Abstract
Glucocorticoid regulation of the hypothalamic-pituitary-adrenal (HPA) axis is believed to depend on multiple actions operative within discrete time domains. However, the underlying cellular and molecular mechanism for those glucocorticoid actions remain undetermined. Moreover, there is an absence of in vivo studies examining whether there are multiple glucocorticoid effects on HPA axis related function within an intermediate feedback time-frame (1–3 h after glucocorticoid elevation), and whether those effects depend on de novo protein synthesis. We examined in rats the effects of protein synthesis inhibition on HPA axis response to restraint (15min) after 1 and 3 h phasic corticosterone (CORT) pretreatment. We measured HPA axis hormones (ACTH and CORT) and gene expression in the paraventricular nucleus (c-fos and crh genes), as well as gene expression in the anterior and intermediate pituitary (c-fos and pomc genes). Both CORT pretreatment intervals produced inhibition of stress-induced ACTH secretion, but no inhibition was seen in the presence of protein synthesis inhibition. CORT pretreatment produced inhibitory effects on stress-induced gene expression that varied for each gene depending on the anatomical site, pretreatment time and protein synthesis dependency. Taken together, the ACTH and gene expression patterns support the presence of multiple independent glucocorticoid actions initiated during the intermediate glucocorticoid negative feedback phase. Moreover, we conclude that those effects are exerted predominantly on the intrinsic anatomical elements of the HPA axis, and some of those effects depend on CORT induction of the expression of one or more regulatory gene products.
Keywords: ACTH, CRH, POMC, cfos, HPA-axis
Introduction
Based largely on a number of innovative in vitro and in vivo studies conducted in the 1960’s and 1970’s, the existence and importance of glucocorticoid negative feedback control of the hypothalamic pituitary adrenal (HPA) axis became established. Most of those studies relied on indirect measures of hormone levels, and the in vivo studies were typically conducted under non-physiological conditions (e.g. animals were anesthetized or adrenalectomized). A model of glucocorticoid negative feedback function emerged from those studies that featured the hypothesis that glucocorticoids exert multiple cellular effects that vary in their time course and anatomical site of action. Dallman and Yates (1969) were the first researchers to provide clear demonstration that negative feedback actions of glucocorticoids operate in temporally distinct phases(Dallman and Yates 1969). This emerging model of glucocorticoid negative feedback function was presented in two seminal review papers by Dallman and colleagues (Dallman, et al. 1987; Keller-Wood and Dallman 1984). Those reviews described the empirical basis for three separate phases of glucocorticoid negative feedback that could be distinguished by time course: 1) fast feedback (< 10 min onset with short lasting duration of approximately 5–15 min), 2) intermediate feedback (onset between 0.5–2 h and duration of approximately 6–12 h), and 3) slow feedback (onset after constant glucocorticoid exposure for 12 h or more and duration that may last for days). Those three phases of feedback are also believed to be separable by mechanism of action. Some supporting evidence is that fast feedback does not require altered gene transcription and de novo protein synthesis, whereas intermediate and slow feedback do (Brattin and Portanova 1977; Dayanithi and Antoni 1989; Hinz and Hirschelmann 2000; Keller-Wood and Dallman 1984). Intermediate and slow feedback have been proposed to differ according to whether glucocorticoids inhibit stimulus-induced hormone secretion (intermediate feedback) or both hormone secretion and hormone production (slow feedback) (Keller-Wood and Dallman 1984).
Surprisingly, there has been very little advance in the subsequent 25 years in determination of the underlying molecular mechanisms by which glucocorticoids produce negative feedback regulation of the HPA axis. For example, it remains undetermined whether each of those phases of feedback function are operative at both the hypothalamic paraventricular nucleus (PVN) and anterior pituitary elements of the HPA axis (intrinsic negative feedback). Glucocorticoid negative feedback also appears to depend on glucocorticoid alteration of neural input to the PVN (extrinsic negative feedback), however the time-course for that influence has not been explored. Although the mechanism of slow feedback is believed to be largely due to glucocorticoid inhibition of corticotrophin releasing hormone (CRH) and adrenocorticotropin hormone (ACTH) production, the molecular mechanism(s) of intermediate feedback remain unknown (Keller-Wood and Dallman 1984). Moreover, it remains to be determined whether intermediate feedback depends on a single or multiple glucocorticoid effects. The prospect of multiple glucocorticoid effects within the intermediate feedback time frame has been questioned based on in vitro study of corticotrophs (Dayanithi and Antoni 1989). There is also an absence of in vivo studies that clearly illustrate within the same study and experimental conditions the presence of separate dissociable glucocorticoid intermediate negative feedback effects. In addition, in vivo studies have not examined whether de novo protein synthesis is required for glucocorticoid inhibition within the intermediate feedback time frame. Because, intermediate glucocorticoid inhibitory effects are evident within 1 h after treatment (Ginsberg, et al. 2003; Ginsberg, et al. 2006), establishing the requirement for de novo protein synthesis is warranted. Determination of the mechanisms of glucocorticoid negative feedback has clinical importance. There is considerable evidence for altered negative feedback function associated with a wide range of clinical disorders (e.g. depression, post traumatic stress disorder, type II diabetes, chronic fatigue syndrome, fibromyalgia and chronic facial pain) and associated precursor conditions (e.g. obesity and systemic hypertension) (Bruehl, et al. 2007; Galli, et al. 2009; Jerjes, et al. 2007; Mattsson, et al. 2009; Pariante and Miller 2001; Wingenfeld, et al. 2007; Wirtz, et al. 2007; Yehuda, et al. 2004).
The goal of this study was to determine whether a phasic increase in corticosterone (CORT) comparable to a moderate stressor, would produce over the course of several hours (1–3 h) multiple glucocorticoid negative feedback effects. Specifically, we sought to determine if separate glucocorticoid effects could be identified by anatomical site of action, time-course and dependence on de novo protein synthesis. The key strategy that we adopted for these studies was to not only monitor effects of corticosterone (CORT) on stress-induced HPA axis hormone secretion, but also to monitor CORT effects on stress-induced gene expression. We and others have found that a number of genes are rapidly induced (within 15 min) by acute stress within the cellular elements of the HPA axis (Imaki, et al. 1995; Kovács and Sawchenko 1996; Pace, et al. 2009). Some of those genes (e.g. c-fos) function as immediate early genes in a wide range of neuronal and endocrine cell populations. Other genes (e.g. crh gene and pro-opiomelanocortin, pomc, gene) are rapidly induced within a restricted population of cells. Rapid induction of the crh and pomc genes in the PVN and anterior pituitary, respectively, can be observed when measuring levels of their primary transcript (hnRNA) (Autelitano, et al. 1989; Herman, et al. 1992; O’Connor, et al. 2005).
In this study we examined the effect of CORT pretreatment on stress-induced c-fos, crh and pomc gene expression, under normal conditions and in the presence of systemic protein synthesis blockade. Both crh and pomc genes are believed to be direct targets for glucocorticoid receptor (GR) mediated CORT repression (Dostert and Heinzel 2004). On the other hand, the c-fos gene appears to not be directly repressed by CORT (Ginsberg et al. 2003; Ginsberg et al. 2006), although its expression can be inhibited by long-term glucocorticoid treatment (Umemoto, et al. 1997). In previous studies we have found that 1 h glucocorticoid pretreatment is not sufficient to suppress subsequent restraint-induced c-fos mRNA in the PVN or anterior pituitary (Ginsberg et al. 2003; Ginsberg et al. 2006). It appears then that crh, pomc and c-fos gene expression all reflect aspects of recent stress-induced HPA axis cellular excitation. However, c-fos gene expression may only reflect glucocorticoid actions as they alter stress-induced intercellular and intracellular signals that converge on the c-fos gene promoter. Thus, we may be able to determine the influence of phasic CORT on stress-induced excitatory input to the PVN (i.e. extrinsic feedback) by examining c-fos mRNA. In contrast, the expression of crh and pomc genes appear to integrate information about both the direct presence of activated glucocorticoid receptors (GR) and upstream signaling events.
