Abstract
Glucose-6-phosphatase-β (G6Pase-β or G6PC3) deficiency, also known as severe congenital neutropenia syndrome 4, is characterized not only by neutropenia but also by impaired neutrophil energy homeostasis and functionality. We now show the syndrome is also associated with macrophage dysfunction, with murine G6pc3−/− macrophages having impairments in their respiratory burst, chemotaxis, calcium flux, and phagocytic activities. Consistent with a glucose-6-phosphate (G6P) metabolism deficiency, G6pc3−/− macrophages also have a lower glucose uptake and lower levels of G6P, lactate, and ATP than wild-type macrophages. Furthermore, the expression of NADPH oxidase subunits and membrane translocation of p47phox are down-regulated, and G6pc3−/− macrophages exhibit repressed trafficking in vivo both during an inflammatory response and in pregnancy. During pregnancy, the absence of G6Pase-β activity also leads to impaired energy homeostasis in the uterus and reduced fertility of G6pc3−/− mothers. Together these results show that immune deficiencies in this congenital neutropenia syndrome extend beyond neutrophil dysfunction.
Introduction
Glucose-6-phosphatase-β (G6Pase-β or G6PC3) deficiency,1–3 also known as severe congenital neutropenia syndrome type 4 (SCN4),4 is an autosomal recessive disorder. The G6PC3 gene maps to human chromosome 17q21 and encodes the enzyme G6Pase-β that catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose and phosphate.5,6 Although a key clinical characteristic of the disease is neutropenia, it has a distinct phenotype of increased visibility of superficial veins, congenital heart defects, and urogenital malformations.2–4 Recently, we have shown that, beyond the neutropenia, there is also a significant neutrophil dysfunction.3,7,8
The neutrophil dysfunction arises from impairments in neutrophil G6P metabolism and energy homeostasis, which are directly related to the absence of G6Pase-β activity.8 Between meals, when there is no external source of glucose, glucose homeostasis depends on the activity of a complex composed of 2 endoplasmic reticulum (ER) membrane proteins: glucose-6-phosphatase (G6Pase) and G6P transporter (G6PT).1,9,10 There are 2 G6Pase activities, G6Pase-α and G6Pase-β. Blood glucose homeostasis depends on the activity of a liver/kidney/intestine-restricted enzyme G6Pase-α.11 Mutation of this enzyme results in the metabolic disorder glycogen storage disease type Ia (GSD-Ia).1,9 In the G6Pase-α/G6PT complex, both proteins are functionally codependent. Therefore, mutation of G6PT also leads to a near-identical metabolic disorder, GSD-Ib. The primary difference between these 2 disorders is that in GSD-Ib there is an additional phenotype of neutrophil dysfunction.1,9,10 This has been shown to arise as a result of the ubiquitous expression of G6PT interfering with the activity of the G6Pase-β/G6PT complex. Indeed, G6Pase-β is also expressed ubiquitously, suggesting that, in tissues outside of the liver/kidney/intestine where there may be increased demands for glucose, the G6Pase-β/G6PT complex plays a critical role. In neutrophils, where these needs exist for activities, such as respiratory burst, chemotaxis, calcium flux, and phagocytic activities, loss of G6PT activity results in impairments of these functions.1,9,10 Moreover, monocyte/macrophage dysfunction has also been shown in patients deficient for G6PT (GSD-Ib).12,13 Consistent with the functional codependence between G6Pase-β and G6PT, in G6Pase-β deficiency, there is also neutrophil dysfunction, but in the absence of the systemic metabolic abnormalities in GSD-Ib.1,7,8
We have now investigated whether similar macrophage dysfunction occurs in G6pc3−/− mice. Macrophages play key roles in innate immunity, inflammation, and tissue remodeling.14,15 During pregnancy, macrophages also influence the homeostasis of the developing placenta and are important in preventing premature fetal rejection.16–18 We hypothesized that this dysfunction would manifest as macrophage-mediated pregnancy-associated complications. In GSD-Ib this issue has been difficult to address because of the loss of blood glucose homeostasis. In this study, we show that G6pc3−/− macrophages do exhibit impairments in respiratory burst, calcium flux, chemotaxis, and phagocytic activities. As predicted, glucose uptake and levels of G6P, lactate, and ATP are markedly lower in G6pc3−/− macrophages, compared with the controls. The G6pc3−/− macrophages also exhibit reduced NADPH oxidase activity. The macrophage trafficking in vivo is depressed in G6pc3−/− ascites during an inflammatory response, and, consistent with our hypothesis, the number of decidual macrophages in G6pc3−/− matings is significantly lower than in wild-type matings. Finally, in pregnant G6pc3−/− mothers, the uterus exhibits impaired energy homeostasis, and, overall, the G6pc3−/− mice exhibit reduced fertility. Together these findings suggest that the G6Pase-β deficiency leads to cellular dysfunction that extends beyond neutrophils to macrophages and can affect multiparous reproductive health, highlighting the importance of endogenous glucose production in other tissues outside of the gluconeogenic organs.
Methods
Isolation of mouse macrophages and cytokine assays
Animal studies were conducted under a protocol approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee. BM cells were isolated from the femurs and tibiae of 6- to 8-week-old wild-type and G6pc3−/− mice in the C57BL/6J background,8 and blood samples were collected from the heart with the use of EDTA-containing CAPIJECT tubes (Terumo Medical Co). Erythrocytes were removed by lysis with the use of Ack lysing buffer (Quality Biologicals), and BM and blood leukocyte counts were determined with the Guava Viacount reagent in a Guava EasyCyte Mini System (Millipore). The proportion of monocytes (SSCloGr-1−/lo/+CD11b+) and macrophages (F4/80+CD11b+) in BM and peripheral blood leukocytes were analyzed by flow cytometry in the Guava EasyCyte Mini System (Millipore) after staining leukocytes with anti–Gr-1/anti-CD11b Abs for monocytes or anti-F4/80/anti-CD11b (eBiosciences) Abs for macrophages. For the isolation of intraperitoneal macrophages and fluids, wild-type and G6pc3−/− mice were injected intraperitoneally with 3% thioglycollate broth (1 mL/25 g of body weight) 3 days before peritoneal lavage with 7 mL of precooled PBS. The lavage was centrifuged at 600g for 10 minutes at 4°C to pellet cells, and the supernatant fluid used for cytokine analysis. The proportion of macrophages in the cell pellet was estimated to be 80%-90% by flow cytometric analysis with the use of anti-F4/80/anti-CD11b Abs in the Guava EasyCyte Mini System (Millipore). For functional analysis, macrophages were further purified with CD11b Microbeads (Miltenyi Biotec). Briefly, freshly isolated wild-type and G6pc3−/− macrophages were incubated with CD11b Microbeads for 15 minutes at 4°C, and the mixture was passed through a MACS cell separation column (Miltenyi Biotec). The macrophages were eluted with PBS, supplemented with 0.5% (wt/vol) BSA and 2mM EDTA, pelleted, resuspended in the appropriate buffer, and used for functional assays. The purity of the enriched macrophages was analyzed by flow cytometry with the use of anti-F4/80 and anti-CD11b Abs in the Guava EasyCyte Mini System (Millipore).
