Abstract
Context
More than 1.5 million US adults use stimulants and other medications labeled for treatment of attention deficit hyperactivity disorder (ADHD). These agents can increase heart rate and blood pressure, raising concerns about their cardiovascular safety.
Objective
Examine whether current use of medications used primarily to treat ADHD is associated with increased risk of serious cardiovascular events in young and middle-aged adults.
Design
Retrospective, population-based cohort study
Setting
Computerized health records from 4 study sites (OptumInsight Epidemiology, Tennessee Medicaid, Kaiser Permanente California, and the HMO Research Network), starting in 1986 at one site and ending in 2005 at all sites, with additional covariate assessment using 2007 survey data.
Participants
Adults aged 25–64 years with dispensed prescriptions for methylphenidate, amphetamine, or atomoxetine at baseline. Each medication user (n=150,359) was matched to two non-users on study site, birth year, sex, and calendar year (total users and non-users=443,198).
Main Outcome
Serious cardiovascular events, including myocardial infarction (MI), sudden cardiac death (SCD), or stroke. Comparison between current or new users and remote users to account for potential healthy user bias.
Results
During 806,182 person-years of follow-up (median 1.3 years per person), 1357 cases of MI, 296 cases of SCD, and 575 cases of stroke occurred. There were 107,322 person-years of current use (median 0.33 years), with a crude incidence per 1000 person-years of 1.34 (95% CI, 1.14–1.57) for MI, 0.30 (95% CI, 0.20–0.42) for SCD, and 0.56 (95% CI, 0.43–0.72) for stroke. The multivariable adjusted rate ratio (RR) of serious cardiovascular events for current use vs non-use of ADHD medications was 0.83 (95% CI 0.72–0.96). Among new users of ADHD medications, the adjusted RR was 0.77 (95% CI 0.63–0.94). The adjusted RR was 1.03 (95% CI, 0.86–1.24) for current use vs remote use, and was 1.02 (95% CI, 0.82–1.28) for new use vs remote use.
Conclusion
Among young and middle-aged adults, current or new use of ADHD medications, compared with non-use or remote use, was not associated with an increased risk of serious cardiovascular events. Apparent protective associations likely represent healthy user bias.
Introduction
Between 2001 and 2010, use of medications labeled for treatment of Attention Deficit Hyperactivity Disorder (ADHD) increased even more rapidly in adults than in children(1). According to a US Food and Drug Administration (FDA) advisory committee briefing on the safety of ADHD medications held in 2006, more than 1.5 million US adults were taking stimulants in 2005, and adults received approximately 32% of all issued prescriptions (2). The increase in ADHD diagnoses is likely the primary cause of increased prescribing (3,4), although stimulants also are approved for treatment of narcolepsy(5) and may be used off-label to treat obesity(6) and fatigue related to depression(7), stroke(8), or traumatic brain injury(9). Adults with ADHD are commonly treated with the stimulant classes methylphenidate and amphetamine and increasingly a non-stimulant agent, atomoxetine.
Placebo-controlled studies in children and adults indicate stimulants and atomoxetine elevate systolic blood pressure levels by approximately 2–5 mm Hg and diastolic blood pressure by 1–3 mm Hg, and also lead to increases in heart rate(10,11). While these effects would be expected to slightly increase risk for myocardial infarction (MI), sudden cardiac death (SCD), and stroke(12), clinical trials have not been large enough to assess risk of these events.
In a summary from the FDA Adverse Event Reporting System, cardiac arrest, MI, and death were among the top 50 adverse events reported after use of amphetamine and methylphenidate(2). Although one study among children suggested markedly elevated risks of SCD(13), cardiovascular safety data from pharmacoepidemiologic studies are limited and inconsistent(13–16), especially among adults(17,18).
The aim of this study was to examine whether current use of medications used primarily to treat ADHD is associated with increased risk of MI, SCD, or stroke in adults aged 25–64 years. Study drugs included all agents with a labeled indication for treatment of ADHD in either children or adults as of December 31, 2005.
Methods
The study was conducted in parallel with a study of ADHD drug use and serious cardiovascular events in youths aged 2–24 years(19).
Data sites
Study sites included Vanderbilt University (Tennessee State Medicaid data), Kaiser Permanente (KP) California (Northern and Southern KP regions), OptumInsight Epidemiology (data from a large health insurance plan) and the HMO Research Network (Harvard Pilgrim Health Care; Fallon Community Health Plan; Group Health Cooperative of Puget Sound; HealthPartners; KP Georgia; KP Northwest; and KP Colorado). The selected sites provide geographic and sociodemographic diversity and have similar computerized data structures.
The start date for the availability of computerized data differed across study sites, ranging from 1986 to 2002. Follow-up concluded at the end of 2005 so that mortality searches could be conducted using complete state death records and the National Death Index (NDI). The study was approved by institutional review boards (IRB) at each participating institution and the FDA Research in Human Subjects Committee. The requirement for participant informed consent was waived.
Study participants
Eligible individuals were aged 25–64 years with at least 12 months of continuous health plan coverage and pharmacy benefits before cohort entry (denoted as t0). Individuals were excluded if they had one or more of the following diagnoses (based on ICD-9/10 codes) within 365 days before t0: sickle cell disease, cancer (other than non-melanoma skin cancer), HIV infection, organ transplant, liver failure/hepatic coma, end-stage renal disease, respiratory failure, or congestive heart failure. When these diagnoses occurred after t0, follow-up time was censored.
At each contributing site, we assembled the eligible members and time periods when all eligibility criteria were met. For each exposed period (ie, at least one ADHD prescription), starting with the earliest, we randomly selected two unexposed periods from all members with no ADHD medication use on t0 and the same sex and birth year.
Study medications and exposure categories
Medication use was based on prescription fills from electronic pharmacy records. ADHD medications included stimulant class medications (methylphenidate, amphetamines, pemoline), and atomoxetine, a selective norepinephrine reuptake inhibitor. Amphetamines included dextroamphetamines and amphetamine salts. Although infrequently used and not structurally similar to the other stimulants, pemoline was included because of its labeled indication for ADHD. Each person-day of follow-up was classified into mutually exclusive exposure categories according to ADHD drug use, based on prescription dispensing dates and days supply. Current use was the period between prescription start date and end of days supply (including up to a 7-day carryover from previous prescriptions). Indeterminate use was the first 89 days after end of current use. Former use began at 90 days after end of current use and ended at 364 days after last current use. Greater than 364 days since end of last days supply was considered remote use. Non-use referred to person-days with no current use and no past use (back to 365 days before t0). Past users and non-users could become current users during follow-up, and when this occurred, their person-time was classified as described above. Less than 1% of non-users became users after baseline. Current use was further categorized based on specific medications (amphetamines, methylphenidate, atomoxetine, multiple ADHD drugs, or pemoline) and on pre-specified duration categories (1–30 days, 31–90 days, 91–182 days, 183–365 days, 366+ days).
We consider current use the most etiologically relevant exposure. Risk during current use was compared to risk during non-use. In addition, to account for potential selection bias or unmeasured confounding that could arise from users being more or less healthy than non-users, we restricted some analyses to ever users of ADHD medications. We compared rates during periods of current use to rates during periods 365 days or more after use ended (i.e., remote use). These analyses are less influenced by potential confounders that are unmeasured and stable over time, but these analyses assume no medication effects that remain after discontinuation.
Study endpoints
Potential endpoints were identified from claims and vital records (diagnoses and ICD codes provided in eTable 1). For members with death not identified from these sources and whose health plan enrollment ended prior to end of study period, we performed NDI searches.