Materials and Methods
Animals
Young adult male Sprague Dawley rats (270–315 g) were purchased from a commercial vendor (Harlan Labs, Indianapolis, IA, USA) and were given a 2 week period of acclimation to the University of Colorado animal facility and housing conditions before experimental use. The housing room lights were regulated on a 12 hour light/dark cycle (lights on at 0700 h), and room temperature maintained at 22 ± 1 °C. Rats were housed 2 per cage. Food (Teklad Rodent Diet 8640; Harlan Labs) and tap water were available ad libitum. The housing and testing area consisted of a procedure area surrounding 4 separate interior home rooms that were each sound attenuated with independent air supply and exhaust. This arrangement minimized the extent to which rats while in their home room were exposed to any extraneous sounds or odors associated with the various procedures on the test day. Procedures for ethical treatment of animals conformed to the guidelines found in the “Guide for the Care and Use of Laboratory Animals,” (DHHS Publication No. NIH 80–23, revised 1996 ed.) and all procedures were approved by the University of Colorado Institutional Animal Care and Use Committee.
Drugs
Protein synthesis inhibitor cycloheximide (CX; 100 mg/ml) was dissolved in sterile 0.9% saline on the test day. CX was purchased from Sigma-Aldrich (St. Louis, MO, USA). Corticosterone (CORT; 2.5 mg/ml) was dissolved on the test day in a mixture of 10% ethanol, 30% propylene glycol, and 60% saline. CORT was purchased from Steraloids (Newport, RI, USA).
Experimental procedure
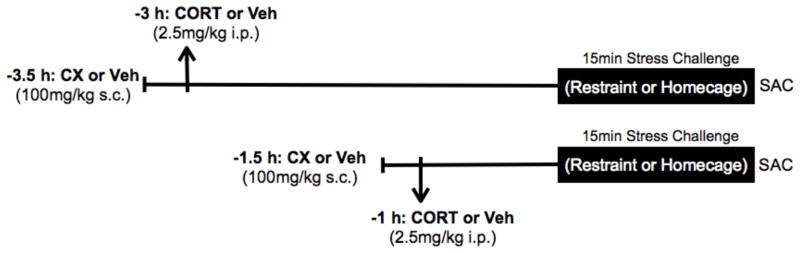
The experiment was comprised of 16 separate treatment groups (2 × 2 × 2 × 2 factorial between subjects design; n = 6; N = 96 rats). The four treatment factors were: 1) protein synthesis inhibition (CX or vehicle), 2) CORT pretreatment (CORT or vehicle), 3) time of CORT pretreatment (1 or 3 h), and 4) stress challenge (restraint or homecage) (Fig. 1). On the test day rats were injected with CX (100 mg/kg, s.c.) or vehicle 30 min before injection with CORT (2.5 mg/kg, i.p.) or vehicle. This dose of CX has been reported to not alter in the PVN stress-induced crh gene expression or activation of the cyclic response element binding protein (CREB), while effectively blocking 90% of stress-induced Fos protein expression (Kovács, et al. 1998). This finding indicates that this systemic dose of CX has effective inhibitory effects within the central nervous system on stress induced protein production within the PVN. This exogenous CORT treatment procedure produces plasma CORT levels in rats that closely match the endogenous CORT levels and time-course associated with a moderate intensity stressor, such as restraint (Pace et al. 2009). After each injection rats were returned to their home cage and home room. Rats were then challenged 1 h or 3 h later with stress (15 min restraint) or remained in their home cage. For restraint challenge, rats were placed in clear plexiglas tubes (23.5 cm in length and 7 cm in diameter; with multiple air holes) with their tails protruding. The size of the tube restricted lateral, forward and backward movement, but did not interfere with breathing. Rats were decapitated immediately after 15 min restraint or at a comparable time after drug pretreatment for the no stress comparison. Stress challenged rats were restrained in an area adjacent to their home room, whereas non-stressed rats remained in their home cage and home room until decapitation. Paired rats were placed into the same stress condition to minimize any disturbance that may be caused by removing a rat from its cage mate. This ensured that blood and tissue samples were collected under the same conditions. Trunk blood collection and brain extraction were rapidly performed after decapitation in an area adjacent to their home room. Test day procedures occurred between 0800 and 1300 h, and time of day was counterbalanced across treatment groups. Rats were habituated to both subcutaneous and intraperitoneal injections by poking rats with the blunt end of a 1 ml syringe (no needle attached) for 2 min over a 2 day period before testing. Experimentation was divided into two separate cohorts of rats, n = 3 per treatment group for each cohort, and data were then pooled for the two cohorts.
Figure 1.
Test Day timeline for CX and CORT pretreatment of stress challenged rats. Rats were pretreated with CORT or CORT-vehicle either 3 h or 1 h before restraint challenge. CX or CX-vehicle treatment was administered 0.5 h before CORT or CORT-vehicle. Restraint challenged rats were killed (SAC) immediately after 15 min restraint. No stress control rats were killed at the comparable time-point. n = 6; N = 96 rats.
ACTH and CORT assays
Trunk blood was collected into EDTA containing vacutainer tubes from Becton-Dickinson (Franklin Lakes, New Jersey, USA)., placed on wet ice and then centrifuged for 15 min (4 °C). Plasma was then aliquoted into storage microfuge tubes and snap-frozen on dry ice. The entire procedure was completed within 45 min after blood collection. ACTH (pg/ml) was determined in duplicate (100 μl plasma) by radioimmunoassay as adapted and described previously from a single-stage assay protocol (Nicholson, et al. 1984; Pace et al. 2009). Radio-labeled 125I ACTH was obtained from DiaSorin (Minneapolis, MN, USA). Primary ACTH antiserum Rb 7 (diluted to a final concentration of 1:30,000) was kindly donated by Dr. William Engeland (University of Minnesota). Sensitivity for the ACTH assay was approximately 15 pg/ml. The intra-assay coefficient of variability was 6% and inter-assay coefficient of variability was 6%.
Measurement of plasma corticosterone (μg/100 ml) was conducted in duplicate using 20μl of plasma with an enzyme immunoassay kit (Assay Design, Ann Arbor, MI, USA) according to manufacturer’s instructions. Sensitivity for the CORT assay was approximately 0.13 μg/100mL. The intra-assay coefficient of variability was 4% and inter-assay coefficient of variability was 6%.
In situ hybridization histochemistry
Brains and pituitaries were rapidly removed and collected after decapitation and frozen in chilled isopentane (at temperature held between −30 and −40 °C), and stored at −80°C. Coronal brain sections (12 μm thick) were collected through the extent of the PVN approximately 1.80mm posterior to bregma (Paxinos, et al. 1980). Horizontal pituitary sections (12 μm thick) were collected from the middle portion of the tissue. All tissue was sectioned on a cryostat (Leica Microsystems, model 1850, Bannockburn, IL, USA). Both brain and pituitary sections were thaw mounted onto poly-L-lysine coated slides and stored at −80 °C. For in situ hybridization, tissue was postfixed in buffer solution containing 4% paraformaldehyde for 1 h, at room temperature and then processed as previously published (Girotti, et al. 2006). Generation of 35S-UTP labeled cRNA probes for crh hnRNA, cfos mRNA, and pomc hnRNA were generated from cDNA subclones in transcription vectors using standard in vitro transcription methodology (Promega, Madison, WI, USA)(Girotti et al. 2006). Rat c-fos cDNA clone was obtained from Dr. T. Curran, St. Jude Children’s Research Hospital, Memphis, TN. A portion of rat crh intron was provided courtesy of Dr. R. T. Thompson, University of Michigan. A portion of rat pomc intron provided courtesy of Dr. Stanley Watson, University of Michigan. The identity of all cloned DNA was verified by DNA sequencing (University of Colorado Molecular, Cellular and Developmental Biology sequencing facility). After the hybridization assay procedure, slides were exposed to x-ray film (Kodak Biomax MR film): 3 weeks for crh hnRNA, 10 days for cfos mRNA and 12 h (for intermediate lobe analysis) or 2 days (for anterior lobe analysis) for pomc hnRNA. Separate in situ hybridization assays were performed for each experimental cohort.
