Abstract
Objectives
Meningoencephalitis caused by Cryptococcus gattii is associated with significant morbidity and the need for aggressive therapy, and often necessitates neurosurgical intervention. We adapted a previously described murine model of cryptococcal meningoencephalitis due to Cryptococcus neoformans to that caused by C. gattii.
Methods
Mice were inoculated intracranially with either C. gattii (genotype VGIIa) or C. neoformans. In virulence studies, different C. gattii infecting inocula were compared with a fixed inoculum of C. neoformans, and differences were assessed by survival, brain tissue fungal burden, serum antigen titres and histopathological changes within brain tissue. For treatment, fluconazole or posaconazole (10 mg/kg orally twice daily) was initiated 24 h post-inoculation.
Results
C. gattii was more virulent than C. neoformans, as evident by shorter median survival, earlier histopathological changes and higher serum antigen titres. However, no differences in fungal burden or dissemination to other organs were observed among the various groups. In treatment studies, both fluconazole and posaconazole improved the median survival of mice infected with either species. However, neither regimen improved the percentage of animals surviving to the predetermined study endpoint.
Conclusions
These results demonstrate the virulence of C. gattii meningoencephalitis and the potential of this model for the assessment of new treatment strategies.
Keywords: cryptococcal meningoencephalitis, C. gattii, Cryptococcus neoformans, posaconazole, fluconazole
Introduction
Cryptococcosis is an invasive fungal infection most commonly caused by one of two species of encapsulated yeast: Cryptococcus neoformans and Cryptococcus gattii. C. gattii has emerged as an important cause of infection in both immunocompetent and immunocompromised hosts, as illustrated by the recent outbreak on Vancouver Island, BC, Canada, and the north-west USA.1 These recent reports of infections caused by C. gattii illustrate significant differences between C. gattii and C. neoformans in their epidemiology, susceptibility patterns, chronicity of infection, and frequency of neurosurgical intervention the last of which is higher for C. gattii than C. neoformans, which may be due to the propensity of C. gattii to form cryptococcomas.2,3 These species differences have prompted the search for antifungal agents with greater activity against C. gattii.
Prior murine studies of C. gattii have focused on infection occurring via the intraperitoneal,4 intranasal5 or intratracheal route.6 These models are effective for the evaluation of in vivo drug response to pulmonary infection and the immunological response, but they have several limitations in the evaluation of meningoencephalitis due to cryptococcosis. Pulmonary infection in murine models will disseminate to the CNS, yet human infection is infrequently synchronous. Pulmonary infection in humans has typically resolved at the onset of meningitis;7 thus, an effective evaluation of the response of meningitis to therapy may be obscured by concurrent pulmonary infection in murine models.
Our objectives were to adapt an established murine model of cryptococcal meningoencephalitis to infections caused by C. gattii, to assess differences between invasive diseases caused by C. gattii and C. neoformans, and to determine whether infections caused by C. gattii in this model would respond to antifungal therapy.
Materials and methods
Isolates
Two clinical isolates [C. gattii 06-3908 (serotype C, genotype VGIIa) and C. neoformans USC1597 (serotype A, genotype VNI)] were obtained from the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio. Our group has previously found the MICs for both isolates to be equivalent for fluconazole (2 mg/L) and posaconazole (0.25 mg/L) and the isolates were chosen for this reason.8 Isolates were serotyped using a previously described multiplex PCR method9 and genotyped using a consensus multilocus typing scheme.10 Each isolate was subcultured at least twice on Sabouraud dextrose agar (SDA) (Remel, Inc., Lenexa, KS, USA) prior to in vitro testing or mouse inoculation. Prior to inoculation, organisms were washed three times in sterile saline and the concentrations verified with a haemocytometer, and viability was confirmed by plating serial dilutions and enumeration of colonies.
