Abstract
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is increasingly recognized as a central regulator of multiple signaling pathways in inflammation and cancer, and the ability to use chemical biological tools to investigate its biological effects is very attractive. A peptide comprising a TAT-conjugated Nrf2 sequence is shown to activate Nrf2 and its downstream target gene heme-oxygenase-1 (HO-1) in a dose-dependent manner in intact human THP-1 monocytes. Levels of Nrf2 protein peak after 3 h, whereas HO-1 mRNA and protein peak after 6 and 12 h, respectively. The peptide is also shown to inhibit the production of the pro-inflammatory cytokine TNF. The TAT-14mer constitutes a useful chemical biology tool with potential therapeutic applications.
Keywords: cell-penetrating peptide, Nrf2, Keap-1, protein−protein interaction, inflammation, antioxidant response
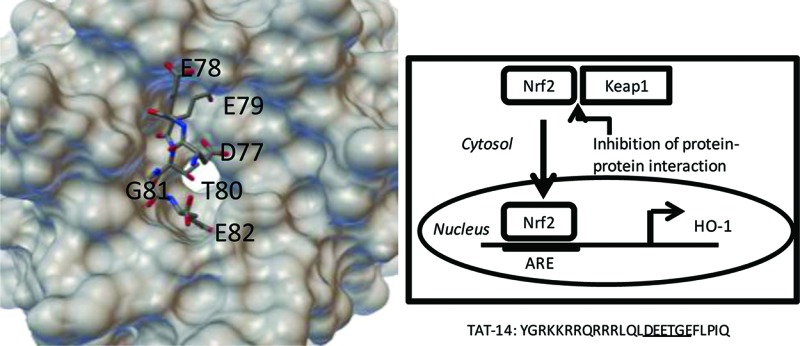
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a transcription factor and member of the Cap'N'collar family of proteins.1 Nrf2 plays a central role in the response to various types of stress by activating a myriad of cellular antioxidants,2 detoxification enzymes,3 drug efflux pumps,4 and other cytoprotective proteins.5 As such, it is closely controlled in most cells by the presence of a protein called Kelch-like ECH-associated protein 1 (Keap1).6 Keap1 binds to Nrf2 and induces ubiquitination and proteasome-mediated degradation, thus maintaining low levels of the protein under normal conditions. In response to oxidative stimuli, the Nrf2-Keap1 interaction is perturbed, and Nrf2 translocates into the nucleus where it forms a dimer with small Maf proteins7 and binds to the antioxidant response element (ARE) in the regulatory regions of its target genes (Figure 1A). This results in the production of proteins that have a protective effect for the cell. These include the cellular antioxidants heme-oxygenase 1 (HO-1),8 proteins that regulate glutathione biosynthesis,9 and the phase II enzyme NAD(P)H:quinone oxidoreductase 1 (NQO1).10
Figure 1.