Image Analysis
Semiquantitative analyses were performed on digitized images from x-ray films using the linear range of the gray values obtained from an acquisition system (Northern Light lightbox, model B 95; CCD camera, model XC-77, Sony, Tokyo, Japan; image capture with National Institutes of Health scion Image v1.59 software) as previously described (Campeau, et al. 2002). Signal pixels of the region of interest were defined as those with a gray value of 3.5 standard deviation above the mean gray value of background (lateral hypothalamus or corpus callosum). The PVN region was determined by matching digitized rat hypothalamic structures with rat brain atlas images. The product of the number of pixels and the average pixel values above the set background was then computed to give an integrated mean gray level measure. Between 4–8 brain sections and 6–8 anterior pituitary sections were used for analysis of each rat for all genes. The data were normalized for each cohort by expressing relative integrated gray levels as a percent of the average value for the restraint challenged rats that had received vehicle injections 3 and 3.5 hours earlier.
Statistical Analysis
Statistical analyses were conducted using the SPSS statistical analysis program 10.5 (IL, Chicago, USA) for Macintosh operating system. In order to minimize heteroscedasticity, the data were log transformed for inferential statistical analyses. The data were first analyzed by 4-way analysis of variance (ANOVA) in order to determine whether there was a main effect of CX treatment and interaction between CX and the other treatment factors. Separate 3-way ANOVAs were then conducted in order to assess the effects of stress, corticosterone, and pretreatment time within non-CX (vehicle) or CX treated rats. In cases where there was an overall significant F-test, pairwise comparisons of interest were assessed by Fisher’s least significant difference test (FLSD), alpha = 0.05. Graphed data depict the non-log transformed group means ± standard error of the mean.
Results
Hormone levels
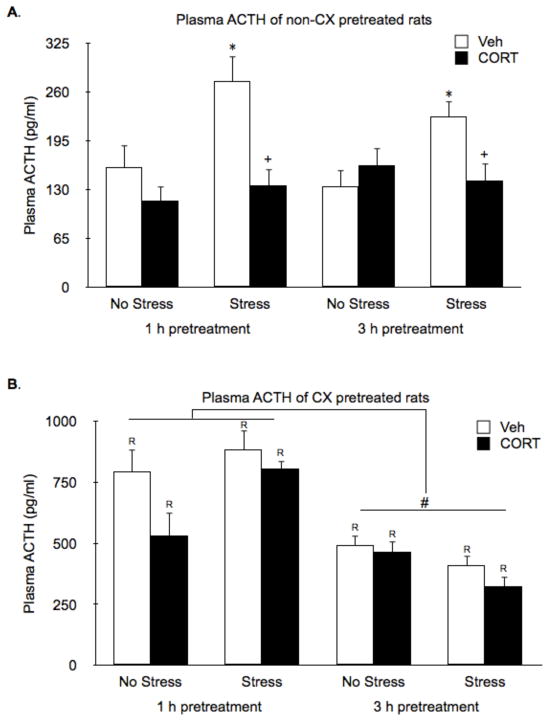
As expected, without CX pretreatment, 15 min of restraint significantly increased plasma ACTH levels (Stress effect: F1,40 = 7.6, P < 0.01), and this was suppressed by both 1 h and 3 h CORT pretreatment (CORT Pretreatment effect: F1,40 = 11.8, P < 0.01; Fig. 2A). Systemic pretreatment with CX induced high secretion of ACTH (CX effect: F1,84 = 327.2, P < 0.01; Fig. 2B) that declined some over the 3.5 h after treatment, but nevertheless remained higher than levels produced by restraint (CX by Time effect: F1,84 = 17.3, P < 0.01). Neither acute stress or CORT pretreatment had an effect on the high ACTH levels present after CX treatment. Thus, CX pretreatment prevented the suppressive effect of 1 h and 3 h CORT pretreatment on plasma ACTH levels.
Figure 2.
Effects of CORT pretreatment on basal and stress-induced ACTH plasma hormone levels of non-CX or CX pretreated rats. (A) In the absence of CX pretreatment, restraint significantly increased plasma ACTH levels and this was suppressed by both 1 h and 3 h CORT pretreatment. (B) Systemic pretreatment with CX induced high secretion of ACTH that declined some over the 3.5 h after treatment. CX prevented the suppressive effect of CORT pretreatment on plasma ACTH levels. *, represents a significant stress effect within same drug condition and pretreatment time point (p < 0.05, FLSD); +, represents a significant CORT effect compared to vehicle treated rats within the same pretreatment time and stress condition (p < 0.05, FLSD); #, represents a significant difference in ACTH secretion between 1 h and 3 h pretreatments within CX treated rats (p < 0.05, FLSD); R, represents a significant difference between CX pretreated rats (B) compared to the corresponding non-CX treated rats (A) (p < 0.05, FLSD).
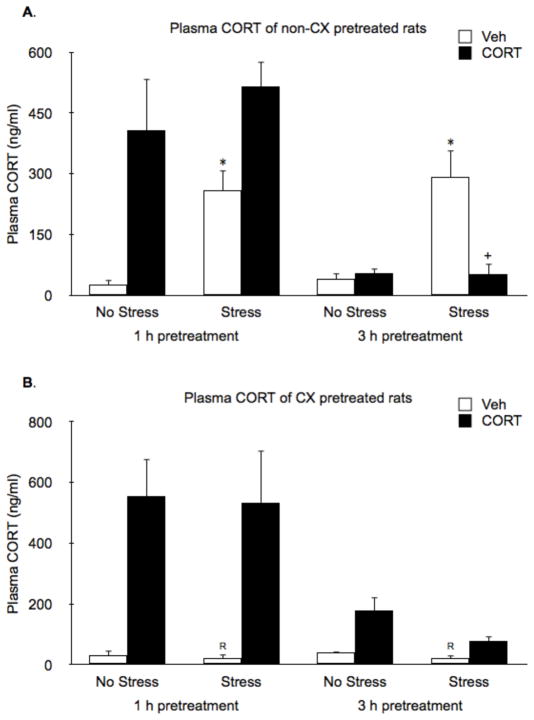
In the absence of CX pretreatment, endogenous CORT levels were also significantly increased by 15 min of restraint (evident in vehicle pretreatment groups; Fig. 3A). CORT pretreatment 3 h prior to restraint suppressed stress induced endogenous CORT production (Stress by CORT interaction: F1,40 = 11.28, P < 0.01, followed by FLSD post-hoc test, P < 0.01). We could not determine whether 1 h CORT pretreatment also suppressed endogenous CORT production because exogenous CORT levels resulting from the 1 h pretreatment injection had not yet cleared from the systemic blood circulation (see high CORT levels in no-stress rats 1 h, but not 3 h, after CORT treatment; Fig. 3A). Strikingly, endogenous CORT (Fig. 3B) production was very low in CX treated rats despite the high levels of ACTH secretion (CX by CORT interaction: F1,84 = 20.7, P < 0.01; Fig. 2B).
Figure 3.
Effects of CORT pretreatment on basal and stress-induced CORT levels of non-CX or CX pretreated rats. (A) In the absence of CX pretreatment, endogenous CORT levels were significantly increased by 15 min of restraint (evident in vehicle pretreatment groups). Exogenous CORT levels cleared from the circulation by 3 h after treatment, at which time it was evident that stress-induced endogenous CORT was suppressed. (B) Systemic pretreatment with CX prevented stress-induced production of endogenous CORT, and appeared to interfer with the rate of clearance of exogenous CORT. *, represents a significant stress effect within same drug condition and pretreatment time point (p < 0.05, FLSD); +, represents a significant CORT effect compared to vehicle treated rats within the same pretreatment time and stress condition (p < 0.05, FLSD); R, represents a significant difference between CX pretreated rats (B) compared to the corresponding non-CX treated rats (A) (p < 0.05, FLSD).
