Abstract
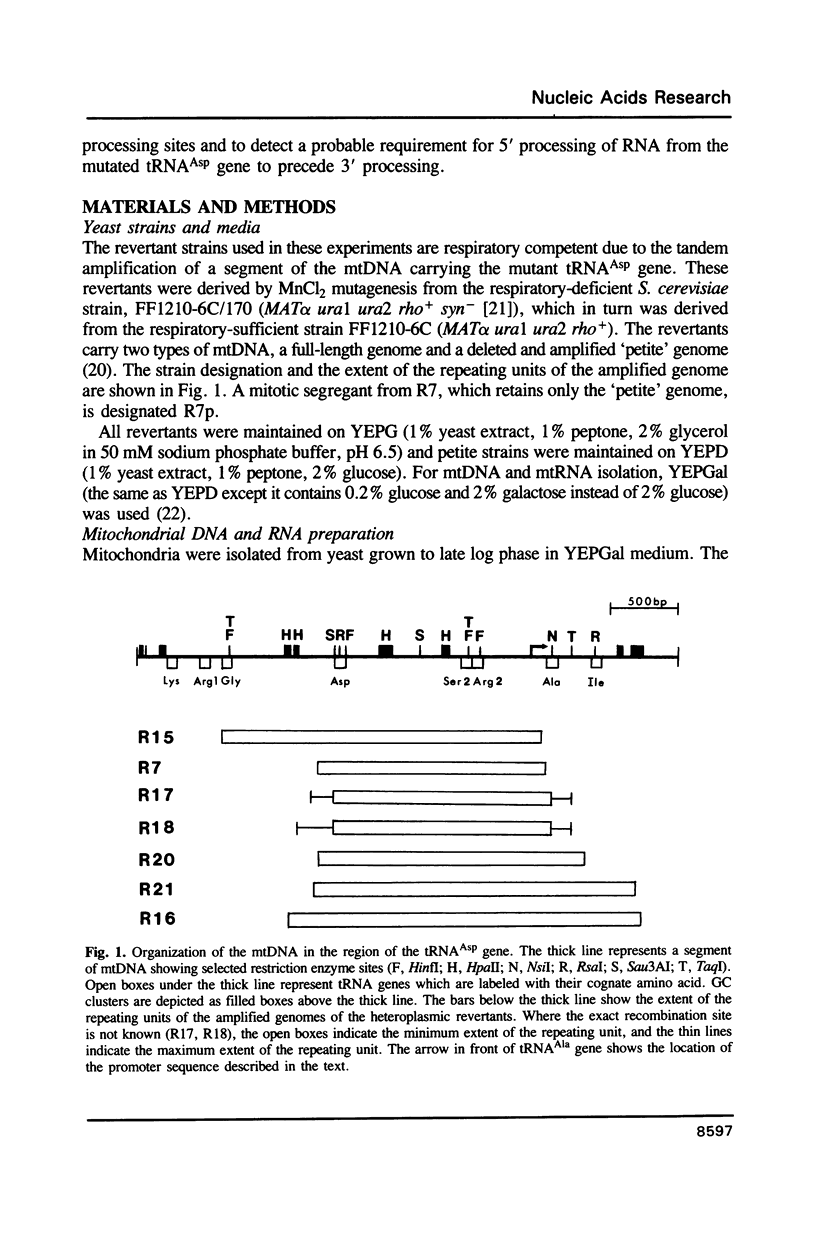
Saccharomyces cerevisiae strain FF1210-6C/170 is respiratory deficient due to a mutation of the penultimate base of the mitochondrial tRNA(Asp) gene. We have identified a number of progeny from this strain which have reverted to respiratory sufficiency by the excision and tandem amplification of a small region of the mitochondrial (mt) DNA carrying the tRNA(Asp) gene, while also maintaining the full-length mtDNA. We have studied the structure of the mtDNA and mitochondrial transcription in a number of these heteroplasmic strains. The exact site of the recombination involved in the excision of the repeating unit of the amplified mtDNA has been determined for five of the revertants. Recombination occurs between identical sequences 4-13 base pairs in length. Each of the different repeating units of the amplified DNA retains an active promoter which has been moved to a site just upstream of the tRNA(Asp) gene by the excision/amplification. Transcripts from the heteroplasmic strains have been characterized to determine the sites of mitochondrial RNA termini. We find that in addition to the 5' and 3' processing of the tRNAs, many of the transcripts terminate at a position about 300 base pairs downstream of the gene for tRNA(Asp). We also find that 3' processing of tRNA(Asp) precursors is absent in petite strains which lack 5' processing indicating that 5' processing of tRNA(Asp) may be a prerequisite for 3' processing in this mutant.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. S., Getz G. S. Identification of a new promoter within the tRNA gene cluster of the mitochondrial DNA of Saccharomyces cerevisiae. Nucleic Acids Res. 1987 Nov 25;15(22):9309–9324. doi: 10.1093/nar/15.22.9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequences of yeast mitochondrial tRNA genes. J Biol Chem. 1980 Oct 10;255(19):9075–9081. [PubMed] [Google Scholar]
- Bordonné R., Dirheimer G., Martin R. P. Transcription initiation and RNA processing of a yeast mitochondrial tRNA gene cluster. Nucleic Acids Res. 1987 Sep 25;15(18):7381–7394. doi: 10.1093/nar/15.18.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Cohen M., Rabinowitz M., Fukuhara H., Getz G. S. Hybridization of mitochondrial transfer RNA's with mitochondrial and nuclear DNA of grande (wild type) yeast. J Mol Biol. 1972 Feb 14;63(3):431–440. doi: 10.1016/0022-2836(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Castaño J. G., Tobian J. A., Zasloff M. Purification and characterization of an endonuclease from Xenopus laevis ovaries which accurately processes the 3' terminus of human pre-tRNA-Met(i) (3' pre-tRNase). J Biol Chem. 1985 Jul 25;260(15):9002–9008. [PubMed] [Google Scholar]
- Chen J. Y., Martin N. C. Biosynthesis of tRNA in yeast mitochondria. An endonuclease is responsible for the 3'-processing of tRNA precursors. J Biol Chem. 1988 Sep 25;263(27):13677–13682. [PubMed] [Google Scholar]
- Christianson T., Rabinowitz M. Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J Biol Chem. 1983 Nov 25;258(22):14025–14033. [PubMed] [Google Scholar]
- Dieckmann C. L., Koerner T. J., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP1, a yeast nuclear gene involved in 5' end processing of cytochrome b pre-mRNA. J Biol Chem. 1984 Apr 25;259(8):4722–4731. [PubMed] [Google Scholar]