Medical records, including hospitalizations, reports from emergency medical services, autopsies, and death certificates, were requested on all potential SCDs (n=411) and strokes (n=980) and on a random 31% sample of potential MIs (n=433) for assessment by trained adjudicators (primary care physicians for MI and SCD, neurologists for stroke. Of the 371 MI cases with sufficient records available, 353 (95%) were confirmed by adjudication. MI was defined as an acute event involving hospitalization with characteristic cardiac enzyme changes, and either symptoms or characteristic electrocardiographic changes(20,21). SCD was defined as witnessed sudden death in a community setting preceded by typical symptoms of cardiac ischemia. Deaths were excluded when documentation suggested a non-cardiac cause (eg, motor vehicle accident) or if clinically severe heart disease was present and sudden cardiac death was not unexpected (eg, end-stage congestive heart failure). Stroke was defined as an acute neurologic deficit of sudden onset that persisted more than 24 hours, corresponded to a vascular territory, and was not explained by other causes such as trauma, infection, vasculitis, extracranial hemorrhage leading to hypotension, or profound hypotension from another cause. Strokes that occurred during a hospitalization were excluded.
All MIs, other than those determined by adjudication to be non-cases (n=18), were included in analyses. For potential SCD cases without available or adequate hospital or autopsy records (n=203), we used an ICD-9/10 code-based definition with a previously reported positive predictive value (PPV) of 86%(22). SCD cases based on the code-based definition (n=157), as well as those confirmed by clinical adjudication (n=139), were included in primary analyses. For potential strokes with insufficient hospital or autopsy records for clinical adjudication (n=179) or for whom records were unavailable (n=69), we used a code-based definition to identify probable strokes. Probable strokes had ICD-9/10 codes with a positive predictive value (PPV) of 80% or greater, based on those strokes for whom records were available. Strokes confirmed by adjudication (n=451) and those with insufficient records meeting the diagnostic code-based definition (n=124), were included as events in primary analyses (eTables 2a, 2b). In secondary analyses, we included all electronically identified SCDs or strokes except those confirmed as non-events by adjudication.
Confounders
To control for potential differences in cardiovascular disease (CVD) risk between exposed and unexposed individuals, we constructed a summary cardiovascular risk score (CRS)(23,24). The CRS was based on inpatient and outpatient diagnoses (from claims or encounter databases) and pharmacy records and included CVD and medications, mental health conditions (excluding ADHD) and psychotropic medications, other health conditions (e.g., diabetes mellitus, obesity, smoking-related) and medications, and health care utilization (see Table 1 and eTable 3 for details). For each endpoint (MI, SCD, stroke or any serious cardiovascular event), a separate score was created from a Poisson regression model among all patients, adjusted for ADHD medications and matching variables (age, sex, data site, calendar year at cohort entry). The score was the linear predictor from the coefficients of the resulting regression model, excluding the coefficients for ADHD medications and the matching variables. In primary analyses, several CRS variables not thought to be on the causal pathway between medication use and our outcomes were treated as time-varying (eTable 3). In secondary analyses, all CRS variables were based on diagnoses/medication use in the 365 days prior to t0 and fixed at baseline. For the new user analyses, we used the CRS for comparisons of current vs remote use and constructed a propensity score (PS)(25) for current vs. non-use of ADHD medications at t0 using variables included in the CRS.
Table 1.
Selected characteristics of study cohort at baseline
| Characteristic | Current Use | Nonuse | ||
|---|---|---|---|---|
| Number of unique individuals | 150,359 | 292,839 | ||
| Number of membership periodsa | 152,852 | 293,749 | ||
| Median year of cohort entry | 2003 | 2003 | ||
| Person-years during follow-upb | 107,322 | 533,540 | ||
| Demographics | ||||
| Median age in years (interquartile range) | 42 | 34–49 | 42 | 34–49 |
| Male sex (%) | 70,245 | 46.0% | 135,002 | 46.0% |
| Medicaid enrollment (%) | 14,786 | 9.7% | 29,171 | 9.9% |
| ADHD Medication | ||||
| Amphetamines | 57,824 | 37.8% | 0 | 0% |
| Methylphenidate | 70,923 | 46.4% | 0 | 0% |
| Atomoxetine | 19,283 | 12.6% | 0 | 0% |
| Pemoline | 3,973 | 2.6% | 0 | 0% |
| Multiple | 849 | 0.6% | 0 | 0% |
| Cardiovascular disease within past yearc | ||||
| Acute myocardial infarction | 340 | 0.2% | 689 | 0.2% |
| Ischemia | 3,998 | 2.6% | 6,857 | 2.3% |
| Coronary revascularization | 253 | 0.2% | 643 | 0.2% |
| Congestive heart failure | 1,112 | 0.7% | 1,759 | 0.6% |
| Arrhythmia | 3,560 | 2.3% | 5,076 | 1.7% |
| Stroke/transient ischemic attack | 1,826 | 1.2% | 2,075 | 0.7% |
| Congenital heart disorder | 331 | 0.2% | 556 | 0.2% |
| Coronary artery anomaly | 66 | 0.0% | 89 | 0.0% |
| Peripheral vascular disease | 1,225 | 0.8% | 1,651 | 0.6% |
| Hypertension | 22,562 | 14.8% | 39,011 | 13.3% |
| Hyperlipidemiad | 28,613 | 18.7% | 42,601 | 14.5% |
| Mental health claims within past year | ||||
| ADHD | 46,356 | 30.3% | 455 | 0.2% |
| Major depression | 61,417 | 40.2% | 23,296 | 7.9% |
| Bipolar disorder | 11,196 | 7.3% | 2,682 | 0.9% |
| Anxiety | 30,472 | 19.9% | 15,670 | 5.3% |
| Psychotic disorders | 2,494 | 1.6% | 1,833 | 0.6% |
| Other selected medical conditions within past year | ||||
| Diabetes d | 8,972 | 5.9% | 15,862 | 5.4% |
| Obesity | 9,119 | 6.0% | 11,439 | 3.9% |
| Smoking | 11,579 | 7.6% | 14,717 | 5.0% |
| Alcohol/substance abuse | 7,965 | 5.2% | 4,514 | 1.5% |
| Suicide attempt | 795 | 0.5% | 410 | 0.1% |
| Injury | 30,655 | 20.1% | 37,559 | 12.8% |
| Seizure | 3,062 | 2.0% | 2,854 | 1.0% |
| Asthma | 11,627 | 7.6% | 12,432 | 4.2% |
| Use of cardiovascular drug within past yearc | ||||
| Loop diuretic | 4,328 | 2.8% | 4,932 | 1.7% |
| Digoxin | 587 | 0.4% | 1,130 | 0.4% |
| Nitrates | 1,941 | 1.3% | 3,298 | 1.1% |
| Anticoagulant | 1,768 | 1.2% | 2,421 | 0.8% |
| Platelet inhibitor | 996 | 0.7% | 1,675 | 0.6% |
| Anti-arrhythmic agents | 556 | 0.4% | 631 | 0.2% |
| ACE inhibitor | 10,719 | 7.0% | 19,796 | 6.7% |
| Angiotensin receptor blocker | 3,652 | 2.4% | 5,988 | 2.0% |
| Beta-blocker | 12,431 | 8.1% | 19,091 | 6.5% |
| Calcium-channel blocker | 7,028 | 4.6% | 12,233 | 4.2% |
| Thiazide diuretic | 12,471 | 8.2% | 20,008 | 6.8% |
| Other antihypertensive | 1,668 | 1.1% | 2,192 | 0.7% |
| Use of psychotropic medications within past year | ||||
| Antipsychotic, any | 14,618 | 9.6% | 5,371 | 1.8% |
| Tricyclic antidepressant | 14,224 | 9.3% | 9,907 | 3.4% |
| Antidepressants, other or SSRI/SNRI | 81,639 | 53.4% | 36,962 | 12.6% |
| Benzodiazepines | 43,695 | 28.6% | 25,956 | 8.8% |
| Lithium | 4,177 | 2.7% | 1,002 | 0.3% |
