerno G.L., Sánchez R.A. Are phytochrome-mediated effects on leaf growth, carbon partitioning and extractable sucrose-phosphate synthase activity the mere consequence of stem-growth responses in light-grown mustard? J. Exp. Bot. 1995b;46:753–757. [Google Scholar]
- Yu J.W., Rubio V., Lee N.Y., Bai S., Lee S.Y., Kim S.S., Liu L., Zhang Y., Irigoyen M.L., Sullivan J.A., Zhang Y., Lee I., Xie Q., Paek N.C., Deng X.W. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol. Cell. 2008;32:617–630. doi: 10.1016/j.molcel.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Liu H., Klejnot J., Lin C. The cryptochrome blue light receptors. The Arabidopsis Book. 2010;8:e0135. doi: 10.1199/tab.0135. doi:10.1199/tab.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Maruhnich S.A., Folta K.M. Green light induces shade avoidance symptoms. Plant Physiol. 2011;157:1528–1536. doi: 10.1104/pp.111.180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Hanada A., Yamaguchi S., Kamiya Y., Beers E.P. The Arabidopsis Myb genes MYR1 and MYR2 are redundant negative regulators of flowering time under decreased light intensity. Plant J. 2011;66:502–515. doi: 10.1111/j.1365-313X.2011.04508.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Christensen S.K., Fankhauser C., Cashman J.R., Cohen J.D., Weigel D., Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]