Abstract
Background.
Reduced glomerular filtration rate and albuminuria are associated with an increased risk for stroke. Their association with stroke symptoms is not known.
Methods.
The incidence of stroke symptoms was determined in 20 386 participants ≥45 years of age in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study who were free of a history of stroke, transient ischemic attack and stroke symptoms at baseline. Six stroke symptoms were assessed via telephone interviews at baseline and every 6 months. Participants were followed over a median of 2.1 years (maximum follow-up of 6 years). Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation and the albumin-to-creatinine ratio from spot urine samples.
Results.
The incidence of any stroke symptom (n = 2548 cases) was 10.8, 12.9, 18.2 and 20.7% among participants with an eGFR ≥90, 60–89, 45–59 and <45 mL/min/1.73m2, respectively, and 10.8, 14.4, 17.0 and 18.8 for participants with albumin-to-creatinine ratios <10, 10–29, 30–299 and ≥300 mg/g, respectively (each P-trend < 0.001). The multivariable-adjusted hazard ratio (95% confidence interval) for any stroke symptom was 1.02 (0.91–1.14), 1.22 (1.01–1.48) and 1.26 (0.98–1.62) for those with an eGFR of 60–89, 45–59 and <45 mL/min/1.73m2, respectively, versus ≥90 mL/min/1.73m2 (P-trend = 0.022) and 1.16 (1.03–1.31), 1.29 (1.12–1.50) and 1.11 (0.82–1.49) for those with albumin-to-creatinine ratios of 10–29, 30–299 and ≥300 versus <10 mg/g, respectively (P-trend = 0.005).
Conclusions.
Reduced eGFR and higher albuminuria levels are associated with an increased risk for incident stroke symptoms.
Keywords: albuminuria, chronic kidney disease, stroke, stroke symptoms
Introduction
Chronic kidney disease (CKD), defined by the presence of a reduced estimated glomerular filtration rate (eGFR) or the presence of albuminuria, is associated with an increased prevalence and incidence of stroke [1]. In the US National Health and Nutrition Examination Survey (NHANES) 1999–2004, adults with CKD were five times more likely to report a history of a diagnosis of stroke than their counterparts without CKD [2]. In addition to the higher prevalence of stroke, adults with CKD have a higher prevalence of many stroke risk factors [1]. In an analysis of the Atherosclerosis Risk in Communities (ARIC) study, moderate to advanced CKD (i.e. an eGFR < 60 mL/min/1.73m2) was associated with a multivariable-adjusted relative risk of stroke of 1.82 [95% confidence interval (CI): 1.26–2.01] [3]. Also, in a meta-analysis of 10 published cohort studies involving 140 231 participants and 3266 stroke events, albuminuria was associated with an odds ratio for stroke of 1.71 (95% CI: 1.39–2.10) [4].
Among individuals without a prior diagnosis of stroke, the presence of stroke symptoms is associated with an increased risk for future stroke events [5, 6]. Population-based studies indicate that stroke symptoms are common among individuals without a prior medical diagnosis of stroke or transient ischemic attack (TIA) [7]. Stroke symptoms may be a marker of small cerebral infarcts or clinical symptoms of a minor stroke. However, a previous study reported that ∼60% of individuals seek care for their stroke symptoms [8]. Despite the high risk for stroke among individuals with CKD, few data are available on the prevalence and incidence of stroke symptoms among this population. Given their high stroke risk, if adults with CKD have an increased prevalence and incidence of stroke symptoms, targeted screening for stroke symptoms in this population may be warranted. The goal of the present analysis was to assess whether individuals with reduced eGFR and albuminuria have a higher burden of stroke symptoms. To do so, we analyzed data from the community-based REasons for Geographic and Racial Differences in Stroke (REGARDS) study. First, we determined the prevalence of stroke symptoms among individuals without a prior diagnosis of stroke or TIA by level of eGFR and albuminuria. Next, we determined the incidence of new stroke symptoms among individuals without stroke, TIA or stroke symptoms at baseline.
Materials and methods
The REGARDS study is a population based investigation of stroke among US adults ≥45 years of age [9]. The study was designed to oversample African-Americans and to provide approximate equal representation of men and women. By design, 56% (goal 50%) of the sample was recruited from the ‘stroke buckle’ (defined as the coastal North Carolina, South Carolina and Georgia areas) and ‘stroke belt’ (remainder of North Carolina, South Carolina and Georgia as well as Alabama, Mississippi, Tennessee, Arkansas and Louisiana), with the remaining 44% of the sample recruited from the other 40 contiguous US states and the District of Columbia. Overall, 30 239 African-American and Caucasian US adults were enrolled between January 2003 and October 2007. For the cross-sectional analyses of prevalent stroke symptoms at baseline, we excluded participants who reported a history of stroke (n = 1932) or TIA (n = 1138) at baseline and individuals missing data on stroke/TIA (n = 212). Additionally, individuals missing data on stroke symptoms (n = 377) and those without serum creatinine or urinary albumin or creatinine measurements (n = 1922) were excluded from the present analyses. After these exclusions, 24 678 participants were available for analysis. For the analysis of incident stroke symptoms, the sample was further restricted to participants without stroke symptoms at baseline and with follow-up interview data available (n = 20 386). The REGARDS protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided informed consent.
