Abstract
Background.
Protein–energy wasting is common in patients on maintenance hemodialysis and is strongly associated with poor quality of life and mortality. However, clinical assessment of protein–energy wasting remains difficult. Predialysis creatinine levels are associated with mortality risk but may be influenced by both muscle mass and dialysis dose. This might be overcome by examining the rate of rise in creatinine between dialysis sessions.
Methods.
We conducted an observational cohort study among 81 patients on maintenance hemodialysis at our Veterans Affairs unit. Predialysis serum creatinine and change in serum creatinine between midweek dialysis sessions served as the predictor variables of interest and clinically available proxies of nutritional status and time to mortality served as the outcome variables. Linear regression and Cox proportional hazards models evaluated relationships, respectively.
Results.
The mean age of the study participants was 63 ± 10 years, 77 (95%) were male, mean body mass index was 27 ± 6 kg/m2 and 69% had diabetes. Median follow-up time was 13 months, during which 12 patients (15%) died. Interdialytic change in serum creatinine showed a strong direct correlation with predialysis serum creatinine (R = 0.96). Higher levels of both markers were associated with younger age, less residual urine volume and higher serum albumin, serum phosphorus and normalized protein catabolic rate (P < 0.05 for all). Both markers were approximately equally strongly associated with mortality. For example, compared to the highest predialysis creatinine tertile, participants in the lowest tertile (<6 mg/dL) had 5.5-fold [95% confidence interval (CI) 1.1, 26.6] higher risk of death. Similarly, participants in the lowest tertile of interdialytic change in creatinine (change <3.7 mg/dL/48 h), had 5.0-fold (95% CI 1.0, 24.4) higher death risk.
Conclusions.
Predialysis creatinine and interdialytic change in creatinine are both strongly associated with proxies of nutritional status and mortality in hemodialysis patients and are highly correlated. Interdialytic change in creatinine provided little additional information about nutritional status or mortality risk above and beyond predialysis creatinine levels alone.
Introduction
End-stage renal disease (ESRD) patients have a 5-year survival of only 34%, with 7- to 8-fold greater mortality than similarly aged persons in the general population [1]. Protein–energy wasting is highly prevalent in ESRD patients [2] and is strongly associated with depression [3, 4], decreased functional status, diminished quality of life [4, 5] and mortality [5–9]. A significant hurdle in preventing and treating malnutrition is accurate clinical detection, particularly at early stages where interventions may be more effective. Measurement of multiple anthropometric and biochemical parameters is needed to accurately assess nutritional status in ESRD patients [10], and markers used commonly in the general population such as body mass index (BMI) are relatively insensitive to early wasting and are complicated in ESRD due to fluctuations in total body water. Perhaps, the most commonly used marker of nutritional status, serum albumin, may be more reflective of inflammation than nutritional status in ESRD patients [11].
Muscle wasting is a component of protein–energy wasting and is also highly prevalent in ESRD patients [12]. Diminished muscle mass is strongly associated with mortality, independent of body weight, BMI or percent body fat in this setting [7, 8]. Several methods for measuring muscle mass in ESRD patients have been proposed. Mid-arm muscle circumference and skin fold thickness measurements [7], computed tomographic (CT) thigh imaging [13] and measurements of urinary creatinine excretion [8] have been investigated, but all have significant limitations. For example, CT imaging is expensive and requires radiation exposure, and measurement of urine creatinine is not possible for the many ESRD patients who are anuric.
The primary source of serum creatinine is skeletal muscle, and serum creatinine concentrations are elevated in individuals with greater muscle mass, independent of renal function [14]. Predialysis serum creatinine concentrations are strongly correlated with lean body mass in patients requiring maintenance hemodialysis [15] and are associated with improved functional status [16] and lower mortality risk [6, 17, 18]. However, as a small molecule, serum creatinine is efficiently cleared by dialysis and thus predialysis creatinine levels might be influenced not only by muscle mass but also by the dose of dialysis provided at the preceding dialysis session [2, 19]. Thus, individuals with greater dialysis dose may have lower predialysis creatinine, which may render it less useful as an indicator of muscle mass or marker of death risk.