| Modafinil | 4,732 | 3.1% | 383 | 0.1% |
| Insomnia medications | 15,270 | 10.0% | 6,732 | 2.3% |
| Thioridazine | 307 | 0.2% | 181 | 0.1% |
| Mood stabilizers, without seizure | 22,426 | 14.7% | 8,631 | 2.9% |
| Clonidine/guanfacine, without hypertension | 2,000 | 1.3% | 659 | 0.2% |
| Use of other selected medications within past year | ||||
| Beta-agonist | 18,971 | 12.4% | 20,835 | 7.1% |
| Epinephrine | 1,342 | 0.9% | 1,274 | 0.4% |
| Asthma medications, other | 39,645 | 25.9% | 45,102 | 15.4% |
| Seizure medications, any | 24,139 | 15.8% | 10,397 | 3.5% |
| Theophylline compounds (asthma med) | 960 | 0.6% | 1,200 | 0.4% |
| COX-2 inhibitors | 10,666 | 7.0% | 10,838 | 3.7% |
| Other drugs to improve blood flow | 216 | 0.1% | 250 | 0.1% |
| Clonidine | 2,602 | 1.7% | 1,787 | 0.6% |
| pde5 inhibitors | 5,183 | 3.4% | 4,504 | 1.5% |
| Triptans | 7,164 | 4.7% | 5,298 | 1.8% |
| Oral contraceptives | 18,379 | 12.0% | 28,590 | 9.7% |
| Hormones, menopausal | 18,026 | 11.8% | 23,388 | 8.0% |
| Utilization within past year | ||||
| Cardiovascular visits | ||||
| Emergency, 1+ | 5,728 | 3.7% | 7,697 | 2.6% |
| Inpatient, 1+ | 6,022 | 3.9% | 7,130 | 2.4% |
| Physician, 1–4 | 43,474 | 28.4% | 65,256 | 22.2% |
| Physician, 5+ | 13,242 | 8.7% | 17,713 | 6.0% |
| Psychiatric visitse | ||||
| Emergency, 1+ | 4,417 | 2.9% | 2,897 | 1.0% |
| Inpatient, 1+ | 7,761 | 5.1% | 3,827 | 1.3% |
| Physician, 1–4 | 43,538 | 28.5% | 26,703 | 9.1% |
| Physician, 5+ | 40,176 | 26.3% | 11,048 | 3.8% |
| Other visits | ||||
| Emergency, 1+ | 7,885 | 5.2% | 9,594 | 3.3% |
| Inpatient, 1+ | 5,812 | 3.8% | 5,595 | 1.9% |
| Physician, 1+ | 55,386 | 36.2% | 69,134 | 23.5% |
| Number of different medicationsf | ||||
| 1 | 24,309 | 15.9% | 61,193 | 20.8% |
| 2+ | 108,955 | 71.3% | 116,680 | 39.7% |
Abbreviations: ACE, angiotensin-converting enzyme; SSRI, selective serotonin reuptake inhibitors; SNRI, serotonin and norepinephrine reuptake inhibitors; COX, cyclooxygenase; pde5, phosphodiesterase type 5
Percents are based on membership periods. There were 299 indeterminate and former users at baseline.
Follow-up time based on combined endpoint (MI, SCD, or stroke).
Including medications
Variables used to define history of CVD for subgroup analyses in Figure 2.
Excluding ADHD visits
Excluding ADHD medications
All variables in table included in CRS, except demographics and ADHD
Unmeasured confounders
To examine the possible extent and direction of unmeasured confounding by risk factors for cardiovascular disease on which information was not or was inconsistently available in the electronic health care records, we conducted sensitivity analyses using information on potential confounders from two sources. Race/ethnicity, smoking, obesity, history of CVD and drug abuse were obtained from the adjudicated records of SCD, MI and stroke cases. In addition, race/ethnicity, income, education, smoking, obesity, and family history of CVD were available on approximately 200,000 KP Northern California members aged 25–64 years who completed a mailed survey for a different study in 2007 (eMethods 1). Electronic pharmacy records for ADHD medications were obtained on survey participants.
We used multivariable logistic regression to examine the association between potential confounders (from either survey or chart reviews) and use of ADHD medications. For variables associated with use of ADHD medications, we assessed the extent of their potential confounding effect on our rate ratios (RR) for MI, SCD or stroke associated with ADHD medications using external adjustment methods(26–28). This approach assumed associations in our study population were similar to our external samples and did not address joint confounding by several unmeasured covariates.
Statistical approach
Follow-up began at cohort entry and ended at one of the four endpoints (MI, SCD, stroke, or any of these serious cardiovascular events), death, end of insurance coverage/pharmacy benefit, day before 65th birthday, or end of study period (December 2005), whichever came first. Poisson regression was used to estimate the association of ADHD medication use with risk of serious cardiovascular events, while adjusting for potentially confounding variables. Covariates in the full model included study site, age (5-year dummy categories), sex, calendar year (1986–1992, 1993–1999, 2000–2001, 2002–2003, 2004–2005), and CRS (specified as decile dummies). Matching variables (site, age, sex, calendar year at cohort entry) were included in the full model because, while matching assured balance with respect to these variables at baseline, it did not assure balance during follow-up.
To minimize biases related to underascertainment of events occurring early in therapy (29), we also conducted analyses restricting to new users of ADHD medications (no use in the year prior to baseline). In these analyses, risk during current use was compared to risk during follow-up classified as remote use. Current use among new users also was compared to non-use (in their matches).
To examine whether associations could be influenced by prior disease conditions, we conducted subgroup analyses. In one analysis, users were restricted to those with a prior diagnosis of ADHD and compared to their matched non-users. Additional subgroups were based on prior CVD, prior non-ADHD psychiatric diagnoses or medication use, or age (25–44 vs. 45–64 years) during follow-up, and data site.
When examining rates of any serious cardiovascular event in the full cohort, we had 80% power to detect RRs of 1.23 for current use vs. non-use and 1.30 for current use vs. remote use. In new user analyses, the least detectable RRs were 1.31 for current use vs. non-use, and 1.38 for current vs. remote use. All analyses were done with SAS version 9.1. For all RR estimates, 95% confidence limits were reported.
Results
The study included a total of 443,198 adults, of whom 150,359 were users of ADHD medications at baseline. Methylphenidate accounted for 45% of current use; amphetamine, atomoxetine and pemoline accounted for 44%, 8%, and 3%, respectively.
Characteristics of study population
Baseline characteristics of users and non-users are shown in Table 1; characteristics of person-time by medication use are presented in the Appendix (eTable 3). Cardiovascular diseases were generally uncommon and similar or modestly more prevalent in users than non-users. As expected, ADHD was substantially more common among current users than non-users. This also was true for other psychiatric conditions. The prevalences of cardiovascular risk factors were modestly higher during periods of remote use than during periods of current use – or non-use.
Number of events and rate ratios in the full cohort
During 806,182 person-years of follow-up (median 1.3, interquartile range 0.6–2.6 years per person), 1357 cases of MI, 296 cases of SCD, and 575 cases of stroke occurred. There were 107,322 person-years of current use (median 0.33, range 0.0–13.5, years per user), with a crude incidence per 1,000 person-years of 1.34 (95% confidence interval (CI) 1.14–1.57) for MI, 0.30 (95% CI 0.20–0.42) for SCD, and 0.56 (95% CI 0.43–0.72) for stroke.