Animal model
A previously described murine model of cryptococcal meningoencephalitis was utilized for all studies.11,12 Immunocompetent outbred ICR mice (Harlan) weighing approximately 25 g were housed five mice per cage and had access to food and water ad libitum. In the initial virulence studies, mice (n = 10 per inoculum group) were anaesthetized using isoflurane and randomized to intracranial inoculation with 225, 500 or 2000 cfu/animal of C. gattii or 2000 cfu/animal of C. neoformans. Inoculation was delivered as a 0.06 mL volume through a 27 gauge needle fastened to a tuberculin syringe with a cuff to prevent penetration of >1 mm. A midline puncture through the cranial vault ∼6 mm posterior to the orbit was made and the inoculum was injected, after which the mice were allowed to recover. In treatment studies animals were divided into three groups: untreated controls and groups treated with either fluconazole 10 mg/kg or posaconazole 10 mg/kg by oral gavage twice daily. Because of the higher virulence observed with C. gattii during the initial virulence studies, different infecting inocula of each species (225 cfu/mouse for C. gattii and 2000 cfu/mouse for C. neoformans) were used in the treatment studies to control for this difference. Antifungal treatment was initiated 1day after inoculation and continued until day 7 in fungal burden experiments (n = 20 mice per treatment group) and day 10 in survival studies. In survival studies mice were monitored off therapy at days 21 and 30 post-inoculation and any animal that appeared moribund was humanely euthanized (n = 20–22 mice per group when monitored to day 21, n = 10 per group when monitored to day 30). The animal protocol was approved by the University Institutional Animal Care and Use Committee and all animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care.13
Serum antigen and tissue fungal burden
During the initial inoculation experiments serum from different sets of three mice per inoculum group was drawn on days 3, 5 and 7 post-infection. In addition, brains, lungs, livers, kidneys and spleens were collected from five mice per inoculum group on day 7. In treatment studies, brain tissue was collected on day 8 post-inoculation in order to measure fungal burden. This timepoint was used in order to control for antifungal carry-over as antifungal therapy was stopped after the doses on day 7. Tissues were weighed and then homogenized in sterile saline with a tissue homogenizer. Serial dilutions were prepared in sterile saline and plated on SDA. Following 24 h of incubation, colonies were counted and cfu per gram of tissue for each animal was calculated. Cryptococcal serum antigen was measured using a commercially available kit according to the manufacturer’'s instructions (Premier Cryptococcal Antigen EIA, Meridian Biosciences).
Histopathology
In the initial inoculation experiments, brains were collected from three untreated mice per inoculum group on days 7 and 10 post-inoculation and placed into phosphate-buffered formalin. The tissues were processed and embedded in paraffin wax and 5 μm sections were made. The sections were then stained with mucicarmine, which stains the inner capsule of the yeast red, or haematoxylin and eosin (H&E) and viewed by light microscopy.14
Statistics
Survival was plotted by Kaplan–Meier analysis, and log-rank and Fisher’s exact tests were used to determine whether differences in median survival and percentage survival, respectively, were significant. Differences in serum antigen and tissue fungal burden were assessed using analysis of variance with Tukey’s post test. A P value of ≤0.05 was considered statistically significant.
Results
Virulence of C. gattii compared with C. neoformans
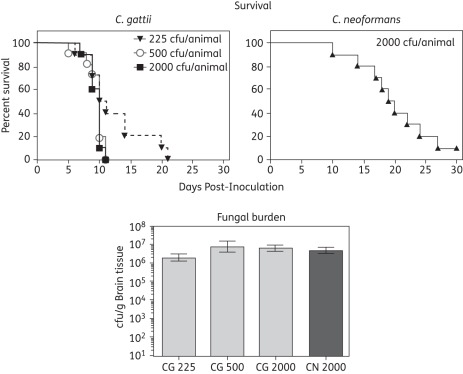
To evaluate the virulence of C. gattii in this murine model of cryptococcal meningoencephalitis, mice were inoculated intracranially with three different inocula of this species and survival was compared with that observed for a fixed inoculum of C. neoformans.11,12 Although there were no significant differences in the percentage of untreated animals among the different groups surviving to day 21 post-inoculation, mice inoculated with C. gattii succumbed to infection quicker than those infected with C. neoformans. As shown in Figure 1, the median survival for animals inoculated with C. gattii at 225, 500 or 2000 cfu ranged between 10 and 10.5 days, and these periods were significantly less than those observed for C. neoformans (19.5 days; P < 0.01). Measurement of brain fungal burden on day 7 post-inoculation revealed no differences among the different groups. The mean fungal burden ranged from 6.4 to 7.1 log10 cfu/g for the three C. gattii inocula groups, which were not significantly different from those observed in mice infected with C. neoformans (6.7 log10 cfu/g; Figure 1).
Figure 1.
Kaplan–Meier survival curves and brain tissue fungal burden in mice infected with different inocula of Cryptococcus gattii (CG) and C. neoformans (CN). Mice were infected by intracranial inoculation with C. gattii at 225, 500 or 2000 cfu/animal or C. neoformans at 2000 cfu/animal. Each inoculum level was used in 10 animals. Animals were followed until day 30 post-inoculation. In a separate group, mice were euthanized on day 7 post-inoculation, the brain tissue was collected and fungal burden was measured by cfu enumeration. Fungal burden data are reported as mean ± SEM.