We hypothesized that the absolute change in serum creatinine between hemodialysis sessions might provide a more specific marker of muscle mass than the predialysis creatinine concentrations alone. We measured both pre- and postdialysis creatinine levels and examined the absolute change (in mg/dL/48 h) rather than the % change (a marker of dialysis dose) in an attempt to mitigate the effect of clearance by dialysis. Thus, we hypothesized that a greater absolute interdialytic change in creatinine would be more strongly associated with proxies of nutritional status and with lower mortality risk compared to predialysis creatinine levels. To test this hypothesis, we compared the associations of interdialytic creatinine change and predialysis creatinine with proxies of nutritional status and mortality in 81 outpatient maintenance hemodialysis patients at our Veterans Affairs (VA) affiliated center.
Methods
Participants
We identified all patients who underwent maintenance hemodialysis at the VA San Diego Healthcare System between May 2009 and July 2010 (N = 98). Patients were included if they had routine surveillance blood laboratory measurements in three consecutive months during the study period. Exclusions were for peritoneal dialysis (n = 1), discontinuation of hemodialysis due to recovery of native kidney function (n = 2), transfer to another dialysis unit (n = 9) or death (n = 5) prior to 3 months of routine labs resulting in a final analytic sample of 81 patients for this analysis. The study protocol was reviewed and approved by the University of California San Diego Institutional Review Board and the VA Research Service. Requirement for patient consent was waived due to the use of retrospective data and minimal participant risk.
Measurements
Predictor Variables.
Serum creatinine was measured using the enzymatic method by a Roche Diagnostics Cobas 6000 machine. Coefficients of variation were <2%. Both pre- and postdialysis creatinine concentrations were measured monthly during the first midweek dialysis session (Wednesday or Thursday) in conjunction with routine monthly laboratory assessments. Specimens were delivered directly to the clinical laboratory on site at the same facility as our dialysis unit, and creatinine measurements were made within 3 h of collection. Predialysis serum creatinine values were averaged over 3 months. Similarly, the absolute interdialytic change in serum creatinine was determined by subtracting the postdialysis creatinine from the predialysis creatinine and averaging the results over 3 months.
Outcome.
Patient deaths during the study period were determined from review of the VA-computerized medical records. Deaths that occur at outside hospitals or at home, in addition to dates and circumstances of death, were reported to the VA and are recorded in the medical record.
Other measurements.
Age, sex and race were determined by self report. Dialysis vintage was determined from date of maintenance hemodialysis initiation recorded in the VA dialysis unit records. Predialysis weights were measured by scale at each dialysis session. Ultrafiltration volume was determined from computerized hemodialysis machine output. Postdialysis weights were determined from scale measurement. Height was obtained from the most recent value recorded in the medical record. BMI was calculated by postdialysis mass in kilograms divided by the square of height in meters. It is the practice of the attending nephrologist and renal pharmacist to ask patients about the volume of residual urine output semiannually and record these data in the patient record. If that information was not available, a 24-h urine collection obtained while a patient was on maintenance hemodialysis or <2 months prior to initiation of hemodialysis, patient estimation recorded in the medical record at hospital admission or measurement of 24-h urine output while a patient was hospitalized were used. Amputation status was determined from review of the medical record and categorized as either a below- or above-the-knee amputation. Normalized protein catabolic rate (nPCR) was calculated as follows:
where blood urea nitrogen (BUN) was measured during routine monthly labs before and after hemodialysis [20].
Dialysis dose (Kt/V) was determined using single-pool variable-volume estimation:
where R is the ratio of postdialysis BUN to predialysis BUN [21]. We also evaluated the urea reduction ratio (URR), which was calculated as:
Albumin, phosphorus and bicarbonate were measured by routine clinical analyzers using the first month’s surveillance blood sample during the study period.
