Abstract
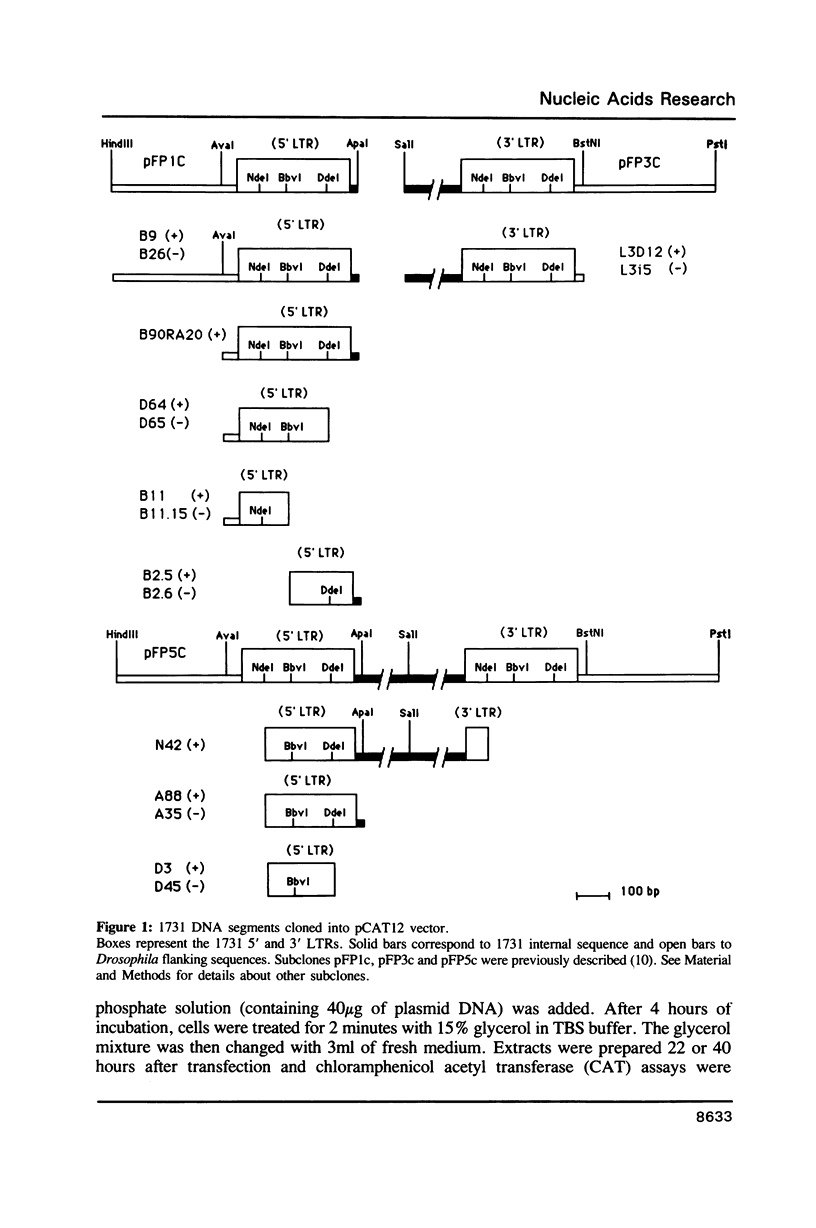
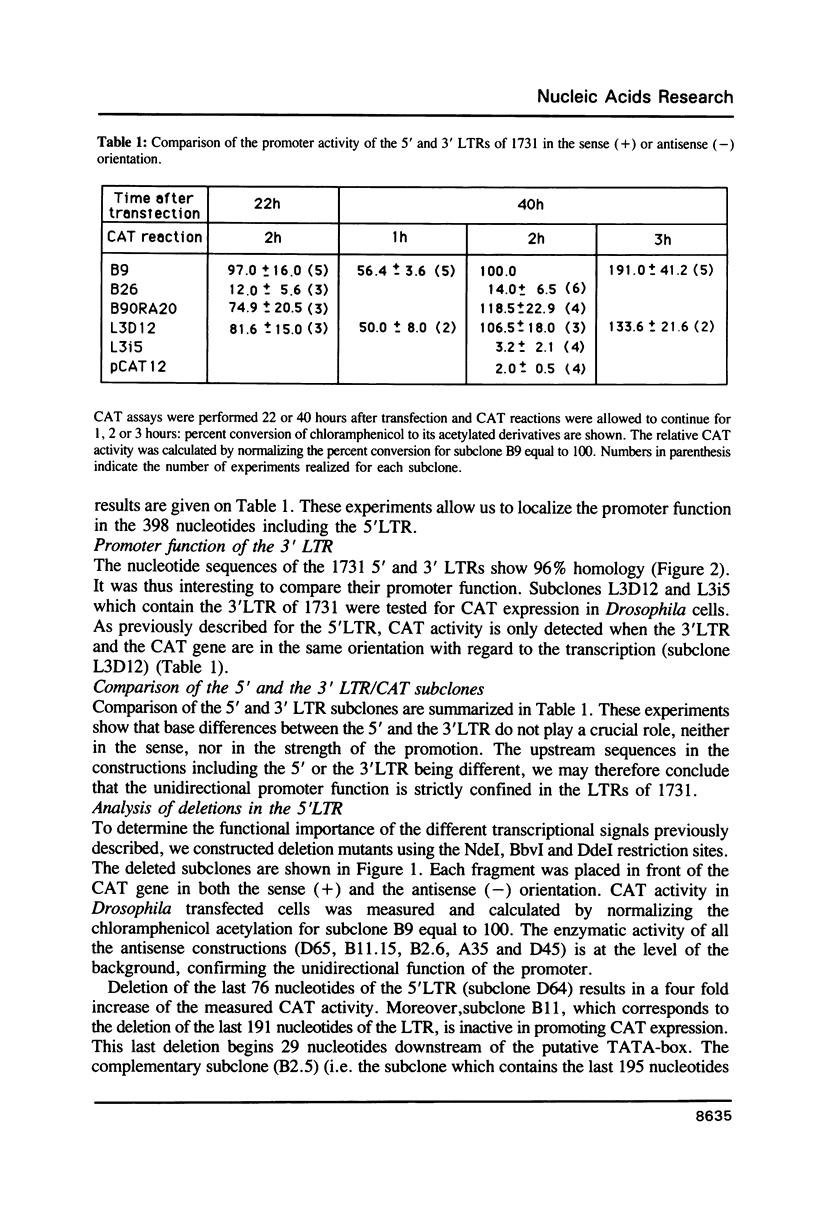
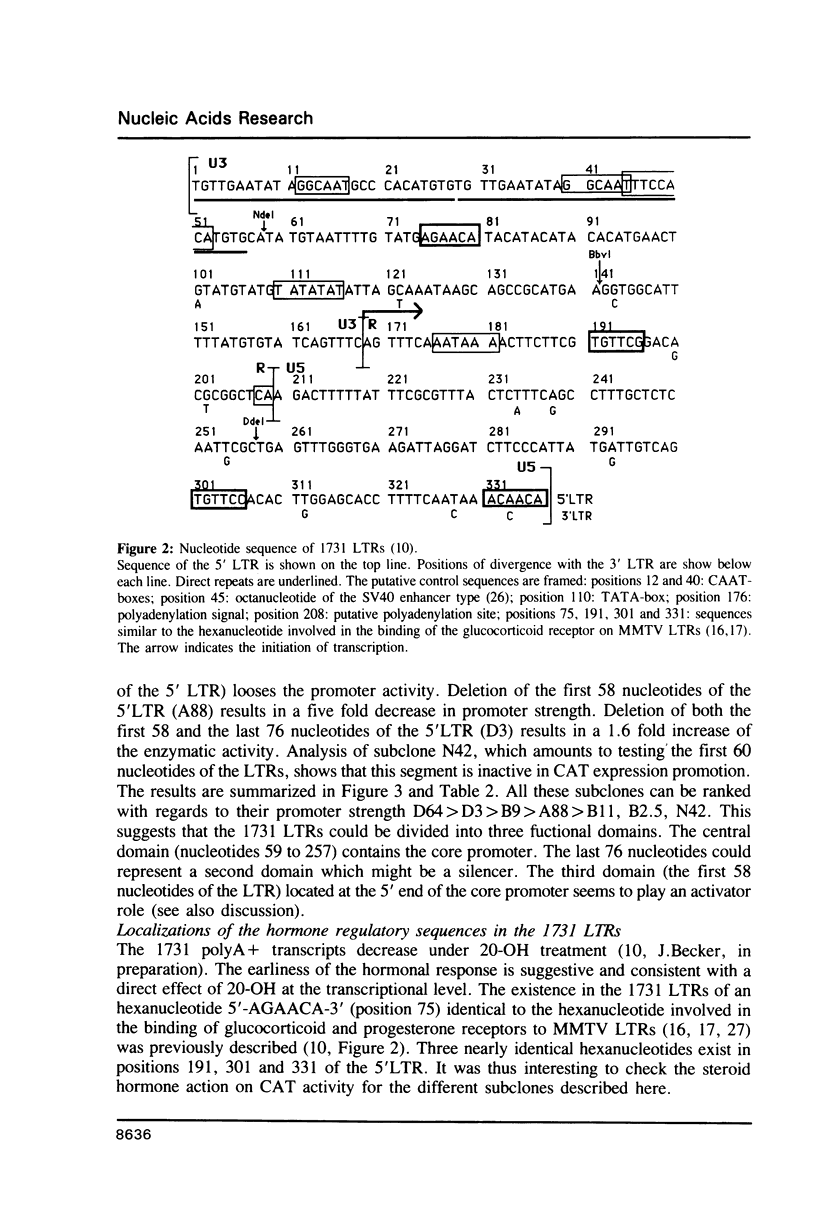
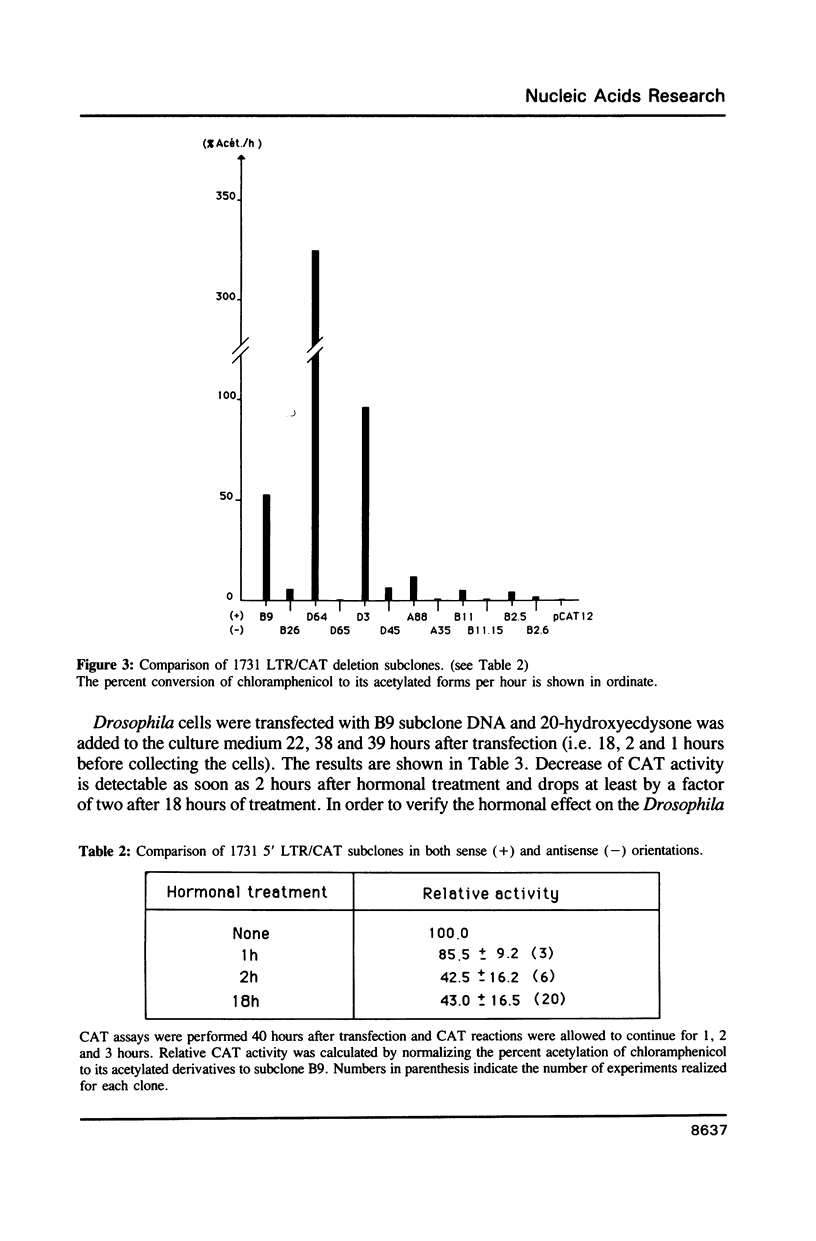
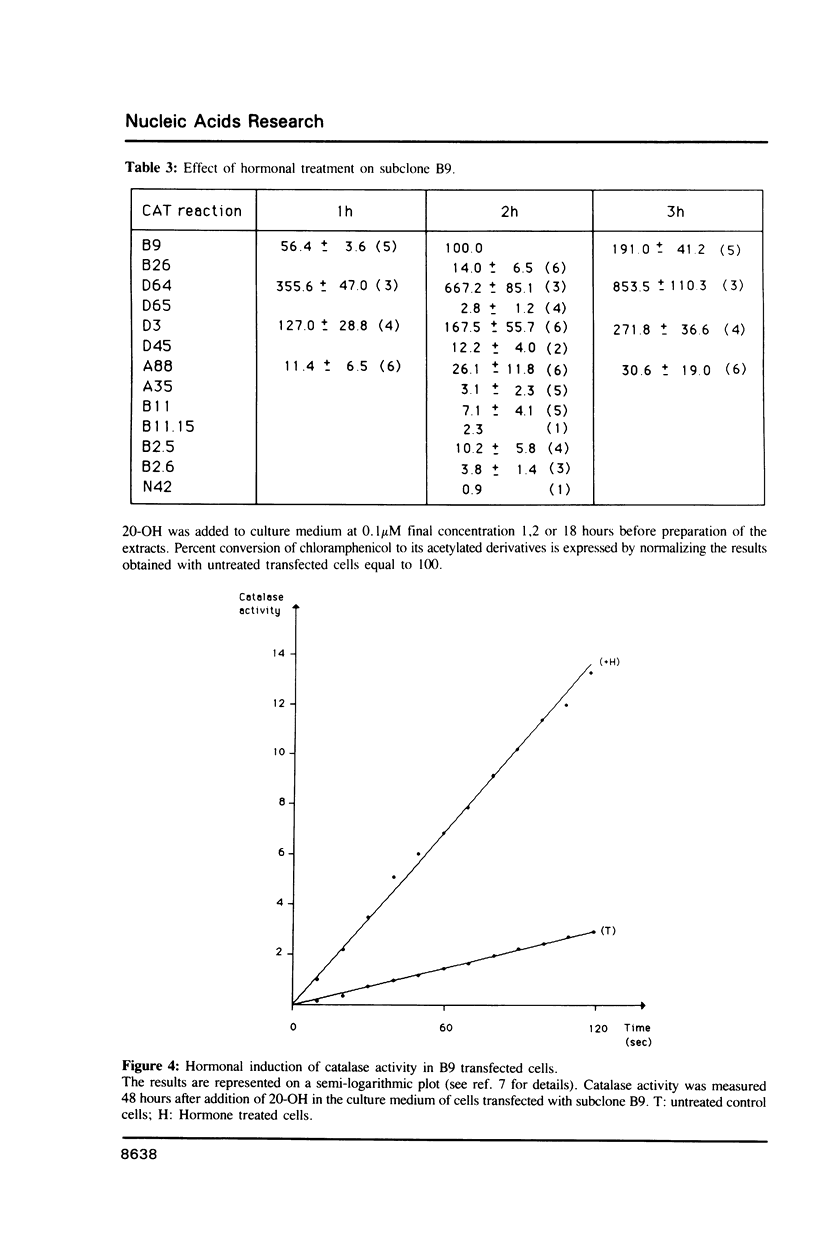
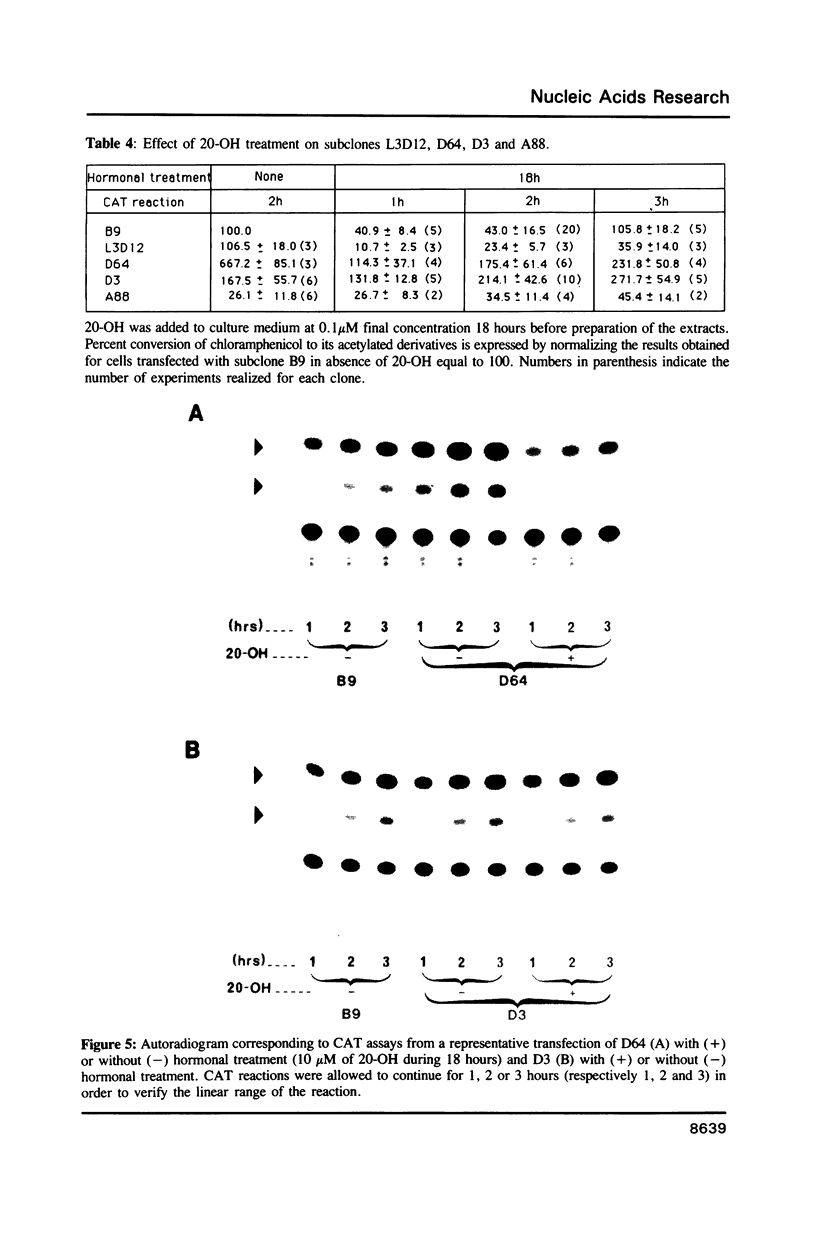
1731 is a Drosophila retrotransposon whose transcripts decrease in Drosophila cells after treatment by the steroid hormone 20-hydroxyecdysone (20-OH). Several constructions have been made where the bacterial chloramphenicol acetyltransferase (CAT) gene is put under the control of either the 5' or the 3' long terminal repeats (LTRs) of 1731. CAT activity assays in transfected Drosophila cells show that either the 5' or the 3'LTR constitutes a unidirectional promoter. Analysis of partially deleted LTR suggests the presence of so-called silencer and activator regions in these LTRs. Moreover, the first 260 bp of the LTR are sufficient to provoke 20-OH inhibition whereas the first 58 bp are necessary for hormonal responsiveness. These 58 bp contain sequences showing similarities with the targets of trans-acting factors such as Octal-c and NFkB.