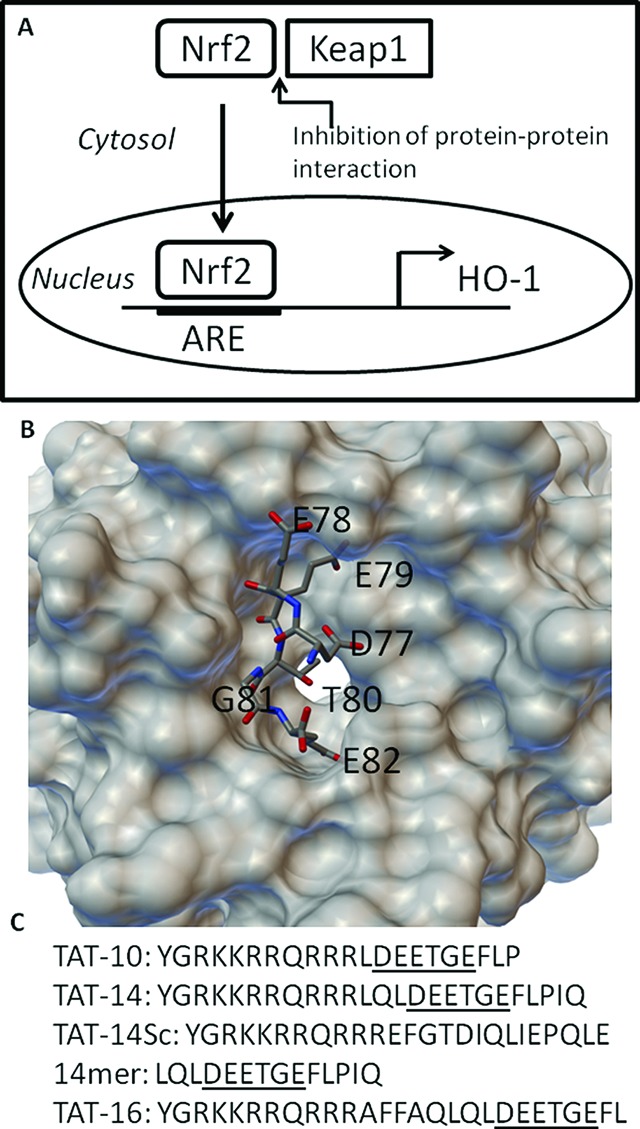
(A) Nrf2 is constitutively expressed but normally binds to Keap1 and is degraded. Inhibition of Nrf2/Keap1 through interaction with TAT-14 leads to nuclear translocation and activation of downstream proteins such as HO-1. (B) Structure of the conserved DEETGE sequence binding to the Kelch domain and Keap1.15 (C) The peptide sequences used in this study.
Recently, Nrf2 has been recognized as an important regulator of inflammation. Nrf2 knockout mice are more susceptible to inflammation than wild-type mice, and Nrf2 exerts anti-inflammatory effects in cells in vitro.11 This makes Nrf2 an interesting target for the development of compounds with potential anti-inflammatory activity. To date, most effort has focused on either the triterpenoid compounds CDDO-Im and CDDO-Me or on covalent inhibitors that bind to cysteines on the surface of Keap1 that have been suggested to be important for the protein–protein interactions. CDDO-Im and CDDO-Me are synthetic analogues of a triterpene and have been shown to activate the Nrf2 pathway and induce high levels of antioxidant enzymes.12 Both have protective effects in animal models of inflammation, with CDDO-Im demonstrating protection in an animal model of sepsis (a blood-borne infection causing acute inflammation and affecting several tissues). In addition, CDDO-Me is currently in phase III clinical trials in type II diabetics with chronic kidney disease.13 More recently, Gray and co-workers described the identification of a small molecule that inhibited the Nrf2/Keap1 interaction through covalent modification of Cys151 of the human Keap1 and led to activation of Nrf2.14 The authors built a structure–activity relationship for the relatively simple compound AI-1 that acts as a good lead for further development. However, covalent modification may be less desirable in a drug due to off-target effects in vivo. Electrophiles that interact with the cysteines on Keap1, particularly with such a simple structure as AI-1, may well interact with the cysteines or other nucleophiles on other proteins and may also be substrates for the reaction with, for example, high levels of glutathione.
These observations suggest that an approach involving simpler compounds than CDDO-Me that interact to block Nrf2/Keap1 through noncovalent interactions are desirable and have great therapeutic potential. Hannink and co-workers have shown that short peptides based upon the Nrf2 binding region to the Kelch domain of Keap1 can bind with high affinity where the central DEETGE site is maintained.15 Three peptides of 10, 14, and 16 amino acid length were designed, and the 16mer and 14mer showed high affinity binding, while the 10mer also bound but with lower affinity. The structure of the 16mer peptide bound to the Kelch domain is shown in Figure 1B. While peptides are not ideal drug candidates, they are very useful as chemical tools, particulary when conjugated to a cell-penetrating peptide (CPP) such as the trans-activating transcriptional activator (TAT) peptide derived from HIV.16 A peptide targeted at the protein–protein interaction between the PSD95 and the NR2B subunit of the NMDA receptor has proved to be an effective intracellular regulator of the interaction, useful in stroke.17 Although it has been suggested that TAT-peptides targeting Nrf2/Keap1 do not function18 and more recently that even shorter peptides can be used to target the interaction,19 we focused on the conjugation of TAT to the Hannink peptides, for which there is structural data for binding.
