Abstract
Background.
The interrelationship of left ventricular hypertrophy (LVH) with ejection fraction (EF) and their impact on mortality in non-dialysis-dependent chronic kidney disease (NDD-CKD) is unclear.
Methods.
We examined the associations of EF and LVH with all-cause mortality in a historic cohort of 650 male US veterans with moderate-to-advanced NDD-CKD. EF and LVH were examined both separately and after categorizing patients according to their concomitant EF and presence/absence of LVH. Associations with mortality were examined in Cox models with adjustments for demographics, blood pressure, comorbidities, smoking status, medication use and biochemical characteristics.
Results.
EF <30 and 30–50% were associated with higher all-cause mortality compared to EF >50% even after multivariable adjustments [multivariable adjusted hazard ratio, 95% confidence interval (CI): 2.83 (1.86–4.30) and 1.38 (1.06–1.78), P < 0.001 for linear trend]. LVH in itself was not associated with mortality [multivariable adjusted hazard ratio, 95% CI: 0.83 (0.66–1.05), P = 0.12], but the presence of LVH combined with an EF <50% was associated with the highest mortality [multivariable adjusted hazard ratios, 95% CI in patients with EF >50% + LVH, EF ≤50%−LVH and EF ≤50% + LVH, compared to EF >50%−LVH: 0.84 (0.63–1.13), 1.36 (1.00–1.83) and 1.62 (1.07–2.46)].
Conclusions.
Low EF is associated with higher mortality in patients with NDD-CKD. In the presence of a low EF, LVH is also associated with higher mortality. Clinical trials are needed to determine if interventions targeting patients with low EF and LVH can lower mortality in NDD-CKD.
Keywords: chronic kidney disease, echocardiography, ejection fraction, left ventricular hypertrophy, mortality
Introduction
The mortality rate in chronic kidney disease (CKD) patients is very high, including patients with end-stage renal disease (ESRD) on hemodialysis (HD) [1–3] and patients with non-dialysis-dependent (NDD)-CKD [3, 4]. Cardiovascular mortality accounts for most of these deaths, emphasizing the importance of investigating and treating identifiable cardiovascular abnormalities in CKD and ESRD. Echocardiography has been a useful tool, as it provides information on both functional and anatomical characteristics such as left ventricular hypertrophy (LVH) and ejection fraction (EF). LVH is very common in patients with ESRD; it has been found to be present in 46–74% of patients at the time of initiation of dialysis therapy [5–7], compared to 51% in patients with coronary artery disease (CAD) and 12–20% in patients with hypertension [8, 9]. This high prevalence of LVH in ESRD can be explained by the confluence of various factors in this patient population, including afterload-dependent (arterial resistance and renin–angiotensin activation), preload-dependent (volume overload, anaemia and arterio-venous fistulas) and non-preload- or afterload-dependent factors (hyperparathyroidism, hyperphosphatemia, hyperhomocysteinemia, cytokine aberrations, hyperaldosteronism and vitamin D deficiency) [6, 7, 10–13]. Additionally, LVH has also been recognized as a compensatory mechanism in patients with left ventricular (LV) dilation and with high afterload. The presence of LVH has been associated with increased mortality in some studies of ESRD patients [14–16] but another study examining the predictive ability of LVH in the context of LV function suggested the presence of an interaction between these two characteristics, and patients with both LVH and decreased LV function had the highest mortality [17]. The same study established low LV function as a significant predictor of increased cardiovascular mortality independent of LVH or other classic or novel cardiovascular risk factors in ESRD [17], and the importance of LV function was further emphasized by the finding that longitudinal changes in this parameter were also shown to be significant predictors of cardiovascular morbidity and mortality [18]. The complex relationship between LVH and LV function was also highlighted by an earlier study in ESRD patients with normal systolic function showing that despite both high LV mass and high LV volume being independent predictors of late mortality, prognosis was unaffected when both lesions were noted [19], possibly because the prognostic effects of LVH may be dependent on concomitant EF. Similarly, detailed descriptions of the characteristics and consequences of LVH and LV function in patients with NDD-CKD have not been previously performed, in spite of the larger numbers of patients with this condition and their high mortality [20]. Since the development of LVH in patients with normal and with decreased EF may be related to fundamentally different pathophysiological processes, it is important to consider both characteristics when examining outcomes associated with them [21].
We conducted a study to assess the association of echocardiographic abnormalities (LVH and reduced EF) with mortality in a large historical cohort of male US veterans with NDD-CKD from a single medical center.