Data collection
Socio-demographic and clinical data were collected at baseline through a telephone interview, a self-administered questionnaire and an in-home examination at baseline. Follow-up telephone interviews were conducted at 6-month intervals to ascertain the occurrence of stroke symptoms and outcome events. Diabetes at baseline was defined as a serum glucose ≥ 126 mg/dL for participants who had fasted ≥8 h prior to sampling, serum glucose ≥200 mg/dL for those who had not fasted or self-report of a prior diagnosis of diabetes with current use of insulin or oral hypoglycemic medications. Dyslipidemia was defined as serum total cholesterol ≥ 240 mg/dL or low-density lipoprotein cholesterol ≥160 mg/dL or high density lipoprotein cholesterol <40 mg/dL or self-reported use of cholesterol- lowering medications. Elevated C-reactive protein was defined as levels ≥2 mg/L.
Using isotope-dilution mass spectrometry-traceable serum creatinine, eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [10]. Using a random spot urine collection, albumin-to-creatinine ratio was determined. Urinary albumin was measured at the Department of Laboratory Medicine and Pathology at the University of Minnesota, using the BN ProSpec Nephelometer from Dade Behring (Marburg, Germany). Urinary creatinine was measured with a rate-blanked Jaffe procedure, using the Modular-P analyzer (Roche/Hitachi; Indianapolis, IN). eGFR and albuminuria were categorized using previously accepted cut-points (<45, 45–59, 60–89 and ≥90 mL/min/1.73m2) and (<10, 10–29, 30–299 and ≥300 mg/g), respectively [11, 12].
Outcomes
Stroke symptoms were assessed at baseline and during bi-annual follow-up telephone interviews. Using the Questionnaire for Verifying Stroke-Free Status (QVSFS), participants were asked about the sudden onset of each of six stroke symptoms (Supplementary Appendix) [13]; at baseline, the QVSFS asked ‘have you ever had’ while the follow-up interview asks ‘since the last time we talked with you’. The QVSFS is a validated questionnaire proposed as a quick screening instrument for identification of stroke-free individuals in the general population [14, 15]. The prospective analysis of incident stroke symptoms includes telephone interviews conducted through June 2009, and the occurrence of the first reported stroke symptom was analyzed. For this analysis, participants completed up to 12 follow-up interviews (median = 7 follow-up interviews).
Statistical analysis
For the cross-sectional analysis of baseline data, characteristics of participants and the prevalence of stroke symptoms were calculated by levels of eGFR and albuminuria, separately. Next, the odds ratios for each stroke symptom, as well as any stroke symptom, associated with eGFR and albuminuria levels separately, were calculated using logistic regression models. Initial regression models included adjustment for age, race, sex and region of residence (stroke belt, stroke buckle or other region) with subsequent models including additional adjustment for education, household income, current smoking, alcohol consumption, body mass index (BMI), systolic blood pressure, antihypertensive medication use, dyslipidemia, diabetes and elevated C-reactive protein. For the analysis of incident stroke symptoms, participant characteristics and the percent of participants developing each stroke symptom and the total number of stroke symptoms developed was calculated by eGFR and albuminuria levels, separately. The hazard ratio for developing each, as well as any, stroke symptom during follow-up was calculated for each eGFR and albuminuria level, separately. As the exact date a participant developed stroke symptoms was not known (i.e. this was assessed at 6-month intervals), we used interval censored regression models to calculate the hazard ratios [16, 17]. Participants who died were right censored on their date of death, those who did not develop stroke symptoms were right censored on the date of their last REGARDS follow-up interview and those developing a stroke symptom were left or interval censored. Participants having one stroke symptom during follow-up remained at risk for the other stroke symptoms. Linear trends across eGFR and albuminuria levels were assessed by including level-specific median values as a continuous variable in the regression models. Finally, analyses were run to assess multiplicative interaction on developing any stroke symptoms between eGFR and albuminuria as well as eGFR and age (<65 years versus ≥65 years), eGFR and race (black versus white), albuminuria and age and albuminuria and race. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Cross-sectional associations—prevalent stroke symptoms
Characteristics of participants included in the cross-sectional analysis of prevalent stroke symptoms are presented by eGFR levels (top panel) and albuminuria levels (bottom panel) in Supplementary Table 1. At baseline, 4118 REGARDS study participants reported a stroke symptom. The prevalence of any stroke symptom was 17.1, 15.7, 18.0 and 23.6% among individuals with eGFR levels ≥90, 60–89, 45–59 and <45 mL/min/1.73m2, respectively (P-trend = 0.023; Supplementary Table 2). The prevalence of sudden onset of numbness, sudden loss of vision in one or both eyes and sudden loss of half vision were each more common at lower levels of eGFR (each P-trend < 0.001). The multivariable adjusted odds ratio for any stroke symptom associated with eGFR levels of 60–89, 45–59 and <45 mL/min/1.73m2 versus ≥90 mL/min/1.73m2 were 0.99 (95% CI: 0.91–1.07), 1.01 (95% CI: 0.88–1.17) and 1.24 (95% CI: 1.04–1.48), respectively (P-trend = 0.113). Also, lower eGFR levels were associated with a higher multivariable adjusted odds ratio for sudden loss of vision in one or both eyes and sudden loss of half vision.