The cytokines MCP-1 and M-CSF were quantified with Quantikine ELISA kits (R&D Systems).
Respiratory burst, calcium flux, chemotaxis, and phagocytosis measurements
The respiratory burst of macrophages was monitored by luminal-amplified chemiluminescence with the use of the LumiMax Superoxide Anion (SOA) Detection kit (Stratagene) and Victor Light 1420 Luminescence counter (PerkinElmer Life & Analytical Sciences) as described previously.7,8 Macrophages in LumiMax SOA assay medium were activated with 200 ng/mL phorbol myristate acetate (PMA; Sigma-Aldrich). Intracellular calcium concentration in peritoneal macrophages suspended in HBSS, containing 20mM HEPES, pH 7.4, was measured with the FLIPER calcium 3 assay kit component A (Molecular Devices). The ligand, leukotriene D4,19 was added to macrophages to a final concentration of 10−7M, and the fluorescence intensity ws recorded every 0.5 seconds as the ratio of fluorescence at 515 nm relative to 485 nm with the use of a Flexstation II Fluorometer (Molecular Devices). Macrophage chemotaxis in response to MCP-1 (R&D Systems) and M-CSF (PeproTech) was performed with the CytoSelect 96-well Cell Migration Assay kit (Cell Biolabs) according to the manufacturer's protocol. Briefly, 5 × 105 macrophages in 5.6mM glucose-containing RPMI-1640 medium was placed in the upper chamber and separated from cytokines in the lower chamber by a polyethylene terephthalate membrane with 5-μm pores. After incubation at 37°C for 2 hours, macrophages that had migrated into the lower chamber were lysed in the presence of CyQUANT GR dye, and their fluorescent intensity was measured by excitation at 480 nm and emission at 530 nm with the use of a Flexstation II Fluorometer (Molecular Devices).
The phagocytic activity of macrophages was measured by the uptake of red fluorescent pHrodo Escherichia coli bioparticles (Invitrogen) as described by Wan et al20 with modification. Briefly, 5 × 106 macrophages were suspended in 100 μL of HBSS containing 20mM HEPES, pH 7.4, and mixed with 20 μL of pHrodo E coli bioparticles. The mixture was incubated for 30 minutes at either 37°C for uptake activity or 0°C for background activity. After incubation, macrophages were washed twice with component C (Invitrogen), resuspended in 500 μL of component C, and analyzed by flow cytometry with the Guava EasyCyte Mini System (Millipore).
Analysis of apoptosis, active caspase-3, and membrane-bound GLUT1, GLUT3, or p47phox
Peritoneal macrophages were cultured in vitro for 36 hours in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 5.6mM glucose, in the absence or presence of 500 ng/mL brefeldin A21 (BFA; Epicentre). Macrophage apoptosis was assessed using the annexin V–FITC apoptosis detection kit (BioVision) and analyzed by flow cytometry in the Guava EasyCyte Mini System (Millipore) with the use of CytoSoft version 4.2.1 (Millipore) and FlowJo Version 7 (TreeStar). Annexin V–binding detects early apoptotic cells. Propidium iodide (PI) staining was used to detect late apoptotic or dead cells. Apoptotic macrophages include annexinV+PI− and annexinV+PI+ cells. To analyze active caspase-3, 105 macrophages were fixed in 3.75% formaldehyde, permeabilized in 0.2% Triton X-100, then stained with a rabbit polyclonal Ab against cleaved caspase-3 (Asp175) conjugated with Alexa Fluor 488 (Cell Signaling). To analyze membrane-bound glucose transporter 1 (GLUT1), GLUT3, or p47phox, 105 freshly isolated macrophages were stained serially: first with a rabbit polyclonal against GLUT1, GLUT3, or p47phox (Santa Cruz Biotechnology) and then by an anti-rabbit IgG Ab conjugated with Alexa Fluor 555. Stained cells were analyzed by flow cytometry with the Guava EasyCyte Mini System (Millipore).
Quantitative real-time RT-PCR and Western blot analyses
Total RNAs were isolated from peritoneal macrophages and uterine tissues with the use of the TRIzol Reagent (Invitrogen). The mRNA expression was quantified by real-time RT-PCR in an Applied Biosystems 7300 Real-Time PCR System. The following TagMan probes were used: M-CSF, Mm00432686_m1; MCP-1, Mm00441242_m1; gp91phox, Mm01287743_m1; p22phox, Mm00514478_m1; p47phox, Mm00447921_m1; GLUT1, Mm00441480_m1; GLUT3, Mm00441483_m1; and β-actin, Mm00607939_s1. Data were analyzed with the SDS Version1.3 software (Applied Biosystems) and normalized to β-actin RNA.
For Western blot analysis, macrophage or uterine lysates in RIPA lysis buffer (Thermo Scientific) containing Halt Protease and Phosphatase Inhibitor Cocktails (Thermo Scientific) were electrophoresed through 4%-12% polyacrylamide gels and trans-blotted onto polyvinylidene fluoride membranes (Invitrogen). The membranes were incubated with the relevant Abs, namely, a mouse monoclonal Ab against KDEL (Assay Designs) or gp91phox (BD Biosciences); a rabbit polyclonal Ab against GLUT1, GLUT3, p22phox, p47phox (Santa Cruz Biotechnology), or GRP17022; or a mouse polyclonal Ab against β-actin (Santa Cruz Biotechnology). The membranes were then incubated with an appropriate HRP-conjugated secondary Ab, and the immunocomplex was visualized with the SuperSignal Dura West Pico Chemiluminescent substrate (Pierce). Protein expression was quantified by densitometry analysis with the use of Quality One Version 4.6.5 (Bio-Rad). At least 3 separate experiments were conducted for each protein in which each mouse was assessed individually.