Materials and methods
Study population and data collection
We reviewed the medical records of all patients who were referred for NDD-CKD evaluation at Salem Veterans Affairs Medical Center (VAMC) between 1 January 1990 and 30 June 2005 [22, 23]. Of 1012 total patients, 661 (65%) underwent echocardiography for clinical indications. Patients with available echocardiography were similar in age, race and gender to patients without echocardiographic studies, but they were significantly more likely to have cardiovascular disease and diabetes mellitus (data not shown). Seven female patients and four patients whose race was other than white or black were excluded. The final study population consisted of 650 patients.
Clinical characteristics recorded at the time of the patients’ undergoing echocardiography were extracted retrospectively, including demographic and anthropometric characteristics, comorbid conditions and laboratory results. Medication use including that of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARB), aspirin and beta blockers was assessed at baseline and also over the entire follow-up period. Glomerular filtration rate (GFR) was estimated using the abbreviated equation developed for the Modification of Diet in Renal Disease Study (MDRD) [24] and categorized according to the staging system introduced by the Kidney/Dialysis Outcome Quality Initiative (K/DOQI) Clinical Practice Guidelines for CKD: Evaluation, Classification, and Stratification [25]. All the biochemical measurements were performed in a single laboratory at the Salem VAMC. Echocardiography reports were reviewed for the level of EF and for the presence/absence of LVH. If only a qualitative assessment was available for EF in the report, then ‘normal EF’ was categorized as >50%, ‘mild’ or ‘moderate decrease in EF’ as 30–50% and ‘severe decrease in EF’ as <30%. For analyses that considered EF as a continuous variable arbitrary EF values of 55, 45, 35 and 25% were assigned to correspond with the above qualitative categories.
Statistical analyses
Data points were missing for comorbidity index (1.2% missing), body mass index (21.7% missing), smoking status (5.4% missing), blood pressure (15.7% missing), serum albumin (0.8% missing), phosphorus (0.6% missing), blood cholesterol (2% missing), haemoglobin (1% missing), white blood cell count (WBC, 0.5% missing), percent lymphocytes in WBC (0.8% missing) and 24-h urine protein (2.8% missing). Missing data points were not imputed in primary multivariable analyses. Imputations were performed for sensitivity analyses by using linear regression with all the other patient characteristics as independent variables for continuous variables and by creating dummy categories corresponding for the missing data points for categorical variables.
Outcomes analysis
The start time for survival analysis was the date when the echocardiogram was performed. Patients were considered lost to follow-up if no contact was documented with them for >6 months, and they were censored at the date of the last documented contact. The outcome measure of interest was all-cause mortality (ascertained from VA electronic records). The associations of EF, LVH and the combination of EF and LVH with all-cause mortality were evaluated in Cox models with adjustment for potential confounders. Selection of variables to be included in the final multivariable models was done a priori by determining probable confounders [26] based on differences in baseline characteristics between patients with different EF levels and presence/absence of LVH and based on theoretical considerations. Models were thus constructed to assess unadjusted (Model 0), age, race, comorbidity (CAD, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, malignancies and Charlson comorbidity index), medication use and smoking-adjusted (Model 1) and Model 1 + estimated GFR, albumin, cholesterol, calcium, phosphorus, haemoglobin, WBC count, percent of lymphocytes in WBC count and 24 h urine protein-adjusted (Model 2) associations. Since blood pressure could be a confounder but could also be in the path of the outcomes associated with EF and/or LVH, we constructed additional models to include further adjustments for baseline systolic and diastolic blood pressures (Model 3). EF was examined both as a continuous variable and after categorizing it according to prespecified cutoffs (<30, 30–50 and >50%). The concomitant effects of EF and LVH were examined in patients who were categorized according to their EF level and the concomitant presence or absence of LVH: EF >50% and no LVH (referent category), EF >50% and LVH, EF ≤50% and no LVH and EF ≤50% and LVH. Prespecified subgroup analyses were performed in patients categorized by their age, race, systolic and diastolic blood pressure, presence/absence of CAD and estimated GFR. Sensitivity analyses were performed by imputing missing values of independent variables and by adjusting for future medication use analogous to intent to treat analyses. The proportionality assumption was tested by using Schoenfeld residuals. P-values of <0.05 were considered significant. Statistical analyses were performed using STATA statistical software version 11 (STATA Corporation, College Station, TX). The study protocol was approved by the Research and Development Committee at the Salem VAMC.