The prevalence of any stroke symptoms was 15.8, 16.6, 20.4 and 26.5% among individuals with albuminuria levels <10, 10–29, 30–299 and ≥300 mg/g, respectively (Supplementary Table 3; P-trend < 0.001). The prevalence and age, race, sex and region adjusted odds ratio for each stroke symptom was higher at higher albuminuria levels (each P-trend < 0.001). The multivariable adjusted odds ratios for any stroke symptoms for individuals with albuminuria levels of 10–29, 30–299 and ≥300 mg/g versus <10 mg/g were 0.99 (95% CI: 0.91–1.08), 1.13 (95% CI: 1.02–1.26) and 1.34 (95% CI: 1.11–1.63), respectively (P-trend = 0.006). Albuminuria levels ≥300 mg/g were associated with an increased multivariable adjusted odds ratio for sudden loss of vision in one or both eyes, sudden loss of half vision and suddenly losing the ability to express oneself verbally or in writing.
Prospective associations—incident stroke symptoms
Characteristics of participants included in the prospective analysis of incident stroke symptoms are presented by eGFR levels (top panel) and albuminuria levels (bottom panel) in Table 1. Over a median of 2.1 years of follow-up (maximum follow-up = 6 years), 2548 participants developed stroke symptoms. Supplementary Table 4 shows the number of incident stroke symptoms that developed by eGFR levels and albuminuria levels. The proportion of participants developing incident stroke symptoms was higher at lower levels of eGFR (Figure 1, top panel). Also, the incidence of multiple stroke symptoms was more common among those with lower eGFR and higher albuminuria levels (Figure 2, top panel). Among individuals with an eGFR < 45 mL/min/1.73m2, 11.7, 5.9 and 3.1% developed 1, 2 and ≥3 stroke symptoms during follow-up, respectively, compared to 7.4, 2.2 and 1.2%, respectively, for their counterparts with an eGFR ≥ 90 mL/min/1.73m2. After adjustment for age, race, sex and region of residence, hazard ratios for developing each stroke symptom increased with progressively lower eGFR levels (Table 2). After further adjustment for education, household income, current smoking alcohol consumption, BMI, systolic blood pressure, antihypertensive medication use, dyslipidemia, diabetes and elevated C-reactive protein, significant trends of higher hazard ratios at lower eGFR levels were present for any stroke symptom, the sudden onset of weakness, the sudden loss of vision in one or both eyes, the sudden loss of the ability to understand people and the sudden loss of ability to express oneself verbally or in writing.
Table 1.
Baseline characteristics of REGARDS study participants included in the prospective analysis of incident stroke symptoms by eGFR level (top panel) and albuminuria level (bottom panel)a
| Level of eGFR, mL/min/1.73m2 |
||||||
| ≥90 (n = 9431) | 60–89 (n = 9053) | 45–59 (n = 1321) | <45 (n = 581) | P-trend | ||
| Age, years | 60.2 (7.5) | 67.2 (8.8) | 71.9 (8.4) | 72.3 (9.0) | <0.001 | |
| Women, % | 58.8 | 49.7 | 54.8 | 54.0 | <0.001 | |
| African-American, % | 43.7 | 30.5 | 36.9 | 46.3 | <0.001 | |
