Abstract
We show that loss of p85α inhibits the growth and maturation of mast cells, whereas loss of p85β enhances this process. Whereas restoring the expression of p85α in P85α−/− cells restores these functions, overexpression of p85β has the opposite effect. Consistently, overexpression of p85β in WT mast cells represses KIT-induced proliferation and IL-3–mediated maturation by inhibiting the expression of Microphthalmia transcription factor. Because p85α and p85β differ in their N-terminal sequences, chimeric proteins consisting of amino or carboxy-terminal of p85α and/or p85β do not rescue the growth defects of p85α−/− cells, suggesting cooperation between these domains for normal mast cell function. Loss of p85β impaired ligand induced KIT receptor internalization and its overexpression enhanced this process, partly because of increased binding of c-Cbl to p85β relative to p85α. In vivo, loss of p85β resulted in increased mast cells, and bone marrow transplantation of cells overexpressing p85β resulted in significant reduction in some tissue mast cells. Overexpression of p85β suppressed the growth of oncogenic KIT-expressing cells in vitro and prolonged the survival of leukemic mice in vivo. Thus, p85α and p85β differentially regulate SCF and oncogenic KIT-induced signals in myeloid lineage-derived mast cells.
Introduction
Mast cells are effector cells of the immune system that originate from multipotent stem cells in the bone marrow (BM).1,2 These cells regulate both innate and adaptive immunity3,4 and have been implicated in a variety of inflammatory diseases, including multiple sclerosis, atherosclerosis, rheumatoid arthritis, coronary artery disease, inflammatory bowel disease, and angiogenesis.5 In the BM, growth and differentiation of mast cells are critically dependent on signals regulated by the KIT and IL-3 receptors.6–12 KIT belongs to type 3 receptor tyrosine kinase subfamily and is encoded by the W locus.13 Loss of function of KIT because of mutations at the W locus results in ablation of KIT tyrosine kinase activity, leading to defective mast cell growth and severe mast cell deficiency in all tissues.14 Although normal KIT signaling is vital for various mast cell related functions, abnormal KIT signaling because of activating mutations in the KIT receptor have been described in germ cell tumors,15 gastrointestinal stromal tumors,16 lymphomas,17 acute myeloid leukemia,18 and systemic mastocytosis.19 For some of these mutations, including the KITD816V mutation found in patients with acute myeloid leukemia and systemic mastocytosis, no good therapies are currently available.
In addition to KIT and its ligand SCF, IL-3 is also critical for the development, survival, and function of tissue mast cells,11,12 in particular under conditions of immunologic stress.20 Although it is known for some time that KIT and IL-3 receptor–induced signals are essential for mast cell growth and differentiation, the nature of intracellular signals downstream from these receptors in regulating both growth and maturation of these cells is poorly understood. To this end, studies by Fukao et al have shown that some PI3 kinase (PI3K) signaling components may contribute to mast cell development.21
PI3K is a lipid kinase composed of a heterodimer made up of p85 regulatory subunit(s) and p110 catalytic subunit(s). In hematopoietic cells, 4 regulatory (p85α, p85β, p55α, and p50α) and 3 catalytic (p110α, p110β, and p110δ) subunits of class IA PI3K are expressed.22 The regulatory subunits mediate the binding, activation, and localization of the PI3K enzyme.23 Whereas regulatory subunits p85α and p85β are encoded by separate genes, Pik3r1 and Pik3r2, respectively,24,25 regulatory subunits p85α, p55α, and p50α are splice variants of a single gene, Pik3r126 Although p85α and p85β subunits share near 80% identity in the C-terminus, only 40% identity exists in the N-terminus.24 In contrast, p55α and p50α subunits completely lack N-terminal end sequences, including the SH3 and the BH domains.27 Thus, although these subunits are highly related in their C-terminus, they differ significantly with respect to the N-terminal sequences. Although the current dogma in the field of PI3K signaling suggests that all regulatory subunits of PI3K function in a similar manner, we provide genetic and biochemical evidence to suggest that p85 regulatory subunits differentially regulate maturation, growth, and survival of mast cells and contribute in a unique manner to the regulation of myeloproliferative disease (MPD).
Methods
Cytokines, antibodies, and reagents
Recombinant murine IL-3 and SCF were purchased from PeproTech. PE-conjugated KIT antibody, FITC-conjugated IgE receptor antibody, PE–annexin V antibody, and 7-amino actinomycin D (7-AAD) were purchased from BD Biosciences. Rabbit anti-KIT antibody was purchased from Santa Cruz Biotechnology. Mouse anti-hemagglutinin (anti-HA) antibody, rabbit anti-p85 pan antibody, and anti-p85α specific antibody were purchased from Upstate Biotechnology. Rabbit anti–phospho-AKT and anti–phospho-ERK were purchased from Cell signaling Technology. Anti-p85β antibody was purchased from Abcam. Rabbit anti–phospho-c-Cbl antibody was purchased from Epitomics. Protein A-Sepharose beads were purchased from GE Healthcare. Retronectin was purchased from Takara. IMDM was purchased from Invitrogen. [3H]Thymidine was purchased from PerkinElmer Life and Analytical Sciences.
Mice
C57BL/6 mice and C3H/HeJ mice were purchased from The Jackson Laboratory. Mice deficient of p85α (P85α−/−) or p85β (P85β−/−) have been previously described.28,29 All mice were maintained under specific pathogen-free conditions at the Indiana University Laboratory Animal Research Center. All animal experiments were approved by the Indiana University Institutional Animal Care and Use Committee.
Cell lines
The murine IL-3 dependent myeloid cell line (32D) was used to express KIT and p85 regulatory subunits of PI3Kinase.
Construction of p85 plasmids
RNA was isolated and cDNA was prepared from murine spleen. After synthesis of cDNA, the following primers were used for p85α PCR: forward, 5′-GAATTCATGTACCCATACGATGTTCCAGATTACGCTATGAGTGCAGAGGGCTACCAG; reverse, 5′-CTCGAGTCATCGCCTCTGTTGTGCATATAC. pGEX-4T3 plasmid containing full-length p85β gene of mouse was purchased from Addgene, and the full-length p85β was amplified using primers: forward, 5′-CAAGAATTCATGTACCCATACGATGTTCCAGATTACGCTGCAGGAGCCGAG and reverse, 5′-CACCTCGAGTCAGCGTGCTGCAG ACGG.
For cloning of chimeric constructs p85αβ (which has the amino-terminal SH3 and BH domain of p85α, and the carboxy-terminal containing 2 SH2 and inter SH2 domain of p85β) and p85βα (which has the amino-terminal SH3 and BH domain of p85β, and carboxy-terminal containing 2 SH2 and inter SH2 domain of p85α), the amino-terminal half of p85α was amplified using full-length p85α as a template and amplified using primers: forward, 5′-CAAGAATTCATGTACCCATACCCATACGATGTTCCAGATTACGCTAGTGCAGAG and reverse, 5′-CCCCAGTACCATTCAGC. The carboxy-terminal half of p85β was amplified using full-length p85β as a template and amplified using primers: forward, 5′-TGGTACTGGGGGGACATC and reverse, 5′-CAACTCGAGTCAGCGTGCTGCAG. The 2 products were linked together using another PCR reaction of these amplified products using primers: forward, 5′-CAAGAATTCATGTACCCATACCCATACGATGTTCCAGATTACGCTAGTGCAGAG and reverse, 5′-CAACTCGAGTCAGCGTGCTGCAG. Similarly, for construction of p85βα clone, the amino-terminal half of p85β was amplified using primers: forward, 5′-CAAGAATTCATGTACCCATACGATGTTCCAGATTACGCTGCAGGAGCCCGA and reverse: 5′-GTCTCCCCAGTACCACTCTGCATCCTGAAGC and full-length p85β as the template. The carboxy-terminal p85α half was amplified from full-length p85α using primers: forward, 5′-GAGTGGTACTGGGGAGACATCTCAAGG; and reverse, 5′-CACCTCGAGTCATCGCCTCTGTTGTGC and using full-length p85α as a template. The 2 amplified products were linked together by another set of PCR reaction using the primers: forward, 5′-CAAGAATTCATGTACCCATACGATGTTCCAGATTACGCTGCAGGAGCCCGA; and reverse, 5′-CACCTCGAGTCATCGCCTCTGTTGTGC. PCR was performed using the following conditions: an initial denaturation step at 94°C for 2 minutes followed by 23 cycles of 94°C for 30 seconds, 60°C for 1 minute, and 72°C for 2 minutes, with a final step of 72°C for 7 minutes. All the constructs have an HA tag at the amino terminus and were cloned into the EcoRI/XhoI site upstream of an internal entry site and the enhanced green fluorescence (EGFP) protein containing bicistronic retroviral vector MIEG3.