Statistical analyses
Patients were grouped into tertiles based on interdialytic creatinine change and predialysis creatinine levels. Baseline demographic, nutritional and dialysis-related variables were compared across tertiles using analysis of variance or Kruskal–Wallis tests for continuous variables and the χ2 test or Fisher’s exact test for categorical variables, as appropriate. Pearson correlation coefficients were used to evaluate the relationship of interdialytic and predialysis creatinine levels with measures of dialysis dose (URR and Kt/V).
Time-to-death was calculated from the date of the baseline laboratory assessment until the first occurrence of death, discontinuation of dialysis for recovery of renal function, transplantation, transfer to another unit or study closeout (July 1, 2010). Cox proportional hazards models were used to evaluate the associations of interdialytic creatinine change and predialysis creatinine with mortality. Sequential models were developed. An initial model was unadjusted. A subsequent model adjusted for age and race, and a final model adjusted for age, race, BMI, albumin, nPCR, phosphorus, ultrafiltration and residual urine output. All analyses were conducted using STATA statistical software version 11.0 (College Station, TX).
Results
The average age of the 81 maintenance hemodialysis patients was 65 ± 11 years. Only four patients were female, reflecting the gender distribution of our VA population. Fifty-three percent were white, 17% were black, 10% were Hispanic and 11% were Asian. The median dialysis vintage was 22 months (interquartile range 12–53 months). When averaged over the first 3 months of the study, the mean URR was 73.1 ± 8.3% (range 50.7–95.4%). Median follow-up time was 13 months (interquartile range 8–13 months), during which 12 patients (15%) died.
Compared to patients with predialysis creatinine levels in the highest tertile, those in lower tertiles were younger, had lower serum albumin, lower nPCR and lower phosphorus levels (Table 1). They also had higher residual urine output and lower volume of ultrafiltration on dialysis. Individuals with lower predialysis creatinine levels were less frequently black although this result did not reach statistical significance.
Table 1.
Baseline nutritional and dialysis characteristics by tertile of predialysis creatininea
| Tertile (range in mg/dL) | Highest (8.4–13.7) | Middle (6.0–8.3) | Lowest (1.7–6.0) | P-value |
| Age (years) | 61 ± 9 | 62 ± 8 | 69 ± 13 | 0.01 |
| Black (%) | 22 | 22 | 7 | 0.25 |
| Male sex (%) | 96 | 96 | 96 | 0.99 |
| Albumin (g/dL)b | 4.0 (3.9, 4.2) | 3.7 (3.2, 4.1) | 3.6 (3.4, 4.0) | 0.01 |
| Postdialysis weight (kg) | 81 ± 21 | 86 ± 18 | 81 ± 22 | 0.62 |
| BMI (kg/m2) | 26.4 ± 6.1 | 27.5 ± 6.2 | 25.6 ± 5.6 | 0.55 |
| nPCR (g/kg/day)b | 0.96 (0.78, 1.05) | 0.87 (0.72, 0.96) | 0.74 (0.67, 0.90) | 0.03 |
| Phosphorus (mg/dL)b | 5.2 (4.6, 7.1) | 5.4 (4.7, 7.2) | 4.6 (3.8, 4.9) | <0.01 |
| Bicarbonate (mmol/L) | 26.5 ± 3.3 | 25.3 ± 2.3 | 26.5 ± 3.2 | 0.13 |
| Kt/V | 1.71 ± 0.71 | 1.73 ± 0.71 | 1.73 ± 0.57 | 0.99 |
| Ultrafiltration (L) | 3.9 ± 1.3 | 3.1 ± 1.2 | 2.8 ± 1.2 | <0.01 |
| Residual urine volume (mL/day)b | 0 (0, 350) | 200 (0, 500) | 600 (200, 1000) | <0.01 |
| Amputation (%) | 11 | 7 | 11 | 0.87 |
± values are means ± SDs.
Median (25th percentile, 75th percentile).