Results
The mean (± SD) age of the cohort was 68 ± 10 years, 23% of patients were black and the mean estimated GFR was 37 ± 21 mL/min/1.73m2. Most patients had CKD Stages 3 (46%) and 4 (26%) with fewer patients categorized as CKD Stages 1 (2%), 2 (11%) and 5 (14%). A total of 455 patients died [mortality rate: 154/1000 patient-years, 95% confidence interval (CI): 140–169] over a median follow-up of 3.7 years. Thirty-two patients (5%) were lost to follow-up; their characteristics were not significantly different (data not shown).
Baseline characteristics in patients divided by categories of EF and by the presence/absence of LVH are shown in Table 1 and Table 2. Patients with lower EF had lower systolic blood pressure, cholesterol and proteinuria, higher Charlson index, were more likely to have CAD and to use ACEI/ARB and were less likely to have LVH to be active smokers and to have malignancies. Patients with LVH were more likely to be black and less likely to be active smokers and to have CAD had a lower EF and estimated GFR and had higher serum phosphorus and proteinuria.
Table 1.
Baseline characteristics of individuals stratified by their EFa
| EF <30% (N = 51) | EF 30–50% (N = 194) | EF >50% (N = 405) | P for trend | |
| Age (years) | 69 ± 9 | 68 ± 10 | 68 ± 11 | 0.4 |
| Race (Black) | 11 (22) | 33 (17) | 105 (26) | 0.06 |
| BMI (kg/m2) | 27.9 ± 4.9 | 29.3 ± 5.2 | 29.6 ± 6.1 | 0.2 |
| Active smoking | 5 (11) | 42 (24) | 115 (29) | 0.007 |
| SBP (mmHg) | 134.3 ± 20.2 | 145.7 ± 26.2 | 146.7 ± 26.0 | 0.051 |
| DBP (mmHg) | 71.1 ± 13.1 | 70.8 ± 16.1 | 69.2 ± 15.7 | 0.4 |
| Charlson comorbidity index | 2.9 ± 1.3 | 3.0 ± 1.8 | 2.4 ± 1.7 | 0.001 |
| DM | 34 (67) | 125 (65) | 238 (59) | 0.1 |
| CAD | 32 (63) | 119 (62) | 183 (46) | <0.001 |
| CVA | 7 (14) | 22 (11) | 67 (17) | 0.16 |
| PVD | 19 (37) | 52 (27) | 102 (25) | 0.13 |
| LVH | 13 (25) | 56 (29) | 209 (52) | <0.001 |
| Hyperlipidemia | 24 (47) | 92 (47) | 204 (51) | 0.4 |
| Malignancy | 6 (12) | 27 (14) | 85 (21) | 0.017 |
| Liver disease | 3 (6) | 6 (3) | 27 (7) | 0.3 |
| Pulmonary disease | 16 (31) | 40 (21) | 95 (24) | 0.7 |
| eGFR (mL/min/1.73m2) | 37.7 ± 17.2 | 37.2 ± 23.0 | 36.6 ± 20.1 | 0.7 |
| Serum albumin (g/dL) | 3.3 ± 0.7 | 3.4 ± 0.6 | 3.5 ± 0.7 | 0.13 |
| Serum cholesterol (mg/dL) | 163 ± 54 | 181 ± 49 | 187 ± 55 | 0.006 |
| Serum calcium (mg/dL) | 9.1 ± 0.6 | 9.0 ± 0.7 | 9.03 ± 0.68 | 0.9 |
| Serum phosphorus (mg/dL) | 4.1 ± 1.5 | 3.9 ± 1.0 | 3.9 ± 1.0 | 0.3 |
| Blood Hgb (g/dL) | 12.4 ± 2.0 | 12.1 ± 2.1 | 12.2 ± 2.1 | 0.8 |
| Blood WBC (1000/mm3) | 7.8 ± 3.4 | 8.0 ± 3.1 | 8.4 ± 4.3 | 0.16 |
| Blood lymphocytes (% WBC) | 23.6 ± 25.7 | 20.2±9.4 | 22.2±15.5 | 0.7 |
| Bicarbonate (mmol/L) | 26.4 ± 4.2 | 25.8 ± 4.2 | 25.6 ± 4.0 | 0.2 |
| Proteinuria (mg/24 h) | 500 (329–760) | 925 (738–1158) | 968 (835–1123) | 0.02 |
| Statin use | 19 (37) | 98 (51) | 182 (45) | 0.9 |
| ACEI/ARB use | 41 (80) | 128 (66) | 245 (61) | 0.007 |
| ASA use | 30 (59) | 107 (55) | 199 (49) | 0.09 |
| Beta blocker use | 27 (53) | 103 (53) | 208 (51) | 0.7 |
Data are presented as means ± SD, number (% of total) or geometric means (95% CI). BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; CVA, cerebrovascular accident; PVD, peripheral vascular disease; eGFR, estimated GFR; Hgb, haemoglobin; ASA, amino salicylic acid. Comparisons were made by chi-square test for linear trend.