Glucose uptake and G6P, lactate, and ATP determination
Glucose uptake was measured by the rate of uptake of 2-deoxy-d-[1,2-3H]-glucose (2-DG; 33 Ci/mmol [12.21 ×1011 Bq/mmol]; MP Biomedicals).8 For macrophage intracellular G6P and lactate determination,107 macrophages were suspended in 500 μL of ice-cold PBS, then disrupted and deproteinized by adding perchloric acid to a final concentration of 14% (vol/vol).8 G6P in the deproteinized macrophage lysate was determined by oxidation to 6-phosphogluconolactone in the presence of NADP+ with yeast G6P dehydrogenase (Sigma-Aldrich). The NADPH generated in a 1:1 molar ratio with G6P oxidation was measured by excitation at 340 nm and emission at 450 nm in a Flexstation II Fluorometer (Molecular Devices).8 Lactate in deproteinized macrophage lysates was analyzed with a Lactate Assay Kit (BioVision), and the fluorescence intensities were measured by excitation at 535 nm and emission at 575 nm with the use of a Flexstation II Fluorometer (Molecular Devices). Total ATP in macrophage was measured with an ATP Assay Kit (BioVision).8 Briefly, macrophages (5 × 105) were lysed in 50 μL of ATP assay buffer and centrifuged, and the supernatant fluid (5 μL) was mixed with ATP Probe, ATP Converter, Developer Mix, and incubated for 30 minutes at room temperature in the dark. The fluorescence intensity was determined by excitation at 535 nm and emission at 587 nm with the use of a Flexstation II Fluorometer (Molecular Devices). To determine uterine G6P, lactate, and ATP contents, gestation day 15 uterine tissues were homogenized in RIPA buffer (Thermo Scientific), and G6P in the uterine lysates was measured with a kit from BioVision. Uterine levels of lactate and ATP were determined with the lactate and ATP Assay kits (BioVision) as describe earlier.
IHC analysis
Mouse placentas were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 10-μm thickness, and rehydrated. The endogenous tissue peroxidases were quenched with 0.3% hydrogen peroxide in methanol for 30 minutes, and the endogenous avidin/biotin was blocked with the Avidin/Biotin Blocking Kit (Vector Laboratories). For staining, blocked sections were incubated with a rat monoclonal Ab against F4/80 (Abcam), followed by biotinylated anti–rat IgG (Vector Laboratories), and the resulting complexes were detected with an ABC kit and DAB Substrate, from Vector Laboratories, according to the manufacturer's instructions. Sections were counterstained with hematoxylin before mounting. Sections were visualized using a Zeiss Axioskop2 plus microscope equipped with 20×/0.75NA or 40×/0.50NA objectives (Carl Zeiss MicroImaging). Images were acquired using a Nikon DS-Fil digital camera and NIS-Elements F3.0 imaging software (Nikon).
Statistical analysis
The unpaired t test was performed with the Prism Version 4 (GraphPad Software). Values were considered statistically significant at P < .05.
Results
G6pc3−/− macrophages exhibit impaired trafficking
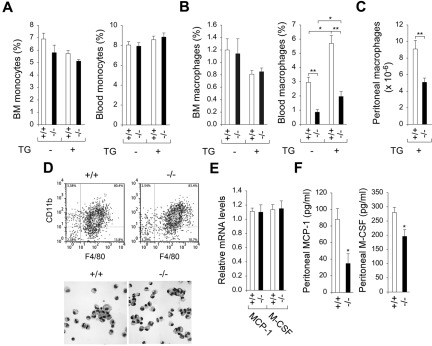
We examined whether monocyte or macrophage counts are reduced in G6pc3−/− mice. In the BM, in the absence of external stimuli, monocyte counts of 6- to 8-week-old wild-type and G6pc3−/− mice were statistically (P = .17) similar (Figure 1A). Similarly, BM macrophage counts, although low, were also indistinguishable between the 2 populations (Figure 1B). In the blood, although monocyte counts did not differ between wild-type and G6pc3−/− mice, macrophage counts for G6pc3−/− mice were significantly lower, being only 33.8% of wild type (Figure 1B). This suggests a trafficking defect in the G6pc3−/− macrophages. After the induction of experimental peritonitis by peritoneal injection of thioglycollate,23 monocyte counts in both BM and blood remained statistically similar between wild-type and G6pc3−/− mice (Figure 1A). In contrast, although the BM macrophage counts remained unchanged statistically (P = .86), blood macrophage counts increased, but proportionately, with the counts in G6pc3−/− mice still remaining 34.4% of those of the control mice (Figure 1B). As expected, the number of macrophages in the peritoneal space increased significantly on induction with thioglycollate in both the wild-type and G6pc3−/− mice. However, the trafficking defect was still clear, with 9.1 ± 1.0 × 106 cells in the peritoneal space of wild-type mice compared with 5.1 ± 0.5 × 106 cells in the peritoneal space of G6pc3−/− mice (Figure 1C). Despite the differences in peritoneal macrophage accumulation, flow cytometric analysis with F4/80 and CD11b Abs showed that both wild-type and G6pc3−/− peritoneal cell populations were similar, consisting of 80%-90% macrophages (Figure 1D). Both populations were also structurally mature macrophages (Figure 1D).
Figure 1.
G6pc3−/− macrophages exhibit impaired trafficking in vivo. Macrophages were isolated from 6- to 8-week-old wild-type (+/+) and G6pc3−/− (−/−) littermates. Monoctyes (SSCloGr-1−/lo/+/CD11b+) and macrophages (F4/80+/CD11b+) were analyzed by flow cytometry. (A) BM and blood monocyte counts in wild-type (n = 8) and G6pc3−/− (n = 8) mice in the absence and presence of thioglycollate (TG). (B) BM and blood macrophage counts in wild-type (n = 8) and G6pc3−/− (n = 8) mice in the absence and presence of TG. (C) The total peritoneal macrophage counts in wild-type (n = 8) and G6pc3−/− (n = 8) mice challenged with TG. (D) Flow cytometric analysis of peritoneal macrophages in control and G6pc3−/− mice and Hema 3-stained cytospins of peritoneal macrophages at magnification of ×400. (E) Quantification of MCP-1 and M-CSF mRNA in peritoneal macrophages by real-time RT-PCR. Expression is normalized to β-actin and measured relative to one wild-type mouse arbitrarily defined as 1. (F) The levels of MCP-1 and M-CSF in peritoneal exudates of wild-type (n = 8) and G6pc3−/− (n = 8) mice after intraperitoneal injection of TG. Data represent the mean ± SEM. **P < .005 and *P < .05.
The recruitment of monocytes/macrophages to a site of injury or inflammation is mediated by cytokines, including MCP-124,25 and M-CSF.26 These cytokines are produced both by monocyte/macrophages and by peritoneal tissues.27–31 When experimental peritonitis was induced by peritoneal injection of thioglycollate, quantitative real-time RT-PCR analysis showed that the expression of transcripts for MCP-1 and M-CSF were similar for both wild-type and G6pc3−/− peritoneal macrophages (Figure 1E), suggesting no impairment of cytokine expression by these cells. However, measurement of cytokine protein concentrations in the peritoneal exudates showed a difference, with the levels of MCP-1 and M-SCF in G6pc3−/− mice being 38.6% and 70%, respectively, of that in the control mice (Figure 1F), suggesting peritoneal tissue expression is modified by the G6Pase-β deficiency. Therefore, impairment in peritoneal macrophage recruitment in G6pc3−/− mice might result from reduced local production of MCP-1 and M-CSF as well as reduced numbers of peritoneal macrophages.
