Abstract
Background.
Dietary phosphorus intake is usually restricted in dialysis patients but the associations of dietary phosphorus intake with mortality in moderate chronic kidney disease (CKD) are unknown. Therefore, we examined these associations in National Health and Nutrition Examination Survey III.
Methods.
Dietary phosphorus intake was estimated from 24-h dietary recalls administered by trained personnel. CKD was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Time to mortality was examined by Cox regression models taking into account the complex survey design.
Results.
1105 adults with CKD were studied. Phosphorus intake was 1033 ± 482 mg/day (mean ± SD), eGFR was 49.3 ± 9.5 mL/min/1.73 m2 and serum phosphorus was 3.5 ± 0.5 mg/dL. Compared to those in the lowest tertile of phosphorus intake (mean 532 ± 161 mg/day), those in the highest third (1478 ± 378 mg/day) had similar serum phosphorus levels (3.6 ± 0.5 versus 3.5 ± 0.6 mg/dL, P = 0.113) and modestly higher eGFR (50.0 ± 8.1 versus 47.5 ± 12.0 mL/min/1.73 m2, P = 0.014). After adjustment for demographics, comorbidity, eGFR, physical activity, energy intake and nutritional variables, phosphorus intake was not associated with mortality [hazard ratio (HR) 0.98 per 100 mg/dL increase, 0.93–1.03].
Conclusions.
High dietary phosphorus intake is not associated with increased mortality in moderate CKD, presumably because serum phosphorus levels are maintained in the normal range at this level of GFR. Interventional trials are needed to define optimal phosphorus intake in moderate CKD.
Keywords: dietary phosphorus intake, mortality, moderate chronic kidney disease
Introduction
Phosphorus is a critically important mineral, playing a vital role in energy metabolism, cellular signaling, nucleic acid metabolism, platelet aggregation and bone mineralization. An average adult contains <1 kg of phosphorus in the body. About 85% of that is present in bones and teeth in the form of apatitie, and the most of the remainder is inside cells in soft tissues. Only ∼0.1% of body phosphate circulates in the blood. A well-fed healthy adult consumes ∼1.5 g of phosphorus/day and about two-thirds of that is excreted in the urine and the rest in the feces.
The serum phosphorus level is influenced by dietary intake, gastrointestinal absorption, distribution across the body compartments including uptake into bone and excretion [1]. Regulation of serum phosphorus levels involves vitamin D, parathyroid hormone and fibroblast-derived growth factor-23 (FGF-23). Increased serum phosphorus concentrations are associated with vascular calcification [2] and mortality in dialysis patients [3–6]. Reduction in dietary phosphorus intake by avoiding foods high in phosphorus such as colas or dairy products is considered critical in controlling serum phosphorus in dialysis population. Thus, the national guidelines recommend reduction in dietary phosphorus intake in dialysis patients.
As glomerular filtration rate (GFR) declines to about 40–45 mL/min/1.73 m2, urinary excretion of phosphorus decreases and serum levels of phosphorus begin to increase [7, 8]. More recently, in non-dialysis-dependent chronic kidney disease (CKD) patients, higher serum phosphorus levels have been shown to be associated with increased mortality [9, 10]. These data raise the possibility that higher dietary phosphorus intake might be associated with increased mortality in non-dialysis CKD. To our knowledge, there are no prior studies on the associations of dietary phosphorus with mortality in moderate CKD. Therefore, we examined these associations in the moderate CKD population in the National Health and Nutrition Examination Survey (NHANES) III data.
Methods
Study population and baseline data
The NHANES is an ongoing series of the surveys of the non-institutionalized civilian population in the USA conducted by the National Center for Health Statistics. From 1988 to 1994, NHANES III, a cross-sectional survey of the US population was carried out. It used a complex, multistage sampling design to obtain a sample that is representative of the non-institutionalized civilian US population of early 1990s [11].
Briefly, participants provided informed consent and underwent a structured home interview conducted by trained personnel to ascertain self-reported medical history of conditions such as myocardial infarction, stroke, congestive heart failure and diabetes. This was followed by a physical examination, which included blood pressure measurement, extensive anthropometric and physiological assessments and blood draw for laboratory testing [11] at the NHANES Mobile Examination Center. The time of the blood draw and the number of hours of fasting before the blood draw were recorded. As detailed below, during this visit, participants also underwent a detailed diet interview.