Histopathology
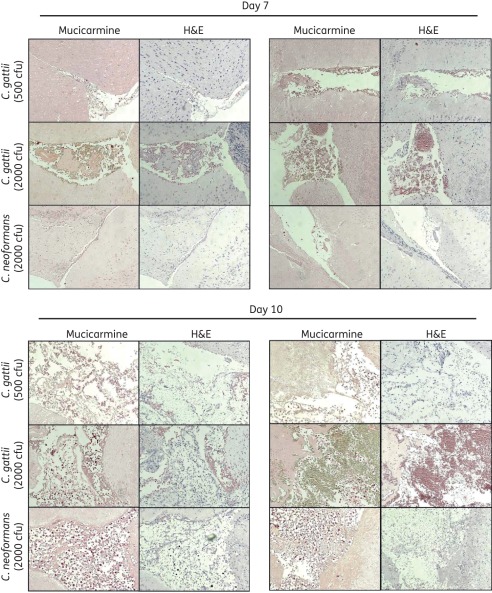
Histopathology revealed marked damage within the brains of mice inoculated with C. gattii. As shown in the mucicarmine-stained samples in Figure 2, cryptococcal cells were observed on day 7 within the brain tissue of animals infected with either species. Qualitatively, for each inoculum the damage caused by C. gattii, as evident by characteristic soap bubble cystic lesions in both the mucicarmine- and H&E-stained samples, appeared to be greater by day 7 post-inoculation compared with the damage caused by C. neoformans. However, by day 10 the damage within the brain appeared to be similar, as these histopathological lesions were observed for each species.
Figure 2.
Representative histopathological sections (stained with mucicarmine or H&E) of mouse brain tissue. Mice were inoculated intracranially with C. gattii at 500 or 2000 cfu/animal or C. neoformans at 2000 cfu/animal. Three mice per inoculum group were humanely euthanized on days 7 and 10 post-inoculation and brain tissue was collected and processed for histopathology. Sections were viewed by light microscopy at 100× magnification.
Serum antigenaemia and dissemination to other organs
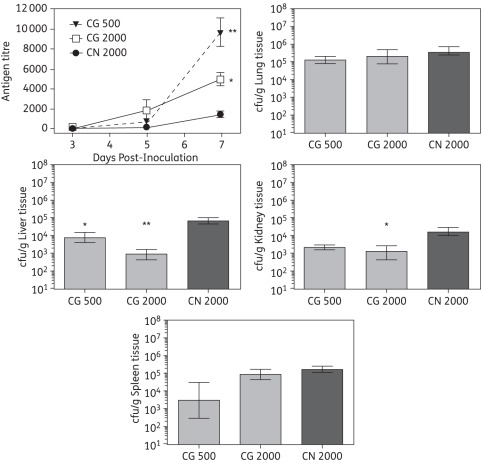
In order to measure the extent of dissemination from the CNS, serum antigen levels and tissue fungal burden in other organs were assessed. Cryptococcal serum antigen levels were similar among mice inoculated with C. gattii and those inoculated with C. neoformans on day 3 post-inoculation, with titres ranging between 1 and 176 (Figure 3). By day 5 antigen titres were highest in mice inoculated with C. gattii 2000 cfu (1779 ± 1138). At this timepoint animals inoculated with C. gattii 500 cfu (616 ± 375) or C. neoformans (200 ± 51) had similar values. Interestingly, by day 7 the highest titres were observed in mice infected with C. gattii 500 cfu, and the antigen levels were significantly higher for both C. gattii inocula levels (9613 ± 2823 and 4971 ± 1198 for 500 and 2000 cfu, respectively) compared with C. neoformans (1482 ± 705; P < 0.05). Despite the elevated serum antigen levels in mice infected with C. gattii, tissue fungal burden levels were significantly higher within the liver and kidneys, but not the lungs and spleens, of mice infected with C. neoformans. Brain fungal burdens did not differ significantly between mice infected with C. gattii or C. neoformans.
Figure 3.