| Region | ||||||
| Other region, % | 43.4 | 45.2 | 46.9 | 46.3 | Ref | |
| Stroke belt, % | 35.6 | 34.2 | 34.0 | 32.2 | 0.004 | |
| Stroke buckle, % | 21.0 | 20.6 | 19.2 | 21.5 | 0.048 | |
| High school education, % | 90.7 | 90.6 | 85.8 | 80.0 | <0.001 | |
| Annual income | ||||||
| <$20 000 | 15.9 | 15.7 | 21.9 | 29.0 | Ref | |
| $20 000–$34 999 | 23.8 | 27.2 | 33.9 | 36.3 | <0.001 | |
| $35 000–$74 999 | 37.3 | 36.8 | 32.6 | 25.9 | <0.001 | |
| ≥$75 000 | 23.0 | 20.4 | 11.5 | 8.8 | <0.001 | |
| Current smoking, % | 16.7 | 10.1 | 9.0 | 11.9 | <0.001 | |
| Consumes alcohol, % | 57.3 | 55.0 | 45.2 | 40.1 | <0.001 | |
| BMI, kg/m2 | 29.6 (6.4) | 28.6 (5.6) | 29.5 (6.3) | 29.7 (6.6) | <0.001 | |
| Systolic blood pressure, mmHg | 126.1 (16.2) | 127.0 (16.2) | 131.8 (17.7) | 132.0 (17.8) | <0.001 | |
| Antihypertensive medication use, % | 40.8 | 48.5 | 71.2 | 80.9 | <0.001 | |
| Dyslipidemia, % | 52.6 | 59.0 | 67.9 | 70.5 | <0.001 | |
| Diabetes, % | 17.9 | 15.5 | 25.8 | 38.7 | <0.001 | |
| Elevated C-reactive protein, % | 52.0 | 47.5 | 56.7 | 62.5 | 0.029 | |
| Level of Albuminuria, mg/g | ||||||
| <10 (n = 13183) | 10–29 (n = 4556) | 30–299 (n = 2213) | ≥300 (n = 434) | P-trend | ||
| Age, years | 63.3 (8.8) | 66.2 (9.3) | 66.8 (9.7) | 66.8 (8.9) | <0.001 | |
| Women, % | 53.3 | 60.2 | 50.8 | 43.1 | 0.060 | |
| African-American, % | 35.2 | 37.5 | 45.7 | 62.4 | <0.001 | |
| Region | ||||||
| Other region, % | 44.7 | 43.7 | 44.8 | 46.5 | Ref | |
| Stroke belt, % | 34.7 | 34.8 | 35.7 | 33.0 | 0.923 | |
| Stroke buckle, % | 20.7 | 21.6 | 19.5 | 20.5 | 0.613 | |
| High school education, % | 91.7 | 88.6 | 84.6 | 85.5 | <0.001 | |
| Income | ||||||
| <$20 000 | 13.9 | 19.8 | 23.8 | 27.0 | Ref | |
| $20 000–$34 999 | 24.6 | 29.0 | 29.1 | 34.8 | <0.001 | |
| $35 000–$74 999 | 38.0 | 34.3 | 33.1 | 29.8 | <0.001 | |
| ≥$75 000 | 23.5 | 16.9 | 14.0 | 8.4 | <0.001 | |
| Current smoking, % | 12.1 | 13.5 | 16.7 | 22.4 | <0.001 | |
| Consumes alcohol, % | 57.1 | 53.5 | 48.2 | 44.0 | <0.001 | |
| BMI, kg/m2 | 28.9 (5.8) | 29.2 (6.3) | 30.2 (6.6) | 30.9 (7.1) | <0.001 | |
| Systolic blood pressure, mmHg | 124.4 (14.9) | 129.7 (16.9) | 133.3 (18.7) | 141.0 (19.7) | <0.001 | |
| Antihypertensive medication use, % | 42.1 | 53.2 | 60.2 | 79.0 | <0.001 | |
| Dyslipidemia, % | 55.4 | 57.7 | 61.4 | 73.2 | <0.001 | |
| Diabetes, % | 12.6 | 22.4 | 33.0 | 55.5 | <0.001 | |
| Elevated C-reactive protein, % | 47.7 | 52.9 | 60.1 | 65.0 | <0.001 | |
Ref, reference for evaluating trends. Numbers in table are mean (SD) or percentage. Dyslipidemia defined as total cholesterol ≥240 mg/dL, high-density lipoprotein-cholesterol <40 mg/dL, low-density lipoprotein-cholesterol ≥160 mg/dL or lipid-lowering medication use. Elevated C-reactive protein defined as levels ≥2 mg/L.
Fig. 1.
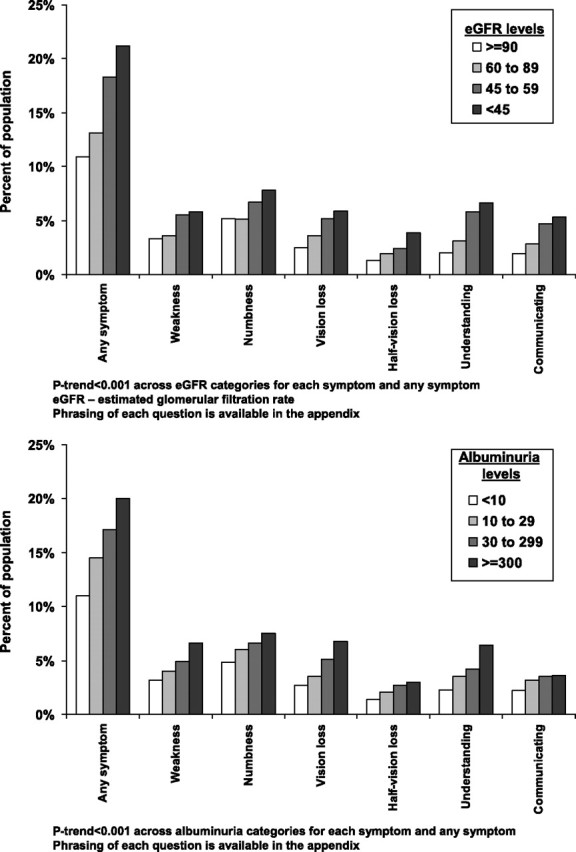
Percent of REGARDS study participants developing incident stroke symptoms over a median follow-up of 2.1 years by eGFR (top panel) and level of albuminuria (bottom panel). The maximum follow-up available was 6 years.