G6pc3−/− macrophages exhibit impaired function
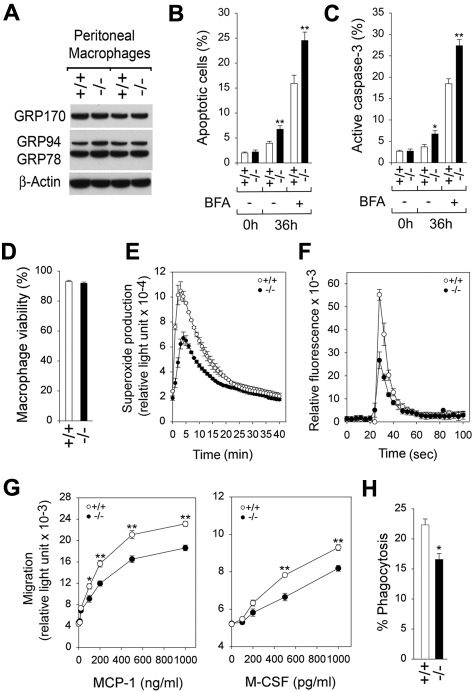
Previous studies have shown that G6pc3−/− neutrophils exhibit enhanced ER stress and apoptosis.7,8 We therefore examined the expression of molecular chaperones in the unfolded protein response signal transduction pathway32 in freshly isolated peritoneal macrophages from G6pc3−/− and control mice. Western blot analysis showed that G6pc3−/− and control macrophages expressed similar levels of molecular chaperons, GRP78,32 GRP94,32 and GRP17022,32 (Figure 2A), indicating that freshly isolated G6pc3−/− macrophages do not exhibit accelerated ER stress. As expected by that finding, the viability of freshly isolated G6pc3−/− and control macrophages estimated by annexin V and PI binding were statistically similar (Figure 2B). Studies have shown that macrophages are inherently resistant to apoptosis.33,34 We therefore examined survival of G6pc3−/− and control macrophages after culturing in vitro for 36 hours in the absence or presence of the apoptotic stimulus, BFA.21 Although both G6pc3−/− and wild-type macrophages underwent an increased rate of apoptosis during culturing in vitro, G6pc3−/− macrophages exhibited an accelerated rate of apoptosis, compared with wild-type macrophages both in the absence or presence of BFA (Figure 2B). Consistent with the enhanced rates of G6pc3−/− macrophage apoptosis, levels of active caspase-335 were consistently higher in G6pc3−/− macrophages than levels in control macrophages during culturing in vitro both in the absence or presence of BFA (Figure 2C).
Figure 2.
Analysis of ER stress, apoptosis, and functionality in G6pc3−/− macrophages. Peritoneal macrophages were isolated from 6- to 8-week-old wild-type (+/+) and G6pc3−/− (−/−) littermates. Freshly isolated macrophages were used to examine ER stress and apoptosis. For functional analysis, macrophages were further enriched by CD11b microbeads binding. (A) Western blot analysis of protein extracts of macrophages with the use of Abs against GRP170, GRP78/GRP94, or β-actin. Data from 2 pairs of littermates are shown, and each lane contains 50 μg of protein. (B) Quantification of apoptotic cells in macrophages of control (n = 6) and G6pc3−/− (n = 6) mice during culturing in vitro in the absence or presence of BFA. Results represent the mean ± SEM. (C) Quantification of active caspase-3 in macrophages of control (n = 4) and G6pc3−/− (n = 4) mice during culturing in vitro in the absence or presence of BFA. (D) The viability of CD11b-enriched macrophages from wild-type (n = 10) and G6pc3−/− (n = 10) mice, estimated by flow cytometry. Results represent the mean ± SEM. (E) Macrophage respiratory burst activity in response to 200 ng/mL PMA. (F) Macrophage calcium flux in response to 10−7M of leukotriene D4. (G) Macrophage concentration-dependent chemotaxis in response to MCP-1 or M-CSF. (H) Macrophage phagocytosis activity. Quantification of bioparticle-positive macrophages in control and G6pc3−/− mice; the numbers reflect the percentage of total macrophage that have engulfed particles. Data represent the mean ± SEM of 3 independent experiments. **P < .005 and *P < .05.
For functional studies, the peritoneal macrophages were enriched by CD11b selection. The viability of the CD11b-enriched macrophages from control and G6pc3−/− mice, estimated by annexin V and PI binding, were indistinguishable (Figure 2D). In wild-type macrophages, superoxide production was markedly increased by exposure to PMA, whereas in G6pc3−/− macrophages the PMA-stimulated superoxide production was markedly reduced (Figure 2E), implying G6Pase-β expression in macrophages is important for the respiratory burst activity. Similarly, mobilization of calcium in response to leukotriene D419 was impaired in G6pc3−/− peritoneal macrophages relative to controls (Figure 2F). Moreover, peritoneal macrophages from wild-type mice exhibited a greater dose-dependent chemotactic response to exogenous MCP-1 or M-CSF than peritoneal macrophages from G6pc3−/− mice (Figure 2G).
The phagocytic activity of the peritoneal macrophages was examined by measuring the uptake of pHrodo E coli bioparticles. The particles, which are not fluorescent at the neutral pH of growth media, fluoresce bright red when phagocytosed into the acidic intracellular environment.20 Flow cytometric analysis showed that the bioparticles were internalized 1.34-fold more efficiently by wild-type macrophages than the G6pc3−/− macrophages (Figure 2H), consistent with an impairment in phagocytosis.
G6pc3−/− macrophages exhibit reduced glucose uptake and levels of G6P, lactate, and ATP
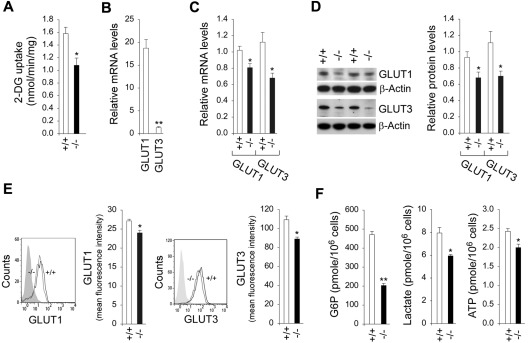
One role of G6Pase-β is to regulate the amount of available cytoplasmic glucose/G6P.8 The CD11b-enriched peritoneal macrophages from wild-type mice took up 2-DG from the culture medium at a rate 1.5-fold greater than macrophages from G6pc3−/− mice (Figure 3A). Macrophages express both GLUT1 and GLUT3.36 Quantitative real-time RT-PCR analysis showed that the expression of GLUT1 was ∼ 16-fold higher than that of GLUT3, indicating GLUT1 is the main glucose transporter in peritoneal macrophages (Figure 3B). The mRNA levels of GLUT1 and GLUT3 in macrophages of G6pc3−/− mice were, on average, 79% and 61%, respectively, of the levels in macrophages of their age-matched control littermates (Figure 3C). Western blot analysis confirmed the reduction in total macrophage GLUT1 and GLUT3 protein (Figure 3D). Moreover, flow cytometric analysis showed that the membrane-associated GLUT1 and GLUT3 were also decreased in G6pc3−/− macrophages, compared with the controls (Figure 3E). Consistent with the reduced 2-DG uptake, intracellular levels of G6P, lactate, and ATP in G6pc3−/− macrophages were 43.1%, 74.8%, and 82.4%, respectively, of the levels in control macrophages (Figure 3F). In summary, G6pc3−/− macrophages exhibit impaired glucose uptake and harbor reduced intracellular levels of G6P, lactate, and ATP, compared with control macrophages.