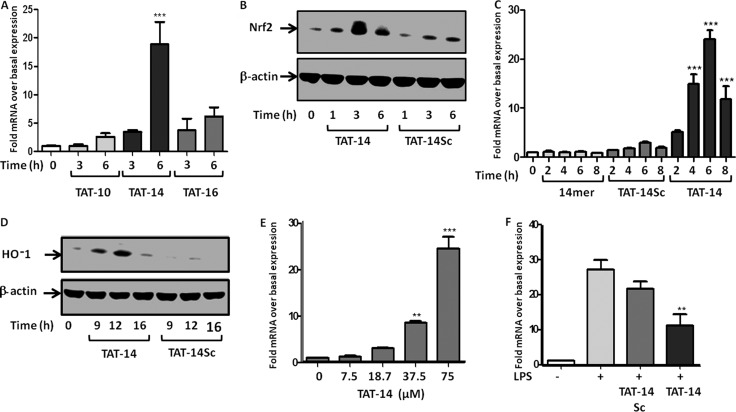
Using standard Fmoc chemistry and automated synthesis, we generated three peptides with the TAT sequence located at the amine terminus of the 10-, 14-, and 16mer peptides (TAT-10, TAT-14, and TAT-16, Figure 1C). The peptides were purified by HPLC to >90% pure and were then screened for their ability to activate HO-1 gene expression in intact THP-1 cells (a human monocytic cell line), previously validated as a model for the study of the Nrf2 pathway in human monocytes.20 HO-1 is a downstream product of the activation of Nrf2. Only the TAT-14 peptide was seen to activate HO-1 expression, suggesting that the conjugation of the TAT peptide to the 10- and 16mer peptide was either not enhancing uptake into cells or was inhibiting the binding of the peptides to the Kelch domain (Figure 2A). Activation by the TAT-14 compound was significant, however, and this conjugated product was chosen for further analysis.
Figure 2.
(A) Induction of HO-1 by the three TAT peptides. THP-1 cells were treated with the peptide for the time indicated, and levels of HO-1 mRNA were measured using qRT-PCR and normalized to the expression of the housekeeping gene GAPDH (see the Supporting Information for full experimental details). Means ± SEMs, n = 3, ***p < 0.001. (B) Western blot analysis of the induction of Nrf2 protein following incubation with the TAT-14 peptide or the TAT-14sc peptide. (C) Induction of HO-1 by the nonconjugated 14mer, the TAT-14sc, and TAT-14. THP-1 cells were treated with the peptide for the time indicated, and levels of HO-1 mRNA were analyzed using qRT-PCR and normalized to GAPDH expression. Means ± SEMs, n = 3, ***p < 0.001. (D) Western blot analysis of HO-1 protein levels following treatment with TAT-14 and TAT-14sc. Representative experiment of n = 3. (E) Dose dependence of HO-1 induction as assessed by qRT-PCR and normalized to GAPDH mRNA expression. Means ± SEMs, n = 3, **p < 0.01, ***p < 0.001. (F) TNFα induction following stimulation with LPS in the presence of either TAT-14 or TAT-14Sc. mRNA levels were analyzed using qRT-PCR and normalized to GAPDH. Means ± SEMs, n = 3, **p < 0.01.