Generation of in vitro BMMCs from WT, P85α−/−, and P85β−/− mice
To generate BM-derived mast cells (BMMCs), low density mononuclear cells (LDMNCs) were isolated from WT, P85α−/−, and P85β−/− mice and cultured in IMDM supplemented with 10% FBS, 2% penicillin/streptomycin, and 10 ng/mL of IL-3 for 3 to 4 weeks. These cells were used at different stages for growth, differentiation, and biochemical experiments.
Expression of p85 constructs in 32D cells and MCps
To express different regulatory subunits in 32D cells, cells were infected with 2 mL of high titer retroviral supernatants in the presence of 8 μg/mL polybrene and 10 ng/mL IL-3. To express different regulatory subunits in mast cell progenitors (MCps), LDMNCs were collected from WT and P85α−/− mice and prestimulated in IMDM supplemented with 20% FBS, 2% penicillin/streptomycin, and cytokines (100 ng/mL SCF and 10 ng/mL IL-3) for 48 hours before retroviral infection on fibronectin fragments (Retronectin) in nontissue culture plates. On the third day, MCps were infected with 4 mL of high-titer retroviral supernatants for various constructs. A second shot of viral infection was given 24 hours later. At 48 hours after the second infection, cells expressing EGFP were sorted and used to perform all experiments.
Proliferation assay
Proliferation assays were performed as previously described.30
Analysis of cell death
BMMCs from WT, P85α−/−, and P85β−/− mice were washed twice with warm IMDM to remove growth factors (IL-3) and starved in IMDM containing 0.2% BSA for 6 to 7 hours. A total of 2 × 105 cells were plated in 24-well plates in the presence and absence of SCF (50 ng/mL). At 48 hours after treatment, cells were washed with PBS, and the percentage of cell death was determined by annexin V and 7-AAD staining.
Mast cell maturation
Maturation of BMMCs from WT and various knockout mice was analyzed by examining the expression of KIT and IgE receptor using flow cytometry. WT and knockout MCps (1 × 106) were washed with PBS containing 0.2% BSA (Sigma-Aldrich) and incubated with 1 μg of PE–anti-KIT and FITC–anti-IgE antibodies for 30 minutes at 4°C. Cells were washed to remove unbound antibodies with PBS containing 0.2% BSA and analyzed by flow cytometry (BD Biosciences).
Mast cell degranulation assay
Mast cells from WT, P85α−/−, or P85β−/− were washed and starved in RPMI containing 0.5% BSA for 2 to 4 hours and then resuspended in Tyrode buffer (10mM HEPES buffer, pH 7.4, 130mM NaCI, 5mM KCI, 1.4mM CaCI2, 1mM MgCI2, 5.6mM glucose, and 0.1% BSA). For measuring degranulation in response to IgE, cells were sensitized with 10 mg/mL anti-dinitrophenyl (anti-DNP) IgE mAb SPE-7 (Sigma-Aldrich) for 1 hour, washed twice with 23°C Tyrode buffer, equilibrated in Tyrode buffer to 37°C for 5 minutes, and then treated for 15 minutes with DNP-human serum albumin (Sigma-Aldrich). For measuring degranulation in response to SCF, cells were incubated with 100 ng/mL SCF for 15 minutes at 37°C. The degree of degranulation was determined by measuring the release of β-hexosaminidase.31
KIT receptor internalization and degradation experiments
Rate of internalization of KIT was determined after SCF (100 ng/mL) stimulation for the indicated periods of time at 37°C by flow cytometry by staining cells with a PE–anti-KIT antibody. For protein degradation experiments, cells were incubated with cycloheximide (100 μg/mL) for 2 hours at 37°C to inhibit protein synthesis. Cells were then stimulated with SCF (100 ng/mL) for indicated periods at 37°C. Samples were assessed by SDS-PAGE and Western blotting with an antibody against KIT.
Mast cell reconstitution studies
A single dose of 5-fluorouracil (150 mg/kg body weight) was injected intraperitoneally into WT C57BL/6 and P85α−/− mice. After 3 days of injection, LDMNCs were isolated. LDMNCs were prestimulated in IMDM supplemented with 20% FBS and murine cytokines (10 ng/mL IL-6, 100 ng/mL SCF, 50 ng/mL Flt-3L, and 100 ng/mL thrombopoietin) for 2 days. Prestimulated cells were transduced with a retrovirus encoding empty vector, full-length p85α, or full-length p85β. After 2 rounds of infection, EGFP-expressing cells were purified by FACS. A total of 1 × 106 transduced and sorted cells were mixed with 1 × 105 supporting fresh splenocytes from Wsh mice and administrated intravenously by tail vein injection into lethally irradiated Wsh recipient mice (1100-cGy split dose with 4-hour interval).
Tissue distribution of mast cells
Ear, skin, and small gastrointestinal tract (stomach, duodenum, jejunum, ileum, and colon) were harvested from WT, P85α−/−, and P85β−/− mice and fixed in 10% buffered formalin, sectioned, and stained with toluidine blue (Sigma-Aldrich). For each sample, mast cells stained in purple were counted in 10 to 12 fields under 200× magnification using Leica Microsystems. An average numbers of mast cells in a given field are represented. For identification of mast cells in the peritoneal cavity, 5 mL of sterile PBS free of Ca2+/Mg+ was injected intraperitoneally into mice, and fluid was allowed to equilibrate in the peritoneum for 5 minutes. A total of 3 mL of lavage was retrieved, and total cells were counted after red cell lysis.
Mouse leukemia induction
For C3H/HeJ mouse leukemia model, 32D cells coinfected with WT KIT or KITD814V and p85α or p85β subunit were washed 3 times with PBS. A total of 1 × 106 cells in 200 μL PBS were injected into the mouse through tail vein. Injected mice were monitored for leukemia development and survival for 12 weeks.
Statistics
All graphical data were evaluated by paired Student t test, and results were considered significantly different with P value < .05. All data are represented as mean values plus or minus SD. Survival probability of transplanted mice cohorts were compared using a Kaplan-Meier survival analysis in which statistical significance was determined as P values less than .05 by log-rank test.