Serum creatinine, albumin, calcium and phosphorus were analyzed on a Hitachi 737 automated analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN) using reagents from Boehringer Mannheim Diagnostics. Serum creatinine measurements obtained using a kinetic rate Jaffe method in NHANES III were recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, OH) as standard creatinine = −0.184 + 0.960 × NHANES III measured serum creatinine [12]. GFR was estimated as 175 × (standardized serum creatinine)−1.154 × (age)−0.203 × 0.742 (if the individual is woman) × 1.212 (if the individual is African-American) [13]. CKD was defined as GFR <60 mL/min/1.73 m2.
Dietary assessment
Details about dietary assessment methodology have been published elsewhere [11]. A computer-based interview system developed by the University of Minnesota’s Nutrition Coordinating Center (Regents of the University of Minnesota) was used to conduct a 24-h dietary recall. The 24-h dietary recall was conducted by trained interviewers. The US Department of Agriculture’s Survey Nutrient Data Base was used to calculate macro- and micronutrient content of the foods consumed during the 24-h recall period for each respondent.
Nutritional assessment
A trained technician carried out anthropometric measurements, while another trained technician assisted and recorded the measures. Weight was measured to the nearest 0.01 kg on an electronic scale. Standing height was measured to the nearest 0.1 cm with a stadiometer. Bioimpedance analysis (BIA) model 1990B (Valhalla Scientific, San Diego, CA) was used for the measurement of whole body electrical resistance and impedance. A standardized protocol for BIA procedure was carried out by trained observers and physicians in a standardized environment. Lean body mass (LBM) was estimated from prediction equations that were validated and cross-validated for men and women separately and for blacks and whites between the ages of 12 and 94 years. [men: LBM (kg) = −10.68 + (0.65 × S2/resistance) + (0.26 × weight) + (0.02 × resistance) and women: LBM (kg) = −9.53 + (0.69 × S2/resistance)+ (0.17 × weight) + (0.02 × resistance) where S2/resistance is stature squared divided by resistance (cm2/Ω)] [14].
Follow-up data
The National Center for Health Statistics created an NHANES III Linked Mortality File that contains mortality follow-up data from the date of NHANES III survey participation (1988–1994) through December 31, 2000. This information was based upon the results from a probabilistic match between NHANES III and National Death Index death certificate records [15].
Statistical analyses
NHANES III utilized a complex multistage probability sample design. Several aspects of the NHANES design must be taken into account in data analysis, including the sampling weights and clustered sampling. We used the svy suite of commands in Stata 11 (Stata 11, College station, TX) and followed the analytical guidelines for NHANES data proposed by the Centers for Disease Control [16]. The svy commands in Stata accounts for the elements of NHANES sampling design to calculate the expected means and proportions of the entire US non-institutionalized civilian CKD population, which are presented with the estimated values and with associated 95% confidence intervals.
Using gender-specific tertiles, unadjusted associations of dietary phosphorus intake with baseline characteristics including dietary variables (calorie, protein, calcium and magnesium intake), nutritional variables (serum albumin and LBM) and serum calcium, phosphorus and calcium–phosphorus product were examined using chi-square contingency table analysis for categorical variables and analysis of variance for continuous variables.
In a multivariable linear regression model, the association of dietary phosphorus with serum phosphorus was examined adjusted for demographics (age, gender and race), dietary variables (calorie intake and percent calories from protein), time of the day (morning: 7:00–11:59 AM, afternoon: 12:00–4:49 PM and evening: 5:00–11:00 PM) when the blood was drawn and the hours of fasting before the blood was drawn.
Survival analyses
The unadjusted association of dietary phosphorus intake with mortality was first examined in a Cox proportional hazards regression model without covariate adjustment. Next, this model was adjusted for age, gender and race to examine the extent to which demographic variables confound this association. A third Cox regression model was performed with further covariate adjustment for comorbid conditions (history of myocardial infarction, stroke, congestive heart failure, cancer and diabetes), lifestyle factors (smoking, alcohol use and physical activity), dietary variables (calorie intake and percent calories from protein) and estimated glomerular filtration rate (eGFR). Finally, as higher phosphorus intake might be associated with better nutritional status, the effects of further adjusting for nutritional variables [serum albumin, body mass index (BMI) and LBM] were examined in a fourth Cox regression model.