Figure 3.
Analysis of 2-DG uptake, the expression of GLUTs, and intracellular G6P, lactate, and ATP levels in G6pc3−/− macrophages. The CD11b-enriched macrophages used for function analysis were isolated from 6- to 8-week-old wild-type (+/+) and G6pc3−/− (−/−) littermates. For 2-DG uptake and quantitative real-time RT-PCR, the data represent the mean ± SEM of 4 independent experiments. (A) Uptake of 2-DG in macrophages. (B) Quantification of GLUT1 and GLUT3 mRNA in wild-type macrophages by real-time RT-PCR. Expression is normalized to β-actin and measured relative to 1 wild-type GLUT3 arbitrarily defined as 1. (C) Quantification of GLUT1 and GLUT3 mRNA in wild-type and G6pc3−/− macrophages by real-time RT-PCR. Expression is normalized to β-actin and measured relative to 1 wild-type mouse arbitrarily defined as 1. (D) Western blot analysis of protein extracts of peritoneal macrophages with the use of Abs against GLUT1, GLUT3, or β-actin. Each lane contains 50 μg of protein. The relative GLUT1 and GLUT3 protein levels were quantified by densitometry of 4 separate pairs of Western blots, and the measurements are relative to β-actin. (E) Quantitative flow cytometric analysis of membrane-bound GLUT1 and GLUT3 in macrophages. The gray tracing represents the fluorescence background. Data represent the mean ± SEM of 4 independent experiments. (E) Macrophage G6P, lactate, and ATP levels. Data represent the mean ± SEM of 4 independent experiments. **P < .005 and *P < .05.
Impaired expression and activation of NADPH oxidase in G6pc3−/− macrophages
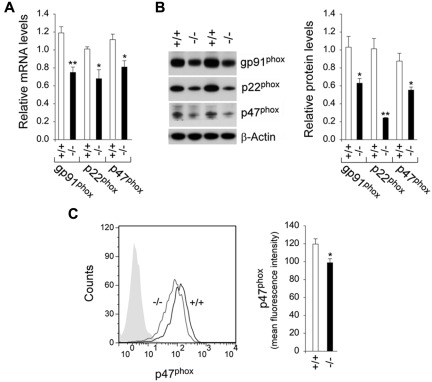
NADPH oxidase, which is required for the respiratory burst,37 is a multicomponent enzyme system composed of 2 transmembrane proteins, gp91phox and p22phox, and several cytosolic proteins, including p47phox.38 Quantitative real-time RT-PCR analysis showed that gp91phox, p22phox, and p47phox mRNA levels in macrophages of G6pc3−/− mice were, on average, 63%, 67%, and 73%, respectively, of the levels in macrophages of their age-matched control littermates (Figure 4A). Western blot analysis confirmed the reduction in gp91phox, p22phox, and p47phox proteins (Figure 4B). Activation of NADPH oxidase requires the translocation of the p47phox subunit to the plasma membrane.39 Flow cytometric analysis showed that the expression of membrane-associated p47phox was decreased in G6pc3−/− macrophages, compared with the controls (Figure 4C). In conclusion, the impaired respiratory burst inherent of the G6pc3−/− macrophages correlates with the impairment of both the expression and activation of NADPH oxidase in the hexose monophosphate shunt pathway.
Figure 4.
Analysis of the expression of NADPH oxidase in G6pc3−/− macrophages. The CD11b-enriched macrophages used for function analysis were isolated from 6- to 8-week-old wild-type (+/+) and G6pc3−/− (−/−) littermates. (A) Quantification of gp91phox, p22phox, and p47phox mRNA in macrophages by real-time RT-PCR. Expression is normalized to β-actin and measured relative to 1 wild-type mouse arbitrarily defined as 1. Data represent the mean ± SEM of 4 independent experiments. (B) Western blot analysis of macrophage protein extracts with the use of Abs against gp91phox, p22phox, p47phox, or β-actin. Data from 2 pairs of littermates are shown, and each lane contains 50 μg of protein. The relative protein levels of gp91phox, p22phox, and p47phox were quantified by densitometry of 4 separate pairs of Western blots. The measurements are relative to β-actin. (C) Quantitative flow cytometric analysis of membrane-bound p47phox in macrophages. The gray tracing represents the fluorescence background. Data represent the mean ± SEM of 4 independent experiments. **P < .005 and *P < .05.
G6pc3−/− mice exhibit reduced fertility
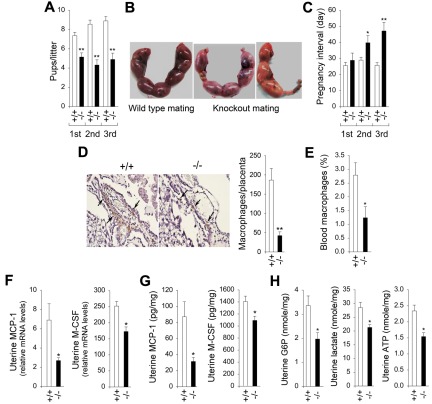
To examine whether macrophage trafficking in vivo was impaired during pregnancy, we conducted a series of controlled mating between 8- to 10-week-old male and female mice. Mice with the appropriate G6pc3 genotype were paired, and each pair was maintained in a monogamous relationship for 3 consecutive pregnancies. Focusing solely on the G6pc3 genotype, the experiment consisted of 10 pairs (female/male) of [−/−/−/−] (G6pc3−/− and G6pc3−/−) matings, 6 pairs of [−/−/+/−] matings, 10 pairs of [+/−/+/−] matings, and 6 pairs of [+/+/+/+] matings. As expected, the fertility of wild-type and heterozygote mice were indistinguishable, and the results of matings between [+/+/+/+] mating and [+/−/+/−] mating were reported together as wild-type mating. Likewise, the fertility of [−/−/−/−] mating and [−/−/+/−] mating were also similar, and the results were reported together as knockout mating.