Results
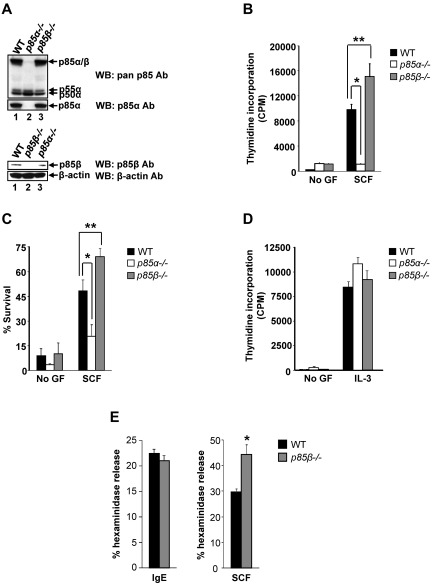
BMMCs express p85α, p85β, p55α, and p50α subunits of PI3K (Figure 1A). Loss of p85α or p85β in P85α−/− and P85β−/− BMMCs was confirmed by Western blotting (Figure 1A). To assess the role of p85α and p85β in proliferation, BMMCs derived from WT, P85α−/− and P85β−/− mice were stimulated with SCF. WT BMMCs demonstrated a significant increase in thymidine incorporation in the presence of SCF relative to unstimulated cells, whereas deficiency of p85α resulted in significant loss of SCF-mediated growth (Figure 1B). To our surprise, loss of p85β in BMMCs significantly enhanced growth relative to WT BMMCs. The changes in SCF induced BMMC growth observed because of the deficiency of p85α or p85β were partly the result of reduced and enhanced survival of these cells, respectively (Figure 1C). In contrast, IL-3–induced growth in 3 genotypes was similar (Figure 1D). Thus, p85α acts as a positive regulator and p85β as a negative regulator of BMMC growth and survival in response to KIT signaling. To further understand the role of p85β subunit in mast cell functions, we performed degranulation studies in response to IgE and SCF. As seen in Figure 1E, no significant difference in IgE-mediated degranulation was observed in P85β−/− mast cells compared with WT controls. In contrast, the deficiency of p85β resulted in a significant increase in SCF-mediated degranulation compared with WT controls. These results suggest that p85β negatively regulates mast cell growth as well as functions.
Figure 1.
Deficiency of p85β regulatory subunit of class IA PI3K results in enhanced BMMC growth and survival. (A) Expression of class IA PI3K regulatory subunits in WT, P85α−/−, and P85β−/− BMMCs. BMMCs were harvested and equal amount of protein extracts were subjected to Western blot analysis using a pan-anti–p85 antibody (this antibody recognizes all regulatory subunits of class IA PI3K), p85α-specific antibody, p85β-specific antibody, and β-actin antibody as indicated. Expression of different regulatory subunits is indicated. (B) PI3K regulatory subunits p85α and p85β differentially regulate proliferation of BMMCs. BMMCs from WT, P85α−/−, and P85β−/− mice were starved for 6 hours in serum- and cytokine-free media and cultured in the presence or absence of SCF (50 ng/mL). After 48 hours, proliferation was evaluated by [3H]thymidine incorporation. Bars represent the mean [3H]thymidine incorporation in BMMCs (CPM + SD) from one representative experiment performed in quadruplicate. Similar results were observed in 6 independent experiments. *P < .01, WT versus P85α−/−.**P < .01, WT versus P85β−/−. (C) Enhanced survival of p85β-deficient BMMCs. BMMCs from WT, P85α−/−, and P85β−/− mice were starved for 6 hours in serum- and cytokine-free media and cultured in the presence or absence of SCF (50 ng/mL). After 48 hours, cells were stained with PE-conjugated annexin V and 7-AAD followed by flow cytometric analysis. Shown is a representative bar graph demonstrating percentage of annexin V and 7-AAD–negative cells in the presence and absence of SCF. Similar results were observed in 4 independent experiments. *P < .05, WT versus P85α−/−. **P < .01, WT versus P85β−/−. (D) Cells described in panel B were subjected to proliferation in the presence of IL-3. Bars represent the mean [3H]thymidine incorporation in BMMCs (CPM + SD) from one representative experiment performed in quadruplicate. (E) p85β deficiency enhances SCF-mediated mast cell granule mediator release. Cells described in panel B were starved of growth factor for 6 hours and washed. Cells were sensitized with anti–DNP IgE and stimulated with DNP-BSA for 30 minutes (left panel) or cells were stimulated with SCF for 30 minutes (right panel). Degranulation was measured as β-hexosaminidase release. Similar results were observed in 2 independent experiments. *P < .05, WT versus P85β−/−.
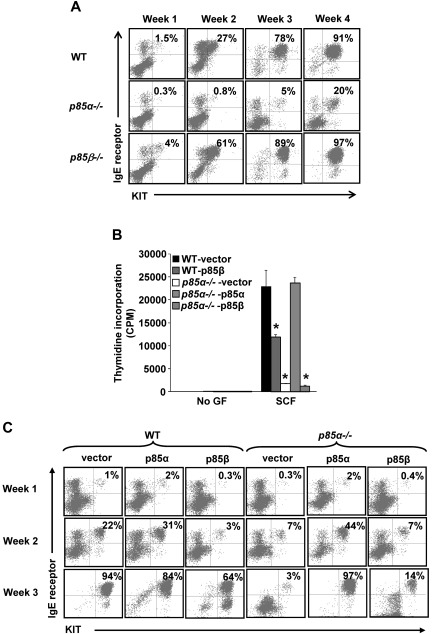
Although the aforementioned studies demonstrated functional dichotomy between the 2 regulatory subunits with respect to KIT-induced growth and survival, we assessed whether these differences could be the result of changes in the maturation of BMMCs, including changes in the expression of KIT. Although WT BMMCs fully matured into KIT and IgE receptor double-positive mast cells by 3 to 4 weeks of culture, p85α-deficient BMMCs showed a significant defect in this process (Figure 2A). In contrast, p85β-deficient BMMCs matured at a significantly faster rate compared with WT BMMCs as assessed by the acquisition of KIT and IgE receptor double-positive cells (Figure 2A). As seen in Figure 2B, restoring the expression of p85α in p85α-deficient MCps completely restored SCF-induced growth. Overexpression of p85β in p85α-deficient cells did not rescue SCF-induced growth, whereas overexpression of p85β in WT MCps significantly reduced SCF-induced growth. Similarly, restoring the expression of p85α in p85α-deficient cells completely corrected the defective maturation of these cells as determined by the acquisition of KIT and IgE receptor double-positive cells (Figure 2C). In contrast, overexpression of p85β in p85α-deficient MCps did not correct the defective maturation. Furthermore, overexpression of p85β in WT MCps resulted in significant reduction in the maturation of BMMCs. These results demonstrate that p85α and p85β play opposite roles in regulating the growth and maturation of BMMCs.
Figure 2.
Overexpression of p85β regulatory subunit of class IA PI3K inhibits BMMC growth and maturation. (A) Deficiency of p85α and p85β alters the maturation of BMMCs. LDMNCs from WT, P85α−/−, and P85β−/− mice were cultured in the presence of IL-3 (10 ng/mL) for 4 weeks. At the indicated time points, maturation was analyzed by staining the cells with antibodies that recognize KIT and IgE receptor followed by flow cytometry. Shown is dot blot profile from one of 4 independent experiments. (B) Reduced growth of WT MCps overexpressing p85β. WT and P85α−/− MCps transduced with vector, p85α, or p85β were sorted to homogeneity and cultured in the presence of IL-3 (10 ng/mL). Cells were starved for 6 hours in serum- and cytokine-free media and cultured in the presence or absence of SCF (50 ng/mL). After 48 hours, proliferation was evaluated by a [3H]thymidine incorporation assay. Bars represent the mean [3H]thymidine incorporation in BMMCs (CPM + SD) from one representative experiment performed in quadruplicate. Similar results were observed in 3 or 4 additional independent experiments. *P < .01, WT-vector versus WT-p85β, WT-vector versus P85α−/−-vector, and WT-vector versus P85α−/− p85β. (C) Overexpression of p85β in WT MCps results in reduced differentiation. WT and P85α−/− MCps transduced with vector, p85α, or p85β were sorted to homogeneity and cultured in the presence of IL-3 (10 ng/mL). Cells were harvested at indicated time points, and maturation was analyzed by staining the cells with antibodies to detect the expression of KIT and IgE receptor by flow cytometry. Shown is a dot blot profile of an independent experiment. Similar findings were observed in 2 to 4 additional independent experiments.