The assumption of proportional hazards was examined by comparing the logarithm of the hazard ratio (HR) for each predictor variable in the first 3 years of follow-up to the logarithm of the HR of the predictor variables after Year 3. No models showed proportional hazards assumption violations with respect to dietary phosphorus intake.
The factors gender and stroke exhibited a significant deviation from proportional hazards (P < 0.05) in at least one of the models. Hence, rather than including these factors as covariates, each of the Cox regressions was stratified by each of these factors to allow separate baseline hazard functions within each stratum. In addition, quadratic terms were tested for each continuous covariate to test for the presence of nonlinear effects of that covariate. No quadratic terms were statistically significant.
Similar analyses were performed treating dietary phosphorus intake as a categorical variable with the lowest dietary phosphorus intake as the reference group.
Results
Of the 16 864 adults with valid data for GFR estimation, the subpopulation of 1105 CKD participants with nonmissing data for dietary intake, nutritional variables and mortality were included in this analysis.
Higher phosphorus intake was associated with younger age and non-African-American race (Table 1). GFR was modestly higher in the higher phosphorus intake group. There was a very small but statistically significant difference in calcium–phosphorus product.
Table 1.
Participant demographics, pertinent lifestyle and medical history characteristicsa
| Lowest phosphorus intake tertile, 532 ± 161 (mg/day) | Middle phosphorus intake tertile, 912 ± 166 (mg/day) | Highest phosphorus intake tertile, 1478 ± 378 (mg/day) | P-value | |
| Age (years) | 70.8 ± 12.3 | 70.4 ± 12.4 | 67.4 ± 13.0 | 0.02 |
| Women (%) | 68 (63–73) | 57 (49–64) | 65 (57–73) | 0.07 |
| African-American (%) | 12 (10–16) | 6 (5–9) | 4 (2–5) | <0.001 |
| Myocardial infarction (%) | 18 (13–25) | 14 (9–21) | 15 (11–19) | 0.41 |
| Congestive heart failure (%) | 14 (9–19) | 12 (8–19) | 12 (8–16) | 0.86 |
| Stroke (%) | 11 (7–16) | 8 (6–12) | 9 (6–13) | 0.60 |
| Diabetes (%) | 18 (14–23) | 21 (16–27) | 22 (18–28) | 0.51 |
| History of malignancy (%) | 11 (7–17) | 9 (6–14) | 12 (8–16) | 0.73 |
| Current smoker (%) | 14 (10–20) | 11 (7–16) | 14 (10–20) | 0.59 |
| Current alcohol user (%) | 20 (14–26) | 32 (24–40) | 35 (26–45) | 0.02 |
| Systolic BP (mmHg) | 143.2 ± 23.5 | 141.6 ± 18.6 | 138.8 ± 20.7 | 0.04 |
| Diastolic BP (mmHg) | 75.6 ± 13.5 | 75.0 ± 11.3 | 74.7 ± 10.5 | 0.72 |
| eGFR (mL/min/1.73 m2) | 47.5 ± 12.0 | 49.9 ± 8.7 | 50.0 ± 8.1 | 0.01 |
| Serum phosphorus (mg/dL) | 3.5 ± 0.6 | 3.4 ± 0.5 | 3.6 ± 0.5 | 0.11 |
| Serum calcium (mg/dL) | 9.3 ± 0.5 | 9.3 ± 0.5 | 9.4 ± 0.5 | 0.09 |
| Calcium–phosphorus product (mg2/dL2) | 32.8 ± 6.2 | 32.1 ± 5.1 | 33.4 ± 5.0 | 0.07 |
Percentages shown as percent (95% CI); continuous measures shown as mean ± SD. BP, blood pressure.
As is expected, higher phosphorus intake was associated with higher intake of protein, energy, calcium and magnesium intake (Table 2). Of the anthropometric measures, higher phosphorus intake was associated with higher estimated LBM but not BMI or serum albumin (Table 2).