Fig. 2.
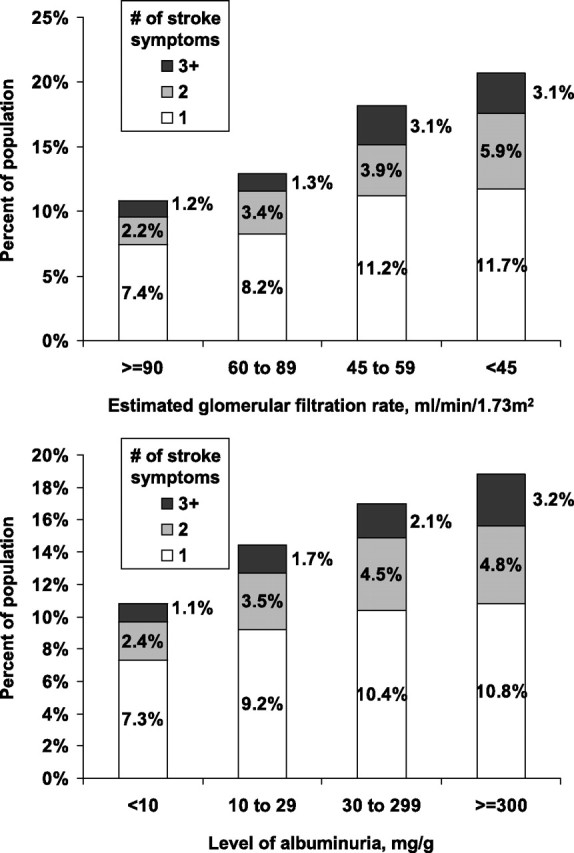
Number of incident stroke symptoms occurring over a median follow-up of 2.1 years by eGFR (top panel) and level of albuminuria (bottom panel). The maximum follow-up available was 6 years.
Table 2.
Hazard ratios for incident stroke symptoms associated with level of eGFRa
| Level of eGFR, mL/min/1.73m2 |
|||||
| ≥90 (n = 9516) | 60–89 (n = 9152) | 45–59 (n = 1335) | <45 (n = 591) | P-trend | |
| Any stroke symptom | |||||
| Hazard ratio 1 | 1 (ref) | 1.01 (0.93–1.11) | 1.26 (1.08–1.46) | 1.40 (1.15–1.70) | <0.001 |
| Hazard ratio 2 | 1 (ref) | 1.01 (0.92–1.11) | 1.18 (1.01–1.38) | 1.21 (0.99–1.48) | 0.017 |
| Symptom—sudden weakness | |||||
| Hazard ratio 1 | 1 (ref) | 1.09 (0.92–1.29) | 1.49 (1.13–1.95) | 1.41 (0.97–2.07) | 0.004 |
| Hazard ratio 2 | 1 (ref) | 1.08 (0.91–1.28) | 1.39 (1.06–1.84) | 1.25 (0.85–1.83) | 0.033 |
| Symptom—sudden numbness | |||||
| Hazard ratio 1 | 1 (ref) | 1.03 (0.9–1.18) | 1.29 (1.02–1.65) | 1.45 (1.05–1.99) | 0.010 |
| Hazard ratio 2 | 1 (ref) | 1.03 (0.9–1.19) | 1.22 (0.96–1.56) | 1.23 (0.89–1.71) | 0.095 |
| Symptom—sudden painless loss of vision in one or both eyes | |||||
| Hazard ratio 1 | 1 (ref) | 1.13 (0.94–1.36) | 1.33 (1.00–1.78) | 1.41 (0.96–2.07) | 0.019 |
| Hazard ratio 2 | 1 (ref) | 1.16 (0.96–1.40) | 1.29 (0.96–1.72) | 1.24 (0.84–1.84) | 0.076 |
| Symptom—sudden loss of half vision | |||||
| Hazard ratio 1 | 1 (ref) | 1.14 (0.88–1.47) | 1.22 (0.80–1.86) | 1.88 (1.16–3.04) | 0.025 |
| Hazard ratio 2 | 1 (ref) | 1.13 (0.87–1.46) | 1.10 (0.72–1.69) | 1.57 (0.96–2.54) | 0.155 |
| Symptom—suddenly lost ability to understand people | |||||
| Hazard ratio 1 | 1 (ref) | 0.98 (0.79–1.20) | 1.44 (1.08–1.93) | 1.59 (1.10–2.31) | 0.002 |
| Hazard ratio 2 | 1 (ref) | 0.96 (0.78–1.18) | 1.32 (0.98–1.78) | 1.37 (0.94–2.00) | 0.024 |
| Symptom—suddenly lost ability to express self verbally or in writing | |||||
| Hazard ratio 1 | 1 (ref) | 1.03 (0.84–1.27) | 1.42 (1.04–1.94) | 1.58 (1.05–2.36) | 0.007 |
| Hazard ratio 2 | 1 (ref) | 1.03 (0.83–1.27) | 1.35 (0.98–1.86) | 1.44 (0.96–2.18) | 0.025 |
Ref, reference category. Numbers in table are hazard ratio (95% CI). Hazard ratio 1 is adjusted for age, race, sex and geographic region. Hazard ratio 2 is adjusted for age, race, sex, geographic region, education, household income, current smoking, alcohol consumption, BMI, systolic blood pressure, antihypertensive medication use, dyslipidemia, diabetes and elevated C-reactive protein.