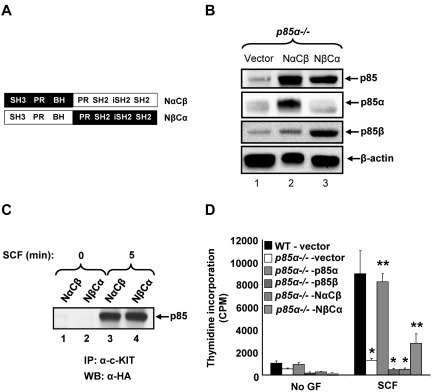
Because p85α and p85β share close to 80% homology in the carboxy-terminal sequences (SH2 domains) and only 40% homology in the amino-terminal sequences (SH3 and BH), we assessed whether the opposite functions of p85α and p85β in BMMC growth and differentiation were the result of differences in amino-terminal sequences and whether both N- and C-terminal sequences were necessary for these functions. To this end, we made mutant p85 constructs consisting of the amino-terminal of p85α and the carboxy-terminal of p85β (NαCβ), and vice versa (NβCα) as shown in Figure 3A. Figure 3B shows the expression of these mutants in p85α-deficient cells, and Figure 3C shows the binding of p85 mutants to KIT on SCF stimulation. As seen in Figure 3D, restoration of p85α in p85α-deficient BMMCs completely corrected the defective proliferation associated with P85α−/− BMMCs, whereas overexpression of p85β did not rescue SCF-induced proliferation. Only one of the chimeric mutants of p85α (NβCα) showed partial correction in SCF-induced growth. These results suggest that cooperation between carboxy- and amino-terminal sequence of p85α is essential for normal SCF-induced BMMC growth.
Figure 3.
Cooperation between carboxy- and amino-terminal sequences of p85 is required for mast cell growth. (A) Schematic of p85 chimera constructs consisting of N-terminal p85α and C-terminal p85β sequences (NαCβ) or N-terminal p85β and C-terminal p85α sequences (NβCα). (B) P85α−/− mast cell progenitors (MCp) transduced with indicated p85 constructs were sorted to homogeneity and cultured in the presence of IL-3 (10 ng/mL). To detect the expression of p85 subunits, cells were harvested and subjected to Western blot analysis using anti-p85 PAN, anti-p85α, anti-p85β, and β-actin antibodies. (C) Murine myeloid 32D cells were coinfected with WT KIT and p85 chimera mutants (NαCβ or NβCα) and starved for 8 hours in serum- and cytokine-free media followed by SCF stimulation (100 ng/mL) for 5 minutes. An equal amount of protein (500 μg) was subjected to immunoprecipitation using an anti-KIT antibody followed by Western blotting with an anti-HA antibody. (D) WT and P85α−/− BMMCs transduced with vector or p85 constructs were sorted to homogeneity and cultured in the presence of IL-3 (10 ng/mL). Cells were starved for 6 hours in serum- and cytokine-free media and cultured in the presence or absence of SCF (50 ng/mL). After 48 hours, proliferation was evaluated by [3H]thymidine incorporation. Bars represent the mean [3H]thymidine incorporation in BMMCs (CPM + SD) from one representative experiment performed in quadruplicate. Similar results were observed in 6 independent experiments. *P < .01, WT-vector versus P85α−/−-vector versus P85α−/−-p85β versus P85α−/− -NαCβ. **P < .01, P85α−/−-vector versus P85α−/−-p85α versus P85α−/− -NβCα.
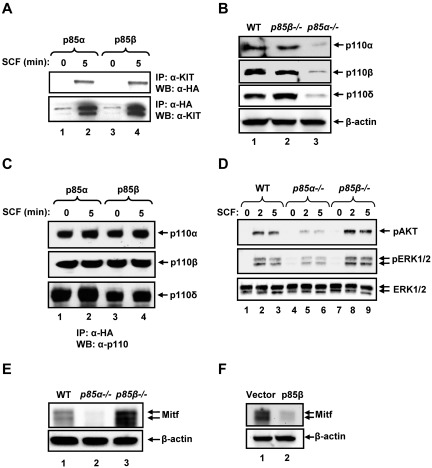
In an effort to further determine the mechanism(s) behind the differential regulation of BMMC growth and maturation by p85α and p85β, we assessed whether p85α and p85β bound to KIT to the same extent. As seen in Figure 4A, p85α and p85β bind similarly to KIT in response to SCF stimulation. We next assessed the expression of p110 catalytic subunits in BMMCs and the ability of p85α and p85β to bind p110 catalytic subunits. Although expression of the catalytic subunit p110α, p110β, and p110δ was significantly reduced in P85α−/− BMMCs, their expression in P85β−/− BMMCs was similar to WT BMMCs (Figure 4B). Furthermore, both p85α and p85β are equally capable of binding p110α, p110β, and p110δ catalytic subunits of PI3K (Figure 4C). These results suggest that p85α is the major subunit involved in regulating the stability of p110 catalytic subunits in BMMCs, and both p85α and p85β are equally capable of interacting with the catalytic subunits. We next examined the activation of AKT and ERK MAP kinase in WT, p85α, and p85β deficient BMMCs to assess whether perturbation in the activation of these molecules may explain the enhanced growth observed in P85β−/− BMMCs in response to SCF compared with WT BMMCs. As seen in Figure 4D, a significant increase in the activation of AKT and ERK1/2 was observed in P85β−/− BMMCs relative to WT. Thus, differential regulation of BMMC growth and survival by p85α and p85β is probably the result of differential activation of AKT and ERK MAP kinase downstream from KIT.
Figure 4.
p85α and p85β differentially regulate signaling in BMMCs. (A) p85α and p85β bind to KIT in response to SCF. Cells (32D) expressing WT KIT and p85α or p85β were starved for 8 hours and stimulated with SCF (100 ng/mL) for 5 minutes. Equal amount of cell lysates (500 μg) were subjected to immunoprecipitation with an anti-KIT antibody followed by Western blot analysis with an anti-HA antibody or immunoprecipitated with an anti-HA antibody followed by Western blot analysis with an anti-KIT antibody. (B) Deficiency of p85β in BMMCs does not impair the expression of p110 catalytic subunits of PI3K. Cell lysates from WT, p85α-, and p85β-deficient BMMCs were subjected to Western blot analysis using antibodies that specifically recognize the indicated forms of p110 catalytic subunits. Expression of various catalytic subunits is indicated. (C) p85α and p85β regulatory subunits of PI3K bind p110α, p110β, and p110δ catalytic subunits with equal efficiency. Cells (32D) expressing p85α or p85β subunit of PI3K were starved and stimulated with SCF and subjected to immunoprecipitation using an anti-HA antibody followed by Western blot analysis with an anti-p110α, anti-p110β, or anti-p110δ antibody. The amount of immunoprecipitated protein in each lane is indicated. (D) Enhanced activation of AKT and ERK MAP kinase in p85β-deficient BMMCs. WT, P85α−/−, and P85β−/− BMMCs were starved overnight in serum- and cytokine-free media and stimulated with SCF (100 ng/mL) for 2 and 5 minutes. Equal amount of protein lysates were subjected to Western blot analysis using an anti–phospho-AKT or anti–phospho-ERK1/2 antibody. Total ERK1/2 protein in each lane is indicated. Similar findings were observed in 2 independent experiments. (E) Deficiency of p85β in BMMCs results in enhanced expression of Mitf. WT, P85α−/−, or P85β−/− BMMCs were lysed, and lysates subjected to Western blot analysis using an anti-Mitf antibody. Expression of Mitf and β-actin in each lane is indicated. A representative Western blot is shown. Similar results were obtained in 3 independent experiments. (F) Overexpression of p85β in WT BMMCs inhibits the expression of Mitf. WT BMMCs expressing empty vector or p85β were lysed and lysates subjected to Western blot analysis using an anti-Mitf antibody. A representative Western blot is shown. Similar results were obtained in 2 independent experiments.