Table 2.
Baseline characteristics of individuals stratified by the presence or absence of LVHa
| LVH (N = 278) | No LVH (N = 369) | P-value | |
| Age (years) | 67.6 ± 10.3 | 68.3 ± 10.6 | 0.4 |
| Race (Black) | 86 (31) | 61 (17) | <0.001 |
| BMI (kg/m2) | 29.2 ± 5.9 | 29.2 ± 5.9 | 0.9 |
| Active smoking | 79 (30) | 81 (23) | 0.045 |
| SBP (mmHg) | 149.3 ± 27.0 | 145.4 ± 25.5 | 0.09 |
| DBP (mmHg) | 71.1 ± 16.5 | 70.2 ± 15.3 | 0.5 |
| Charlson comorbidity index | 2.6 ± 1.7 | 2.6 ± 1.7 | 0.8 |
| DM | 166 (60) | 229 (62) | 0.5 |
| CAD | 130 (47) | 203 (56) | 0.033 |
| CVA | 44 (16) | 51 (14) | 0.5 |
| PVD | 70 (25) | 103 (28) | 0.4 |
| EF | 46 ± 12 | 51 ± 10 | <0.001 |
| Hyperlipidemia | 129 (47) | 190 (52) | 0.2 |
| Malignancy | 46 (17) | 72 (20) | 0.3 |
| Liver disease | 15 (5) | 20 (5) | 0.9 |
| Pulmonary disease | 62 (22) | 89 (24) | 0.6 |
| eGFR (mL/min/1.73m2) | 34.9 ± 19.9 | 38.4 ± 21.3 | 0.03 |
| Serum albumin (g/dL) | 3.4 ± 0.6 | 3.5 ± 0.7 | 0.7 |
| Serum cholesterol (mg/dL) | 186.3 ± 55.1 | 181.0 ± 52.4 | 0.2 |
| Serum calcium (mg/dL) | 9.1 ± 0.6 | 9.0 ± 0.7 | 0.12 |
| Serum phosphorus (mg/dL) | 4.0 ± 1.0 | 3.8 ± 1.1 | 0.03 |
| Blood Hgb (g/dL) | 12.0 ± 2.1 | 12.3 ± 2.1 | 0.14 |
| Blood WBC (1000/mm3) | 8.2 ± 3.43 | 8.3 ± 4.2 | 0.6 |
| Blood lymphocytes (% WBC) | 22.4 ± 13.9 | 21.2 ± 16 | 0.3 |
| Bicarbonate (mmol/L) | 25.9 ± 4.2 | 25.6 ± 4.0 | 0.4 |
| Proteinuria (mg/24 h) | 1054 (885–1256) | 804 (684–946) | 0.027 |
| Statin use | 123 (44) | 174 (47) | 0.5 |
| ACEI/ARB use | 178 (64) | 234 (63) | 0.8 |
| ASA use | 141 (51) | 191 (53) | 0.7 |
| Beta blocker use | 145 (52) | 191 (52) | 0.9 |
Data are presented as means ± SD, number (% of total) or geometric means (95% CI). BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; CVA, cerebrovascular accident; PVD, peripheral vascular disease; eGFR, estimated GFR; Hgb, haemoglobin; ASA, amino salicylic acid. Comparisons were made by t-test or chi-square test.