Table 2.
Association of phosphorus intake with dietary intake and nutritional statusa
| Lowest phosphorus intake tertile, 532 ± 161 (mg/day) | Middle phosphorus intake Tertile 912 ± 166 (mg/day) | Highest phosphorus intake tertile 1478 ± 378 (mg/day) | P-value | |
| Calories (kcal/day) | 1041 ± 411 | 1570 ± 521 | 2103 ± 698 | <0.001 |
| Dietary protein(% kcal) | 15.3 ± 6.5 | 15.7 ± 4.4 | 17.3 ± 5.3 | 0.01 |
| Dietary calcium (mg/day) | 306 ± 164 | 568 ± 204 | 992 ± 413 | <0.001 |
| Dietary magnesium (mg/day) | 146 ± 56 | 230 ± 71 | 346 ± 113 | <0.001 |
| Serum albumin (g/dL) | 4.01 ± 0.4 | 4.04 ± 0.3 | 4.06 ± 0.4 | 0.51 |
| BMI (kg/m2) | 27.1 ± 5.8 | 28.0 ± 5.5 | 27.7 ± 5.3 | 0.40 |
| LBM (%)b | 48.9 ± 10.3 | 53.2 ± 11.8 | 52.8 ± 10.8 | <0.001 |
Continuous measures shown as mean ± SD.
LBM estimated from bioimpedance measurements.
Dietary phosphorus was weakly associated with serum phosphorus after adjustment for demographics, calorie intake, percent calories from protein intake, time of blood draw and duration of fasting. A 100 mg/day increase in dietary phosphorus was associated with a corresponding change in serum phosphorus of 0.009 mg/dL (95% confidence limits: 0.006–0.011 mg/dL, Table 3).
Table 3.
Adjusted associations of dietary phosphorus with serum phosphorus
| Regression coefficient (95% CI) | P-value | |
| Each 100 mg/day increase of dietary phosphorus | 0.009 (0.006, 0.011) | <0.001 |
| Each year increase in age | −0.003 (−0.004, −0.003) | <0.001 |
| Men | −0.157 (−0.1733, −0.141) | <0.001 |
| African-American race | 0.017 (−0.000, 0.034) | 0.056 |
| Each 100 kcal/day increase | −0.004 (−0.005, −0.002) | <0.001 |
| Each increase in 5% kcal from dietary protein | −0.002 (−0.012, 0.007) | 0.620 |
| Time of blood draw | ||
| Morning blood draw (7:00 AM–11:59 AM) | Reference | |
| Afternoon blood draw (12:00 PM–16:59 PM) | 0.148 (0.129, 0.166) | <0.001 |
| Evening blood draw (1700–2300) | 0.195 (0.171, 0.218) | <0.001 |
| Duration of fasting (h) | −0.008 (−0.010, −0.006) | <0.001 |
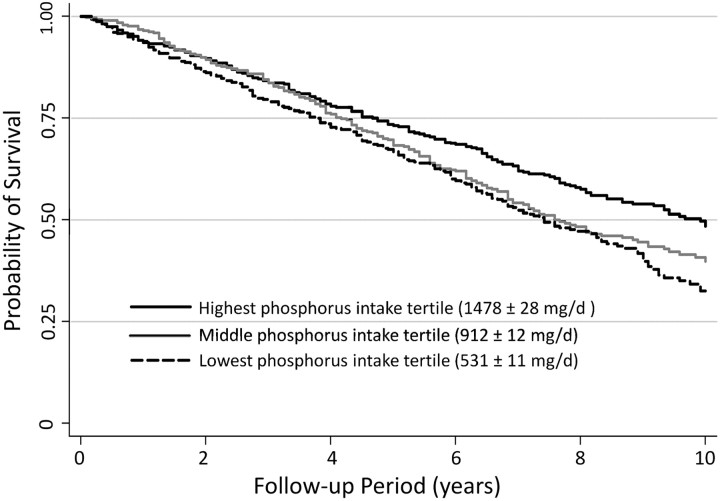
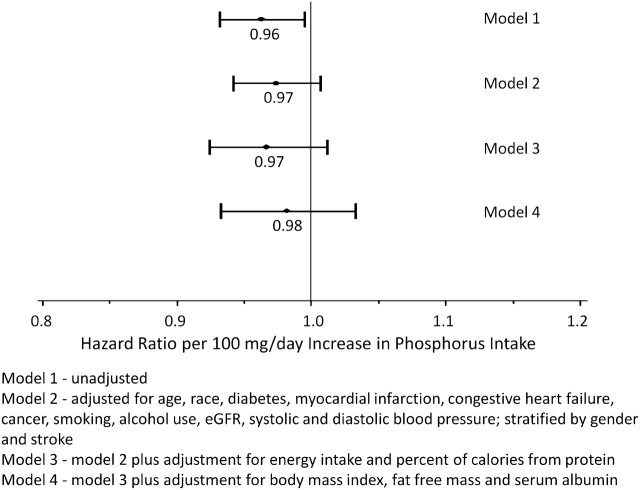
There were 592 (54%) deaths over an average of 6.5years of follow-up. The unadjusted hazard of death was lower with higher phosphorus intake (Figure 1). As shown in Figure 2, adjustment for age, gender and race attenuated the decrease in the HRs associated with higher phosphorus intake. Adjustment for comorbid conditions, eGFR, smoking, alcohol use, physical activity levels and energy intake further attenuated this association. Adjustment for nutritional status eliminated the lower hazard of mortality associated with high phosphorus intake.