Investigation of the effect of the TAT-14 peptide on Nrf2 protein expression was then studied using Western blot analysis. Cells were incubated with the peptide and a version of the TAT-14 peptide scrambled in the 14mer region (TAT-14Sc), which would affect binding to the Kelch domain but the peptide would still enter the cell (see Figure 1C). Levels of Nrf2 protein reached a peak after 3 h of incubation with the TAT-14 peptide and showed no enhancement with the TAT-14sc peptide (Figure 2B). The TAT-14 peptide had no effect on Nrf2 mRNA expression, measured by qRT-PCR (data not shown), suggesting that the increased levels of protein were due to the interaction of TAT-14 with Keap1 rather than through a stress-induced increase in basal levels of the transcription factor. This is also supported by the lack of effect of the scrambled peptide.
As noted above, the initial screen for the TAT-14 peptide involved investigation of its effect on expression of HO-1, a downstream product of the binding of Nrf2 to the ARE. To clearly define the timing of this effect and also investigate the effects on HO-1 protein, experiments were carried out using both qRT-PCR (for mRNA) and Western blotting (for protein). Effects on HO-1 mRNA by the TAT-14 peptide were compared with the nonconjugated peptide (14mer) and also the TAT-14sc peptide. The effects of the TAT-14 peptide (75 μM) on HO-1 mRNA expression were significant with an average 24-fold induction at the peak incubation time of 6 h (Figure 2C). The induction then gradually returned to basal levels. Neither the 14mer nor the TAT-14sc peptides had any significant effect on the levels of HO-1 mRNA. HO-1 protein expression was similarly investigated in comparison with the TAT-14sc peptide, and this peaked at 12 h, with the TAT-14sc having no effect (Figure 2D).
The high levels of HO-1 expression allowed the examination of the dose response of the peptide (Figure 2E). The effect of the peptide was assessed at 7.5, 18.75, 37.5, and 75 μM and gave a clear dose response with significant activation also occurring at the 37.5 μM concentration.
Nrf2 deficiency results in susceptibility to inflammation in animal models, including bacteria-induced sepsis in mice, and this is due to an excessive production of pro-inflammatory cytokines including tumor necrosis factor α (TNF) in the Nrf2 knockout mice. We have previously shown that bacterial lipopolysaccharide (LPS)-induced TNF production is a valid cell model of sepsis. Monocytes play a central role in the inflammatory response during sepsis. Inhibition of Nrf2 using siRNA leads to the increased production of TNF following stimulation of THP-1 monocytes by LPS.20 This suggests that conversely the release of Nrf2 from Keap1 and its resultant binding to the ARE may inhibit the production of the cytokine following a similar stimulation by LPS in these cells. In turn, this would have an anti-inflammatory effect in this model for septic shock. THP-1 cells were rested for 24 h to generate stress-free conditions and then treated with TAT-14 or TAT-14sc for 4 h prior to stimulation with LPS for 3 h. After this, the cells were lysed and analyzed for TNF mRNA expression by qRT-PCR (Figure 2F). TAT-14 significantly suppressed LPS-induced TNF expression, with an average of 61% inhibition of the cytokine, signifying that the TAT-14 peptide has an anti-inflammatory effect.
These results demonstrate that the conjugation of a CPP to a sequence targeting the Nrf2 binding site on Keap1 generates a chemical biological tool that is able to enter cells and inhibit the protein–protein interaction, thereby activating Nrf2. Such a construct also has some biological activity and can act as an anti-inflammatory. Importantly, this study also demonstrates that carefully designed “druglike” small molecules that target the same site on Keap1 could have a similar activity. These compounds have potential in the treatment of diseases where resolution of inflammatory processes can play a beneficial role. The progression of CDDO-Me into the clinic suggests that the Nrf2/Keap1 interaction is an important target and that chemical tools such as the TAT-14 peptide will have a role to play in understanding the biological processes involved in this pathway, as well as defining a clear therapeutic target.