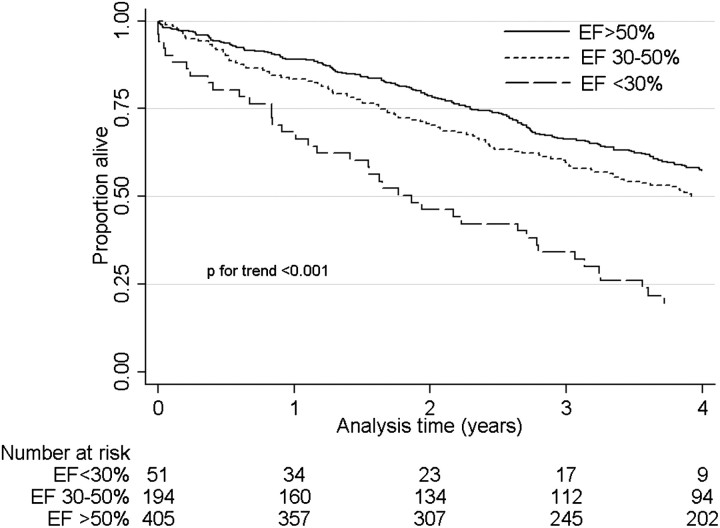
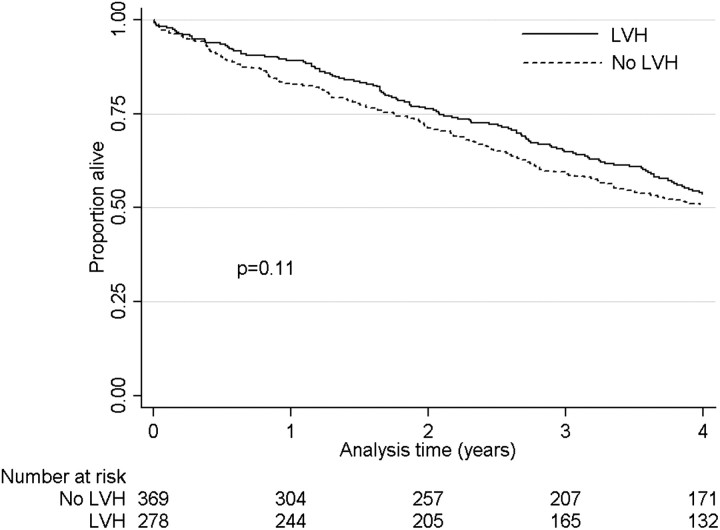
Figure 1 shows Kaplan–Meier survival curves in patients with different levels of baseline EF, indicating significantly higher mortality in patients with lower EF. In unadjusted Cox models, a 10% lower EF was associated with a hazard ratio (95% CI) of mortality of 1.26 (1.16–1.37), P < 0.001, which remained consistent after adjustments for confounders: 1.19 (1.09–1.30), P < 0.001 (Model 1), 1.24 (1.14–1.35), P < 0.001 (Model 2) and 1.25 (1.15–1.36), P < 0.001 (Model 3). Compared to EF >50%, an EF of 30–50 and <30% were associated with higher mortality even after sequential adjustments for various potential confounders (Table 3). Figure 2 shows Kaplan–Meier survival curves in patients with and without LVH on echocardiography, indicating similar mortality in the two groups. LVH was not associated with mortality in any of the multivariable adjusted models, except in Model 2, in which it was associated with significantly lower mortality (Table 3). Table 4 shows hazard ratios (95% CI) of all-cause mortality in patients grouped according to their EF level and the concomitant presence or absence of LVH. Patients with EF >50% had similar hazard ratios for mortality irrespective of the presence or absence of LVH. Patients with EF ≤50% had significantly higher hazard ratios of mortality compared to patients with EF >50% irrespective of the presence or absence of LVH. The presence of LVH combined with an EF <50% was associated with the highest hazard ratio of mortality after adjustment for blood pressure levels in Model 3 (Table 4).
Fig. 1.
Kaplan–Meier survival curves of patients categorized according to their EF level. Comparisons were made by the log rank test for equality of survivor function.
Table 3.
Hazard ratios (95% CIs) of all-cause mortality associated with various levels of EF and with the presence of LVHa
| EF <30% N = 51 | EF 30–50% N = 194 | EF >50% (referent) N = 405 | P for trend | LVH N = 278 | No LVH (referent) N = 369 | P | |
| Model 0 | 2.57 (1.87–3.54) | 1.25 (1.02–1.53) | 1 | <0.001 | 0.86 (0.71–1.04) | 1 | 0.11 |
| Model 1 | 2.25 (1.57–3.23) | 1.16 (0.93–1.45) | 1 | <0.001 | 0.88 (0.71–1.07) | 1 | 0.2 |
| Model 2 | 2.62 (1.78–3.87) | 1.25 (0.99–1.57) | 1 | <0.001 | 0.80 (0.65–0.99) | 1 | 0.04 |
| Model 3 | 2.83 (1.86–4.30) | 1.38 (1.06–1.78) | 1 | <0.001 | 0.83 (0.66–1.05) | 1 | 0.12 |
Associations were examined in unadjusted Cox models (Model 0) and with adjustments for age, race, CAD, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, malignancies, Charlson index, baseline medication use and smoking (Model 1), for Model 1 variables + estimated GFR, albumin, cholesterol, calcium, phosphorus, haemoglobin, WBC count, percent of lymphocytes in WBC count and 24 h urine-adjusted (Model 2) and for Model 2 variables + systolic and diastolic blood pressure.