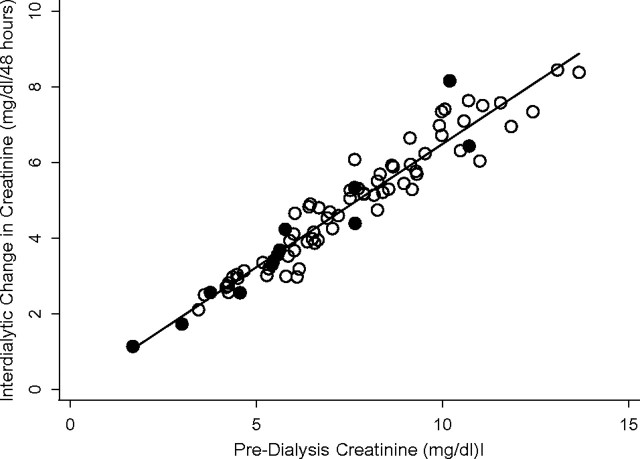
Interdialytic creatinine change had a strong direct correlation with predialysis creatinine concentrations (Pearson correlation r = 0.96; Figure 1). Accordingly, associations with proxies of nutritional status were similar to those observed for predialysis creatinine. Patients in the lower tertiles of interdialytic creatinine change had lower levels of serum albumin and phosphorus, lower nPCR, lower volumes of ultrafiltration and larger volumes of residual urine output (Table 2). They also tended to be older and were less likely to be black, but these results did not reach statistical significance.
Fig. 1.
Predialysis creatinine vs. interdialytic change in creatinine (Pearson correlation: γ = 0.96). Filled circles indicate patients who died during the study period.
Table 2.
Baseline, nutritional and dialysis characteristics by tertile of interdialytic creatinine changea
| Tertile (range in mg/dL/48 h) | Highest (5.4–8.4) | Middle (3.9–5.3) | Lowest (1.1–3.7) | P-value |
| Age (years) | 62 ± 10 | 62 ± 9 | 68 ± 12 | 0.06 |
| Black (%) | 30 | 7 | 15 | 0.09 |
| Male sex (%) | 96 | 93 | 100 | 0.35 |
| Albumin (g/dL)b | 4.0 (3.9, 4.2) | 3.8 (3.2, 4.1) | 3.5 (3.4, 3.7) | <0.001 |
| Postdialysis weight (kg) | 79 ± 19 | 85 ± 19 | 87 ± 25 | 0.40 |
| BMI (kg/m2) | 26.1 ± 5.9 | 27.0 ± 6.0 | 27.9 ± 6.9 | 0.59 |
| nPCR (g/kg/day)b | 0.87 (0.78, 1.03) | 0.89 (0.72, 1.08) | 0.74 (0.67, 0.85) | <0.01 |
| Phosphorus (mg/dL)b | 5.3 (4.6, 7.1) | 5.1 (4.7, 7.2) | 4.6 (3.8, 4.9) | <0.01 |
| Bicarbonate (mmol/L) | 26.2 ± 3.2 | 25.4 ± 2.5 | 26.7 ± 2.7 | 0.24 |
| Kt/V | 1.78 ± 0.71 | 1.70 ± 0.66 | 1.68 ± 0.62 | 0.85 |
| Ultrafiltration (L) | 3.8 ± 1.3 | 3.0 ± 1.3 | 3.1 ± 1.3 | 0.05 |
| Residual urine volume (mL/day)b | 50 (0, 300) | 100 (0, 750) | 600 (350, 1000) | <0.001 |
| Amputation (%) | 11 | 4 | 15 | 0.38 |
± values are means ± SDs.
Median (25th percentile, 75th percentile).
While interdialytic creatinine change and predialysis creatinine concentrations were highly correlated with one another, neither measure was correlated with Kt/V or URR when all participants were evaluated together (Table 3). However, these correlations may have been influenced by residual renal function. While predialysis creatinine and interdialytic change in creatinine remained highly correlated among the subgroup of 24 participants who were completely anuric, inverse correlations between predialysis creatinine and Kt/V or URR emerged (Pearson correlations r = −0.41 and r = −0.46, respectively). Modest correlations also emerged between interdialytic change in creatinine with Kt/V and URR, albeit correlations were weaker and not statistically significant (r = −0.25 and r = −0.21, respectively, Table 4).