Across the entire study, the average number of pups per litter for wild-type matings was statistically larger than those of the knockout matings (Figure 5A). In the virgin mating, wild-type and knockout matings yielded 7.4 ± 0.4 and 5.1 ± 0.4 pups per litter, respectively (Figure 5A). Over the next 2 serial matings the trend remained consistent, the wild-type matings yielding 8.6 ± 0.4 and 8.9 ± 0.5 pups per litter, respectively, whereas the knockout matings maintained an average of 4.3 ± 0.5 and 4.9 ± 0.6 pups per litter (Figure 5A). The uterine sacs reflected this difference with each wild-type uterine sac containing an embryo (Figure 5B), whereas for knockout matings, ≥ 1 placentas had no embryos (Figure 5B).
Figure 5.
G6pc3−/− mice exhibit reduced fertility. (A-C) Pup size and pregnancy intervals in wild-type and knockout matings. Sixteen pairs of wild-type and 16 pairs of knockout matings were set up with 8- to 10-week-old unaffected (+/+) and G6pc3−/− (−/−) littermates. The pregnancy outcome was examined for 3 consecutive pregnancies. (A) The litter size. (B) Representative placentas at gestation day 15 in wild-type and knockout matings. (C) The intervals between the first 3 serial pregnancies. Values represent mean ± SEM. Data were measured for each pregnancy. (D) Representative IHC analysis of macrophages in the decidual region of the gestation day 15 placenta in wild-type (n = 8) and G6pc3−/− (n = 8) matings at magnifications of ×400 and quantification of macrophage counts. Values represent mean ± SEM. Arrows denote macrophages. In wild-type placenta, numerous macrophages accumulated along the deciduas, and in G6pc3−/− placenta few macrophages were seen. (E) Blood macrophage counts in wild-type (n = 8) and G6pc3−/− (n = 8) mothers at gestation day 15, expressed as a percentage of total white blood cells. (F) Quantification of uterine MCP-1 and M-CSF mRNA in wild-type (n = 8) and G6pc3−/− (n = 8) mothers at gestation day 15 by real-time RT-PCR. Levels are expressed as a ratio of uterine tissue to peritoneal macrophages. (G) Quantikine ELISA analysis of the levels of uterine MCP-1 and M-CSF in wild-type (n = 8) and G6pc3−/− (n = 8) mothers at gestation day 15. (H) Uterine G6P, lactate, and ATP levels in wild-type (n = 8) and G6pc3−/− (n = 8) mothers at gestation day 15. Data represent the mean ± SEM. **P < .005 and *P < .05.
The initial rate of conception for wild-type and knockout matings were statistically similar, averaging 26 ± 2 and 29 ± 5 days, respectively, to conception. For wild-type matings, the intervals between subsequent pregnancies also remained similar, averaging 29.0 ± 2 and 26 ± 2 days for the next 2 pregnancies (Figure 5C). In contrast, the knockout fertility decreased progressively, from 29 days for the virgin mating to 40 and 47 days, respectively, for the first and second interval between pregnancies (Figure 5C).
Reduced uterine production of cytokine, G6P, lactate, and ATP in G6pc3−/− pregnancies
When macrophages recruited to the placental deciduas at 15 days of gestation were compared, IHC analysis showed wild-type matings accumulated almost 5-fold more F4/80+ macrophages than the knockout matings (Figure 5D). Macrophage recruitment to the blood was unaffected by pregnancy, which is reflected by the similar levels of reduction in blood macrophage counts in G6pc3−/− mice at gestation day 15 (Figure 5E).
Both MCP-1 and M-CSF are expressed at high levels in the uterus during pregnancy.40,41 To determine whether uterine tissue expression and/or macrophage expression plays the main role in recruiting macrophages to the decidua during pregnancy we undertook quantitative real-time RT-PCR analysis. Transcript levels of each cytokine measured in uterine tissue was normalized to the respective transcript level in macrophages isolated during thioglycollate-induced peritonitis. The MCP-1 transcripts in uterine tissues of wild-type mice at day 15 of gestation were ∼ 2.6-fold higher than the levels in G6pc3−/− mice (Figure 5F). In both wild-type and knockout mice, the uterine MCP-1 transcripts were 6.9- and 2.7-fold higher, respectively, than the macrophage transcripts. The M-CSF transcripts in uterine tissues of wild-type mice at day 15 of gestation were ∼ 1.5-fold higher than the levels in G6pc3−/− mice (Figure 5F). In both wild-type and knockout mice, the uterine M-CSF transcripts were 252- and 173-fold higher, respectively, than the macrophage transcripts. ELISA analysis confirmed that the decrease in uterine MCP-1 and M-CSF protein levels in G6pc3−/− mice, compared with the controls (Figure 5G).
G6Pase-β, which is ubiquitously expressed, regulates the amount of available cytoplasmic glucose/G6P that affects cellular energy homeostasis in all tissues outside the gluconeogenic organs.8 We hypothesized that during pregnancy, when demand for glucose is increased, the uterus in pregnant G6pc3−/− mothers could exhibit impairment in energy homeostasis. Indeed, intracellular levels of G6P, lactate, and ATP in uterine tissues of G6pc3−/− mice at day 15 of gestation were 61.4%, 74.6%, and 66.0%, respectively, of the levels in the uterine tissues of wild-type mice at day 15 of gestation (Figure 5H).