In addition to increased KIT-induced growth, deficiency of p85β also results in enhanced IL-3 induced BMMC maturation, including KIT expression. BMMC maturation, including KIT expression, is largely regulated by the transcription factor Mitf. To determine whether enhanced and reduced BMMC maturation resulting from loss or gain of p85β expression, respectively, impacts Mitf expression in BMMCs, we performed Western blot analysis on WT, P85α−/−, and P85β−/− BMMCs as well as in vector-expressing WT or p85β-overexpressing WT BMMCs. As seen in Figure 4E, whereas P85α−/− BMMCs showed significantly reduced Mitf expression, loss of p85β resulted in increased Mitf expression relative to controls. Consistently, overexpression of p85β in WT BMMCs resulted in reduced Mitf expression (Figure 4F). These results suggest that p85β regulates BMMC maturation, including KIT expression in part by regulating the expression of Mitf.
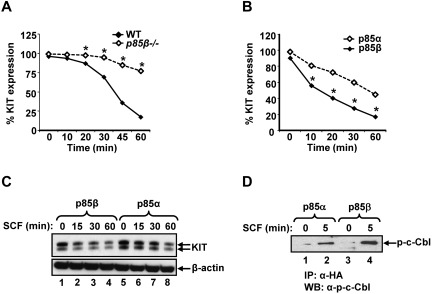
Activation of KIT receptor by its ligand SCF is followed by its internalization and degradation.32,33 To determine the mechanism(s) behind increased growth and activation of downstream signaling events from KIT receptor in p85β-deficient BMMCs, we examined the rate of internalization of the KIT receptor in response to SCF. As seen in Figure 5A, deficiency of p85β in BMMCs reduces SCF induced KIT receptor internalization compared with WT controls at all time points examined. Consistently, overexpression of p85β significantly enhances SCF induced KIT receptor internalization compared with p85α-expressing cells (Figure 5B). Likewise, significantly enhanced KIT receptor protein degradation was observed in p85β-overexpressing cells compared with p85α-overexpressing cells in response to SCF stimulation (Figure 5C).
Figure 5.
Reduced KIT receptor internalization in p85β-deficient BMMCs compared with WT in response to SCF. (A) WT or P85β−/− BMMCs were starved overnight and incubated with cycloheximide (100 ng/mL) for 2 hours. After cycloheximide treatment, cells were stimulated with SCF (100 ng/mL) for indicated time points, and KIT receptor internalization was studied by staining the cells with PE-conjugated anti-KIT receptor antibody followed by flow cytometric analysis. Shown is one of 4 independent experiments performed in triplicate. *P < .01, WT versus P85β−/−. (B) KIT receptor internalization is enhanced in cells expressing p85β compared with p85α on SCF stimulation. Cells (32D) coinfected with KIT and p85α or p85β were starved for 8 hours and incubated with cycloheximide (100 ng/mL) for 2 hours. After cycloheximide treatment, cells were stimulated with SCF (100 ng/mL) for indicated time points, and KIT receptor internalization was studied by staining the cells with PE-conjugated anti-KIT receptor antibody followed by flow cytometric analysis. Shown is one of 6 independent experiments performed in triplicate. *P < .01, p85α versus p85β. (C) Enhanced KIT receptor degradation in cells expressing p85β compared with p85α on SCF stimulation. Cells (32D) expressing WT KIT and p85α or p85β subunits were starved for 8 hours and incubated with cycloheximide (100 ng/mL) for 2 hours. After cycloheximide treatment, cells were stimulated with SCF (100 ng/mL) for indicated time points, and equal amount of protein lysates were subjected to Western blot analysis using an anti-KIT receptor antibody. Similar results were observed in 4 independent experiments. (D) p85β preferentially binds to c-Cbl compared with p85α in response to SCF stimulation. 32D cells coinfected with KIT and p85α or p85β were starved for 8 hours and stimulated with SCF (100 ng/mL) for 5 minutes. Equal amount of cell lysates (500 μg) were immunoprecipitated with an anti-HA antibody followed by Western blotting with a phospho-c-Cbl antibody. Data are from one of 4 independent experiments.
Previous studies have shown that KIT internalization and degradation are regulated in large part by an E3 ubiquitin ligase c-Cbl.34,35 We hypothesized that perhaps p85α and p85β differentially regulate the activation and binding of c-Cbl in response to KIT activation. To test this, we examined the association of p85α and p85β with the phosphorylated form of c-Cbl. Immunoprecipitation experiments in 32D cells expressing p85α or p85β and stimulated with SCF followed by Western blot analysis using an anti–phospho-c-Cbl antibody showed significantly enhanced binding of phospho-c-Cbl to p85β compared with p85α (Figure 5D). Taken together, these results suggest that p85β negatively regulates KIT receptor signaling in part by regulating its internalization and degradation.
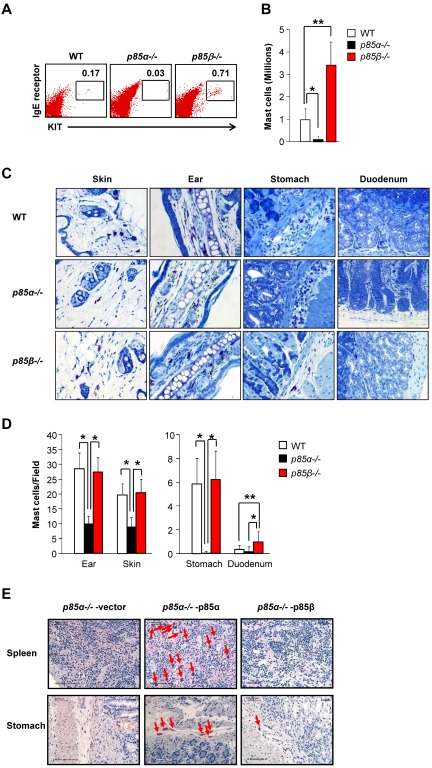
To determine whether the increased growth and maturation of P85β−/− BMMCs in vitro is associated with increased numbers of mast cells in vivo, we harvested cells from the peritoneal cavity of WT and P85β−/− mice, counted and stained the cells with anti-KIT and anti-IgE receptor antibodies followed by flow cytometric analysis. As seen in Figure 6A and B, loss of p85β resulted in significantly enhanced percentage and number of mast cells in the peritoneal cavity of P85β−/− mice relative to WT controls. To further determine the distribution of mast cells in various tissues, we harvested, embedded, sectioned, and stained various tissues from WT, P85α−/−, and P85β−/− mice. Figure 6C shows representative tissue sections from 3 genotypes. As seen in Figure 6D, P85α−/− mice show significantly reduced mast cells in ear, skin, and stomach compared with WT mice. In contrast, no significant difference in the number of mast cells was observed in ear, skin, and stomach of P85β−/− mice compared with WT mice. Furthermore, P85β−/− mice show a significant increase in the number of mast cells in duodenum compared with WT mice. Thus, consistent with our in vitro observations using BMMCs derived from the BM of P85β−/− mice, in vivo deficiency of p85β also results in increased number of mast cells in some tissues, but not all.