Fig. 2.
Kaplan–Meier survival curves of patients categorized according to the presence or absence of LVH. Comparisons were made by the log rank test for equality of survivor function.
Table 4.
Hazard ratios (95% CIs) of all-cause mortality in patients categorized according to their EF and the concomitant presence or absence of LVHa
| EF >50%, No LVH (referent) N = 196 | EF >50%, LVH N = 209 | EF ≤50%, No LVH N = 176 | EF ≤50%, LVH N = 69 | |
| Model 0 | 1 | 0.92 (0.73–1.17) | 1.38 (1.08–1.75) | 1.33 (0.96–1.83) |
| Model 1 | 1 | 0.92 (0.71–1.19) | 1.25 (0.96–1.62) | 1.25 (0.88–1.79) |
| Model 2 | 1 | 0.80 (0.61–1.04) | 1.22 (0.92–1.60) | 1.31 (0.91–1.90) |
| Model 3 | 1 | 0.84 (0.63–1.13) | 1.36 (1.00–1.83) | 1.62 (1.07–2.46) |
Associations were examined in unadjusted Cox models (Model 0) and with adjustments for age, race, CAD, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, malignancies, Charlson index, baseline medication use and smoking (Model 1), for Model 1 variables + estimated GFR, albumin, cholesterol, calcium, phosphorus, haemoglobin, WBC count, percent of lymphocytes in WBC count and 24 h urine-adjusted (Model 2) and for Model 2 variables + systolic and diastolic blood pressure.
Similar associations were detected in subgroup analyses. The results also did not change significantly in sensitivity analyses using imputed values for missing covariates and by adjusting for future medication usage (data not shown).
Discussion
We describe an independent association of lower EF with higher mortality in patients with moderate and advanced NDD-CKD. Importantly, in patients with higher EF, the presence of LVH had no impact on mortality (and was even associated with a trend towards a protective effect in adjusted models), but in those with lower EF, the presence of LVH was associated with higher mortality.
LVH has been shown to predict mortality independent of clinical risk factors in the general population and in hypertensive patients [15], and systolic dysfunction and LV dilation have also been associated with increased cardiovascular risk and mortality in the general population and patients with ESRD [19]. Furthermore, in an earlier prospective cohort of 254 ESRD patients, Zoccali et al. [17] described low LV function (measured with three different methods) as a significant predictor of cardiovascular mortality independent of the presence of LVH and described a significant interaction between LVH and low LV function in that patients who had both of these characteristics experienced the highest mortality. The importance of low LV function as both a predictor of poor outcomes and also as a potential therapeutic target was further emphasized by a subsequent study based on the same prospective cohort showing that a decrease in LV function on repeated evaluation was associated with poor cardiovascular outcomes, but an improvement was associated with significantly improved outcomes [18]. Our results corroborate some of these findings and extend them to patients with NDD-CKD, suggesting that low EF is a significant predictor of outcomes in them, and that the complex interaction between LVH and LV function extends to this much larger group of patients. LVH is both a target organ response to hemodynamic as well as non-hemodynamic stimuli and a risk factor responsible for cardiac events. Clinical factors responsible for generation of LVH are not physiologically homogenous and this may underlie the differences in outcomes associated with LVH in different settings including reduced versus normal EF. CKD patients have many factors associated with the generation of LVH [6, 7, 10–13], and it is possible that LVH in this setting may be a distinct lesion and may have distinct cardiac sequelae from LVH generated in the context of other disease states. It has previously been shown that the generation of LVH due to pressure versus volume overload has a distinct underlying signaling mechanism and different patterns and mechanisms of growth [21]. LVH is a compensatory mechanism, but it is not perfectly regulated via a typical feedback loop. As a result, while the initially developing LVH may be compensatory, adverse remodeling often develops whereby the ventricle becomes more dilated and ultimately diastolic and systolic dysfunction ensue. Patients with preserved EF and LVH may represent those who have a more balanced and hence purely adaptive stage of LVH, which could explain their lack of association with mortality.