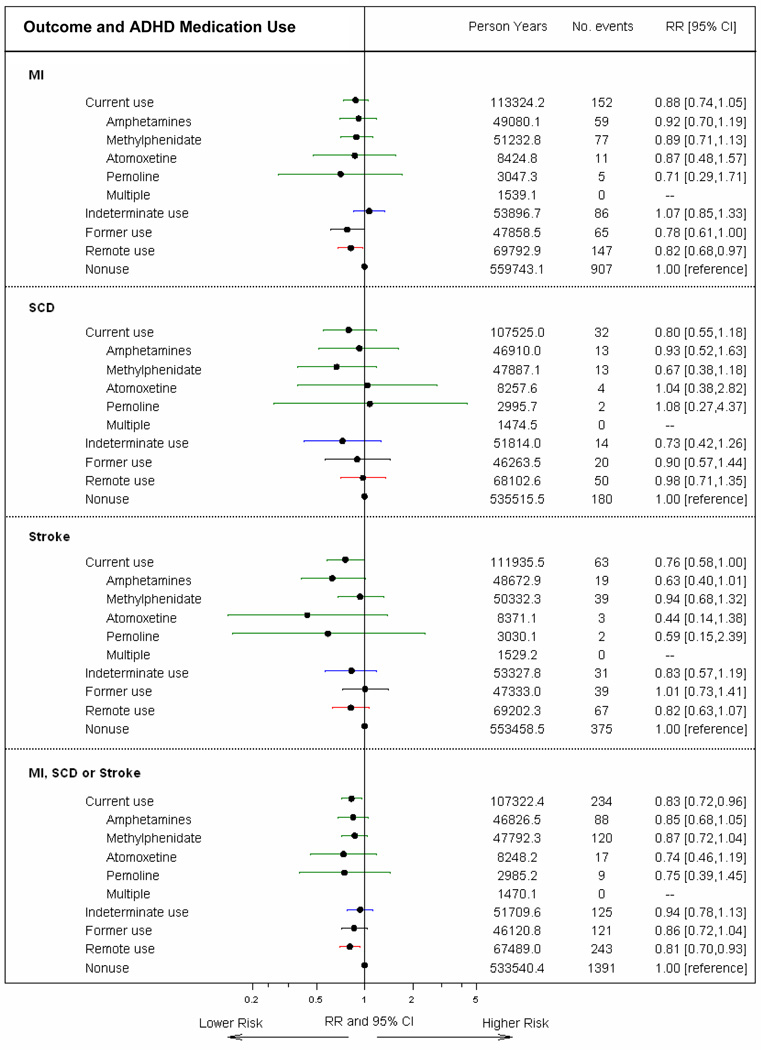
In analysis adjusted for matching variables only, the rate ratio (RR) of MI, SCD, or stroke for current vs. non-use of ADHD medications was 0.97 (95% CI 0.84–1.12). After also adjusting for the CRS, the RR was modestly lower (RR=0.83, 95%CI 0.72–0.96). Results were similar for specific medications and across endpoints (Figure 1). RRs also were similar for ischemic or hemorrhagic stroke (eTables 4a and 4b). Findings for SCD and stroke changed only minimally when all electronically identified cases were included except those adjudicated as non-cases (eTables 5a and 5b). Overall results were essentially unchanged when all variables in the CRS were fixed at baseline (eTable 6).
Figure 1. Adjusted rate ratios for serious cardiovascular events associated with ADHD medication use.
The figure depicts RRs and 95% CIs for current use, indeterminate use and former use, with nonuse as the reference category. RRs adjusted for site, age, sex, calendar year, and CRS (some variables within score are time-varying)
Analyses restricted to users of ADHD medications (remote use comparison)
Among ever users of ADHD medications, the adjusted RRs of serious cardiovascular events was nearly the same during periods of current use as it was during follow-up periods more than one year after use ended, RR=1.03 (95% CI, 0.86–1.24) (Table 2). This 1.24 estimated upper bound for the RR would correspond to an absolute risk difference of 0.17 serious cardiovascular events per 1000 person years in adults ages 25–44 years (ages at which the absolute risk was only 0.87 per 1000 person years); and 0.68 serious cardiovascular events per 1000 person years in adults ages 45–64 years (when the absolute risk during current use was 3.5 per 1000 person years).
Table 2.
Adjusted rate ratios for serious cardiovascular events, associated with ADHD medication use, remote use comparison
| Medication status | Person- yrs |
Number Events |
Rate/1,000 person-yrs |
Unadjusted RR |
Adjusted matching variables RRa |
Adjusted RRb |
95% CI |
|---|---|---|---|---|---|---|---|
| MI | |||||||
| Current use | 113,324.2 | 152 | 1.34 | 0.64 | 0.98 | 1.08 | 0.86 – 1.36 |
| Amphetamines | 49,080.1 | 59 | 1.20 | 0.57 | 0.99 | 1.12 | 0.83 – 1.52 |
| Methylphenidate | 51,232.8 | 77 | 1.50 | 0.71 | 1.00 | 1.10 | 0.83 – 1.45 |
| Atomoxetine | 8,424.8 | 11 | 1.31 | 0.62 | 0.99 | 1.06 | 0.57 – 1.96 |
| Pemoline | 3,047.3 | 5 | 1.64 | 0.78 | 0.87 | 0.87 | 0.36 – 2.12 |
| Multiple | 1,539.1 | 0 | 0.00 | -- | -- | -- | -- |
| Indeterminate use | 53,896.7 | 86 | 1.60 | 0.76 | 1.19 | 1.31 | 1.00 – 1.71 |
| Former use | 47,858.5 | 65 | 1.36 | 0.64 | 0.92 | 0.96 | 0.71 – 1.28 |
| Remote use | 69,792.9 | 147 | 2.11 | 1.00 | 1.00 | 1.00 | reference |
| SCDc | |||||||
| Current use | 107,525.0 | 32 | 0.30 | 0.41 | 0.79 | 0.82 | 0.52 – 1.29 |
| Amphetamines | 46,910.0 | 13 | 0.28 | 0.38 | 0.85 | 0.94 | 0.51 – 1.76 |
| Methylphenidate | 47,887.1 | 13 | 0.27 | 0.37 | 0.66 | 0.68 | 0.37 – 1.27 |
| Atomoxetine | 8,257.6 | 4 | 0.48 | 0.66 | 1.14 | 1.06 | 0.38 – 2.95 |
| Pemoline | 2,995.7 | 2 | 0.67 | 0.91 | 1.11 | 1.10 | 0.27 – 4.56 |
| Multiple | 1,474.5 | 0 | 0.00 | -- | -- | -- | -- |
| Indeterminate use | 51,814.0 | 14 | 0.27 | 0.37 | 0.70 | 0.74 | 0.41 – 1.35 |
| Former use | 46,263.5 | 20 | 0.43 | 0.59 | 0.94 | 0.92 | 0.55 – 1.55 |
| Remote use | 68,102.6 | 50 | 0.73 | 1.00 | 1.00 | 1.00 | reference |
| Stroked | |||||||
| Current use | 111,935.5 | 63 | 0.56 | 0.58 | 0.90 | 0.93 | 0.65 – 1.31 |
| Amphetamines | 48,672.9 | 19 | 0.39 | 0.40 | 0.70 | 0.77 | 0.46 – 1.29 |
| Methylphenidate | 50,332.3 | 39 | 0.77 | 0.80 | 1.15 | 1.15 | 0.77 – 1.72 |
| Atomoxetine | 8,371.1 | 3 | 0.36 | 0.37 | 0.55 | 0.54 | 0.17 – 1.71 |
| Pemoline | 3,030.1 | 2 | 0.66 | 0.68 | 0.74 | 0.72 | 0.18 – 2.96 |
| Multiple | 1,529.2 | 0 | 0.00 | -- | -- | -- | -- |
| Indeterminate use | 53,327.8 | 31 | 0.58 | 0.60 | 0.96 | 1.01 | 0.65 – 1.54 |
| Former use | 47,333.0 | 39 | 0.82 | 0.85 | 1.24 | 1.23 | 0.83 – 1.83 |
| Remote use | 69,202.3 | 67 | 0.97 | 1.00 | 1.00 | 1.00 | reference |
| MI, SCD or strokec,d | |||||||
| Current use | 107,322.4 | 234 | 2.18 | 0.61 | 0.96 | 1.03 | 0.86 – 1.24 |
| Amphetamines | 46,826.5 | 88 | 1.88 | 0.52 | 0.93 | 1.05 | 0.82 – 1.34 |
| Methylphenidate | 47,792.3 | 120 | 2.51 | 0.70 | 1.01 | 1.07 | 0.86 – 1.34 |
| Atomoxetine | 8,248.2 | 17 | 2.06 | 0.57 | 0.90 | 0.92 | 0.56 – 1.50 |
| Pemoline | 2,985.2 | 9 | 3.01 | 0.84 | 0.95 | 0.93 | 0.48 – 1.82 |
| Multiple | 1,470.1 | 0 | 0.00 | -- | -- | -- | -- |
| Indeterminate use | 51,709.6 | 125 | 2.42 | 0.67 | 1.09 | 1.17 | 0.94 – 1.45 |
| Former use | 46,120.8 | 121 | 2.62 | 0.73 | 1.06 | 1.07 | 0.86 – 1.33 |
| Remote use | 67,489.0 | 243 | 3.60 | 1.00 | 1.00 | 1.00 | reference |
Abbreviations: MI, myocardial infarction; SCD, sudden cardiac death; RR, rate ratio; CI, confidence interval; CRS, cardiovascular risk score; KP, Kaiser Permanente
Adjusted for site, age, sex, and calendar year (ie, matching variables).