Fig. 1.
Kaplan–Meier plot of unadjusted survival by phosphorus intake tertiles in moderate CKD.
Fig. 2.
HR of death associated with phosphorus intake in moderate CKD.
The associations of phosphorus intake as a categorical variable with mortality were similar (Table 4).
Table 4.
Associations of phosphorus intake as a categorical variable with mortality in the CKD subpopulation using Cox regression modelsa
| Phosphorus intake tertiles | Model 1 | Model 2 | Model 3 | Model 4 |
| Lowest (531 ± 11 mg/day) | Reference | Reference | Reference | Reference |
| Middle (912 ± 12 mg/day) | 0.88 (0.68–1.14) | 1.03 (0.76–1.40) | 1.07 (0.77–1.50) | 1.25 (0.87–1.78) |
| Highest (1478 ± 28 mg/day) | 0.64 (0.46–0.90) | 0.84 (0.60–1.18) | 0.92 (0.61–1.40) | 1.07 (0.67–1.70) |
Model 1—unadjusted; Model 2—adjusted for age, race, diabetes, myocardial infarction, congestive heart failure, cancer, smoking, alcohol use, eGFR, systolic and diastolic blood pressure; stratified by gender and stroke; Model 3—model 2 plus adjustment for energy intake and percent of calories from protein and Model 4—model 3 plus adjustment for BMI, LBM and serum albumin.
Discussion
The results of the current study suggest that high dietary phosphorus levels are associated with very modest increase in serum phosphorus levels but not with increased mortality in early-stage III CKD. However, high dietary phosphorus is positively associated with LBM and intake of energy, protein, calcium and magnesium.
Higher levels of serum phosphorus was associated with increased risk of mortality in advanced CKD [9, 10] as well as hemodialysis patients [3, 5]. Furthermore, higher serum phosphorus levels were associated with lower ankle-brachial index (a marker of peripheral arterial disease) in individuals with normal kidney function or moderate kidney disease [17], increased risk of cardiovascular disease in individuals with eGFR >90 mL/min/1.73 m2 and without proteinuria [18], and increased risk of stroke and death in healthy community-dwelling adults [19]. Therefore, there is much interest in strategies to reduce serum phosphorus (either by decreasing dietary intake of phosphorus or use of phosphorus binders) not only in dialysis population but also in those with CKD [20–22].