Another possible explanation for the discordant association of LVH with mortality is an imbalance in LV functional and anatomical characteristics. LV dysfunction may manifest as systolic dysfunction (fractional shortening <25%), LVH (LV mass >131 g/m2) or LV dilation (LV volume >90 mL/m2) [27, 28]. Each of these abnormalities may occur independently or in combination. Thus, a patient may have LV dilation with normal or reduced fractional shortening or LVH with normal or reduced fractional shortening. An earlier prospective study in ESRD patients on maintenance hemodialysis who all had normal EF, a high LV mass was associated with adverse prognosis only in those with normal cavity volume but not in those with LV dilation [15]. Volume overload and LV dilation is common in CKD patients. Even though we did not have LV cavity volume measurements available for our analyses, it is possible that a high prevalence of LV dilation in our patients with normal EF may explain our results. As LV dilation results in greater wall tension, LVH would decrease wall tension, oxygen utilization, improve perfusion gradient and reduce subendocardial ischemia, based on the law of LaPlace [29].
Our study had a number of limitations. Because our study was a retrospective study, causality cannot be implied. The study examined patients over an extended period of time during which secular trends in applied treatments and in echocardiographic methods could have changed and thus could have affected our findings. This was a single center study done in an entirely male cohort; hence, the results may not apply to the entire population with CKD. Additionally, only 65% of the available patients underwent echocardiography, most likely as a result of selection bias, as echocardiograms in clinical practice are most often performed after clinical events or symptoms. The excluded patients indeed had a lower prevalence of diabetes mellitus and cardiovascular disease, it is hence likely that many or most of them would have had no or mild echocardiographic abnormalities, and hence their inclusion in the study is unlikely to have altered the results significantly. Echocardiography was used to define LVH, which has been shown to overestimate LVH in the dialysis population [30]. However, this was a retrospective study and echocardiography is the most practical way of evaluating cardiac function and LV geometry. Additionally with the increasing awareness of gadolinium-induced nephrogenic systemic fibrosis, magnetic resonance imaging is less often used in patients with CKD, and hence, it is likely that echocardiography will remain the preferred clinical tool for routine noninvasive evaluation of cardiac function and structure. We had limited information derived from the echocardiographic studies obtained for clinical purposes. EF was not always quantified and hence we made assumptions about its level based on qualitative wording from the interpreter (normal, mild, moderate and severe); however, such interpretation is fairly standard in Cardiology practice. The lack of data on LV volume and geometry limits our ability to explore pathophysiological mechanisms that explain our findings. More detailed echocardiographic parameters allow for the differentiation of eccentric and concentric LVH and for the calculation of other parameters describing LV function, such as midwall fractional shortening. Due to lack of pertinent data, we could not extend our analyses to examine these additional parameters, but a study in ESRD patients suggested that the predictive ability of EF was similar to that of midwall fractional shortening [17]. Our lack of data on LV mass also prevented us from refining our analyses by indexing LV mass for either body surface area or for height2.7, the latter method having been shown to make LV mass a more powerful predictor of cardiovascular outcomes in ESRD patients [31]. We attempted to correct for several potential confounders, but residual confounding by unmeasured factors cannot be excluded. We speculate that the associations of echocardiographic abnormalities with all-cause mortality is due to higher rates of cardiovascular deaths in the affected patients, but we did not have data on causes of death to test this hypothesis.
Low EF is a robust and independent predictor of increased mortality in patients with NDD-CKD. LVH is also associated with adverse prognosis in those with reduced EF but has no association with mortality in those with normal EF. Further studies in this area are needed to replicate our findings and to better describe echocardiographic characteristics, including assessment of LV volume and LV mass indexed to body size. Clinical trials are needed to test the hypothesis that interventions targeting patients with low EF can lead to improved outcomes in the NDD-CKD population.
Acknowledgments
Parts of this material were presented as an abstract at the American Society of Nephrology Renal Week 2010, November 17–21, 2010, Denver, CO. This study is supported by grant 1R01DK078106-01 to C.P.K. and K.K.Z. C.P.K. is an employee of the Department of Veterans Affairs.
Transparency declaration. None of the authors have declared any competing financial interests.