Table 3.
Correlation matrix of predialysis and interdialytic change in creatinine with Kt/V and URR in all 81 participantsa
| Predialysis creatinine | Predialysis BUN | Interdialytic change in creatinine | Kt/V | URR | |
| Predialysis creatinine | 1.00 | ||||
| Predialysis BUN | 0.34* | 1.00 | |||
| Interdialytic change in creatinine | 0.96* | 0.30* | 1.00 | ||
| Kt/V | −0.05 | 0.07 | 0.12 | 1.00 | |
| URR | −0.14 | −0.07 | 0.06 | 0.89* | 1.00 |
Data show Pearson correlations.
*P < 0.05.
Table 4.
Correlation matrix of predialysis and interdialytic change in creatinine with Kt/V and URR in all 24 anuric participantsa
| Predialysis creatinine | Predialysis BUN | Interdialytic change in creatinine | Kt/V | URR | |
| Predialysis creatinine | 1.00 | ||||
| Predialysis BUN | 0.36 | 1.00 | |||
| Interdialytic change in creatinine | 0.93* | 0.21 | 1.00 | ||
| Kt/V | −0.40 | −0.20 | −0.25 | 1.00 | |
| URR | −0.46* | −0.43 | −0.21 | 0.97* | 1.00 |
Data show Pearson correlations.
*P < 0.05.
Although there were only 12 deaths during follow-up, we observed strong associations of both lower predialysis creatinine and interdialytic creatinine change with mortality. For predialysis creatinine, two subjects in the highest tertile and two in the middle tertile died, while eight subjects in the lowest tertile died during follow-up. Accordingly, compared to the highest tertile, patients in the lowest tertile had a 5-fold higher risk of death, and each 1 mg/dL lower predialysis creatinine was associated with a 40% greater risk of death in unadjusted analysis (Table 5). While the point estimate remained similar, 95% confidence intervals (CIs) widened and the association was rendered no longer statistically significant with multivariable adjustment.
Table 5.
Association of predialysis serum creatinine concentrations with risk of death in 81 maintenance hemodialysis patientsa
| Tertile (range in mg/dL) | Highest (8.4–13.7) | Middle (6.0–8.3) | Lowest (1.7–6.0) | Per 1 mg/dL lower | P-value |
| Deaths/total patients | 2/27 | 2/27 | 8/27 | N/A | N/A |
| Unadjusted | 1.00 (Ref) | 1.21 (0.17, 8.60) | 5.47 (1.13, 26.55) | 1.44 (1.06, 1.94) | 0.02 |
| Age and race adjusted | 1.00 (Ref) | 1.13 (0.15, 8.34) | 3.56 (0.64, 19.80) | 1.30 (0.95, 1.78) | 0.10 |
| Fully adjustedb | 1.00 (Ref) | 0.45 (0.04, 5.28) | 12.60 (0.66, 241.35) | 1.19 (0.78, 1.81) | 0.43 |
Hazard ratio (95% CI).
Adjusted for age, race, BMI, albumin, nPCR, phosphorus, ultrafiltration and residual urine output.
Results for the association of interdialytic creatinine change with mortality were similar, with the lowest tertile of interdialytic creatinine change also having an ∼5-fold higher risk of death compared with the highest and each 1 mg/dL/48 h lower change in interdialytic creatinine was associated with a 60% higher risk of death in unadjusted analysis (Table 6). The point estimate remained relatively unchanged in multivariable models, although the CIs widened, and the association was rendered no longer statistically significant in the multivariable models.
Table 6.