Even though the above studies of serum phosphorus provide a strong rationale for targeting dietary phosphorus in advanced stages of kidney disease, there is a paucity of data on whether dietary phosphorus by itself is a risk factor for poor outcomes in those with normal kidney function or mild kidney dysfunction. In an earlier analysis of NHANES III data, there were no associations of dietary phosphorus with serum phosphorus in non institutionalized US general population [23]. Antoniucci et al. [24] conducted a rigorous 4-week interventional study of dietary phosphorus in 13 healthy men. In that study, participants were admitted to a General Clinical Research Center and consumed a constant diet that provided 500 mg of phosphorus per day, which was supplemented to achieve three phosphorus intakes, each of 9 days duration:control = 1500 mg/day, supplemented = 2300 mg/day and restricted = 625 mg/day. Intakes of calcium, sodium, potassium, magnesium, and energy were constant. Dietary phosphorus intervention had no effects on serum phosphorus levels. Therefore, there is no clear evidence to support the notion that dietary phosphorus manipulation will impact on serum phosphorus levels in those with normal kidney function or those with moderate CKD.
Nonetheless, independent of serum phosphorus levels, high dietary phosphorus could have deleterious consequences through other mechanisms. In the phosphorus intervention study described above, high dietary phosphorus intake resulted in increased serum levels of FGF-23 [24]. Increased serum levels of FGF-23 have been shown to be independently associated with endothelial dysfunction [25], coronary artery disease [26], left ventricular hypertrophy [27] and mortality [28] in CKD or dialysis patients. Apart from its effects on serum FGF-23 levels, high dietary phosphorus could affect outcomes through other mechanisms. For instance, in vitro, bovine aortic endothelial cells exposed to a phosphorus load increased production of reactive oxygen species and decreased nitric oxide production [29]. Furthermore, flow-mediated dilation of the brachial artery was significantly decreased by dietary phosphorus load in healthy volunteers [29]. Apart from cardiovascular disease, high dietary phosphorus intake could also increase progression of kidney disease as uremic rats fed with low phosphorus diet had slower progression of kidney disease on renal histology [30].
Taken together, the above literature could be interpreted as evidence that high dietary phosphorus intake could be deleterious in those with moderate kidney disease. Indeed, in maintenance hemodialysis patients, high dietary phosphorus was associated with increased hazard of death [31]. However, to our knowledge, there are no published data on whether high dietary phosphorus intake is indeed associated with increased mortality in moderate CKD.
As GFR declines to about 40–45 mL/min/1.73 m2, urinary excretion of phosphorus decreases and serum levels of phosphorus begins to increase [7, 8]. In this sample of people with moderate kidney disease (eGFR, 49.3 ± 9.5 mL/min/1.73 m2), higher reported dietary phosphorus intake was associated with lower hazard of death in unadjusted models (Table 3 and Figure 2). With adjustment, dietary phosphorus intake was not significantly associated with mortality. It is possible that the magnitude of the Cox regression coefficients for dietary phosphorus were attenuated due to effects of measurement error [32] or short-term variation in diet. However, because the estimated HRs corresponding to higher phosphorous levels were <1 in each model considered (Figure 2), it is unlikely that attenuation due to measurement error obscured a direct association of higher phosphorus with increased mortality. Thus, the results of this study suggest that spontaneously higher phosphorus intake is not associated with increased mortality at this level of eGFR. It is still conceivable that dietary phosphorus restriction might improve outcomes in those with more advanced CKD with decreased ability to excrete phosphorus. Interventional studies are warranted to conclusively establish the potential benefit or harm of low phosphorus diet in early-stage III CKD.
Another therapeutic strategy is the use of phosphorus binders to decrease dietary phosphorus absorption in those with early stages of CKD and normal serum phosphorus levels [33]. In individuals with advanced CKD (eGFR, 26.3 ± 15.6 mL/min) and normal serum phosphorus (4.5 ± 0.7 mg/dL), therapy with sevelamer was associated with decreased progression of coronary calcification compared to individuals treated with a low phosphorus diet alone [34]. Since low phosphorus diet was associated with increased coronary calcification in that study, the decreased calcification with sevelamer may be through mechanisms independent of its dietary phosphorus-binding effect. Therefore, it is unclear whether dietary phosphorus restriction would improve coronary calcification and cardiovascular outcomes in CKD. In a retrospective analysis of hemodialysis patients, patients treated with phosphorus binders were matched with those who were not treated with phosphorus binders by their baseline serum phosphate levels and propensity score of receiving phosphorus binders during the first 90 days [33]. In that study, therapy with phosphorus binders was independently associated with lower mortality. This was presumably because phosphorus binders suppressed serum FGF-23 levels. Similarly, in men with moderate to advanced non-dialysis CKD, phosphorus binder therapy was associated with decreased risk of all-cause mortality which was most pronounced in men with higher baseline serum phosphate levels [22]. However, in a small randomized controlled trial of CKD patients, the use of lanthanum, a phosphorus binder was associated with decrease in urinary phosphorus excretion but not that of serum FGF-23 levels [35]. Thus, it is unclear whether the use of phosphorus binders in early-stage CKD patients with normal serum phosphorus levels would impact on hard end-points.