The incidence of each and multiple stroke symptoms was more common at higher levels of albuminuria (Figure 1, bottom panel and Figure 2 bottom panel). The incidence of 1, 2 and ≥3 stroke symptoms was 10.8, 4.8 and 3.2%, respectively, for individuals with albuminuria ≥ 300 mg/g and 7.3, 2.4 and 1.1%, respectively, for their counterparts with an albuminuria < 10 mg/g. After adjustment for age, race, sex and region of residence, the hazard ratios for developing any incident stroke symptoms among individuals with albuminuria levels of 10–29, 30–299 and ≥300 mg/g, versus <10 mg/g, were 1.27 (95% CI: 1.13–1.43), 1.54 (95% CI: 1.33–1.78), 1.62 (95% CI: 1.23–2.15), respectively (Table 3). This association was attenuated but the trend remained statistically significant after further multivariable adjustment. After adjustment for age, race, sex and region of residence, significant trends of higher hazard ratios at higher albuminuria levels were present for each stroke symptom. These associations were attenuated by further multivariable adjustment and trends remained present only for sudden loss of vision in one or both eyes and sudden inability to understand people. The test for multiplicative interaction between eGFR and albuminuria on incident stroke symptoms was not statistically significant (P = 0.161). Additionally, there were no statistically significant differences in the association of eGFR and albuminuria on incidence stroke symptoms by race or sex (each P > 0.1).
Table 3.
Hazard ratios for incident stroke symptoms associated with level of albuminuriaa
| Albuminuria category, mg/g |
|||||
| <10 (n = 13310) | 10–29 (n = 4598) | 30–299 (n = 2246) | ≥300 (n = 440) | P-trend | |
| Any stroke symptom | |||||
| Hazard ratio 1 | 1 (ref) | 1.22 (1.11–1.34) | 1.43 (1.28–1.61) | 1.49 (1.19–1.87) | <0.001 |
| Hazard ratio 2 | 1 (ref) | 1.13 (1.03–1.25) | 1.25 (1.11–1.41) | 1.10 (0.87–1.40) | 0.002 |
| Symptom—sudden weakness | |||||
| Hazard ratio 1 | 1 (ref) | 1.16 (0.98–1.39) | 1.41 (1.14–1.74) | 1.48 (1.00–2.21) | <0.001 |
| Hazard ratio 2 | 1 (ref) | 1.11 (0.93–1.33) | 1.23 (0.99–1.53) | 1.09 (0.71–1.67) | 0.133 |
| Symptom—sudden numbness | |||||
| Hazard ratio 1 | 1 (ref) | 1.25 (1.08–1.44) | 1.34 (1.12–1.61) | 1.26 (0.87–1.82) | <0.001 |
| Hazard ratio 2 | 1 (ref) | 1.17 (1.01–1.36) | 1.16 (0.96–1.40) | 0.92 (0.62–1.37) | 0.334 |
| Symptom—sudden painless loss of vision in one or both eyes | |||||
| Hazard ratio 1 | 1 (ref) | 1.15 (0.95–1.38) | 1.55 (1.25–1.93) | 1.79 (1.20–2.67) | <0.001 |
| Hazard ratio 2 | 1 (ref) | 1.04 (0.86–1.25) | 1.31 (1.04–1.64) | 1.29 (0.84–1.96) | 0.029 |
| Symptom—sudden loss of half vision | |||||
| Hazard ratio 1 | 1 (ref) | 1.30 (1.00–1.68) | 1.70 (1.26–2.29) | 1.51 (0.82–2.79) | <0.001 |
| Hazard ratio 2 | 1 (ref) | 1.13 (0.87–1.48) | 1.39 (1.02–1.89) | 0.95 (0.49–1.83) | 0.222 |
| Symptom—suddenly lost ability to understand people | |||||
| Hazard ratio 1 | 1 (ref) | 1.28 (1.06–1.56) | 1.46 (1.15–1.86) | 2.34 (1.57–3.49) | <0.001 |
| Hazard ratio 2 | 1 (ref) | 1.19 (0.99–1.42) | 1.26 (1.01–1.58) | 1.70 (1.16–2.50) | 0.002 |
| Symptom—suddenly lost ability to express self verbally or in writing | |||||
| Hazard ratio 1 | 1 (ref) | 1.25 (1.03–1.54) | 1.36 (1.05–1.75) | 1.35 (0.80–2.28) | 0.005 |
| Hazard ratio 2 | 1 (ref) | 1.17 (0.94–1.46) | 1.20 (0.91–1.58) | 1.02 (0.57–1.82) | 0.289 |
Ref, reference category. Numbers in table are hazard ratio (95% CI). Hazard ratio 1 is adjusted for age, race, sex and geographic region. Hazard ratio 2 is adjusted for age, race, sex, geographic region, education, current smoking, alcohol consumption, BMI, systolic blood pressure, antihypertensive medication use, dyslipidemia, diabetes and elevated C-reactive protein.