Association of interdialytic change in creatinine with risk of death in 81 maintenance hemodialysis patientsa
| Tertile (range in mg/dL/48 h) | Highest (5.4–8.4) | Middle (3.9–5.3) | Lowest (1.1–3.7) | Per 1 mg/dL/48 h lower | P-value |
| Deaths/total patients | 2/27 | 3/27 | 7/27 | N/A | N/A |
| Unadjusted | 1.00 (Ref) | 1.19 (0.17, 8.45) | 5.04 (1.03, 24.42) | 1.64 (1.06, 2.55) | 0.03 |
| Age and race Adjusted | 1.00 (Ref) | 1.28 (0.18, 9.25) | 3.57 (0.70,18.23) | 1.43 (0.92, 2.24) | 0.11 |
| Fully adjustedb | 1.00 (Ref) | 0.88 (0.08, 9.13) | 18.23 (1.18, 280.65) | 1.39 (0.75, 2.58) | 0.30 |
Hazard ratio (95% CI).
Adjusted for age, race, BMI, albumin, nPCR, phosphorus, ultrafiltration and residual urine output.
Discussion
The primary finding of this study is that measurement of interdialytic change in creatinine is strongly associated with markers of nutritional status and with mortality risk in patients on maintenance hemodialysis. Since we hypothesized that predialysis creatinine levels might be influenced by dialysis clearance in addition to muscle mass, we examined whether interdialytic change in creatinine might provide a more specific marker of muscle mass and nutritional status and might therefore be a more useful marker of mortality risk. We observed no significant association of predialysis creatinine with dialysis clearance in the entire study sample and only a moderate correlation in the subset who were anuric. Furthermore, predialysis creatinine and interdialytic change in creatinine were highly correlated with one another irrespective of the presence or absence of residual renal function, and their relative strengths of association with proxies of nutritional status and mortality risk were very similar. These findings suggest that, compared to the influence of muscle mass, dialysis clearance may have a relatively modest effect on predialysis creatinine concentrations. Accordingly, interdialytic creatinine change provided little additional information about nutritional status or death risk compared to predialysis creatinine levels alone.
We hypothesized that predialysis creatinine levels would be influenced not only by muscle mass but also by dialysis clearance. This principle differentiates between the relative versus the absolute change in creatinine with each dialysis session. For example, let us compare two hypothetical hemodialysis patients; the first has a postdialysis creatinine of 3 mg/dL and a predialysis creatinine level of 10 mg/dL, while the second has a postdialysis creatinine of 12 mg/dL and a predialysis creatinine of 40 mg/dL. Both individuals have a 70% relative reduction in creatinine with dialysis, reflecting similar small molecule clearance. However, in absolute terms, the first individual only produces 7 mg/dL of creatinine between dialysis session, whereas the second produces 28 mg/dL. In the second individual, both the predialysis serum creatinine and the absolute interdialytic change in creatinine suggest greater muscle mass. We hypothesized that lower dialysis dose may render the predialysis creatinine level higher than it would otherwise be, irrespective of muscle mass. Thus, our goal was to investigate the absolute change in creatinine between dialysis sessions as an indicator of muscle mass. However, we observed no association of dialysis dose (Kt/V or URR) with predialysis creatinine levels or with interdialytic change in creatinine in the entire study sample and only a moderate correlation in the subset who were anuric. Predialysis creatinine and interdialytic change in creatinine remained highly correlated irrespective of residual renal function. This suggests that predialysis creatinine largely provides the same information as the interdialytic change in creatinine with respect to muscle mass, and the latter measure adds little additional information about nutritional status.
Shinzato et al. [19] used creatinine kinetics to model creatinine generation rate in a small sample of anuric ESRD patients in Japan. They subjected 16 patients to a marked increase in dialysis dose, by simultaneous increases in dialysis membrane area, treatment time and blood flow rate. They observed that dialysis dose had essentially no effect on estimated creatinine generation rate, whereas there was a modest decline in the predialysis creatinine level. The correlation of predialysis creatinine levels and estimated creatinine generation rate was not reported. These findings are generally similar to the data presented here. We observed strong relationships of both predialysis creatinine and interdialytic change in creatinine with proxies of nutritional status. Furthermore, while we observed no correlation of dialysis dose to either predialysis creatinine or interdialytic change in creatinine in the overall study sample, this finding was modified in subjects who where anuric, where we observed inverse correlations with dialysis dose. However, these correlations were modest in comparison to that of predialysis creatinine and interdialytic change in creatinine with one another. Collectively, these data demonstrate that the amount of muscle mass is the predominant determinant of both predialysis creatinine levels and interdialytic change in creatinine levels irrespective of residual renal function.