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M., Arnemann J., Chalepakis G., Slater E., Willmann T. Gene regulation by steroid hormones. J Steroid Biochem. 1987;27(1-3):9–14. doi: 10.1016/0022-4731(87)90288-3. [DOI] [PubMed] [Google Scholar]
- Beato M. Induction of transcription by steroid hormones. Biochim Biophys Acta. 1987 Nov 20;910(2):95–102. doi: 10.1016/0167-4781(87)90060-1. [DOI] [PubMed] [Google Scholar]
- Best-Belpomme M., Courgeon A. M. Ecdysterone and acetylcholinesterase activity in cultured Drosophila cells. Inducible, non-inducible and constitutive clones or lines. FEBS Lett. 1977 Oct 15;82(2):345–347. doi: 10.1016/0014-5793(77)80617-0. [DOI] [PubMed] [Google Scholar]
- Best-Belpomme M., Courgeon A. M., Rambach A. beta-Galactosidase is induced by hormone in Drosophila melanogaster cell cultures. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6102–6106. doi: 10.1073/pnas.75.12.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best-Belpomme M., Ropp M. Catalase is induced by ecdysterone and ethanol in Drosophila cells. Eur J Biochem. 1982 Jan;121(2):349–355. doi: 10.1111/j.1432-1033.1982.tb05793.x. [DOI] [PubMed] [Google Scholar]
- Best-Belpomme M., Sykiotis M., Courgeon A. M. Antisera against ecdysteroid-induced proteins in an established line and a clone of Drosophila melanogaster cells. FEBS Lett. 1978 May 1;89(1):86–88. doi: 10.1016/0014-5793(78)80528-6. [DOI] [PubMed] [Google Scholar]
- Cherbas P., Cherbas L., Williams C. M. Induction of acetylcholinesterase activity by beta-ecdysone in a Drosophila cell line. Science. 1977 Jul 15;197(4300):275–277. doi: 10.1126/science.877552. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Huang R. C. Functional analysis of the long terminal repeats of intracisternal A-particle genes: sequences within the U3 region determine both the efficiency and direction of promoter activity. Mol Cell Biol. 1988 Mar;8(3):1093–1102. doi: 10.1128/mcb.8.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc J. L., Dastugue B. Ecdysterone-induced modifications of protein synthesis in a Drosophila melanogaster cultured cell line. Biochem Biophys Res Commun. 