PVN gene expression
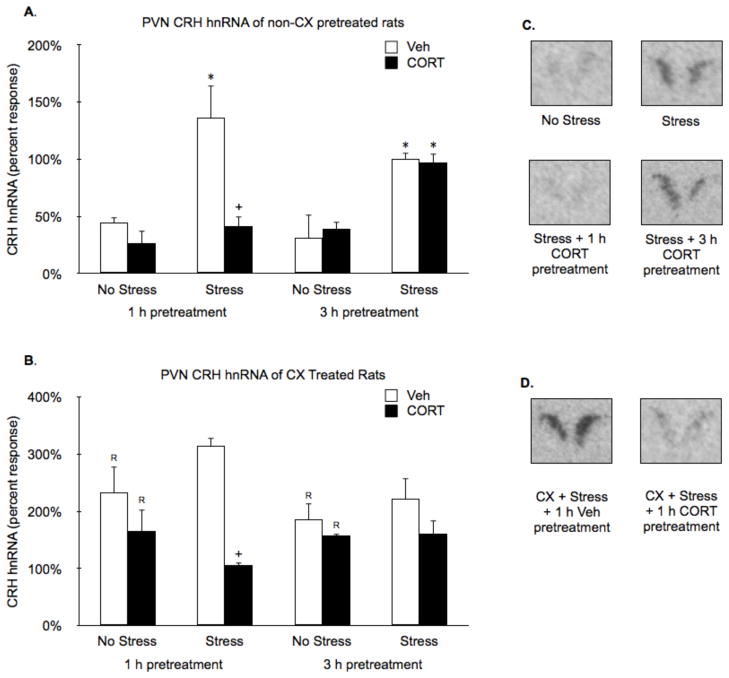
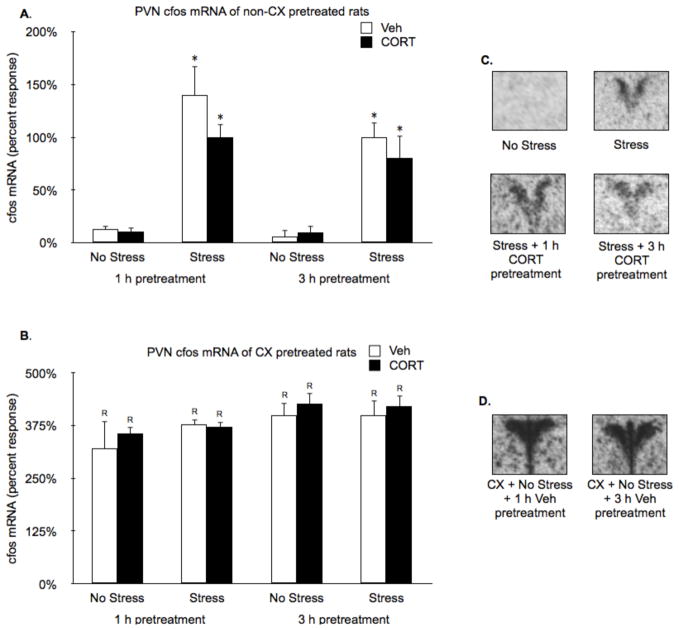
Restraint (15 min) significantly induced PVN crh hnRNA (Stress effect: F1,38 = 35.0, P < 0.01; Fig. 4A and C). Treatment with CORT 1 h, but not 3 h prior to restraint suppressed stress-induced PVN crh hnRNA (Time by CORT interaction: F1,38 = 12.0, P < 0.01; Fig. 4A and C). Systemic pretreatment with CX increased PVN crh hnRNA levels in all groups, irrespective of acute stress or CORT treatment condition (CX effect: F1,71 = 59.2, P < 0.01; Fig. 4B). However, CX pretreatment did not block the suppressive effect of 1 h CORT pretreatment on crh hnRNA levels (CORT effect: F1,38 = 15.2, P < 0.01, followed by FLSD, P < 0.01; Fig. 4B and D).
Figure 4.
Effects of CORT pretreatment on basal and stress-induced PVN crh hnRNA levels of non-CX or CX pretreated rats. (A,B) In the absence of CX pretreatment, restraint significantly induced PVN crh hnRNA and pretreatment with CORT 1 h, but not 3 h, prior to restraint suppressed this effect. (C,D) Systemic pretreatment with CX increased PVN crh hnRNA levels in all groups, but did not block the suppressive effect of 1 h CORT pretreatment on crh hnRNA levels. Panels B and D show representative autoradiographic images of the bilateral PVN portion of coronal brain sections from key comparison conditions depicted in panels A and C, respectively. Values are presented as a percentage of the mean value of the non-CX pretreated stressed 3 h vehicle rats. *, represents a significant stress effect within same drug condition and pretreatment time point (p < 0.05, FLSD); +, represents a significant CORT effect compared to vehicle treated rats within the same pretreatment time and stress condition (p < 0.05, FLSD); R, represents a significant difference between CX pretreated rats (C) compared to the corresponding non-CX treated rats (A) (p < 0.05, FLSD).
Restraint (15 min) also increased PVN c-fos mRNA (Stress effect: F1,37 = 68.7, P < 0.01; Fig. 5A and C). In contrast to crh hnRNA, neither 1 h or 3 h CORT pretreatment significantly suppressed restraint induced PVN c-fos mRNA (Fig. 5A and C). Systemic pretreatment with CX increased PVN c-fos mRNA levels in all groups, irrespective of acute stress or CORT treatment condition (CX effect: F1,76 = 342.9, P < 0.01; Fig. 5B and D).
Figure 5.
Effects of CORT pretreatment on basal and stress-induced PVN cfos mRNA levels of non-CX or CX pretreated rats. (A,B) In the absence of CX pretreatment, restraint induced PVN c-fos mRNA, and neither 1 h or 3 h CORT pretreatment suppressed that induction. (C,D) Systemic pretreatment with CX increased PVN c-fos mRNA levels in all groups, irrespective of acute stress or CORT treatment condition. Panels B and D show representative autoradiographic images of the bilateral PVN portion of coronal brain sections from key comparison conditions depicted in panels A and C, respectively. Values are presented as a percentage of the mean value of the non-CX pretreated stressed 3 h vehicle rats. *, represents a significant stress effect within same drug condition and pretreatment time point (p < 0.05, FLSD); R, represents a significant difference between CX pretreated rats (C) compared to the corresponding non-CX treated rats (A) (p < 0.05, FLSD).
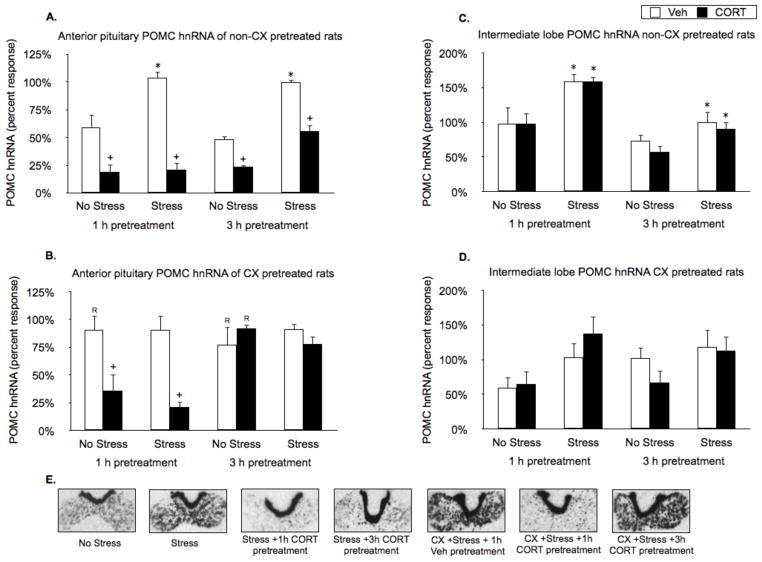
Pituitary pomc gene expression
Restraint (15 min) significantly induced pomc hnRNA in the anterior pituitary (Stress effect: F1,38 = 12.4, P < 0.01; Fig. 6A and E) and in the intermediate lobe of the pituitary (Stress effect: F1,34 = 5.7, P < 0.05; Fig. 6C). CORT pretreatment (1 h and 3 h) suppressed both basal and stress-induced pomc hnRNA levels within the anterior pituitary (CORT effect: F1,38 = 35.9, P < 0.01; Fig. 6A and E), but had no effect on pomc hnRNA levels within the intermediate lobe. Systemic CX pretreatment tended to increase basal, but not stress-induced pomc hnRNA in the anterior pituitary (Fig. 6B and E). CX did not block the suppressive effects of 1 h CORT pretreatment on basal and stress induced anterior pituitary pomc hnRNA expression (FLSD, P < 0.01; Fig. 6B, and E), but CX pretreatment blocked the suppressive effects of 3 h CORT pretreatment on those measures (CORT by Time interaction for CX pretreated rats: F1,38 = 18.4, P > 0.01; Fig. 6B and E). CX pretreatment had no effect on basal or stress-induced pomc hnRNA in the intermediate lobe of the pituitary, figure 6D.