Figure 6.
Loss of p85β regulatory subunit in mice results in increased number of mast cells in vivo. (A) Cells from the peritoneal cavity of WT and p85β-deficient mice were harvested and stained with antibodies against KIT and IgE receptor. Representative dot blots indicate peritoneal cavity-derived mast cells stained with anti-KIT and anti-IgE receptor antibodies from the indicated genotypes. Numbers in the upper right quadrant of dot blot indicate the percentage of peritoneal cells that are double-positive for KIT and IgE receptor expression. (B) Bar graph represents quantitative assessment of the total number of mast cells in 5 mL of peritoneal lavage from the indicated genotypes. n = 4 (mean ± SEM). *P < .05, WT versus P85α−/−. **P < .05, WT versus P85β−/−. (C) Representative photomicrographs of toludine blue-stained tissue sections derived from WT, P85α−/−, and P85β−/− mice. (D) Bar graph represents quantitative assessment of toludine blue-positive mast cells in the indicated tissues. n = 3 to 5 (mean ± SEM). *P < .05. (E) Histologic analysis of stomach and spleen showing the reconstitution of mast cells in Wsh mice. P85α−/− mice were injected intraperitoneally with 5-flurouracil (150 mg/kg body weight), and BM cells were harvested after 72 hours of injection. These cells were transduced with p85α or p85β and sorted to homogeneity on the basis of EGFP expression. Sorted cells (1 × 106) were mixed with recipient Wsh splenocytes (0.1 × 106 cells) and injected into mast cell-deficient Wsh mice. After 4 months of transplantation, mice were killed; different tissues were harvested and analyzed for mast cells by leader staining. Shown are representative sections of spleen and stomach from the indicated genotypes. Arrows indicate mast cells in various tissues.
Because restoring the expression of p85α in p85α deficient BMMCs results in complete rescue of growth and differentiation in vitro, whereas overexpression of p85β in these same cells inhibits growth and maturation, we next determined whether this would indeed be the case in vivo. To do this, we transduced 5-fluorouracil-treated p85α-deficient BM cells with p85α- or p85β-expressing retrovirus, sorted the cells, and transplanted them into lethally irradiated Wsh recipient mice (ie, these mice lack endogenous tissue mast cells). After 4 months, we harvested the spleen and stomach of recipient Wsh mice and analyzed mast cell reconstitution by leader staining. Figure 6E shows representative spleen and stomach sections of Wsh mice transplanted with p85α-deficient cells infected with either p85α- or p85β-encoding retrovirus. Consistent with our in vitro findings, full-length p85α transduced P85α−/− BM cells reconstituted mast cells in Wsh transplanted mice as indicated with red arrows. In contrast, p85β-expressing P85α−/− BM cells showed only minimal reconstitution of mast cells relative to p85α full-length expressing controls. These results confirm the differential regulation of mast cell development by PI3K regulatory subunits of p85α and p85β in vivo in normal mast cell development.
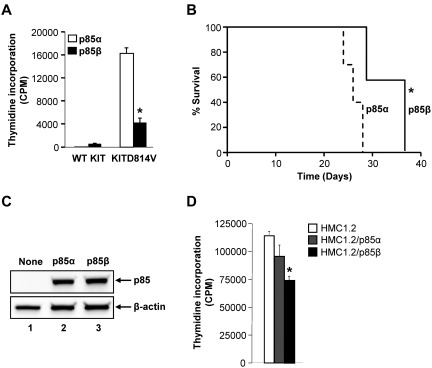
Gain-of-function mutations in KIT receptor (KITD816V) in humans are associated with systemic mastocytosis, gastrointestinal stromal tumors, and acute myelogenous leukemia. We have previously shown that oncogenic KITD814V is sufficient to induce ligand-independent growth in primary hematopoietic stem and progenitor cells (HSC/P), including MCps in vitro as well as transformation in vivo.36 Because p85β negatively regulates mast cell growth and survival by enhancing KIT receptor internalization through c-Cbl, we assessed whether overexpression of p85β would also suppress the constitutive growth of oncogenic KITD814V-expressing cells. We coinfected 32D cells with WT KIT or KITD814V and p85α or p85β. Cells were starved and growth was analyzed in the absence of growth factors by thymidine incorporation assay. As expected, 32D cells bearing WT KIT and p85α or p85β show minimal proliferation in the absence of growth factors (Figure 7A). In contrast, 32D cells expressing oncogenic KITD814V and p85α or p85β showed significantly increased growth in the absence of growth factors. However, approximately 80% reduction in the growth of cells bearing KITD814V and p85β was observed compared with cells bearing KITD814V and p85α (Figure 7A). To determine whether p85β induced ligand-independent suppression of growth observed in vitro results in delayed survival of mice transplanted with oncogenic KITD814V-bearing cells, we transplanted 32D cells coinfected with KITD814V and p85α or p85β subunit into syngenic C3H/HeJ mice, and monitored MPD progression and survival. As seen in Figure 7B, mice transplanted with cells bearing KITD814V and p85β survived significantly longer compared with mice transplanted with cells bearing KITD814V and p85α. These results demonstrate that p85β functions as a negative regulator of cytokine signaling and suppresses the growth of oncogenic KITD814V-bearing cells in vitro and delays the onset of MPD in vivo.
Figure 7.
p85β suppresses the oncogenic potential of activating KITD814V mutation. (A) p85β inhibits the constitutive growth of oncogenic KITD814V-expressing cells. Cells (32D) were coinfected with WT KIT or KITD814V and p85α or p85β subunits, and cells were sorted to homogeneity. Cells were starved in serum- and cytokine-free medium for 6 hours and cultured in the absence of growth factors. After 48 hours, proliferation was evaluated by [3H]thymidine incorporation assay. Bars represent the mean [3H]thymidine incorporation (CPM + SD) from one representative experiment performed in quadruplicate. Similar results were observed in 3 independent experiments. *P < .01, KITD814V and p85α versus KITD814V and p85β. (B) p85β prolongs the survival of mice transplanted with oncogenic KITD814V-expressing cells. Kaplan-Meier survival curves of mice transplanted with 32D cells coinfected with WT KIT or KITD814V and p85α or p85β subunits. Cells (32D; 1 × 106) were injected into syngenic C3H/HeJ mice and mice were monitored for MPD (n = 7). Solid lines represent the survival of p85β-expressing oncogenic KITD814V-transplanted mice; and dotted lines, survival of p85α-expressing KITD814V-transplanted mice. *P < .01, p85β versus p85α. (C) HMC1.2 cells transduced with indicated p85 constructs were sorted to homogeneity based on EGFP expression. To detect the expression of p85 subunits, cells were harvested and subjected to Western blot analysis using anti-HA and β-actin antibodies as indicated. Similar expression of p85α and p85β was observed in transduced cells. (D) HMC1.2 cells transduced with indicated p85 constructs were starved in serum- and cytokine-free medium for 6 hours and cultured in the absence of growth factors. After 48 hours, proliferation was evaluated by [3H]thymidine incorporation assay. Bars represent the mean [3H]thymidine incorporation (CPM + SD) from one representative experiment performed in quadruplicate. Similar results were observed in 3 independent experiments. *P < .05, HMC1.2 versus HMC1.2/p85β. *P < .05, HMC1.2/p85α versus HMC1.2/p85β.