Adjusted for site, age, sex, calendar year, and CRS (some variables within score are time-varying).
Analyses excluded the three HMORN sites (Fallon Community, KP Georgia, KP Northwest) that did not provide data on SCD endpoints.
Analyses excluded the two HMORN sites (Fallon Community, KP Georgia) that did not provide data on stroke endpoints.
New user analyses
In the new user cohort, baseline characteristics of new users of ADHD medication were generally similar to characteristics of all ADHD medication users (eTable 7). Cardiovascular diseases were similar or slightly more prevalent in new users than non-users. ADHD and other psychiatric conditions were substantially more common in the new users than the non-users. In the new user analyses, RRs for current vs. remote use were close to 1.0 for MI, stroke and the combined endpoint (Table 3). Although not statistically significant, RRs for methylphenidate were 1.26, 1.44, and 1.20 for MI, stroke, and the combined endpoint, respectively, somewhat higher than the RRs for the other drugs. For the combined endpoint, there was no pattern of increasing risk with increasing duration of current use or for any window of time. For current use (all durations combined) vs remote use, the RR for the combined endpoint was 1.02. The upper bound of the CI was 1.28; this would amount to an additional 0.19 events per 1000 person years at ages 25–44 years and an additional 0.77 events per 1000 person years at ages 45–64 years.
Table 3.
Adjusted rate ratios for serious cardiovascular events, associated with ADHD medication use, new user cohort, remote use comparison
| Medication status | Person- yrs |
Number Events |
Rate/1,000 person-yrs |
Unadjusted RR |
Adjusted matching variables RRa |
Adjusted RRb |
95%CI |
|---|---|---|---|---|---|---|---|
| MI | |||||||
| Current use | 55,533.9 | 77 | 1.39 | 0.67 | 1.04 | 1.08 | 0.81 – 1.45 |
| Type of medication | |||||||
| Amphetamines | 23,265.6 | 24 | 1.03 | 0.50 | 0.86 | 0.94 | 0.60 – 1.46 |
| Methylphenidate | 23,930.8 | 42 | 1.76 | 0.84 | 1.23 | 1.26 | 0.88 – 1.80 |
| Atomoxetine | 6,475.3 | 9 | 1.39 | 0.67 | 1.06 | 1.06 | 0.54 – 2.10 |
| Pemoline | 1,114.4 | 2 | 1.79 | 0.86 | 0.76 | 0.75 | 0.18 – 3.03 |
| Multiple | 747.7 | 0 | 0.00 | -- | -- | -- | -- |
| Durationc | |||||||
| 1–30 days | 7,526.3 | 11 | 1.46 | 0.70 | 1.26 | 1.31 | 0.70 – 2.43 |
| 31–90 days | 9,656.8 | 11 | 1.14 | 0.55 | 1.01 | 1.06 | 0.57 – 1.96 |
| 91–182 days | 9,556.3 | 8 | 0.84 | 0.40 | 0.71 | 0.75 | 0.36 – 1.53 |
| 183–365 days | 11,221.9 | 16 | 1.43 | 0.68 | 1.13 | 1.19 | 0.70 – 2.01 |
| 366+ days | 16,425.2 | 29 | 1.77 | 0.85 | 1.09 | 1.15 | 0.76 – 1.73 |
| Indeterminate use | 31,090.0 | 52 | 1.67 | 0.80 | 1.27 | 1.32 | 0.95 – 1.84 |
| Former use | 35,087.7 | 55 | 1.57 | 0.75 | 1.08 | 1.08 | 0.78 – 1.49 |
| Remote use | 55,194.2 | 115 | 2.08 | 1.00 | 1.00 | 1.00 | reference |
| SCDd | |||||||
| Current use | 52,203.2 | 15 | 0.29 | 0.34 | 0.61 | 0.62 | 0.34 – 1.11 |
| Type of medication | |||||||
| Amphetamines | 22,002.7 | 3 | 0.14 | 0.16 | 0.34 | 0.38 | 0.12 – 1.22 |
| Methylphenidate | 22,056.6 | 8 | 0.36 | 0.43 | 0.70 | 0.69 | 0.32 – 1.46 |
| Atomoxetine | 6,348.4 | 3 | 0.47 | 0.57 | 0.95 | 0.90 | 0.28 – 2.91 |
| Pemoline | 1,091.6 | 1 | 0.92 | 1.10 | 0.90 | 0.93 | 0.13 – 6.77 |
| Multiple | 703.9 | 0 | 0.00 | -- | -- | -- | -- |
| Durationc | |||||||
| 1–30 days | 7,217.4 | 2 | 0.28 | 0.33 | 0.67 | 0.68 | 0.16 – 2.81 |
| 31–90 days | 9,195.9 | 2 | 0.22 | 0.26 | 0.56 | 0.56 | 0.14 – 2.33 |
| 91–182 days | 9,026.0 | 4 | 0.44 | 0.53 | 1.09 | 1.11 | 0.40 – 3.10 |
| 183–365 days | 10,511.3 | 1 | 0.10 | 0.11 | 0.22 | 0.22 | 0.03 – 1.62 |
| 366+ days | 15,129.8 | 5 | 0.33 | 0.40 | 0.57 | 0.57 | 0.22 – 1.43 |