Figure 6.
Effects of CORT pretreatment on basal and stress-induced pituitary anterior lobe and intermediate lobe pomc hnRNA levels of non-CX or CX pretreated rats. (A) In the absence of CX pretreatment, restraint induced pomc hnRNA in the anterior pituitary. CORT pretreatment (1 h and 3 h) suppressed both basal and stress-induced pomc hnRNA levels. (B) Systemic CX pretreatment tended to increase basal, but not stress-induced pomc hnRNA in the anterior pituitary. CX blocked the suppressive effects of 3 h, but not 1 h CORT pretreatment on those measures. (C) In the absence of CX pretreatment, restraint induced pomc hnRNA in the intermediate lobe of the pituitary, and CORT pretreatment had no effect. (D) CX pretreatment had no significant effect on basal or stress-induced pomc hnRNA in the intermediate lobe of the pituitary. Panel E shows representative autoradiographic images of the pituitaries from key comparison conditions depicted in panels A–D. Values are presented as a percentage of the mean value of the non-CX pretreated stressed 3 h vehicle rats. *, represents a significant stress effect within same drug condition and pretreatment time point (p < 0.05, FLSD); +, represents a significant CORT effect compared to vehicle treated rats within the same pretreatment time and stress condition (p < 0.05, FLSD); R, represents a significant difference between CX pretreated rats (B) compared to the corresponding non-CX treated rats (A) (p < 0.05, FLSD).
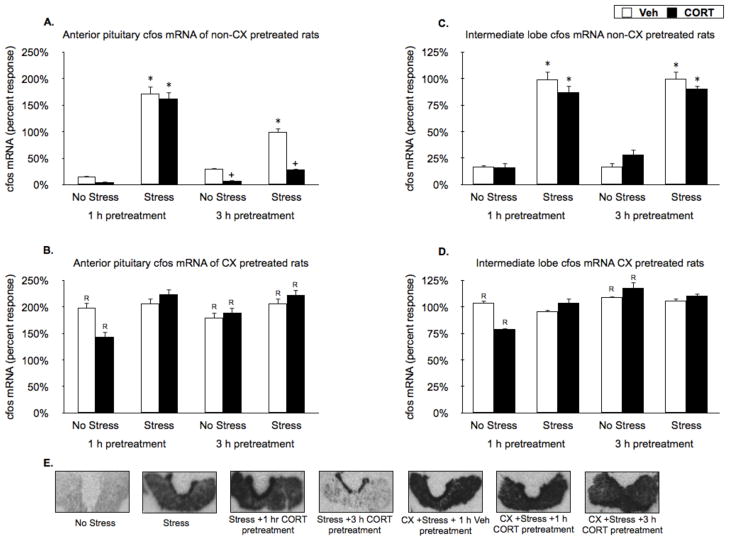
Pituitary c-fos gene expression
Restraint (15 min) produced a large increase in c-fos mRNA within the anterior pituitary (Stress effect: F1,34 = 72.6 P < 0.05; Fig. 7A and E) and in the intermediate lobe of the pituitary (Stress effect: F1,38 = 38.8, P < 0.01). Pretreatment with CORT 3 h, but not 1 h, prior to restraint suppressed stress-induced anterior pituitary c-fos mRNA levels (CORT by Time interaction: F1,34 = 8.4, P > 0.05; Fig. 7A and E), but had no effect on c-fos mRNA levels within the intermediate lobe (Fig. 7C). Systemic pretreatment with CX moderately increased anterior pituitary c-fos mRNA levels regardless of stress and CORT treatment (CX effect: F1,70 = 222, P > 0.05, Fig. 7B and E). CX pretreatment blocked the suppressive effect of 3 h CORT pretreatment on pituitary c-fos mRNA levels (CORT by CX interaction: F1,70 = 8.4, P > 0.05 Fig. 7B and E). CX pretreatment increased basal but not stress-induced c-fos mRNA in the intermediate lobe of the pituitary (Stress by CX interaction: F1,70 = 72.8; Fig. 7D).
Figure 7.
Effects of CORT pretreatment on basal and stress-induced pituitary anterior lobe and intermediate lobe cfos mRNA levels of non-CX or CX pretreated rats. (A) In the absence of CX pretreatment, restraint produced a large increase in c-fos mRNA within the anterior pituitary. Pretreatment with CORT 3 h, but not 1 h, suppressed stress-induced anterior pituitary c-fos mRNA levels. (B) Systemic pretreatment with CX increased basal anterior pituitary c-fos mRNA levels and blocked the 3 h CORT pretreatment effect. (C) In the absence of CX pretreatment, restraint induced c-fos mRNA in the intermediate lobe of the pituitary, and CORT pretreatment had no effect. (D) CX pretreatment increased basal but not stress-induced c-fos mRNA in the intermediate lobe of the pituitary. Panel E shows representative autoradiographic images of the pituitaries from key comparison conditions depicted in panels A–D. Values are presented as a percentage of the mean value of the non-CX pretreated stressed 3 h vehicle rats. *, represents a significant stress effect within same drug condition and pretreatment time point (p < 0.05, FLSD); +, represents a significant CORT effect compared to vehicle treated rats within the same pretreatment time and stress condition (p < 0.05, FLSD); R, represents a significant difference between CX pretreated rats (B) compared to the corresponding non-CX treated rats (A) (p < 0.05, FLSD).
Discussion
We examined the HPA axis negative feedback effects evident 1 h or 3 h after a phasic increase in systemic CORT. Both 1 h and 3 h CORT pretreatment completely suppressed the ACTH secretion response to subsequent restraint challenge, and in both cases there was no suppressive effect in the presence of acute protein synthesis inhibition. CORT also suppressed subsequent stress-induced immediate early gene expression, but the pattern of suppression varied for each gene (crh, pomc or c-fos) depending on anatomical location (PVN, anterior pituitary, intermediate pituitary), time interval (1 h or 3 h) and protein synthesis inhibition.
Stress-induced crh and pomc gene expression in the PVN and anterior pituitary, respectively, was completely suppressed by 1 h CORT pretreatment. In contrast to the suppressive effect of 1 h CORT pretreatment on ACTH secretion, suppression of crh and pomc gene expression occurred even in the presence of protein synthesis inhibition. Our results support other studies indicating that those two genes are subject to direct inhibitory control by glucocorticoids, probably acting through GR (Drouin, et al. 1993; Erdmann, et al. 2008; Guardiola-Diaz, et al. 1996; Reichardt, et al. 1998a; Reichardt, et al. 1998b; Reichardt and Schütz 1998). Glucocorticoid-dependent inhibition of crh and pomc gene expression likely involves both direct gene repression via activated GR binding of DNA (negative glucocorticoid response element) and indirect gene repression via activated GR binding with stimulatory transcription factors (Dostert and Heinzel 2004). The absence of an inhibitory effect of CORT on pomc gene expression in the intermediate lobe of the pituitary can be explained by the fact that in adult rats melanotrophs express very little, if any, GR (Ginsberg et al. 2006; McGimsey, et al. 1991; Ozawa, et al. 1999).