- Francisci S., Palleschi C., Ragnini A., Frontali L. Analysis of transcripts of the major cluster of tRNA genes in the mitochondrial genome of S. cerevisiae. Nucleic Acids Res. 1987 Aug 25;15(16):6387–6403. doi: 10.1093/nar/15.16.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendewey D., Dingermann T., Cooley L., Söll D. Processing of precursor tRNAs in Drosophila. Processing of the 3' end involves an endonucleolytic cleavage and occurs after 5' end maturation. J Biol Chem. 1985 Jan 10;260(1):449–454. [PubMed] [Google Scholar]
- Frontali L., Palleschi C., Francisci S. Transcripts of mitochondrial tRNA genes in Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Nov 25;10(22):7283–7293. doi: 10.1093/nar/10.22.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth M. E., Ainley W. M., Shumard D. S., Butow R. A., Grossman L. I. Location and structure of the var1 gene on yeast mitochondrial DNA: nucleotide sequence of the 40.0 allele. Cell. 1982 Sep;30(2):617–626. doi: 10.1016/0092-8674(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Hudspeth M. E., Shumard D. S., Tatti K. M., Grossman L. I. Rapid purification of yeast mitochondrial DNA in high yield. Biochim Biophys Acta. 1980 Dec 11;610(2):221–228. doi: 10.1016/0005-2787(80)90003-9. [DOI] [PubMed] [Google Scholar]
- Kang Y. W., Miller D. L. Nuclear and mitochondrial revertants of a yeast mitochondrial tRNA mutant. Mol Gen Genet. 1988 Aug;213(2-3):425–434. doi: 10.1007/BF00339612. [DOI] [PubMed] [Google Scholar]
- Locker J. Analytical and preparative electrophoresis of RNA in agarose-urea. Anal Biochem. 1979 Oct 1;98(2):358–367. doi: 10.1016/0003-2697(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Locker J., Rabinowitz M. Transcription in yeast mitochondria: analysis of the 21 S rRNA region and its transcripts. Plasmid. 1981 Nov;6(3):302–314. doi: 10.1016/0147-619x(81)90038-x. [DOI] [PubMed] [Google Scholar]
- Manam S., Van Tuyle G. C. Separation and characterization of 5'- and 3'-tRNA processing nucleases from rat liver mitochondria. J Biol Chem. 1987 Jul 25;262(21):10272–10279. [PubMed] [Google Scholar]
- Martin N. C., Miller D. L., Underbrink K., Ming X. Structure of a precursor to the yeast mitochondrial tRNAMetf. Implications for the function of the tRNA synthesis locus. J Biol Chem. 1985 Feb 10;260(3):1479–1483. [PubMed] [Google Scholar]
- Martin N. C., Miller D., Hartley J., Moynihan P., Donelson J. E. The tRNAAGYSer and tRNACGYArg genes from a gene cluster in yeast mitochondrial DNA. Cell. 1980 Feb;19(2):339–343. doi: 10.1016/0092-8674(80)90508-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Martin N. C. Characterization of the yeast mitochondrial locus necessary for tRNA biosynthesis: DNA sequence analysis and identification of a new transcript. Cell. 1983 Oct;34(3):911–917. doi: 10.1016/0092-8674(83)90548-2. [DOI] [PubMed] [Google Scholar]
- Morimoto R., Rabinowitz M. Physical mapping of the yeast mitochondrial genome: derivation of the fine structure and gene map of strain D273-10B and comparison with a strain (MH41-7B) differing in genome size. Mol Gen Genet. 1979 Feb 16;170(1):25–48. [PubMed] [Google Scholar]
- Müller P. P., Reif M. K., Zonghou S., Sengstag C., Mason T. L., Fox T. D. A nuclear mutation that post-transcriptionally blocks accumulation of a yeast mitochondrial gene product can be suppressed by a mitochondrial gene rearrangement. J Mol Biol. 1984 Jun 5;175(4):431–452. doi: 10.1016/0022-2836(84)90178-5. [DOI] [PubMed] [Google Scholar]