To further determine the relevance of these findings to human disease, we transduced the human mast cell line (HMC1.2) bearing the activating KIT mutation (KITV560G and KITD816V) with p85α or p85β and sorted the transduced cells based on EGFP expression. As seen in Figure 7C, similar expression of p85α and p85β was observed in transduced HMC1.2 cells. In addition, cells bearing p85β showed significantly reduced growth compared with cells bearing p85α subunit of class IA PI3K (Figure 7D). These results are consistent with our observations in murine cells bearing KITD814V and the regulatory subunits of p85 (Figure 7A).
Discussion
Based on recent studies demonstrating enhanced insulin sensitivity in both Pik3r1−/− and Pik3r2−/− mice, it is generally thought that all regulatory subunits of class IA PI3K behave in a functionally redundant manner and that their primary function is to stabilize and activate various catalytic subunits.37–39 Our results in primary hematopoietic cells using both genetic and biochemical approaches clearly demonstrate unique role(s) for regulatory subunits in mast cell growth, maturation, and leukemogenesis. We show that genetic disruption of p85α results in defective IL-3 mediated BMMC differentiation and KIT mediated growth and survival. Reconstitution of P85α−/− BMMCs with p85α, but not p85β, completely corrects both IL-3–mediated differentiation as well as KIT-induced proliferation, suggesting that the phenotypic defects observed in P85α−/− BMMCs are a result of specific loss of p85α and not quantitative reduction in the overall expression of regulatory subunits. The notion of specificity was further demonstrated in BMMCs lacking the p85β subunit of PI3K. Furthermore, our results demonstrate that not only are the amino-terminal domain sequences such as the SH3 and BH domain found within the full-length form of p85α essential for KIT-induced growth and survival, sequences in the carboxy-terminal domain of p85α are also required for this process because chimeric protein consisting of the amino-terminal half of p85α but the carboxy-terminal half of p85β was unable to rescue KIT-induced growth. Although it has been suggested that the amino-terminal domains of p85α and p85β may function as linker domains to allow the p85 subunits to associate with other proteins, their precise role in relevant cell systems under physiologic conditions still remains unknown. The increase in proliferation and reduced cell death of mast cells lacking p85β are consistent with recent reports where T cells lacking p85β subunit show similar increase in proliferation and decrease in death when stimulated with anti-CD3 plus IL-2.40
Activation of KIT by its ligand SCF is followed by internalization and degradation.41 Ubiquitination and subsequent degradation of KIT has been implicated as the key mechanism in regulating the duration and intensity of downstream signaling events.41 Our results show impaired KIT receptor internalization in P85β−/− BMMCs compared with WT controls. Consistently, we show that overexpression of p85β enhances the rate of KIT internalization and degradation compared with cells expressing p85α subunit. These results suggest that the enhanced proliferation and survival of P85β−/− BMMCs in response to SCF are probably the result of reduced KIT receptor internalization leading to prolonged activation of downstream signals, including ERK MAP kinase and AKT. E3 ubiquitin ligases, including Cbl proteins, are involved in binding and degradation of activated KIT leading to down-regulation of KIT receptor signaling.35 We observed enhanced phosphorylation of c-Cbl in cells overexpressing p85β compared with p85α in KIT-stimulated cells, which was associated with enhanced KIT receptor internalization. Thus, p85β through interaction with c-Cbl regulates the internalization of KIT, deficiency of which probably contributes to enhanced KIT-induced growth, survival, and activation of downstream signals.
Recent studies have identified the first c-Cbl mutation in an acute myeloid leukemia patient, called Cbl-R420Q.42 Interestingly, overexpression of Cbl-R420Q with KIT results in cytokine-independent proliferation, survival, and clonogenic growth because of reduced SCF-induced ubiquitination and internalization of KIT in vitro, resulting in generalized mastocytosis and myeloid leukemia in transplanted mice in vivo.43 Interestingly, activating mutations of KIT are known to be involved in several human diseases. Impaired recruitment of Cbl to activated KIT results in deregulated positive signaling, which probably contributes to transformation.44 Because p85β regulates WT KIT receptor internalization through binding to c-Cbl, we tested whether its overexpression will suppress the growth of cells bearing an oncogenic form of KIT (KITD814V). Consistent with our hypothesis, overexpression of p85β significantly suppressed the constitutive growth of cells bearing oncogenic KITD814V in vitro and prolonged the survival of transplanted mice in vivo. These findings are consistent with those recently described with mutated EpoRs found in patients with primary familial and congenital polycythemia, which lack 3 important tyrosines shown not to bind p85 or internalize on stimulation.45 These mutated EpoRs exhibit increased Epo sensitivity and prolonged signaling compared with WT EpoR.45 Interestingly, addition of residues encompassing Y429 and Y431 to these truncated receptors restores the binding of p85β as well as Epo sensitivity. Thus, interactions between p85β and c-Cbl are critical for regulating the internalization and degradation of receptors, deficiency or inability to bind c-Cbl alters the down-regulation of receptors resulting in prolonged downstream signals leading to human malignancies.
Previous studies have shown that the transcription factor Mitf is essential for KIT expression and mice deficient in the expression of Mitf lack mast cells in most tissues.46,47 We observed enhanced expression of Mitf in P85β−/− BMMCs and reduced expression of Mitf in p85β-overexpressing WT BMMCs, which was associated with enhanced and reduced maturation of BMMCs as well as expression of KIT, respectively. Although these are intriguing findings, how precisely p85β represses Mitf expression in BMMCs is unclear. Whether p85β binds to the Mitf promoter directly and represses Mitf expression or inhibits the expression of an intermediary factor is currently under investigation. Taken together, our studies provide genetic and biochemical evidence to demonstrate a differential role for p85 regulatory subunits of PI3K in regulating the growth and maturation of hematopoietic cells as well as in leukemogenesis.
Acknowledgments
The authors thank Marilyn Wales for administrative support.
This work was supported in part by the National Institutes of Health (grants R01HL075816 and R01HL077177, R.K).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.K., R.S.M., B.R., E.S., P.M., J.G., V.M., P.H., and J.D.B. performed research and analyzed data; R.S.M. wrote the paper; and R.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for V.M. is Cancer Immunotherapy and Hematology, Genentech, South San Francisco, CA.
Correspondence: Reuben Kapur, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut St, Rm 168, Indianapolis, IN 46202; e-mail: rkapur@iupui.edu.