| Indeterminate use | 29,752.7 | 13 | 0.44 | 0.52 | 0.94 | 0.96 | 0.52 – 1.79 |
| Former use | 33,877.6 | 16 | 0.47 | 0.57 | 0.89 | 0.88 | 0.50 – 1.56 |
| Remote use | 53,926.6 | 45 | 0.83 | 1.00 | 1.00 | 1.00 | reference |
| Strokee | |||||||
| Current use | 54,569.3 | 41 | 0.75 | 0.73 | 1.10 | 1.09 | 0.72 – 1.64 |
| Type of medication | |||||||
| Amphetamines | 22,965.2 | 10 | 0.44 | 0.43 | 0.72 | 0.77 | 0.39 – 1.53 |
| Methylphenidate | 23,335.7 | 26 | 1.11 | 1.09 | 1.53 | 1.44 | 0.90 – 2.30 |
| Atomoxetine | 6,429.4 | 3 | 0.47 | 0.46 | 0.66 | 0.64 | 0.20 – 2.04 |
| Pemoline | 1,099.7 | 2 | 1.82 | 1.78 | 1.48 | 1.39 | 0.34 – 5.72 |
| Multiple | 739.3 | 0 | 0.00 | -- | -- | -- | -- |
| Durationc | |||||||
| 1–30 days | 7,421.0 | 4 | 0.54 | 0.53 | 0.93 | 0.92 | 0.33 – 2.53 |
| 31–90 days | 9,511.8 | 6 | 0.63 | 0.62 | 1.10 | 1.08 | 0.46 – 2.51 |
| 91–182 days | 9,398.0 | 9 | 0.96 | 0.94 | 1.59 | 1.55 | 0.76 – 3.14 |
| 183–365 days | 11,018.6 | 4 | 0.36 | 0.35 | 0.56 | 0.55 | 0.20 – 1.53 |
| 366+ days | 16,087.6 | 16 | 0.99 | 0.97 | 1.19 | 1.21 | 0.69 – 2.11 |
| Indeterminate use | 30,657.1 | 20 | 0.65 | 0.64 | 1.00 | 1.00 | 0.60 – 1.67 |
| Former use | 34,644.6 | 26 | 0.75 | 0.73 | 1.05 | 1.04 | 0.65 – 1.65 |
| Remote use | 54,702.5 | 56 | 1.02 | 1.00 | 1.00 | 1.00 | reference |
| MI, SCD or stroked,e | |||||||
| Current use | 52,094.6 | 125 | 2.40 | 0.65 | 1.00 | 1.02 | 0.82 – 1.28 |
| Type of medication | |||||||
| Amphetamines | 21,955.8 | 37 | 1.69 | 0.46 | 0.80 | 0.87 | 0.61 – 1.23 |
| Methylphenidate | 22,008.8 | 69 | 3.14 | 0.85 | 1.22 | 1.20 | 0.91 – 1.59 |
| Atomoxetine | 6,340.4 | 14 | 2.21 | 0.60 | 0.93 | 0.91 | 0.53 – 1.56 |
| Pemoline | 1,088.1 | 5 | 4.60 | 1.25 | 1.07 | 1.02 | 0.42 – 2.49 |
| Multiple | 701.4 | 0 | 0.00 | -- | -- | -- | -- |
| Durationc | |||||||
| 1–30 days | 7,215.7 | 16 | 2.22 | 0.60 | 1.08 | 1.10 | 0.66 – 1.83 |
| 31–90 days | 9,191.7 | 19 | 2.07 | 0.56 | 1.04 | 1.05 | 0.66 – 1.69 |
| 91–182 days | 9,018.3 | 18 | 2.00 | 0.54 | 0.96 | 0.97 | 0.60 – 1.57 |
| 183–365 days | 10,494.1 | 20 | 1.91 | 0.52 | 0.85 | 0.86 | 0.54 – 1.37 |
| 366+ days | 15,055.5 | 47 | 3.12 | 0.85 | 1.06 | 1.10 | 0.80 – 1.51 |
| Indeterminate use | 29,694.2 | 82 | 2.76 | 0.75 | 1.19 | 1.22 | 0.94 – 1.58 |
| Former use | 33,774.3 | 97 | 2.87 | 0.78 | 1.13 | 1.11 | 0.87 – 1.41 |
| Remote use | 53,450.1 | 197 | 3.69 | 1.00 | 1.00 | 1.00 | reference |
Abbreviations: MI, myocardial infarction; SCD, sudden cardiac death; RR, rate ratio; CI, confidence interval; CRS, cardiovascular risk score; KP, Kaiser Permanente
Adjusted for site, age, sex, and calendar year (ie, matching variables).
Adjusted for site, age, sex, calendar year, and CRS (some variables within score are time-varying).
Duration does not include pemoline use (pemoline only and pemoline with other ADHD medications).
Analyses excluded the three HMORN sites (Fallon Community, KP Georgia, KP Northwest) that did not provide data on SCD endpoints.
Analyses excluded the two HMORN sites (Fallon Community, KP Georgia) that did not provide data on stroke endpoints.
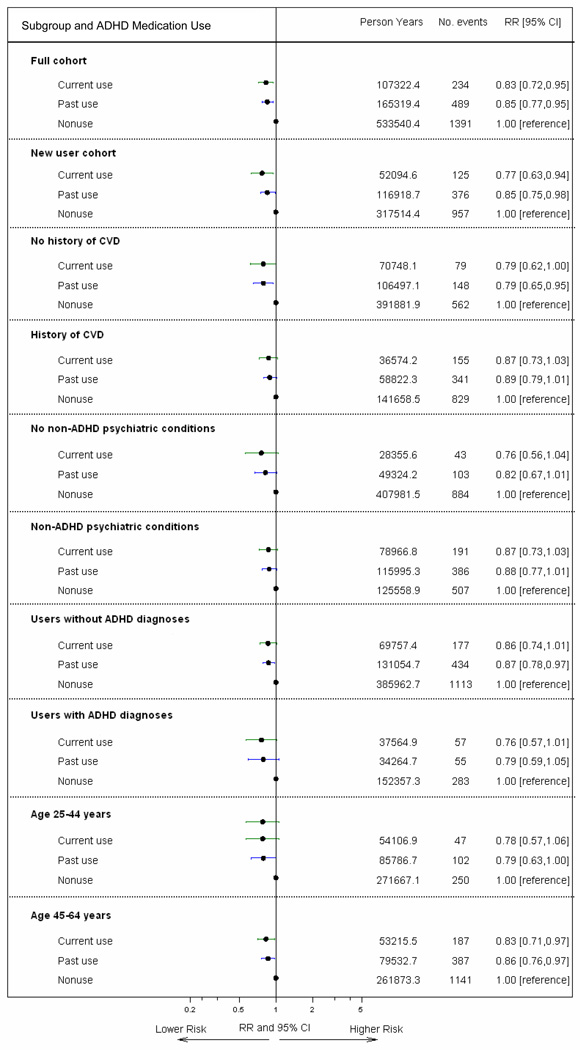
Subgroup analyses
RRs were similar in all subgroup analyses (Figure 2). While we did observe differences in event rates, cohort characteristics, and RRs by data site, RRs for current use were not statistically significantly elevated at any site (eTables 8–10).
Figure 2. Subgroup analyses for combined endpoint (MI, SCD or stroke).
The figure depicts RRs and 95% CIs for current use and past use (indeterminate/former/remote use), with nonuse as the reference category. All RRs adjusted for site, age, sex, calendar year, and CRS (some variables within score are time-varying), except for new users (adjusted for PS).