- Najarian D., Shu H. H., Martin N. C. Sequence and expression of four mutant aspartic acid tRNA genes from the mitochondria of Saccharomyces cerevisiae. Nucleic Acids Res. 1986 Dec 22;14(24):9561–9578. doi: 10.1093/nar/14.24.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nóbrega M. P., Nóbrega F. G. Mapping and sequencing of the wild-type and mutant (G116-40) alleles of the tyrosyl-tRNA mitochondrial gene in Saccharomyces cerevisiae. J Biol Chem. 1986 Mar 5;261(7):3054–3059. [PubMed] [Google Scholar]
- Osinga K. A., De Vries E., Van der Horst G., Tabak H. F. Processing of yeast mitochondrial messenger RNAs at a conserved dodecamer sequence. EMBO J. 1984 Apr;3(4):829–834. doi: 10.1002/j.1460-2075.1984.tb01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleschi C., Francisci S., Zennaro E., Frontali L. Expression of the clustered mitochondrial tRNA genes in Saccharomyces cerevisiae: transcription and processing of transcripts. EMBO J. 1984 Jun;3(6):1389–1395. doi: 10.1002/j.1460-2075.1984.tb01982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Faye G. Organization and processing of the mitochondrial oxi3/oli2 multigenic transcript in yeast. Mol Gen Genet. 1984;196(2):266–274. doi: 10.1007/BF00328059. [DOI] [PubMed] [Google Scholar]
- Thalenfeld B. E., Bonitz S. G., Nobrega F. G., Macino G., Tzagoloff A. oli1 Transcripts in wild type and in a cytoplasmic "petite" mutant of yeast. J Biol Chem. 1983 Dec 10;258(23):14065–14068. [PubMed] [Google Scholar]
- Underbrink-Lyon K., Miller D. L., Ross N. A., Fukuhara H., Martin N. C. Characterization of a yeast mitochondrial locus necessary for tRNA biosynthesis. Deletion mapping and restriction mapping studies. Mol Gen Genet. 1983;191(3):512–518. doi: 10.1007/BF00425771. [DOI] [PubMed] [Google Scholar]
- Wang M. J., Davis N. W., Gegenheimer P. Novel mechanisms for maturation of chloroplast transfer RNA precursors. EMBO J. 1988 Jun;7(6):1567–1574. doi: 10.1002/j.1460-2075.1988.tb02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Santos T., Hamer D. H. TRNA precursor transcribed from a mutant human gene inserted into a SV40 vector is processed incorrectly. Nature. 1982 Feb 11;295(5849):533–535. doi: 10.1038/295533a0. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Martin N. C., Butow R. A. Origins of transcripts of the yeast mitochondrial var 1 gene. J Biol Chem. 1984 May 10;259(9):6019–6027. [PubMed] [Google Scholar]
- Zennaro E., Francisci S., Ragnini A., Frontali L., Bolotin-Fukuhara M. A point mutation in a mitochondrial tRNA gene abolishes its 3' end processing. Nucleic Acids Res. 1989 Jul 25;17(14):5751–5764. doi: 10.1093/nar/17.14.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zamaroczy M., Bernardi G. The GC clusters of the mitochondrial genome of yeast and their evolutionary origin. Gene. 1986;41(1):1–22. doi: 10.1016/0378-1119(86)90262-3. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Bernardi G. The primary structure of the mitochondrial genome of Saccharomyces cerevisiae--a review. Gene. 1986;47(2-3):155–177. doi: 10.1016/0378-1119(86)90060-0. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Gene. 1983 Mar;21(3):193–202. doi: 10.1016/0378-1119(83)90002-1. [DOI] [PubMed] [Google Scholar]