The failure of 3 h CORT pretreatment to suppress subsequent stress-induced PVN crh gene expression is a novel finding and may be accounted for by the complete clearance of exogenous CORT that we observed by that point in time. Thus, there likely was no longer elevated GR activation 3 h after CORT treatment (Freeman and Yamamoto 2001; Meaney, et al. 1988). A down regulation of GR expression in the brain 3 h after CORT injection is not a likely alternative explanation for the lack of an inhibitory effect. A previous study found an absence of changes in GR mRNA or protein expression within this time-frame after injection with a higher dose of CORT (Herman and Spencer 1998). The apparent tight coupling of crh gene expression to activated GR levels may allow for glucocorticoid regulation of PVN crh gene expression to be especially responsive to pulsatile secretion of CORT (Lightman, et al. 2008; Stavreva, et al. 2009). The residual effects that we observed for some other measures 3 h after CORT pretreatment may be a result of CORT alteration of cellular protein levels that was maintained for several hours. In keeping with that notion, all of the inhibitory effects that we observed after 3 h CORT pretreatment were absent in the presence of protein synthesis inhibition. Interestingly, it appears that there was a dual regulatory influence of CORT on corticotroph pomc gene expression—an immediate protein synthesis independent repressive effect that was evident within 1 h, and a delayed protein synthesis dependent inhibitory effect that was evident by 3 h.
In contrast to CORT regulation of crh and pomc gene expression, stress-induced c-fos gene expression in the PVN was not suppressed by 1 h CORT pretreatment. That result replicates our previous findings (Ginsberg et al. 2003; Ginsberg et al. 2006) and indicates that CORT did not inhibit the intercellular and intracellular signals that led to stress-induced c-fos gene induction in the PVN. The c-fos gene is induced by a wide range of intracellular signal transduction pathways (Kovács 1998; Nestler, et al. 2001). Consequently, the absence of an effect of 1 h or 3 h CORT pretreatment on stress-induced c-fos gene expression suggests that there was a corresponding absence of CORT inhibition of the stress-induced stimulatory input to the PVN (i.e. absence of extrinsic negative feedback). Both the hippocampus/ventral subiculum and prefrontal cortex are brain regions that have been implicated as important sites of extrinsic glucocorticoid negative feedback (Feldman and Weidenfeld 1993; Herman and Mueller 2006; Jacobson and Sapolsky 1991). However, it remains to be determined whether manipulation of CORT levels within those brain regions has a short-term (within 3 h) effect on HPA axis activity.
We found in this study that 1 h CORT pretreatment also failed to suppress overall stress-induced c-fos mRNA in the anterior pituitary. A similar inference as discussed above for c-fos gene expression in the PVN could be applied to the anterior pituitary. Thus, the data may indicate that 1 h CORT pretreatment did not inhibit the release of stress-induced ACTH secretagogues. However, we have recently found that microinfusion of CORT directly in the PVN is sufficient to inhibit the ACTH and anterior pituitary pomc hnRNA response to restraint challenge when administered 1 h later (Weiser and Spencer 2009). Most studies have determined that corticotrophs comprise less than 10% of the endocrine cells in the rat anterior pituitary (Levy 2002). Consequently, if there was a selective CORT dependent inhibition of c-fos mRNA within corticotrophs, that inhibition may have been masked by the absence of a suppressive effect on stress-induced c-fos expression in other cell types. Lactotrophs are more abundant than corticotrophs and are also stress reactive in the rat (Freeman, et al. 2000; Takigami, et al. 2008). In contrast to 1 h CORT pretreatment, we found that 3 h CORT pretreatment suppressed overall stress-induced c-fos gene expression in the anterior pituitary, possibly indicating a delayed CORT effect that influenced stress induced activation of lactotrophs and corticotrophs. There is evidence for glucocorticoid suppression of stress-induced prolactin in the rat, although the time-course of that effect has not been determined (Taylor, et al. 1995).
It is noteworthy that stress also led to a rapid increase in pomc and c-fos gene expression in the intermediate lobe of the pituitary, which primarily contains melanotrophs. However, neither gene induction was affected by 1 h or 3 h CORT pretreatment. As noted above, a direct inhibitory effect of CORT on gene expression in the intermediate lobe of the pituitary is not to be expected due to the absence of GR expression. However, our data also suggest that the stress responsive intercellular signals impinging on melanotrophs were not sensitive to CORT pretreatment. The dissociation in CORT responsiveness between cells of the anterior and intermediate pituitary implies either that the observed CORT effects depended on a direct action of CORT on GR expressing cells (anterior pituitary) or depended on an indirect effect on stress-altered secretagogues that target the anterior, but not intermediate, lobe of the pituitary. The leading candidate secretagogues for melanotrophs are CRH and decreased dopamine (Lookingland, et al. 1991; Proulx-Ferland, et al. 1982). Both melanotrophs and lactotrophs are under tonic inhibitory control by dopamine, but there appear to be separate populations of dopamine neurons that regulate those two cell populations, allowing for the possibility of differential glucocorticoid dependence (Goudreau, et al. 1992).
It should be noted when evaluating the results of this study that in contrast to a previous report using a similar systemic protein synthesis inhibition procedure (Kovács et al. 1998), we saw signs of strong generalized activation of the HPA axis (gene expression and hormone levels) after CX treatment. That activation may be due to systemic distress associated with the physiological consequences of long-term protein synthesis inhibition, as described by others(Davis and Squire 1984; Rudy 2008). A key procedural difference between the study by Kovacs and colleagues (Kovács et al. 1998) and our study was the duration of time after CX pretreatment when measurements were made, i.e. 30 min and 1.5–3.5 h, respectively. The relatively long CX pretreatment time in our study (1.5 h or 3.5 h) probably allowed for the toxic effects of CX to fully manifest resulting in the marked HPA axis increases that we report. Plasma ACTH and gene expression in the PVN (crh hnRNA and c-fos mRNA) were much higher after protein synthesis inhibition than those seen after acute restraint. On the other hand, pomc and c-fos gene expression in the anterior pituitary exhibited only modest increases after protein synthesis inhibition, and there was no overall change in gene expression in the intermediate lobe. It appears then that the HPA axis related excitatory effects present after protein synthesis inhibition were primarily due to systemic stress rather than a generalized direct CX effects on cellular function and gene expression. Examination of CORT levels 1.5 h after protein synthesis inhibition also indicates that there was almost complete suppression of endogenous CORT production. Previous studies found that steroidogenesis is tightly coupled to de novo protein synthesis (Crivello and Jefcoate 1978; Davis and Garren 1968).
Although the apparent generalized systemic stress evident after CX treatment limits the extent to which we can draw firm conclusions about the protein synthesis dependent nature of each of the CORT effects that we saw in non-CX treated rats, it does provide some provisional information. Taken together, the results support that intermediate glucocorticoid negative feedback of HPA axis hormone secretion is dependent on glucocorticoid alteration of gene expression. It is not likely, however, that suppression of PVN crh and anterior pituitary pomc gene expression accounts for the decreased stress-induced ACTH secretion present 1 h or 3 h after CORT pretreatment (Watts 2005). Decreased ACTH content in corticotrophs is not observed until more than 6 h after continuous glucocorticoid treatment (Phillips and Tashjian 1982; Shipston 1995a; Shipston 1995b). Consequently, the inhibitory effects of CORT on stress-induced HPA axis hormone secretion within the 3 h time frame appears to be due to induction of a gene and protein product that interferes with the coupling of cellular excitation with hormone secretion. Although 1 h is a fairly short interval of time to see a protein synthesis dependent functional effect of glucocorticoids, other studies have also reported a similar time frame for protein synthesis-dependent glucocorticoid inhibitory effects on corticotroph function in vitro (Clark and Kemppainen 1994; Dayanithi and Antoni 1989; Tierney, et al. 2003; Woods, et al. 1992). The fact that we saw additional protein synthesis dependent inhibitory effects present at 3 h that were not present after 1 h suggests that there may be more than one target gene and cellular mechanism elicited by CORT over that period of time. There are a number of genes that are regulated by glucocorticoids and some of them may participate in glucocorticoid negative feedback such as the calmodulin gene and various phosphatase and potassium-channel genes (Engelbrecht, et al. 2003; Kassel, et al. 2001; Shipston and Antoni 1992a; Shipston and Antoni 1992b; Yamashita, et al. 2009). Shipston and colleagues have provided some evidence in corticotrophs for rapid glucocorticoid suppression of stimulated ACTH secretion to depend on induction of a protein that leads to net activation of large conductance Ca++ and voltage dependent K+ channels (BK channels), thereby reducing corticotroph membrane depolarization (Shipston, et al. 1996; Tian, et al. 2001; Tian, et al. 1998). However, the identity of such a glucocorticoid induced protein remains elusive (Attarzadeh-Yazdi, et al. 2008).