Higher protein intake is associated with higher phosphorus intake. Therefore, zealous restriction of phosphorus intake might decrease protein intake and adversely affect nutritional status [36]. Indeed, in this study, higher phosphorus intake was associated with higher LBM (Table 2). Dietary approaches to maximizing nutritional status while controlling phosphorus intake include maximizing the organic phosphorus to protein ratio and minimizing inorganic sources [31]. An organic Italian Mediterranean diet was reported to both decrease serum phosphorus and increase LBM among patients with Stages 2 and 3 kidney disease [37]. Adjustment of phosphate binder use based on dietary phosphate intake is reported to result in improved phosphorus levels in children without influencing phosphorus intake [22, 33, 35, 38].
Strengths of this study include the representative sample and rigorous methodology of the NHANES study. Limitations of the study include the observational nature, which limits causal inferences. Despite its common use, the Modification of Diet in Renal Disease equation has several limitations when used in specific groups and may result in the over diagnosis of CKD [39]. Furthermore, there were only single baseline measurements of dietary intake with a single 24-h recall at baseline. Conventional nutrient databases may not account for the consumption of food additives laden with highly bioavailable phosphorus [40–43], which might result in underestimation of phosphorus [36]. Imprecise estimates of dietary phosphorus intake might bias the study toward null hypothesis. However, given that the total calorie intake was lower in the lowest phosphorus group and higher in the highest phosphorus group, it is unlikely that there was systematic underestimation of dietary phosphorus in the low phosphorus intake group compared to the high phosphorus intake group. Fasting durations and time of day in blood collection varied between participants and these might affect serum phosphorus levels. However, when adjusted for these variables, dietary phosphorus had statistically significant but clinically not meaningful association with serum phosphorus at this level of eGFR. In addition, there are no data available in this NHANES dataset on serum parathyroid hormone or FGF-23 levels.
We conclude that spontaneously higher dietary phosphorus intake is not associated with increased mortality in moderate CKD in the USA. We conjecture that this absence of association reflects the maintenance of serum phosphorus levels in the normal range at this level of GFR. However, interventional trials are needed to establish the optimal level of dietary phosphorus intake in moderate CKD. Demonstration of sustainable dietary and supplement approaches to maximizing nutritional status and phosphorus metabolism may play a role in improving both clinical outcomes and quality of life of patients with CKD.
Acknowledgments
We thank the NHANES participants, staff and investigators. This investigation is supported by RO1—DK077298 and RO1—DK078112 awarded to S.B. and the University of Utah Study Design and Biostatistics Center, with funding in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources.
Authorship: All the above listed authors had access to the data and played a role in writing this manuscript.
Conflict of interest statement. T.G. currently acts as a consultant for Amgen Inc., Comedix Inc., Eli Lilly and Co., Kery Biopharmaceuticals, and NephroGenex.