1980 Nov 17;97(1):173–181. doi: 10.1016/s0006-291x(80)80151-3. [DOI] [PubMed] [Google Scholar]
- Echalier G. Drosophila retrotransposons: interactions with genome. Adv Virus Res. 1989;36:33–105. doi: 10.1016/s0065-3527(08)60582-5. [DOI] [PubMed] [Google Scholar]
- Emanoil-Ravier R., Mercier G., Canivet M., Garcette M., Lasneret J., Peronnet F., Best-Belpomme M., Peries J. Dexamethasone stimulates expression of transposable type A intracisternal retroviruslike genes in mouse (Mus musculus) cells. J Virol. 1988 Oct;62(10):3867–3869. doi: 10.1128/jvi.62.10.3867-3869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade-Peronnet F., d'Auriol L., Becker J., Galibert F., Best-Belpomme M. Primary structure and functional organization of Drosophila 1731 retrotransposon. Nucleic Acids Res. 1988 Jul 11;16(13):6113–6125. doi: 10.1093/nar/16.13.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Kuwabara M. D., Wu F. K., Garcia J. A., Harrich D., Briskin M., Wall R., Sigman D. S. Repeated B motifs in the human immunodeficiency virus type I long terminal repeat enhancer region do not exhibit cooperative factor binding. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9406–9410. doi: 10.1073/pnas.85.24.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon T. M., Sage B. A., Seeler B. J., O'Connor J. D. Characterization and partial purification of the Drosophila Kc cell ecdysteroid receptor. J Biol Chem. 1988 Apr 5;263(10):4693–4697. [PubMed] [Google Scholar]
- Maroy P., Dennis R., Beckers C., Sage B. A., O'Connor J. D. Demonstration of an ecdysteroid receptor in a cultured cell line of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6035–6038. doi: 10.1073/pnas.75.12.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micard D., Couderc J. L., Sobrier M. L., Giraud G., Dastugue B. Molecular study of the retrovirus-like transposable element 412, a 20-OH ecdysone responsive repetitive sequence in Drosophila cultured cells. Nucleic Acids Res. 1988 Jan 25;16(2):455–470. doi: 10.1093/nar/16.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley K. L., Toohey M. G., Peterson D. O. Transcriptional repression of a hormone-responsive promoter. Nucleic Acids Res. 1987 Sep 11;15(17):6973–6989. doi: 10.1093/nar/15.17.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Peronnet F., Becker J. L., Becker J., d'Auriol L., Galibert F., Best-Belpomme M. 1731, a new retrotransposon with hormone modulated expression. Nucleic Acids Res. 1986 Nov 25;14(22):9017–9033. doi: 10.1093/nar/14.22.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Westphal H. M., Carlson C., Bosshard H., Beato M. Molecular model of the interaction between the glucocorticoid receptor and the regulatory elements of inducible genes. DNA. 1986 Oct;5(5):383–391. doi: 10.1089/dna.1986.5.383. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Thali M., Müller M. M., DeLorenzi M., Matthias P., Bienz M. Drosophila homoeotic genes encode transcriptional activators similar to mammalian OTF-2. Nature. 1988 Dec 8;336(6199):598–601. doi: 10.1038/336598a0. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]