In summary, our study indicates that within the first 3 h after a phasic increase in CORT, CORT produces a protein synthesis dependent inhibitory effect on subsequent HPA axis stress-reactivity that appears to be exerted predominantly on the intrinsic anatomical elements of the HPA axis. In addition, over the course of 3 h, CORT produces multiple molecular effects within the intrinsic HPA axis elements that have different onsets and durations of action. Some of those effects contribute directly to decreased stress-induced HPA axis hormone secretion during that 3 h period. Other effects, such as inhibition of crh and pomc gene expression, may have their primary impact on HPA axis function at later points in time.
Acknowledgments
We thank Erin Jarvis for help with collection of tissue and Vanessa Thompson for help with processing of samples and tissue. This work was supported by National Institute of Mental Health (NIH) grant MH75968.
Abbreviations
- HPA axis
hypothalamic-pituitary-adrenal axis
- PVN
paraventricular nucleus of the hypothalamus
- CRH
corticotropin releasing hormone
- POMC
proopiomelanocortin
- ACTH
adrenocorticotropic hormone
- CX
cycloheximide
- CORT
corticosterone
- FLSD
Fisher’s Least Significant Difference Test
References
- Attarzadeh-Yazdi G, Shipston MJ, Antoni FA. Dex-ras1 and serum- and glucocorticoid-inducible protein kinase 1: regulation of expression by dexamethasone in HEK293 cells. Neurochem Res. 2008;33:609–613. doi: 10.1007/s11064-007-9516-5. [DOI] [PubMed] [Google Scholar]
- Autelitano DJ, Blum M, Roberts JL. Changes in rat pituitary nuclear and cytoplasmic pro-opiomelanocortin RNAs associated with adrenalectomy and glucocorticoid replacement. Mol Cell Endocrinol. 1989;66:171–180. doi: 10.1016/0303-7207(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Brattin WJ, Portanova R. Corticosterone induction of pituitary hyporesponsiveness to CRF: divergent effects of puromycin and cycloheximide. Mol Cell Endocrinol. 1977;7:221–231. doi: 10.1016/0303-7207(77)90054-5. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, Arentoft A, Wolf OT, Convit A. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:2439–2445. doi: 10.1210/jc.2006-2540. [DOI] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson S. c- fos mRNA Induction in Acute and Chronic Audiogenic Stress: Possible Role of the Orbitofrontal Cortex in Habituation. Stress: The International Journal on the Biology of Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TP, Kemppainen RJ. Glucocorticoid negative feedback in sheep corticotrophs: a comparison with AtT-20 corticotroph tumor cells. Am J Physiol. 1994;267:R463–469. doi: 10.1152/ajpregu.1994.267.2.R463. [DOI] [PubMed] [Google Scholar]
- Crivello JF, Jefcoate CR. Mechanisms of corticotropin action in rat adrenal cells. I. The effects of inhibitors of protein synthesis and of microfilament formation on corticosterone synthesis. Biochim Biophys Acta. 1978;542:315–329. doi: 10.1016/0304-4165(78)90027-2. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Yates FE. Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann N Y Acad Sci. 1969;156:696–721. doi: 10.1111/j.1749-6632.1969.tb14008.x. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychological bulletin. 1984;96:518–559. [PubMed] [Google Scholar]
- Davis WW, Garren LD. On the mechanism of action of adrenocorticotropic hormone. The inhibitory site of cycloheximide in the pathway of steroid biosynthesis. J Biol Chem. 1968;243:5153–5157. [PubMed] [Google Scholar]
- Dayanithi G, Antoni F. Rapid as well as delayed inhibitory effects of glucocorticoid hormones on pituitary adrenocorticotropic hormone release are mediated by type II glucocorticoid receptors and require newly synthesized messenger ribonucleic acid as well as protein. Endocrinology. 1989;125:308–313. doi: 10.1210/endo-125-1-308. [DOI] [PubMed] [Google Scholar]
- Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10:2807–2816. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- Drouin J, Sun YL, Chamberland M, Gauthier Y, De Lean A, Nemer M, Schmidt TJ. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. Embo J. 1993;12:145–156. doi: 10.1002/j.1460-2075.1993.tb05640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht Y, de Wet H, Horsch K, Langeveldt CR, Hough FS, Hulley PA. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144:412–422. doi: 10.1210/en.2002-220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann G, Berger S, Schutz G. Genetic Dissection of Glucocorticoid Receptor Function in the Mouse Brain. Journal of Neuroendocrinology. 2008 doi: 10.1111/j.1365-2826.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. The dorsal hippocampus modifies the negative feedback effect of glucocorticoids on the adrenocortical and median eminence CRF-41 responses to photic stimulation. Brain Research. 1993;614:227–232. doi: 10.1016/0006-8993(93)91039-u. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Continuous recycling: a mechanism for modulatory signal transduction. Trends Biochem Sci. 2001;26:285–290. doi: 10.1016/s0968-0004(01)01834-5. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Galli U, Gaab J, Ettlin DA, Ruggia F, Ehlert U, Palla S. Enhanced negative feedback sensitivity of the hypothalamus-pituitary-adrenal axis in chronic myogenous facial pain. Eur J Pain. 2009;13:600–605. doi: 10.1016/j.ejpain.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. Journal of Neuroendocrinology. 2003;15:1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg AB, Frank MG, Francis AB, Rubin BA, O’Connor KA, Spencer RL. Specific and time-dependent effects of glucocorticoid receptor agonist RU28362 on stress-induced pro-opiomelanocortin hnRNA, c-fos mRNA and zif268 mRNA in the pituitary. Journal of Neuroendocrinology. 2006;18:129–138. doi: 10.1111/j.1365-2826.2005.01396.x. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TWW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Goudreau JL, Lindley SE, Lookingland KJ, Moore KE. Evidence that hypothalamic periventricular dopamine neurons innervate the intermediate lobe of the rat pituitary. Neuroendocrinology. 1992;56:100–105. doi: 10.1159/000126214. [DOI] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Kolinske JS, Gates LH, Seasholtz AF. Negative glucorticoid regulation of cyclic adenosine 3′, 5′-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Mol Endocrinol. 1996;10:317–329. doi: 10.1210/mend.10.3.8833660. [DOI] [PubMed] [Google Scholar]
- Herman J, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein. Journal of Neuroscience. 1998 doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behavioural brain research. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest. 1995;96:231–238. doi: 10.1172/JCI118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenal axis. Endocrine Reviews. 1991:118–1134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jerjes WK, Taylor NF, Wood PJ, Cleare AJ. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. 2007;32:192–198. doi: 10.1016/j.psyneuen.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Kassel O, Sancono A, Krätzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20:7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M, Dallman M. Corticosteroid inhibition of ACTH secretion. Endocrine Reviews. 1984 doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kovács K. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Kovács KJ, Arias C, Sawchenko PE. Protein synthesis blockade differentially affects the stress-induced transcriptional activation of neuropeptide genes in parvocellular neurosecretory neurons. Brain Res Mol Brain Res. 1998;54:85–91. doi: 10.1016/s0169-328x(97)00324-0. [DOI] [PubMed] [Google Scholar]
- Kovács KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. Physiological implications of pituitary trophic activity. J Endocrinol. 2002;174:147–155. doi: 10.1677/joe.0.1740147. [DOI] [PubMed] [Google Scholar]
- Lightman S, Wiles C, Atkinson H. The significance of glucocorticoid pulsatility. European Journal of Pharmacology. 2008 doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- Lookingland KJ, Gunnet JW, Moore KE. Stress-induced secretion of alpha-melanocyte-stimulating hormone is accompanied by a decrease in the activity of tuberohypophysial dopaminergic neurons. Neuroendocrinology. 1991;53:91–96. doi: 10.1159/000125703. [DOI] [PubMed] [Google Scholar]