Sensitivity analyses – unmeasured confounding
Information from review of medical records of MI, SCD, and stroke cases and the external survey population suggested that several factors were not or were only very weakly associated with use of ADHD medications and, therefore, were unlikely to be important confounders (obesity, smoking, family history of cardiovascular disease). However, in these data users of ADHD medications more often had some college education compared to nonusers (17% v. 10%, adjusting for age). In addition, 5% of the stimulant users were black or Hispanic versus 12% of the nonusers. If similar patterns for race/ethnicity and education were also present in our full study cohort, and if each of these characteristics independently multiplied the risk of serious cardiovascular events by 2.4, then these two unmeasured factors would yield a “healthy user bias” substantial enough to account for an apparent RR of 0.83 (as in our comparison of current vs non-use) given a true RR of 1.0.
COMMENT
In our population-based cohort of more than 440,000 young and middle-aged adults, including more than 150,000 users of ADHD medications identified through filled prescriptions, we found no evidence of an increased risk of MI, SCD, or stroke associated with current use compared to non-use or remote use of ADHD medications. We also found little support for an increased risk for any specific medication or with longer duration of current use. Results were similar when users were restricted to new users. Rate ratios did not appear to be influenced by prior cardiovascular disease or by prior non-ADHD psychiatric conditions. They also were similar across age groups. As expected, event rates were substantially higher in the Medicaid population; however, the RR for current use was similar to other sites.
Our study has several limitations. Use of ADHD medications was based on electronic pharmacy records of filled prescriptions. Filled prescriptions may not represent medications actually consumed and days supply may not represent actual periods of use. Nonetheless, electronic pharmacy databases have been found to be excellent sources of information on drug use(30). We did not obtain dose data and therefore could not examine if risk varied by this factor. Although we used a strict definition of current use minimizing misclassification of this exposure, there was limited ability to assess medication adherence using standard definitions. Despite its very large size, the study had only moderate power for several comparisons, including current vs. remote use in the new user analyses and in comparisons for individual drugs. The study did not include adults 65 years and older and therefore results cannot be generalized to this age group.
We reviewed the medical records and death certificates to confirm SCD and stroke diagnoses. However, records were unavailable for some of our electronically identified cases. We used an ICD-9/10 code-based definition for these cases and misclassification of some cases may have occurred. If non-differential with respect to ADHD medication use, this misclassification would bias RRs towards the null.
There is limited accuracy of ADHD diagnoses in adults from claims and encounter databases. However, previous studies have validated ICD 9/10 code-based definitions of many important covariates, including diabetes, congestive heart failure, peripheral vascular disease, and hypertension, reporting positive predictive values exceeding 90% for each condition (31–33). Although we adjusted for numerous established and potential cardiovascular risk factors, there were some factors, primarily psychiatric conditions and medications, for which the prevalence was substantial in users of ADHD medications but rare in non-users. Thus, we had limited ability to adjust for these variables. Important residual confounding by psychiatric conditions and medications seems unlikely, since most are not established risk factors for CVD, they were not or were only modestly related to risk in our cohort, and results were similar when we restricted analyses to those with or to those without these psychiatric conditions or medication use.
Importantly, there appears to be a modest amount of healthy user bias influencing our RR comparisons of current vs. non-use. Results are less prone to this bias when analyses are restricted to ever users of ADHD medications, and we compare periods of current use to follow-up periods remote from use. In these comparisons, the RR for serious cardiovascular events was 1.03 in the full cohort and 1.02 in new users, indicating the incidence of these events while currently on ADHD medications is very similar to the incidence during periods while off these medications. In sensitivity analyses, we saw evidence for two potential sources of a modest amount of healthy user bias; a higher percentage of users were white and college educated.
Clinical trials have provided limited information on the cardiovascular safety of ADHD medications, primarily because they have been too small to evaluate serious events such as MI, SCD, or stroke (34,35). Post-marketing surveillance data from AERS (2) and from the National Electronic Injury Surveillance System(36) have suggested a potential elevation in risk of serious cardiovascular events. However, with these surveillance systems, which capture only a small percentage of adverse events, false signals may occur if clinicians suspect, and are thus more likely to report, adverse events for a particular drug.
The findings of the current study were similar to those of our parallel study in youths aged 2–24 years, in which we found no evidence of increased risk for serious cardiovascular events in current users of ADHD medications compared to nonusers (19). To our knowledge, only 2 pharmacoepidemiologic studies of ADHD medications and cardiovascular disease in adults have reported results(17,18). These studies, which were substantially smaller than ours, used electronic pharmacy records and medical encounter data, with similarly limited information on some potentially important risk factors. In one, users of ADHD medications had an over 3-fold higher rate of transient ischemic attacks but a 30% lower rate of cerebrovascular accidents, although the latter was not statistically significant(17). In contrast, no increase in SCDs among children, adolescents or young adults was observed in a second cohort study conducted in the General Practice Research Database (18).
In conclusion, in this cohort of young and middle-aged adults, current or new use of ADHD medications identified from filled prescriptions, compared with non-use or remote use, was not associated with an increased risk of serious cardiovascular events. A modestly elevated risk cannot be ruled out given limited power and a lack of complete information on some potentially important risk factors and other factors related to use of these medications.
Supplementary Material
Acknowledgments
Author Contributions: Dr Habel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chan, Cheetham, Cooper, Fireman, Habel, Ray, Selby, Sox.
Acquisition of data: Andrade, Boudreau, Chan, Cheetham, Cooper, Dublin, Habel, Pawloski, Quinn, Raebel, Selby, Smith, Sox, Uratsu.
Analysis and interpretation of data: Achacoso, Arbogast, Chan, Cheetham, Cooper, Fireman, Go, Habel, Nguyen-Huynh, Quinn, Ray, Selby, Sidney, Sox, Uratsu.
Drafting of the manuscript: Habel
Critical revision of the manuscript for important intellectual content: Achacoso, Andrade, Arbogast, Boudreau, Chan, Cheetham, Cooper, Dublin, Fireman, Go, Nguyen-Huynh, Pawloski, Quinn, Raebel, Ray, Selby, Sidney, Smith, Sox, Uratsu.
Statistical analysis: Achacoso, Arbogast, Cooper, Fireman, Uratsu.
Obtained funding: Chan, Cooper, Habel, Quinn, Selby.
Administrative, technical, or material support: Achacoso, Andrade, Chan, Quinn, Selby, Uratsu.
Study supervision: Andrade, Chan, Cooper, Habel, Quinn, Smith, Sox.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Dr. Chan reported receiving support for travel to meetings for the purpose of the study or other purposes from the FDA. Dr. Chan also reported that he is a part time employee of OptumInsight, a for-profit company that receives funding from medical product manufacturers to provide consultation and conduct research on medical products. As OptumInsight is a part of UnitedHealthGroup, Dr. Chan has received UHG stock options.
Dr. Andrade reported that the Meyers Primary Care Institute has received funding from GlaxoSmithKline and Novartis Pharmaceuticals, manufacturers of medications used to treat ADHD.
Dr. Habel reported receiving grants from Merck for a study of herpes zoster in cancer patients, Takeda for a study of proglitazone and cancer, and Sanofi-Aventis for a study of insulin and cancer.
Dr. Dublin has received a Merck/American Geriatrics Society New Investigator Award for unrelated work. This consisted of an honorarium paid directly to Dr. Dublin.
Funding/Support: This project was funded in part under Contract Numbers HHSA290-2005-0042 (Vanderbilt) and HHSA290-2005-0033 (Harvard Pilgrim Health Care Institute) from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
The project was also funded by the Food and Drug Administration under the following contracts 223-2005-10012 (Kaiser Permanente Northern California), 223-2005-10100C (Vanderbilt), 223-2005-20006C (Ingenix), and 223-2005-10012C (Harvard Pilgrim Health Care Institute).
The project was also funded by National Institute of Aging under the following contract K23AG028954 (Group Health Research Institute).