Discussion
Previous studies have noted a high prevalence and incidence of stroke among individuals with CKD [1, 4, 18]. The relationship between kidney function and stroke symptoms that may or may not reach the point of clinical diagnosis of stroke or transient ischemic attack has been less well characterized. In cross-sectional analyses of the REGARDS study, a higher burden of each of six stroke symptoms studied was present among individuals with lower eGFR and higher levels of albuminuria who reported no prior diagnosis of stroke or transient ischemic attack. Also, in prospective analysis of individuals without stroke symptoms at baseline, those with progressively lower eGFR and higher albuminuria levels were more likely to develop incident stroke symptoms. The findings are robust as noted by the consistency of the cross-sectional and longitudinal findings and the graded relationship between the severity of the kidney disease and stroke symptoms.
It has been suggested that stroke symptoms in the absence of a diagnosis of stroke or transient ischemic attack may reflect the occurrence of a small cerebral infarction [19]. Such infarcts may be analogous to silent myocardial infarctions that are associated with an increased risk for developing clinically pronounced myocardial infarction [20, 21]. In the Rotterdam Scan Study, the presence of silent brain infarcts on brain imaging was associated with a multivariable adjusted hazard ratio of 3.9 (95% CI: 2.3–6.8) for stroke [22]. In that study, the risk for stroke associated with silent brain infarcts was similar to that for individuals with a history of TIA.
The current study did not have access to brain imaging. However, questionnaires are a much more feasible approach for identifying individuals with stroke symptoms in the clinical setting. The QVSFS has been demonstrated to be sensitive (sensitivity = 97%) but not very specific (specificity = 60%) with overall positive and negative predictive values of 71 and 96%, respectively [14]. Although it is estimated that there are 4 million US adults with a history of clinically diagnosed strokes, there may be as many as 10 million adults with prevalent ‘silent’ subclinical strokes in the USA [23]. Given the high burden of stroke symptoms among US adults, further work is needed validating inexpensive and non-invasive approaches to identify individuals with stroke symptoms.
Over the past decade, substantial evidence has accumulated on the increased stroke incidence and mortality associated with CKD prior to the need for dialysis [4, 24]. Given the high prevalence of stroke symptoms and the high incidence of stroke among individuals with CKD, the population-attributable risk for cerebral infarction associated with stroke symptoms may be high. Data from the ARIC and the Renfrew–Paisley studies suggest that stroke symptoms are important indicators for future stroke [5, 6].
Although strong graded associations between eGFR and albuminuria with stroke symptoms were present after adjustment for demographics and region of residence, these associations were attenuated after controlling for other covariables such as diabetes and systolic blood pressure. The attenuation of the hazard ratios is not surprising; CKD is associated with the presence of many traditional and non-traditional risk factors for cardiovascular disease [25, 26]. However, many risk factors may be a consequence, rather than a cause, of CKD [25, 27]. In such a situation, these factors may be intermediates on the pathway between CKD and the incidence of stroke symptoms. For example, CKD can result in blood pressure elevations, which may cause the incidence of stroke symptoms. In such a situation, adjustment for blood pressure (i.e. a factor in the causal pathway between CKD and stroke symptoms) may not be appropriate. Therefore, it is difficult to disentangle the true association between CKD and these symptoms. From an etiological perspective, the role of CKD on stroke symptoms, independent of comorbid conditions may be weaker than suggested by the unadjusted relation. However, from a public health standpoint, screening for stroke symptoms may identify a large group of individuals with a very high stroke risk.
The incidence of stroke symptoms in the current study was high. The presence of stroke symptoms may or may not reflect the occurrence of cerebral infarction and potential alternative explanations for the symptoms exist. For example, individuals with conditions such as migraine with aura or glaucoma may report sudden loss of vision or half vision. While brain imaging for evidence of prior cerebral infarction or an alternative origin for the symptoms may provide insight into the occurrence of stroke symptoms among individuals without stroke or TIA, this was not feasible in the REGARDS study. Therefore, we cannot confirm that the presence of stroke symptoms reflected the presence of infarction on cerebral imaging.