- Mattsson C, Reynolds RM, Simonyte K, Olsson T, Walker BR. Combined receptor antagonist stimulation of the hypothalamic-pituitary-adrenal axis test identifies impaired negative feedback sensitivity to cortisol in obese men. J Clin Endocrinol Metab. 2009;94:1347–1352. doi: 10.1210/jc.2008-2054. [DOI] [PubMed] [Google Scholar]
- McGimsey WC, Cidlowski JA, Stumpf WE, Sar M. Immunocytochemical localization of the glucocorticoid receptor in rat brain, pituitary, liver, and thymus with two new polyclonal antipeptide antibodies. Endocrinology. 1991;129:3064–3072. doi: 10.1210/endo-129-6-3064. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Aitken DH, Bhatnagar S. Stress-induced occupancy and translocation of hippocampal glucocorticoid receptors. Brain research. 1988;445:198–203. doi: 10.1016/0006-8993(88)91093-1. [DOI] [PubMed] [Google Scholar]
- Nestler E, Hyman S, Malenka R. Molecular Neuropharmacology. New York: The McGraw-Hill Companies, Inc; 2001. [Google Scholar]
- Nicholson W, Davis D, Sherrell B. Rapid radioimmunoassay for corticotropin in unextracted human plasma. Clinical Chemistry. 1984 [PubMed] [Google Scholar]
- O’Connor K, Ginsberg A, Maksimova E. Stress-induced sensitization of the hypothalamic-pituitary adrenal axis is associated with alterations of hypothalamic and pituitary gene expression. 2005. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Ito T, Ochiai I, Kawata M. Cellular localization and distribution of glucocorticoid receptor immunoreactivity and the expression of glucocorticoid receptor messenger RNA in rat pituitary gland. A combined double immunohistochemistry study and in situ hybridization histochemical analysis. Cell Tissue Res. 1999;295:207–214. doi: 10.1007/s004410051226. [DOI] [PubMed] [Google Scholar]
- Pace TWW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress. 2009;12:400–411. doi: 10.1080/10253890802530730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Phillips M, Tashjian A. Characterization of an early inhibitory effect of glucocorticoids on stimulated adrenocorticotropin and endorphin release from a clonal strain of mouse pituitary cells. Endocrinology. 1982;110:892–900. doi: 10.1210/endo-110-3-892. [DOI] [PubMed] [Google Scholar]
- Proulx-Ferland L, Labrie F, Dumont D, Cote J, Coy DH, Sveiraf J. Corticotropin-releasing factor stimulates secretion of melanocyte-stimulating hormone from the rat pituitary. Science. 1982;217:62–63. doi: 10.1126/science.6283632. [DOI] [PubMed] [Google Scholar]
- Reichardt H, Kaestner K, Tuckermann J, Kretz O. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998a doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Wessely O, Gass P, Schmid W, Schütz G. Analysis of glucocorticoid signalling by gene targeting. J Steroid Biochem Mol Biol. 1998b;65:111–115. doi: 10.1016/s0960-0760(97)00181-7. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Schütz G. Glucocorticoid signalling--multiple variations of a common theme. Mol Cell Endocrinol. 1998;146:1–6. doi: 10.1016/s0303-7207(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Rudy J. Is there a baby in the bathwater? Maybe: some methodological issues for the de novo protein synthesis hypothesis. Neurobiology of Learning and Memory. 2008 doi: 10.1016/j.nlm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Shipston M. Mechanism (s) of early glucocorticoid inhibition of adrenocorticotropin secretion from anterior. Trends in Endocrinology & Metabolism. 1995a doi: 10.1016/1043-2760(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Shipston M, Antoni F. Inactivation of early glucocorticoid feedback by corticotropin-releasing factor in vitro. Endocrinology. 1992a doi: 10.1210/endo.130.4.1312450. [DOI] [PubMed] [Google Scholar]
- Shipston MJ. Mechanism(s) of early glucocorticoid inhibition of adrenocorticotropin secretion from anterior pituitary corticotropes. Trends Endocrinol Metab. 1995b;6:261–266. doi: 10.1016/1043-2760(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Shipston MJ, Antoni FA. Early glucocorticoid induction of calmodulin and its suppression by corticotropin-releasing factor in pituitary corticotrope tumor (AtT20) cells. Biochem Biophys Res Commun. 1992b;189:1382–1388. doi: 10.1016/0006-291x(92)90227-c. [DOI] [PubMed] [Google Scholar]
- Shipston MJ, Kelly JS, Antoni FA. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J Biol Chem. 1996;271:9197–9200. doi: 10.1074/jbc.271.16.9197. [DOI] [PubMed] [Google Scholar]
- Stavreva D, Wiench M, John S, Conway-Campbell B, McKenna M, Pooley J, Johnson T, Voss T, Lightman S, Hager G. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009 doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigami S, Fujiwara K, Kikuchi M, Yashiro T. In vivo correlation between c-Fos expression and corticotroph stimulation by adrenocorticotrophic hormone secretagogues in rat anterior pituitary gland. Cell Tissue Res. 2008;331:589–594. doi: 10.1007/s00441-007-0547-7. [DOI] [PubMed] [Google Scholar]
- Taylor AD, Cowell AM, Flower RJ, Buckingham JC. Dexamethasone suppresses the release of prolactin from the rat anterior pituitary gland by lipocortin 1 dependent and independent mechanisms. Neuroendocrinology. 1995;62:530–542. doi: 10.1159/000127044. [DOI] [PubMed] [Google Scholar]
- Tian L, Hammond MS, Florance H, Antoni FA, Shipston MJ. Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J Physiol (Lond) 2001;537:57–68. doi: 10.1111/j.1469-7793.2001.0057k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Knaus HG, Shipston MJ. Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. J Biol Chem. 1998;273:13531–13536. doi: 10.1074/jbc.273.22.13531. [DOI] [PubMed] [Google Scholar]
- Tierney T, Christian HC, Morris JF, Solito E, Buckingham JC. Evidence from studies on co-cultures of TtT/GF and AtT20 cells that Annexin 1 acts as a paracrine or juxtacrine mediator of the early inhibitory effects of glucocorticoids on ACTH release. J Neuroendocrinol. 2003;15:1134–1143. doi: 10.1111/j.1365-2826.2003.01111.x. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Kawai Y, Ueyama T, Senba E. Chronic glucocorticoid administration as well as repeated stress affects the subsequent acute immobilization stress-induced expression of immediate early genes but not that of NGFI-A. Neuroscience. 1997;80:763–773. doi: 10.1016/s0306-4522(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Watts A. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Frontiers in Neuroendocrinology. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Weiser M, Spencer RL. Regulation of stress-induced c-fos and corticotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus (PVN) by an acute PVN corticosterone microinjection. Society for Neuroscience Annual Meeting; Chiccago. 2009. [Google Scholar]
- Wingenfeld K, Wagner D, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim C. The low-dose dexamethasone suppression test in fibromyalgia. J Psychosom Res. 2007;62:85–91. doi: 10.1016/j.jpsychores.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, von Känel R, Emini L, Ruedisueli K, Groessbauer S, Maercker A, Ehlert U. Evidence for altered hypothalamus-pituitary-adrenal axis functioning in systemic hypertension: blunted cortisol response to awakening and lower negative feedback sensitivity. Psychoneuroendocrinology. 2007;32:430–436. doi: 10.1016/j.psyneuen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Woods MD, Shipston MJ, Mullens EL, Antoni FA. Pituitary corticotrope tumor (AtT20) cells as a model system for the study of early inhibition by glucocorticoids. Endocrinology. 1992;131:2873–2880. doi: 10.1210/endo.131.6.1332850. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Oki Y, Iino K, Hayashi C, Matsushita F, Faje A, Nakamura H. The role of ether-a-go-go-related gene K(+) channels in glucocorticoid inhibition of adrenocorticotropin release by rat pituitary cells. Regul Pept. 2009;152:73–78. doi: 10.1016/j.regpep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM. The ACTH response to dexamethasone in PTSD. The American journal of psychiatry. 2004;161:1397–1403. doi: 10.1176/appi.ajp.161.8.1397. [DOI] [PubMed] [Google Scholar]