Serum cryptococcal antigen results and tissue fungal burden in lungs, liver and kidneys from mice inoculated intracranially with C. gattii (CG) at 500 or 2000 cfu/animal or C. neoformans (CN) at 2000 cfu/animal. Three mice per inoculum group were humanely euthanized on days 3, 5 and 7, and serum was collected and cryptococcal antigen was measured. *P < 0.05 and **P < 0.01 for C. gattii versus C. neoformans. For fungal burden analysis, five mice per inoculum group were humanely euthanized on day 7 post-inoculation and the lungs, liver and kidneys were removed and homogenized, and serial dilutions of the homogenates were plated onto potato dextrose agar for fungal burden quantification. Data are reported as mean ± SEM. *P < 0.05 and **P < 0.01 for C. gattii versus C. neoformans.
Response to antifungal therapy
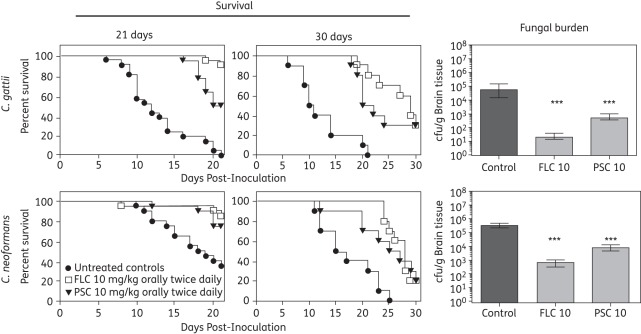
Both fluconazole and posaconazole therapy resulted in a survival benefit compared with untreated controls in mice infected with either C. gattii or C. neoformans (Figure 4). Therapy with either agent improved the median survival in mice infected with either C. gattii (antifungal therapy median survival range 21–29 versus 10.5 days for untreated controls; P < 0.001) or C. neoformans (antifungal therapy range 27.5–28 versus 18.5 days; P < 0.001). However, improvements in percentage survival were time-dependent. At day 21 post-inoculation, 91% of fluconazole-treated mice infected with C. gattii were alive; this was superior to the value in untreated controls (0%; P < 0.0001). Against infections caused by C. neoformans, both fluconazole and posaconazole (85% and 75%, respectively) significantly improved survival at day 21 compared with untreated controls (35%; P < 0.05). Despite the survival advantages at day 21, no survival benefit was observed when mice were monitored to day 30 post-inoculation, as neither fluconazole nor posaconazole significantly improved the percentage of animals surviving to this endpoint (30% each against C. gattii and 20% each against C. neoformans versus 0% for uninfected controls for both species). These results may be due in part to the persistence of residual cryptococcal cells. In separate studies, the brain tissue fungal burden on day 8 post-inoculation in mice infected with C. gattii was significantly reduced by both fluconazole (mean 1.36 ± 0.21 log10 cfu/g) and posaconazole (2.74 ± 0.22 log10 cfu/g) compared with untreated controls (4.69 ± 0.49 log10 cfu/g; P < 0.001). However, neither agent resulted in complete tissue sterilization. Similar results were found in animals infected with C. neoformans and treated with fluconazole (2.86 ± 0.24 log10 cfu/g) or posaconazole (3.95 ± 0.18 log10 cfu/g), both of which differed significantly from the results obtained in untreated controls (5.64 ± 0.11 log10 cfu/g; P < 0.001). Interestingly, the fungal burden data in mice infected with C. gattii was ∼1 log10 cfu/g lower in all groups compared with those inoculated with C. neoformans. This difference may be explained by the lower starting inoculum used for C. gattii (225 cfu/animal) compared with C. neoformans (2000 cfu/animal). Despite the lower inoculum and lower fungal burden measured at day 8 post-inoculation, the overall survival results in the treatment studies were not markedly different between the two species.
Figure 4.
Kaplan–Meier survival curves and brain tissue fungal burden in mice inoculated intracranially with C. gattii (CG) at 225 cfu/animal or C. neoformans (CN) at 2000 cfu/animal. Groups were treated with fluconazole (FLC), posaconazole (PSC) or were untreated controls. Therapy was initiated 1 day post-inoculation and was continued until day 10. In the initial survival studies, mice were monitored off therapy until day 21. In follow-up studies, mice were monitored until day 30 post-inoculation. In separate fungal burden studies, mice were humanely euthanized on day 8 and brain tissue was collected for quantification of cfu. Fungal burden data are reported as mean ± SEM. For controls against C. gattii and C. neoformans, n = 21 and 20, respectively, in the 21 day study. For both fluconazole and posaconazole against C. gattii and C. neoformans, n = 22 and 20, respectively, in the 21 day study. In the 30 day survival study, n = 10 for all groups. ***P < 0.001 versus untreated controls.