References
- 1.Kestenbaum B. Phosphate metabolism in the setting of chronic kidney disease: significance and recommendations for treatment. Semin Dial. 2007;20:286–294. doi: 10.1111/j.1525-139X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Raggi P, Chasan-Taber S, et al. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1489–1496. doi: 10.1093/ndt/gfh125. [DOI] [PubMed] [Google Scholar]
- 3.Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 6.Guerin AP, London GM, Marchais SJ, et al. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–1021. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 7.Levin A, Bakris G, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 8.Pires A, Adragão T, Pais MJ, et al. Inferring. disease mechanisms from epidemiological data in chronic kidney disease: calcium and phosphorus metabolism. Nephron Clin Pract. 2009;112:c137–c147. doi: 10.1159/000214208. [DOI] [PubMed] [Google Scholar]
- 9.Eddington H, Hoefield R, Sinha S, et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:2251–2257. doi: 10.2215/CJN.00810110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Outcomes associated with serum phosphorus level in males with non-dialysis dependent chronic kidney disease. Clin Nephrol. 2010;73:268–275. doi: 10.5414/cnp73268. [DOI] [PubMed] [Google Scholar]
- 11.Hladky JP, Lejeune JP, Singer B, et al. Osteoblastoma of the odontoid process. Pediatr Neurosurg. 1994;21:260–262. doi: 10.1159/000120847. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Sun SS, Chumlea WC, Heymsfield SB, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am J Clin Nutr. 2003;77:331–340. doi: 10.1093/ajcn/77.2.331. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics: The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File: Matching Methodology. 2005. http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf (3 March 2009, date last accessed) [Google Scholar]
- 16.National Center for Health Statistics. Analytical and Reporting guidelines: The Third National Health and Nutrition Examination Survey, 1988–1994. Hyattsville, MD: 1996. (24 April 2005, date last accessed) [Google Scholar]
- 17.Ix JH, De Boer IH, Peralta CA, et al. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4:609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Collins AJ, Ishani A, et al. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez OM, Wolf M. Dietary phosphorus restriction in advanced chronic kidney disease: merits, challenges, and emerging strategies. Semin Dial. 2010;23:401–406. doi: 10.1111/j.1525-139X.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 21.Sigrist MK, Taal MW, Bungay P, et al. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:1241–1248. doi: 10.2215/CJN.02190507. [DOI] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Kuchmak O, Lu JL, et al. Outcomes associated with phosphorus binders in men with non-dialysis-dependent CKD. Am J Kidney Dis. 2010;56:842–851. doi: 10.1053/j.ajkd.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz MI, Sonmez A, Saglam M, et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010;78:679–685. doi: 10.1038/ki.2010.194. [DOI] [PubMed] [Google Scholar]
- 26.Kanbay M, Nicoleta M, Selcoki Y, et al. Fibroblast growth factor 23 and fetuin A are independent predictors for the coronary artery disease extent in mild chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:1780–1786. doi: 10.2215/CJN.02560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusano K, Segawa H, Ohnishi R, et al. Role of low protein and low phosphorus diet in the progression of chronic kidney disease in uremic rats. J Nutr Sci Vitaminol. 2008;54:237–243. doi: 10.3177/jnsv.54.237. [DOI] [PubMed] [Google Scholar]
- 31.Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:683–692. doi: 10.2215/CJN.08601209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll RJ, Ruppert D, Stefanski LA, et al. Measurement Error in Nonlinear Models: A Modern Perspective. 2nd edn. New York, NY: Chapman & Hall; 2006. [Google Scholar]
- 33.Isakova T, Gutierrez OM, Chang Y, et al. Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol. 2009;20:388–396. doi: 10.1681/ASN.2008060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russo D, Miranda I, Ruocco C, et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255–1261. doi: 10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- 35.Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584–591. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinaberger C, Greenland S, Kopple J, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88:1511. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Lorenzo A, Noce A, Bigioni M, et al. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr Pharm Des. 2010;16:814–824. doi: 10.2174/138161210790883561. [DOI] [PubMed] [Google Scholar]
- 38.Ahlenstiel T, Pape L, Ehrich JH, et al. Self-adjustment of phosphate binder dose to meal phosphorus content improves management of hyperphosphataemia in children with chronic kidney disease. Nephrol Dial Transplant. 2010;25:3241–3249. doi: 10.1093/ndt/gfq161. [DOI] [PubMed] [Google Scholar]
- 39.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 40.Benini O, D'Alessandro C, Gianfaldoni D, et al. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J Ren Nutr. 2010 doi: 10.1053/j.jrn.2010.06.021. , doi:10.1053/j.jrn.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr. 2007;17:350–354. doi: 10.1053/j.jrn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez OM, Isakova T, Enfield G, et al. Impact of poverty on serum phosphate concentrations in the Third National Health and Nutrition Examination Survey. J Ren Nutr. 2011;21:140–148. doi: 10.1053/j.jrn.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutierrez OM, Anderson C, Isakova T, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21:1953–1960. doi: 10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]