Discussion
G6Pase-β (or G6PC3) deficiency is classified as a severe congenital neutropenia syndrome, SCN4, although recent work has also unveiled underlying neutrophil dysfunction.3,7,8 However, the enzymatic deficiency in G6Pase-β does not affect blood glucose homeostasis or manifest any of the metabolic disorders seen when the closely related protein, G6Pase-α, is deficient.1,9 This reflects the distinct difference in tissue expression between the 2 proteins. Although G6Pase-β does not contribute significantly to liver, kidney, or intestine G6P metabolism, its ubiquitous expression leads to key roles outside those tissues, in which G6Pase-α is absent. A murine model for G6Pase-β (G6PC3) deficiency has been generated,7 and G6pc3−/− neutrophils have been shown to exhibit impairments in respiratory burst, chemotaxis, calcium mobilization, and phygocytosis activities,7,8 mimicking the myeloid phenotype of GSD-Ib deficient in G6PT.1,9,10 Human G6PC3-deficient neutrophils have also been shown to exhibit impaired respiratory burst activity.3 Both human and murine G6PC3-deficient neutrophils exhibit impaired energy homeostasis, which underlies neutrophil dysfunction.8
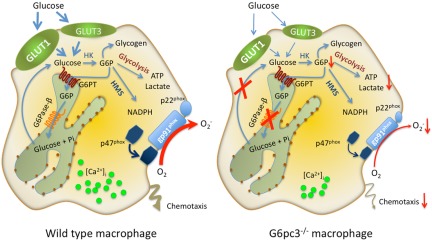
In previous work it was shown that 3 primary pathways compete for intracellular glucose/G6P in neutrophils, namely, glycolysis, the hexose monophosphate shunt, and a recently reported cycling of G6P/glucose between the cytoplasm and ER.8 The latter pathway is mediated by the G6Pase-β/G6PT complex. Functionally, G6Pase-β enzyme activity and G6PT activity are codependent, and an inactivating mutation in one inactivates the other.1 Patients with GSD-Ib, deficient in G6PT, not only manifest neutrophil dysfunction but also macrophage dysfunction.12,13 Therefore, we hypothesized that G6pc3−/− macrophages would also exhibit similar dysfunction and that disruption of ER cycling of G6P/glucose should lead to accumulation of G6P in the ER and prevent release of glucose back to the cytoplasm. This is then predicted to limit cytoplasmic glucose/G6P availability and to affect glycolysis and hexose monophosphate shunt, which in turn should impair additional blood glucose uptake (Figure 6). Moreover, given the key role that macrophages play in ensuring a successful pregnancy,16–18 we predicted that G6PC3 deficiency may also affect fertility and pregnancy. Our findings are consistent with this hypothesis. We showed that G6pc3−/− macrophages have lower intracellular concentrations of G6P, lactate, and ATP, compared with the controls. In normal cells, increasing cytoplasmic lactate and ATP levels stimulate uptake of blood glucose by inducing the translocation of GLUT1 to the plasma membrane.42,43 Consistent with this, we showed that the reverse also holds; namely, the lower levels in G6pc3−/− macrophages impair the expression and membrane translocation of GLUT1/GLUT3, which in turn impairs additional blood glucose uptake. We also predicted that as a result of the lower cytoplasmic G6P concentration, the hexose monophosphate shunt pathway would be affected, and this was seen in the reduction of NADPH oxidase activity in the G6pc3−/− macrophages. The impaired energy metabolism in G6pc3−/− macrophages leads to dysfunction, characterized by impairment in their respiratory burst, calcium flux, chemotaxis, and phagocytic activities, similar to those observed with G6pc3−/− neutrophils.7,8 Macrophages are inherently resistant to apoptosis,33,34 and, unlike G6pc3−/− neutrophils, freshly isolated G6pc3−/− macrophages do not exhibit enhanced apoptosis.7,8 However, when G6pc3−/− macrophages are cultured in vitro they do exhibit an accelerated rate of apoptosis, compared with wild-type macrophages, both in the absence or presence of an apoptotic stimulus, BFA.21
Figure 6.
Proposed pathways for G6P metabolism in wild-type and G6pc3−/− macrophages. Glucose transported into the cytoplasm by GLUT1 and GLUT3 is metabolized by hexokinase (HK) to G6P which can participate in glycolysis, hexose monophosphate shunt (HMS) pathway, glycogen synthesis, or be translocated into the lumen of the ER by the G6PT. In normal macrophages, G6P localized within the ER lumen can be hydrolyzed by G6Pase-β, and the resulting glucose transported back into the cytoplasm to reenter any of the previously mentioned cytoplasmic pathways. However, in G6pc3−/− macrophages, which lack a functional G6Pase-β, ER-localized G6P cannot be recycled to the cytoplasm. Consequently, G6pc3−/− macrophages exhibit reduced glucose uptake and impaired energy homeostasis, leading to impaired functionality. The GLUT1 and GLUT3 transporters, responsible for the transport of glucose in and out of the cell, is shown embedded in the plasma membrane. The G6PT transporter, responsible for the transport of G6P into the ER, and G6Pase-β, responsible for hydrolyzing G6P to glucose and phosphate, are shown embedded in the ER membrane.
BM macrophage counts were similar between wild-type and G6pc3−/− mice, but blood macrophage counts in G6pc3−/− mice were 34% of wild-type counts, suggesting a defect in macrophage trafficking. To investigate the implications for dysfunctional macrophages and their trafficking, we examined 2 different immune challenges: an artificially induced inflammatory response to peritoneal injection of thioglycollate and a naturally induced response to pregnancy. On thioglycollate-induced peritonitis, recruitment of cells into the peritoneal space was significantly inhibited with G6pc3−/− mice accumulating 44% less cells than their wild-type littermates, although for both wild-type and G6pc3−/− mice 80%-90% of recruited cells were macrophages. Likewise, blood macrophage counts in G6pc3−/− mice were only 34.4% of wild-type counts during thioglycollate-elicited peritonitis. A similar effect was observed in physiologic responsiveness during pregnancy, with a 77% decrease in F4/80-reactive macrophages being recruited to the decidual tissue. During recruitment in response to an inflammatory signal, phagocytic cells respond to the presence of inflammatory cytokines. MCP-1 is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages.24,25 M-CSF promotes differentiation and maturation of tissue macrophages and acts as a chemoattractant for these immune cells.26 MCP-1 and M-CSF are produced by a variety of cell types, including endothelial cells, fibroblasts, and epithelial cells, as well as monocytes and macrophages.27–31 Analysis of the exudates in the thioglycollate-induced peritoneal space showed a 61% and 28% decrease in the levels of MCP-1 and M-CSF, respectively, in G6pc3−/− mice compared with wild-type levels, consistent with the lower levels of macrophage recruitment.
During pregnancy, MCP-1 and M-CSF transcripts are expressed in the uterus, and the levels correlate with recruitment of macrophages into the uterus.40,41 In humans, local production of M-CSF is increased in tissues at the maternal-fetal interface during the time of implantation.44 Likewise, the concentrations of M-CSF in the murine uterus increase markedly during pregnancy.45 Moreover, M-CSF–null mice have severely depleted macrophage populations and fertility defects.46 Macrophages play key roles in pregnancy, assisting in decidual homeostasis, placental development, and the prevention of fetal rejection by the effective removal of apoptotic trophoblasts during implantation.16–18 G6Pase-β plays an essential role for energy homeostasis in both neutrophils8 and macrophages. Each of these cell types requires a high level of energy production to support their function. Because macrophages perform essential function in the uterus during a successful pregnancy, we hypothesized that uterine energy homeostasis could also be disturbed in G6pc3−/− mice, leading to decreased production of MCP-1 and M-CSF, which may in turn reduce macrophage recruitment to the deciduas. Consistent with this, we show that uterine levels of G6P, lactate, and ATP in G6pc3−/− mice are significantly lower than those in wild-type mice. Moreover, uterine levels of MCP-1 and M-CSF are reduced in G6pc3−/− mice. Both the dysfunctional macrophages and impaired uterine energy homeostasis correlate with reduced fertility in G6pc3−/− mothers.