We investigated a sample of 81 patients, among whom 12 died during follow-up, providing relatively little statistical power for mortality analysis. Nonetheless, our findings are consistent with previous studies demonstrating that lower muscle mass determined by other methods is associated with mortality in patients on hemodialysis and multiple studies demonstrating that low predialysis creatinine is a powerful risk factor for death [7–9, 22, 23]. Similar findings were recently extended by comparing creatinine levels on dialysis with outcomes after subsequent kidney transplant [24]. We did not have other measures of muscle mass such as CT or DEXA scans in our study. However, a recent study demonstrated that serum creatinine correlates well with lean body mass measured by DEXA and near infrared interactance in patients on hemodialysis and an equation estimating lean body mass from serum creatinine and the URR was developed [15]. Others have shown that higher predialysis creatinine levels are associated with nPCR [25]. Thus, our data provide additional support to the hypothesis that predialysis creatinine concentrations may provide an inexpensive and readily available marker of muscle mass, nutritional status and mortality risk without the cost, burden or radiation exposure required by other methods.
Kalantar-Zadeh et al. [25] recently showed that changes in predialysis serum creatinine levels within individual patients over time (over months) was associated with mortality, independent of changes in the BMI. This suggests that the success of interventions aimed at improving muscle mass, such as exercise or nutrition programs, could be monitored using predialysis creatinine levels. Such a strategy is inexpensive, readily available, renders little or no patient burden and avoids radiation exposure. On the other hand, if such individuals are also losing residual renal function, there may be multiple factors influencing predialysis creatinine levels, which may render it less useful as a surrogate of muscle mass.
This study is novel as it is the first to our knowledge to evaluate interdialytic change in creatinine as a potential surrogate of muscle mass in ESRD. However, the study also has important limitations. Study participants came from one center and were mostly male. Available follow-up time was relatively brief and few deaths were observed. No confirmatory test for muscle mass was available. Nonetheless, the strong correlation of predialysis creatinine with interdialytic change in creatinine suggest that little additional information is garnered by measuring the latter. We measured creatinine levels within 3 h of collection and serum creatinine is known to equilibrate across red blood cell membranes over 6–12 h [26]. Results may differ if samples are stored for longer periods prior to creatinine measurement. Residual renal function was ascertained by asking patients to estimate their daily urine volume in most cases, rather than by collecting timed urine specimens. Associations of dialysis dose with predialysis creatinine were stronger in subjects that were anuric. Whether results would be similar with timed measurements is uncertain, however, it is likely that patient reported urine volumes would be subject to non-differential misclassification and would bias toward the null.
In conclusion, we found that low predialysis creatinine concentrations and low interdialytic change in creatinine are both associated with proxies of poor nutritional status and high mortality risk. Both predialysis creatinine and interdialytic change in creatinine were more strongly correlated with proxies of nutritional status than with dialysis dose. While interdialytic change in creatinine had similar associations with nutritional proxies and mortality, these associations were not stronger and hence measuring interdialytic creatinine change provided little additional information about nutritional status and mortality risk than that garnered by predialysis creatinine concentration in isolation. Future studies should evaluate if predialysis creatinine concentrations represent an inexpensive and readily available surrogate to measure changes in muscle mass over time and to determine whether interventions that improve predialysis creatinine levels are associated with demonstrable improvements in hard health outcomes in maintenance dialysis patients.
Acknowledgments
This study was supported by grants from the National Heart Lung and Blood Institute (NHLBI: R01 HL096851) to Dr. J.H.I.
Conflict of interest statement. None declared.