Glossary
Abbreviations
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- Keap1
Kelch-like ECH-associated protein 1
- ARE
antioxidant response element
- TAT
trans-activating transcriptional activator
- HO-1
heme-oxygenase 1
- CPP
cell-penetrating peptide
- TNF
tumor necrosis factor
Supporting Information Available
Experimental procedures including the peptide synthesis, purification, and biological evaluation. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
R.S. is funded through an EPSRC DTA. E.P. was funded by MRC Grant No. G0801127. J.C. is funded by UEA.
The authors declare no competing financial interest.
Supplementary Material
References
- Moi P.; Chan K.; Asunis I; Cao A.; Kan Y. W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U.S.A. 1994, 91, 9926–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M.; Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Signaling 2005, 7, 385–394. [DOI] [PubMed] [Google Scholar]
- Itoh K.; Chiba T.; Takahashi S.; Ishii T.; Igarashi K.; Katoh Y.; Oyake T.; Hayashi N.; Satoh K.; Htayama I.; Yamamoto Y.; Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [DOI] [PubMed] [Google Scholar]
- Hayashi A.; Suzuki H.; Itoh M.; Yamamoto Y.; Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res. Commun. 2003, 310, 824–829. [DOI] [PubMed] [Google Scholar]
- Chen X. L.; Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: A new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004, 10, 879–891. [DOI] [PubMed] [Google Scholar]
- Itoh K.; Wakabayahsi N.; Katoh Y.; Ishii T.; Igarashi K.; Engel J. D.; Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K.; Igarashi K.; Hayashi N.; Nishizawa M.; Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell. Biol. 1995, 15, 4184–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J.; Stewart D.; Touchard C.; Boinapally S.; Choi A. M.; Cook J. L. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [DOI] [PubMed] [Google Scholar]
- Chan J. Y.; Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim. Biophys. Acta 2000, 1517, 19–26. [DOI] [PubMed] [Google Scholar]
- Venugopal R.; Jaiswal A. K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 14960–14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q.; Battelli L.; Hubbs A. F. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am. J. Pathol. 2006, 168, 1960–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa R. K.; Fuchs R. J.; Malhotra D.; Scollick C.; Traore K.; Bream J. H.; Trush M. A.; Liby K. T.; Spron M. B.; Kensler T. W.; Biswal S. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid. Redox Signaling 2007, 9, 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola P. E.; Raskin P.; Toto R. D.; Meyer C. J.; Huff J. W.; Grossman E. B.; Krauth M.; Ruiz S.; Audhya P.; Christ-Schmidt H.; Wittes J.; Warnock D. G. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [DOI] [PubMed] [Google Scholar]
- Hur W.; Sun Z.; Jiang T.; Mason D. E.; Peters E. C.; Zhang D. D.; Luesch H.; Schultz P. G.; Gray N. S. A small-molecule inducer of the antioxidant response element. Chem. Biol. 2010, 17, 537–547. [DOI] [PubMed] [Google Scholar]
- Lo S. C.; Li X.; Henzl M. T.; Beamer L. J.; Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J. 2006, 3605–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes S.; Morris M. C.; Divita G.; Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell. Mol. Life Sci. 2005, 62, 1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze S. R.; Ho A.; Vocero-Akbani A.; Dowdy S. F. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Redell J. B.; Moore A. N.; Dash P. K. A novel strategy to activate cytoprotective genes in the injured brain. Biochem. Biophys. Res. Commun. 2011, 407, 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R.; Bertrand H. C.; Tsujita T.; Naz S.; El-Bakry A.; Laoruchupong J; Hayes J. D.; Wells G. Peptide inhibitors of the Keap1-Nrf2 protein-protein interaction. Free Radical Biol. Med. 2012, 52, 444–451. [DOI] [PubMed] [Google Scholar]
- Rushworth S. A.; MacEwan D. J.; O'Connell M. A. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J. Immunol. 2008, 181, 6730–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.