Discussion
Meningoencephalitis caused by C. gattii is often difficult to treat. Case–control studies comparing CNS infections caused by this species with those caused by C. neoformans have demonstrated a greater need for neurosurgical intervention, longer hospitalizations, greater duration of amphotericin B therapy and worse outcomes in patients with C. gattii.15 The recent outbreak of C. gattii infections in the Pacific Northwest further illustrates the high morbidity and mortality associated with C. gattii and the need to better understand differences in virulence and therapeutic options for meningoencephalitis caused by this emerging pathogen. The majority of isolates responsible for this outbreak in the Pacific Northwest are of the VGII genotype,16 which has been found to be more virulent than other cryptococcal genotypes.5,17 The C. gattii strain we used was a VGIIa isolate, a genotype found comparable to the major form responsible for the Vancouver island outbreak.17 This subtype has demonstrated increased virulence compared with C. neoformans isolates, other C. gattii genotypes and other VGII subtypes using intranasal models of infection that closely mimic cryptococcal pneumonia.5,17
The results from our model, which more closely mimics cryptococcal meningoencephalitis with reverse dissemination, are consistent with the observations of enhanced virulence with the C. gattii VGIIa genotype compared with infections caused by C. neoformans. Indeed, we observed enhanced virulence, in terms of both a shorter survival period and earlier histopathological changes, within the brain tissue of mice infected with C. gattii at lower inocula compared with those inoculated with C. neoformans. The earlier changes in histopathology are consistent with the propensity for C. gattii to form cryptococcomas,18 and patients infected with C. gattii exhibit a higher incidence of increased intracranial pressure than those infected with C. neoformans.15 Elevations in intracranial pressure and focal cryptococcal lesions may have caused compression necrosis of contiguous tissue and contributed to the histopathological changes we observed.
We also observed higher serum antigen titres in mice infected with C. gattii across all inocula compared with C. neoformans, and, somewhat paradoxically, the highest titres were noted in mice infected with the lowest C. gattii inoculum. Although prior studies have shown a correlation between CSF cryptococcal antigen titres and CSF cryptococcal cfu in C. neoformans-infected patients,19 serum cryptococcal antigen titres have been found to differ significantly between C. gattii and C. neoformans infections. Mitchell et al.15 reported higher serum antigen titres in C. gattii-infected patients than in those infected with C. neoformans, and this difference was apparent despite the lower incidence of positive CSF cultures in patients with C. gattii (78% versus 100%).
The purpose of the antifungal treatment experiments was to determine whether drug therapy would work in the model when adapted for C. gattii, but not specifically to compare fluconazole and posaconazole. Equivalent doses of each agent were therefore used, and the fluconazole dose was based on our previous experience with this drug in the C. neoformans model. We used a fully susceptible VGIIa C. gattii isolate in our study to determine whether mice would respond to treatment. The overall survival benefit at day 30 post-inoculation was similar for the two agents in mice infected with either C. gattii or C. neoformans. This occurred despite marked differences in the infecting inoculum sizes between the two species, which further highlights the virulence of this C. gattii isolate. Antifungal therapy was given only up to day 10, and these results may be reflective of the fungistatic nature of the triazoles as neither agent resulted in sterilization of the brain tissue. Further studies are warranted to determine how therapy would work against different C. gattii isolates with different susceptibility profiles and to determine the optimal pharmacokinetic/pharmacodynamic parameter for the treatment of infections caused by this emerging pathogen.
In conclusion, we have adapted a murine model of cryptococcal meningoencephalitis to C. gattii. This model may potentially be utilized to evaluate the virulence characteristics of the different genotypes of this emerging pathogen, determine the mechanism responsible for differences in antigen titres and compare treatment options for this often moribund infection. Further studies evaluating combination therapy will need to be performed in an effort to provide effective treatment using orally available antifungal agents.
Funding
This work was supported by a contract from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (N01-AI-25475) to J. R. G. and T. F. P.
Transparency declarations
G. R. T. has received research support from Pfizer. N. P. W. has received research support from Pfizer, Schering-Plough, Merck, Basilea and Astellas. J. R. G. has received research support from Pfizer, Merck and Astellas, and has been paid to act as a consultant for Merck. T. F. P. has received research grants from Astellas, Basilea, Merck and Pfizer (to the University of Texas Health Science Center at San Antonio), and has been paid to act as a consultant for Astellas, Basilea, Merck, Pfizer and Toyoma. All other authors: none to declare.