References
- 1.U.S. Renal Data System, USRDS 2009. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: NIDDK, National Institutes of Health; 2009. [Google Scholar]
- 2.Fouque D, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 3.Koo JR, et al. Association of depression with malnutrition in chronic hemodialysis patients. Am J Kidney Dis. 2003;41:1037–1042. doi: 10.1016/s0272-6386(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim S, El Salamony O. Depression, quality of life and malnutrition-inflammation scores in hemodialysis patients. Am J Nephrol. 2008;28:784–791. doi: 10.1159/000131101. [DOI] [PubMed] [Google Scholar]
- 5.Rambod M, et al. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53:298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 7.Huang CX, et al. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int. 2010;77:624–629. doi: 10.1038/ki.2009.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beddhu S, et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, et al. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 10.Fouque D, et al. EBPG guideline on nutrition. Nephrol Dial Transplant. 2007;22(Suppl 2):ii45–ii87. doi: 10.1093/ndt/gfm020. [DOI] [PubMed] [Google Scholar]
- 11.Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. 2010;21:223–230. doi: 10.1681/ASN.2009020213. [DOI] [PubMed] [Google Scholar]
- 12.Mak RH, Rotwein P. Myostatin and insulin-like growth factors in uremic sarcopenia: the yin and yang in muscle mass regulation. Kidney Int. 2006;70:410–412. doi: 10.1038/sj.ki.5001622. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa S, et al. Standardized thigh muscle area measured by computed axial tomography as an alternate muscle mass index for nutritional assessment of hemodialysis patients. Am J Clin Nutr. 2000;71:485–490. doi: 10.1093/ajcn/71.2.485. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald JH, et al. The relationship between estimated glomerular filtration rate, demographic and anthropometric variables is mediated by muscle mass in non-diabetic patients with chronic kidney disease. Nephrol Dial Transplant. 2006;21:3488–3494. doi: 10.1093/ndt/gfl430. [DOI] [PubMed] [Google Scholar]
- 15.Noori N, et al. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis. 2011;57:130–139. doi: 10.1053/j.ajkd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrowes JD, et al. Cross-sectional relationship between dietary protein and energy intake, nutritional status, functional status, and comorbidity in older versus younger hemodialysis patients. J Ren Nutr. 2002;12:87–95. doi: 10.1053/jren.2002.32209. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer JT, et al. Are nutritional status indicators associated with mortality in the Hemodialysis (HEMO) Study? Kidney Int. 2005;68:1766–1776. doi: 10.1111/j.1523-1755.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 19.Shinzato T, et al. New method to calculate creatinine generation rate using pre- and postdialysis creatinine concentrations. Artif Organs. 1997;21:864–872. doi: 10.1111/j.1525-1594.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 20.Jindal KK, Goldstein MB. Urea kinetic modeling in chronic hemodialysis: benefits, problems, and practical solutions. Semin Dial. 1988;1:82–85. [Google Scholar]
- 21.Daugirdas JT. Linear estimates of variable-volume, single-pool Kt/V: an analysis of error. Am J Kidney Dis. 1993;22:267–270. doi: 10.1016/s0272-6386(12)70317-7. [DOI] [PubMed] [Google Scholar]
- 22.Beddhu S, et al. Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol. 2003;14:1000–1005. doi: 10.1097/01.asn.0000057856.88335.dd. [DOI] [PubMed] [Google Scholar]
- 23.Moreau-Gaudry X, et al. Serum creatinine improves body mass index survival prediction in hemodialysis patients: A 1-Year Prospective Cohort Analysis From the ARNOS Study. J Ren Nutr. doi: 10.1053/j.jrn.2010.08.005. 2011; in Press. [DOI] [PubMed] [Google Scholar]
- 24.Streja E, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6:1463–1473. doi: 10.2215/CJN.09131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Descombes E, Perriard F, Fellay G. Diffusion kinetics of urea, creatinine and uric acid in blood during hemodialysis. Clinical implications. Clin Nephrol. 1993;40:286–295. [PubMed] [Google Scholar]