In addition to the lack of brain imaging, the current study needs to be considered within the context of other limitations. These include the reliance on self-reported stroke and TIA at baseline. While the self-reported history of stroke is reasonably accurate, there is a potential for recall bias wherein stroke survivors may not remember receiving a diagnosis. Additionally, follow-up measurements of eGFR and albuminuria were not available. The REGARDS study has many strengths, including the large national sample with systematic evaluation of CKD using both eGFR and albuminuria and identification of those with history of stroke, TIA and stroke symptoms at baseline. In addition, the extensive data collection in the REGARDS study allowed us to assess the relation between CKD and stroke symptoms after adjustment for many potential confounding factors.
In conclusion, lower levels of eGFR and higher levels of albuminuria are associated with a higher prevalence and incidence of stroke symptoms. Prospective studies are needed to better define the association between stroke symptoms on outcomes including clinical stroke and mortality in the context of patients with CKD. Additionally, the assessment of stroke symptoms may provide a means to identify individuals with a high risk of future stroke. In conjunction with the low rate of care seeking among individuals having stroke symptoms previously reported, the current study highlights the need to increase awareness of these symptoms. This is especially important in populations with a high stroke risk such as those with CKD.
Supplementary Material
Acknowledgments
Funding/support of sponsor. This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis or interpretation of the data or the preparation or approval of the manuscript.
Conflict of interest statement. None declared.
References
- 1.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 2.McCullough PA, Li S, Jurkovitz CT, et al. CKD and cardiovascular disease in screened high-risk volunteer and general populations: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51:S38–S45. doi: 10.1053/j.ajkd.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JL, Jurkovitz CT, Vaccarino V, et al. Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: the ARIC Study. Kidney Int. 2003;64:610–615. doi: 10.1046/j.1523-1755.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya T, Perkovic V, Verdon C, et al. Proteinuria and stroke: a meta-analysis of cohort studies. Am J Kidney Dis. 2009;53:417–425. doi: 10.1053/j.ajkd.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 5.Chambless LE, Toole JF, Nieto FJ, et al. Association between symptoms reported in a population questionnaire and future ischemic stroke: the ARIC study. Neuroepidemiology. 2004;23:33–37. doi: 10.1159/000073972. [DOI] [PubMed] [Google Scholar]
- 6.Hart CL, Hole DJ, Smith GD. The relation between questions indicating transient ischaemic attack and stroke in 20 years of follow up in men and women in the Renfrew/Paisley Study. J Epidemiol Community Health. 2001;55:653–656. doi: 10.1136/jech.55.9.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard VJ, McClure LA, Meschia JF, et al. High prevalence of stroke symptoms among persons without a diagnosis of stroke or transient ischemic attack in a general population: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Arch Intern Med. 2006;166:1952–1958. doi: 10.1001/archinte.166.18.1952. [DOI] [PubMed] [Google Scholar]
- 8.Howard VJ, Lackland DT, Lichtman JH, et al. Care seeking after stroke symptoms. Ann Neurol. 2008;63:466–472. doi: 10.1002/ana.21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31:1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 14.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 15.Meschia JF, Lojacono MA, Miller MJ, et al. Reliability of the questionnaire for verifying stroke-free status. Cerebrovasc Dis. 2004;17:218–223. doi: 10.1159/000075794. [DOI] [PubMed] [Google Scholar]
- 16.Allison P. Estimating Parametric Regression Models with PROC LIFEREG. Survival Analysis Using SAS: A Practical Guide. SAS Publishing, Cary, NC: 2008:61–110. [Google Scholar]
- 17.Finkelstein DM. A proportional hazards model for interval-censored failure time data. Biometrics. 1986;42:845–854. [PubMed] [Google Scholar]
- 18.Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 19.Howard G, Safford MM, Meschia JF, et al. Stroke symptoms in individuals reporting no prior stroke or transient ischemic attack are associated with a decrease in indices of mental and physical functioning. Stroke. 2007;38:2446–2452. doi: 10.1161/STROKEAHA.106.478032. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Cupples LA, Gagnon DR. Incidence, precursors and prognosis of unrecognized myocardial infarction. Adv Cardiol. 1990;37:202–214. doi: 10.1159/000418828. [DOI] [PubMed] [Google Scholar]
- 21.Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135:801–811. doi: 10.7326/0003-4819-135-9-200111060-00010. [DOI] [PubMed] [Google Scholar]
- 22.Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 23.Leary MC, Saver JL. Annual incidence of first silent stroke in the United States: a preliminary estimate. Cerebrovasc Dis. 2003;16:280–285. doi: 10.1159/000071128. [DOI] [PubMed] [Google Scholar]
- 24.Khella S, Bleicher MB. Stroke and its prevention in chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1343–1351. doi: 10.2215/CJN.04341206. [DOI] [PubMed] [Google Scholar]
- 25.Muntner P, Hamm LL, Kusek JW, et al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 26.Weiner DE, Tighiouart H, Elsayed EF, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51:212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh NI, Hwang SJ, Larson MG, et al. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884–1891. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