Role of the Sponsors: Individuals from the FDA and AHRQ were on the study Steering Committee and provided input into the study design and conduct of the study, and interpretation of the data. The sponsors had no role in the preparation, review or approval of the manuscript.
Additional Contributions: We would like to also acknowledge and thank the following individuals for their contributions to this project: Andrew Mosholder, MD (FDA), member of Scientific Steering Committee; James Daugherty, MS (Vanderbilt University School of Medicine), programming; Judith Dudley, BS (Vanderbilt University School of Medicine), programming; Chantal Avila, MA (Kaiser Permanente Southern California), project management; Yan Luo, PhD (Kaiser Permanente Southern California), programming; Wansu Chen, MS (Kaiser Permanente Southern California), programming; Mary Kershner, BSN (Kaiser Permanente Colorado), chart reviews and abstraction; April Duddy, MS (Harvard Pilgrim Health Care Institute), project management; Luana Acton, BS (Kaiser Permanente Northern California), project management; Jean Lee, BA (Kaiser Permanente Northern California), medical record retrieval and abstraction; Julie Munneke, BA (Kaiser Permanente Northern California), project management; Monica Highbaugh, BA (Kaiser Permanente Northern California), medical record retrieval and abstraction; Heidi Krause, BA (Kaiser Permanente Northern California), project management. All those in the acknowledgement were compensated for their time.
Reference List
- 1.A report by Medco. [Accessed November 16, 2011];America's State of Mind, 2011. http://medco.mediaroom.com/. Ref Type: Generic.
- 2.Food and Drug Administration. Drug Safety and Risk Management Advisory Committee Meeting. Food and Drug Administration; 2006. Ref Type: Electronic Citation. [Google Scholar]
- 3.Montejano L, Sasane R, Hodgkins P, et al. Adult ADHD: prevalence of diagnosis in a US population with employer health insurance. Curr Med Res Opin. 2011;27(Suppl 2):5–11. doi: 10.1185/03007995.2011.603302. [DOI] [PubMed] [Google Scholar]
- 4.Wilens TE, Morrison NR, Prince J. An update on the pharmacotherapy of attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2011;11(10):1443–1465. doi: 10.1586/ern.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75(7):711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- 6.Leddy JJ, Epstein LH, Jaroni JL, et al. Influence of methylphenidate on eating in obese men. Obes Res. 2004;12(2):224–232. doi: 10.1038/oby.2004.29. [DOI] [PubMed] [Google Scholar]
- 7.Frierson RL, Wey JJ, Tabler JB. Psychostimulants for depression in the medically ill. Am Fam Physician. 1991;43(1):163–170. [PubMed] [Google Scholar]
- 8.Tharwani HM, Yerramsetty P, Mannelli P, et al. Recent advances in poststroke depression. Curr Psychiatry Rep. 2007;9(3):225–231. doi: 10.1007/s11920-007-0023-9. [DOI] [PubMed] [Google Scholar]
- 9.Warden DL, Gordon B, McAllister TW, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23(10):1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 10.Hammerness PG, Surman CB, Chilton A. Adult attention-deficit/hyperactivity disorder treatment and cardiovascular implications. Curr Psychiatry Rep. 2011;13(5):357–363. doi: 10.1007/s11920-011-0213-3. [DOI] [PubMed] [Google Scholar]
- 11.Stiefel G, Besag FM. Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention-deficit hyperactivity disorder. Drug Saf. 2010;33(10):821–842. doi: 10.2165/11536380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 13.Gould MS, Walsh BT, Munfakh JL, et al. Sudden death and use of stimulant medications in youths. Am J Psychiatry. 2009;166(9):992–1001. doi: 10.1176/appi.ajp.2009.09040472. [DOI] [PubMed] [Google Scholar]
- 14.Winterstein AG, Gerhard T, Shuster J, et al. Cardiac safety of central nervous system stimulants in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics. 2007;120(6):e1494–e1501. doi: 10.1542/peds.2007-0675. [DOI] [PubMed] [Google Scholar]
- 15.Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127(6):1102–1110. doi: 10.1542/peds.2010-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winterstein AG, Gerhard T, Shuster J, Saidi A. Cardiac safety of methylphenidate versus amphetamine salts in the treatment of ADHD. Pediatrics. 2009;124(1):e75–e80. doi: 10.1542/peds.2008-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holick CN, Turnbull BR, Jones ME, et al. Atomoxetine and cerebrovascular outcomes in adults. J Clin Psychopharmacol. 2009;29(5):453–460. doi: 10.1097/JCP.0b013e3181b2b828. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy S, Cranswick N, Potts L, et al. Mortality associated with attention-deficit hyperactivity disorder (ADHD) drug treatment: a retrospective cohort study of children, adolescents and young adults using the general practice research database. Drug Saf. 2009;32(11):1089–1096. doi: 10.2165/11317630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Cooper WO, Habel LA, Sox CM, et al. ADHD Drugs and Serious Cardiovascular Events in Children and Young Adults. N Engl J Med. 2011 doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 21.Meier MA, Al Badr WH, Cooper JV, et al. The new definition of myocardial infarction: diagnostic and prognostic implications in patients with acute coronary syndromes. Arch Intern Med. 2002;162(14):1585–1589. doi: 10.1001/archinte.162.14.1585. [DOI] [PubMed] [Google Scholar]
- 22.Chung CP, Murray KT, Stein CM, et al. A computer case definition for sudden cardiac death. Pharmacoepidemiol Drug Saf. 2010;19(6):563–572. doi: 10.1002/pds.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miettinen OS. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104(6):609–620. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- 24.Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res. 2009;18(1):67–80. doi: 10.1177/0962280208092347. [DOI] [PubMed] [Google Scholar]
- 25.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suissa S, Edwardes MD. Adjusted odds ratios for case-control studies with missing confounder data in controls. Epidemiology. 1997;8(3):275–280. doi: 10.1097/00001648-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 28.Schneeweiss S, Glynn RJ, Tsai EH, et al. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005;16(1):17–24. doi: 10.1097/01.ede.0000147164.11879.b5. [DOI] [PubMed] [Google Scholar]
- 29.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 30.West SL, Strom BL, Polle C. Validity of Pharmacoepidemiologic Drug and Diagnosis Data. In: Strom BL, editor. Pharmacoepidemiology. Philadelphia: John Wiley & Sons, Ltd; 2005. pp. 709–765. [Google Scholar]
- 31.Burgos-Lunar C, Salinero-Fort MA, Cardenas-Valladolid J, et al. Validation of diabetes mellitus and hypertension diagnosis in computerized medical records in primary health care. BMC Med Res Methodol. 2011;11:146. doi: 10.1186/1471-2288-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grijalva CG, Chung CP, Stein CM, et al. Computerized definitions showed high positive predictive values for identifying hospitalizations for congestive heart failure and selected infections in Medicaid enrollees with rheumatoid arthritis. Pharmacoepidemiol Drug Saf. 2008;17(9):890–895. doi: 10.1002/pds.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler LA, Zimmerman B, Starr HL, et al. Efficacy and safety of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, double-blind, parallel group, dose-escalation study. J Clin Psychopharmacol. 2009;29(3):239–247. doi: 10.1097/JCP.0b013e3181a390ce. [DOI] [PubMed] [Google Scholar]
- 35.Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl) 2008;197(1):1–11. doi: 10.1007/s00213-007-0996-4. [DOI] [PubMed] [Google Scholar]
- 36.Cohen AL, Jhung MA, Budnitz DS. Stimulant medications and attention deficithyperactivity disorder. N Engl J Med. 2006;354(21):2294–2295. doi: 10.1056/NEJMc060860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.