Conflict of interest statement. None declared.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Garg AX, Clark WF, Haynes RB, et al. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 6.Greaves SC, Gamble GD, Collins JF, et al. Determinants of left ventricular hypertrophy and systolic dysfunction in chronic renal failure. Am J Kidney Dis. 1994;24:768–776. doi: 10.1016/s0272-6386(12)80670-6. [DOI] [PubMed] [Google Scholar]
- 7.Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 8.Ghali JK, Liao Y, Simmons B, et al. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med. 1992;117:831–836. doi: 10.7326/0003-4819-117-10-831. [DOI] [PubMed] [Google Scholar]
- 9.Hammond IW, Devereux RB, Alderman MH, et al. The prevalence and correlates of echocardiographic left ventricular hypertrophy among employed patients with uncomplicated hypertension. J Am Coll Cardiol. 1986;7:639–650. doi: 10.1016/s0735-1097(86)80476-4. [DOI] [PubMed] [Google Scholar]
- 10.Harnett JD, Kent GM, Barre PE, et al. Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J Am Soc Nephrol. 1994;4:1486–1490. doi: 10.1681/ASN.V471486. [DOI] [PubMed] [Google Scholar]
- 11.Ma KW, Greene EL, Raij L. Cardiovascular risk factors in chronic renal failure and hemodialysis populations. Am J Kidney Dis. 1992;19:505–513. doi: 10.1016/s0272-6386(12)80827-4. [DOI] [PubMed] [Google Scholar]
- 12.Silberberg JS, Rahal DP, Patton DR, et al. Role of anemia in the pathogenesis of left ventricular hypertrophy in end-stage renal disease. Am J Cardiol. 1989;64:222–224. doi: 10.1016/0002-9149(89)90462-1. [DOI] [PubMed] [Google Scholar]
- 13.Tucker B, Fabbian F, Giles M, et al. Left ventricular hypertrophy and ambulatory blood pressure monitoring in chronic renal failure. Nephrol Dial Transplant. 1997;12:724–728. doi: 10.1093/ndt/12.4.724. [DOI] [PubMed] [Google Scholar]
- 14.Khan NA, Ma I, Thompson CR, et al. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244–253. doi: 10.1681/ASN.2005030270. [DOI] [PubMed] [Google Scholar]
- 15.Foley RN. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 16.Lindner A, Charra B, Sherrard DJ, et al. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974;290:697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Benedetto FA, Mallamaci F, et al. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol. 2004;15:1029–1037. doi: 10.1097/01.asn.0000117977.14912.91. [DOI] [PubMed] [Google Scholar]
- 18.Zoccali C, Benedetto FA, Tripepi G, et al. Left ventricular systolic function monitoring in asymptomatic dialysis patients: a prospective cohort study. J Am Soc Nephrol. 2006;17:1460–1465. doi: 10.1681/ASN.2005111240. [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Parfrey PS, Harnett JD, et al. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 20.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 21.Carabello BA, Zile MR, Tanaka R, et al. Left ventricular hypertrophy due to volume overload versus pressure overload. Am J Physiol. 1992;263:H1137–H1144. doi: 10.1152/ajpheart.1992.263.4.H1137. [DOI] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Trivedi BK, Anderson JE. Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Adv Chronic Kidney Dis. 2006;13:183–188. doi: 10.1053/j.ackd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, et al. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 26.Thadhani R, Tonelli M. Cohort studies: marching forward. Clin J Am Soc Nephrol. 2006;1:1117–1123. doi: 10.2215/CJN.00080106. [DOI] [PubMed] [Google Scholar]
- 27.Parfrey PS, Collingwood P, Foley RN, et al. Images in nephrology. Left ventricular disorders detected by M-mode echocardiography in chronic uraemia. Nephrol Dial Transplant. 1996;11:1328–1331. [PubMed] [Google Scholar]
- 28.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 29.Sandler H, Dodge HT. Left ventricular tension and stress in man. Circ Res. 1963;13:91–104. doi: 10.1161/01.res.13.2.91. [DOI] [PubMed] [Google Scholar]
- 30.Stewart GA, Foster J, Cowan M, et al. Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int. 1999;56:2248–2253. doi: 10.1046/j.1523-1755.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 31.Zoccali C, Benedetto FA, Mallamaci F, et al. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol. 2001;12:2768–2774. doi: 10.1681/ASN.V12122768. [DOI] [PubMed] [Google Scholar]