References
- 1.Kitamura Y, Shimada M, Hatanaka K, Miyano Y. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977;268(5619):442–443. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- 2.Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271(5250):818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8(6):478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer M, Echtenacher B, Hultner L, et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188(12):2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen BM, Akin C, Gilfillan AM. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br J Pharmacol. 2008;154(8):1572–1582. doi: 10.1038/bjp.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai M, Shih LS, Newlands GF, et al. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo: analysis by anatomical distribution, histochemistry, and protease phenotype. J Exp Med. 1991;174(1):125–131. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai M, Takeishi T, Thompson H, et al. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci U S A. 1991;88(14):6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nocka K, Buck J, Levi E, Besmer P. Candidate ligand for the c-kit transmembrane kinase receptor: KL, a fibroblast derived growth factor stimulates mast cells and erythroid progenitors. EMBO J. 1990;9(10):3287–3294. doi: 10.1002/j.1460-2075.1990.tb07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemura A, Tsai M, Ando A, Wershil BK, Galli SJ. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol. 1994;144(2):321–328. [PMC free article] [PubMed] [Google Scholar]
- 10.Serve H, Yee NS, Stella G, Sepp-Lorenzino L, Tan JC, Besmer P. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J. 1995;14(3):473–483. doi: 10.1002/j.1460-2075.1995.tb07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihle JN, Keller J, Oroszlan S, et al. Biologic properties of homogeneous interleukin 3: I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983;131(1):282–287. [PubMed] [Google Scholar]
- 12.Ihle JN. Interleukin-3 and hematopoiesis. Chem Immunol. 1992;51:65–106. doi: 10.1159/000420755. [DOI] [PubMed] [Google Scholar]
- 13.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52(2):447–452. [PubMed] [Google Scholar]
- 15.Tian Q, Frierson HF, Jr, Krystal GW, Moskaluk CA. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol. 1999;154(6):1643–1647. doi: 10.1016/S0002-9440(10)65419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 17.Choe YS, Kim JG, Sohn SK, et al. c-kit Expression and mutations in peripheral T cell lymphomas, except for extra-nodal NK/T cell lymphomas. Leuk Lymphoma. 2006;47(2):267–270. doi: 10.1080/10428190500281680. [DOI] [PubMed] [Google Scholar]
- 18.Jiao B, Wu CF, Liang Y, et al. AML1-ETO9a is correlated with C-KIT overexpression/mutations and indicates poor disease outcome in t(8;21) acute myeloid leukemia-M2. Leukemia. 2009;23(9):1598–1604. doi: 10.1038/leu.2009.104. [DOI] [PubMed] [Google Scholar]
- 19.Metcalf D. Hematopoietic cytokines. Blood. 2008;111(2):485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lantz CS, Boesiger J, Song CH, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392(6671):90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 21.Fukao T, Yamada T, Tanabe M, et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3(3):295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 22.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 23.Backer JM, Myers MG, Jr, Shoelson SE, et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsu M, Hiles I, Gout I, et al. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- 25.Escobedo JA, Navankasattusas S, Kavanaugh WM, Milfay D, Fried VA, Williams LT. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- 26.Fruman DA, Cantley LC, Carpenter CL. Structural organization and alternative splicing of the murine phosphoinositide 3-kinase p85 alpha gene. Genomics. 1996;37(1):113–121. doi: 10.1006/geno.1996.0527. [DOI] [PubMed] [Google Scholar]
- 27.Pons S, Asano T, Glasheen E, et al. The structure and function of p55PIK reveal a new regulatory subunit for phosphatidylinositol 3-kinase. Mol Cell Biol. 1995;15(8):4453–4465. doi: 10.1128/mcb.15.8.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terauchi Y, Tsuji Y, Satoh S, et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat Genet. 1999;21(2):230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- 29.Ueki K, Yballe CM, Brachmann SM, et al. Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2002;99(1):419–424. doi: 10.1073/pnas.012581799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mali RS, Ramdas B, Ma P, et al. Rho kinase regulates the survival and transformation of cells bearing oncogenic forms of KIT, FLT3, and BCR-ABL. Cancer Cell. 2011;20(3):357–369. doi: 10.1016/j.ccr.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J Immunol. 1997;158(5):2350–2355. [PubMed] [Google Scholar]
- 32.Blume-Jensen P, Claesson-Welsh L, Siegbahn A, Zsebo KM, Westermark B, Heldin CH. Activation of the human c-kit product by ligand-induced dimerization mediates circular actin reorganization and chemotaxis. EMBO J. 1991;10(13):4121–4128. doi: 10.1002/j.1460-2075.1991.tb04989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broudy VC, Lin NL, Liles WC, et al. Signaling via Src family kinases is required for normal internalization of the receptor c-Kit. Blood. 1999;94(6):1979–1986. [PubMed] [Google Scholar]
- 34.Wisniewski D, Strife A, Clarkson B. c-kit ligand stimulates tyrosine phosphorylation of the c-Cbl protein in human hematopoietic cells. Leukemia. 1996;10(9):1436–1442. [PubMed] [Google Scholar]
- 35.Zeng S, Xu Z, Lipkowitz S, Longley JB. Regulation of stem cell factor receptor signaling by Cbl family proteins (Cbl-b/c-Cbl). Blood. 2005;105(1):226–232. doi: 10.1182/blood-2004-05-1768. [DOI] [PubMed] [Google Scholar]
- 36.Munugalavadla V, Sims EC, Borneo J, Chan RJ, Kapur R. Genetic and pharmacologic evidence implicating the p85 alpha, but not p85 beta, regulatory subunit of PI3K and Rac2 GTPase in regulating oncogenic KIT-induced transformation in acute myeloid leukemia and systemic mastocytosis. Blood. 2007;110(5):1612–1620. doi: 10.1182/blood-2006-10-053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fruman DA, Mauvais-Jarvis F, Pollard DA, et al. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85 alpha. Nat Genet. 2000;26(3):379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- 38.Chen D, Mauvais-Jarvis F, Bluher M, et al. p50alpha/p55alpha phosphoinositide 3-kinase knockout mice exhibit enhanced insulin sensitivity. Mol Cell Biol. 2004;24(1):320–329. doi: 10.1128/MCB.24.1.320-329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okkenhaug K, Vanhaesebroeck B. New responsibilities for the PI3K regulatory subunit p85 alpha. Sci STKE. 2001;2001(65):pe1. doi: 10.1126/stke.2001.65.pe1. [DOI] [PubMed] [Google Scholar]
- 40.Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. Enhanced T cell proliferation in mice lacking the p85beta subunit of phosphoinositide 3-kinase. J Immunol. 2004;172(11):6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 41.Miyazawa K, Toyama K, Gotoh A, Hendrie PC, Mantel C, Broxmeyer HE. Ligand-dependent polyubiquitination of c-kit gene product: a possible mechanism of receptor down modulation in M07e cells. Blood. 1994;83(1):137–145. [PubMed] [Google Scholar]
- 42.Sargin B, Choudhary C, Crosetto N, et al. Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood. 2007;110(3):1004–1012. doi: 10.1182/blood-2007-01-066076. [DOI] [PubMed] [Google Scholar]
- 43.Bandi SR, Brandts C, Rensinghoff M, et al. E3 ligase-defective Cbl mutants lead to a generalized mastocytosis and myeloproliferative disease. Blood. 2009;114(19):4197–4208. doi: 10.1182/blood-2008-12-190934. [DOI] [PubMed] [Google Scholar]
- 44.Masson K, Heiss E, Band H, Ronnstrand L. Direct binding of Cbl to Tyr568 and Tyr936 of the stem cell factor receptor/c-Kit is required for ligand-induced ubiquitination, internalization and degradation. Biochem J. 2006;399(1):59–67. doi: 10.1042/BJ20060464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sulahian R, Cleaver O, Huang LJ. Ligand-induced EpoR internalization is mediated by JAK2 and p85 and is impaired by mutations responsible for primary familial and congenital polycythemia. Blood. 2009;113(21):5287–5297. doi: 10.1182/blood-2008-09-179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morii E, Oboki K, Ishihara K, Jippo T, Hirano T, Kitamura Y. Roles of MITF for development of mast cells in mice: effects on both precursors and tissue environments. Blood. 2004;104(6):1656–1661. doi: 10.1182/blood-2004-01-0247. [DOI] [PubMed] [Google Scholar]
- 47.Morii E, Ogihara H, Kim DK, et al. Importance of leucine zipper domain of mi transcription factor (MITF) for differentiation of mast cells demonstrated using mi(ce)/mi(ce) mutant mice of which MITF lacks the zipper domain. Blood. 2001;97(7):2038–2044. doi: 10.1182/blood.v97.7.2038. [DOI] [PubMed] [Google Scholar]