Acknowledgements
This work was presented, in part, at the Forty-eighth Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2008 (Abstract M-1550).
We thank Dr Chris Lambros for his support of this project and Marcos Olivo for his assistance with the animal experiments.
References
- 1.Kidd SE, Hagen F, Tscharke RL, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada) Proc Natl Acad Sci USA. 2004;101:17258–63. doi: 10.1073/pnas.0402981101. doi:10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39:155–68. [PubMed] [Google Scholar]
- 3.Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984;120:123–30. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Rodriguez JM, Morera Y, Baro T, et al. Pathogenicity of Cryptococcus neoformans var. gattii in an immunocompetent mouse model. Med Mycol. 2003;41:59–63. doi: 10.1080/mmy.41.1.59.63. [DOI] [PubMed] [Google Scholar]
- 5.Fraser JA, Giles SS, Wenink EC, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–4. doi: 10.1038/nature04220. doi:10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- 6.Krockenberger MB, Malik R, Ngamskulrungroj P, et al. Pathogenesis of pulmonary Cryptococcus gattii infection: a rat model. Mycopathologia. 2010;170:315–30. doi: 10.1007/s11046-010-9328-z. doi:10.1007/s11046-010-9328-z. [DOI] [PubMed] [Google Scholar]
- 7.Driver JA, Saunders CA, Heinze-Lacey B, et al. Cryptococcal pneumonia in AIDS: is cryptococcal meningitis preceded by clinically recognizable pneumonia? J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:168–71. [PubMed] [Google Scholar]
- 8.Thompson GR, III, Wiederhold NP, Fothergill AW, et al. Antifungal susceptibilities among different serotypes of Cryptococcus gattii and Cryptococcus neoformans. Antimicrob Agents Chemother. 2009;53:309–11. doi: 10.1128/AAC.01216-08. doi:10.1128/AAC.01216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito-Kuwa S, Nakamura K, Aoki S, et al. Serotype identification of Cryptococcus neoformans by multiplex PCR. Mycoses. 2007;50:277–81. doi: 10.1111/j.1439-0507.2007.01357.x. doi:10.1111/j.1439-0507.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyer W, Aanensen DM, Boekhout T, et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol. 2009;47:561–70. doi: 10.1080/13693780902953886. doi:10.1080/13693780902953886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond DM, Bauer M, Daniel BE, et al. Amphotericin B colloidal dispersion combined with flucytosine with or without fluconazole for treatment of murine cryptococcal meningitis. Antimicrob Agents Chemother. 1998;42:528–33. doi: 10.1128/aac.42.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding JC, Bauer M, Diamond DM, et al. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1589–93. doi: 10.1128/aac.41.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 14.Vance AM. The use of the mucicarmine stain for a rapid presumptive identification of Cryptococcus from culture. Am J Med Technol. 1961;27:125–8. [PubMed] [Google Scholar]
- 15.Mitchell DH, Sorrell TC, Allworth AM, et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis. 1995;20:611–6. doi: 10.1093/clinids/20.3.611. doi:10.1093/clinids/20.3.611. [DOI] [PubMed] [Google Scholar]
- 16.Byrnes EJ, III, Li W, Lewit Y, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 2010;6:e1000850. doi: 10.1371/journal.ppat.1000850. doi:10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngamskulrungroj P, Serena C, Gilgado F, et al. Global VGIIa isolates are of comparable virulence to the major fatal Cryptococcus gattii Vancouver Island outbreak genotype. Clin Microbiol Infect. 2011;17:251–8. doi: 10.1111/j.1469-0691.2010.03222.x. doi:10.1111/j.1469-0691.2010.03222.x. [DOI] [PubMed] [Google Scholar]
- 18.Hospenthal DR, Bennett JE. Persistence of cryptococcomas on neuroimaging. Clin Infect Dis. 2000;31:1303–6. doi: 10.1086/317434. doi:10.1086/317434. [DOI] [PubMed] [Google Scholar]
- 19.Brouwer AE, Teparrukkul P, Pinpraphaporn S, et al. Baseline correlation and comparative kinetics of cerebrospinal fluid colony-forming unit counts and antigen titers in cryptococcal meningitis. J Infect Dis. 2005;192:681–4. doi: 10.1086/432073. doi:10.1086/432073. [DOI] [PubMed] [Google Scholar]