In conclusion, we have shown that the loss of G6Pase-β activity in the disorder G6PC3 deficiency/SCN4 has implications beyond neutropenia, neutrophil dysfunction, increased visibility of superficial veins, congenital heart defects, and urogenital malformations reported previously.2–4,7,8 The ER-localized G6Pase-β expression is also important for energy homeostasis both in macrophages and the uterus during pregnancy. The G6Pase-β defect leads to impairment of macrophage respiratory burst, chemotaxis, calcium flux, and phagocytosis and to reduced production of uterine MCP-1 and M-CSF during pregnancy. The macrophage trafficking in vivo both during an inflammatory response and pregnancy is depressed in G6pc3−/− mice, leading to a reduction in fertility in G6pc3−/− mice. Our findings support the hypothesis that G6Pase-β deficiency underlies a broader cell dysfunction. Given the ubiquitous expression of the G6Pase-β/G6PT complex, we anticipate that some of the other secondary defects observed in G6Pase-β deficiency/SCN4 may also reflect loss of glucose metabolism within tissues that require levels of glucose beyond those supplied by the blood.
Acknowledgments
This work was supported by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.S.J. designed and performed the research, analyzed data, and wrote the paper; Y.Y.C. and Y.M.L. performed the research and analyzed data; B.C.M. analyzed data and wrote the paper; and J.Y.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janice Y. Chou, 9000 Rockville Pike, Bldg 10, Rm 9D42, National Institutes of Health, Bethesda, MD 20892-1830; e-mail: chouja@mail.nih.gov.
References
- 1.Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-beta deficiency: etiology and therapy. Nat Rev Endocrinol. 2010;6(12):676–688. doi: 10.1038/nrendo.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boztug K, Appaswamy G, Ashikov A, et al. A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med. 2009;360(1):32–43. doi: 10.1056/NEJMoa0805051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott DH, DeRavin SS, Jun HS, et al. Severe congenital neutropenia due to G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood. 2010;116(15):2793–2802. doi: 10.1182/blood-2010-01-265942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banka S, Chervinsky E, Newman WG, et al. Further delineation of the phenotype of severe congenital neutropenia type 4 due to mutations in G6PC3. Eur J Hum Genet. 2011;19(1):18–22. doi: 10.1038/ejhg.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shieh J-J, Pan C-J, Mansfield BC, Chou JY. Glucose-6-phosphate hydrolase, widely expressed outside the liver, can explain age-dependent resolution of hypoglycemia in glycogen storage disease type Ia. J Biol Chem. 2003;278(47):47098–47103. doi: 10.1074/jbc.M309472200. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh A, Shieh J-J, Pan C-J, Chou JY. Histidine-167 is the phosphate acceptor in glucose-6-phosphatase-beta forming a phosphohistidine-enzyme intermediate during catalysis. J Biol Chem. 2004;279(13):12479–12483. doi: 10.1074/jbc.M313271200. [DOI] [PubMed] [Google Scholar]
- 7.Cheung YY, Kim SY, Yiu WH, et al. Impaired neutrophil activity and increased susceptibility to bacterial infection in mice lacking glucose-6-phosphatase-beta. J Clin Invest. 2007;117(3):784–793. doi: 10.1172/JCI30443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun HS, Lee YM, McDermott DH, et al. Lack of glucose recycling between endoplasmic reticulum and cytoplasm underlies cellular dysfunction in glucose-6-phosphatase-beta-deficient neutrophils in a congenital neutropenia syndrome. Blood. 2010;116(15):2783–2792. doi: 10.1182/blood-2009-12-258491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou JY, Matern D, Mansfield BC, Chen Y-T. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med. 2002;2(2):121–143. doi: 10.2174/1566524024605798. [DOI] [PubMed] [Google Scholar]
- 10.Chou JY, Jun HS, Mansfield BC. Neutropenia in type Ib glycogen storage disease. Curr Opin Hematol. 2010;17(1):36–42. doi: 10.1097/MOH.0b013e328331df85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei K-J, Shelly LL, Pan C-J, Sidbury JB, Chou JY. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993;262(5133):580–583. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick L, Garty BZ, Lundquist KF, et al. Impaired metabolic function and signaling defects in phagocytic cells in glycogen storage disease type 1b. J Clin Invest. 1990;86(1):196–202. doi: 10.1172/JCI114684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCawley LJ, McCawley LJ, Korchak HM, et al. Interferon-gamma corrects the respiratory burst defect in vitro in monocyte-derived macrophages from glycogen storage disease type 1b patients. Pediatr Res. 1993;34(3):265–269. doi: 10.1203/00006450-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Mejia ME, Doseff AI. Regulation of monocytes and macrophages cell fate. Front Biosci. 2009;14:2413–2431. doi: 10.2741/3387. [DOI] [PubMed] [Google Scholar]
- 15.Valledor AF, Comalada M, Santamaría-Babi LF, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation: a question of balance. Adv Immunol. 2010;108:1–20. doi: 10.1016/B978-0-12-380995-7.00001-X. [DOI] [PubMed] [Google Scholar]
- 16.Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr Rev. 2005;26(7):877–997. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- 17.Renaud SJ, Graham CH. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol Invest. 2008;37(5):535–564. doi: 10.1080/08820130802191375. [DOI] [PubMed] [Google Scholar]
- 18.Nagamatsu T, Schust DJ. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci. 2010;17(3):209–218. doi: 10.1177/1933719109349962. [DOI] [PubMed] [Google Scholar]
- 19.Singh RK, Gupta S, Dastidar S, Ray A. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology. 2010;85(6):336–349. doi: 10.1159/000312669. [DOI] [PubMed] [Google Scholar]
- 20.Wan CP, Park CS, Lau BH. A rapid and simple microfluorometric phagocytosis assay. J Immunol Methods. 1993;162(1):1–7. doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee MJ, Park JY, Lee SY, et al. Modulation of constitutive and delayed apoptosis by brefeldin A in human neutrophils. Int Immunopharmacol. 2003;3(6):835–843. doi: 10.1016/S1567-5769(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 22.Lin H-Y, Masso-Welch P, Di Y-P, et al. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell. 1993;4(11):1109–1119. doi: 10.1091/mbc.4.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein O, Dabach Y, Ben-Naim M, et al. Lower macrophage recruitment and atherosclerosis resistance in FVB mice. Atherosclerosis. 2006;189(2):336–341. doi: 10.1016/j.atherosclerosis.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Rutledge BJ, Gu L, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187(4):601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28(5):509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Visser CE, Tekstra J, Brouwer-Steenbergen JJ, et al. Chemokines produced by mesothelial cells: huGRO-alpha, IP-10, MCP-1 and RANTES. Clin Exp Immunol. 1998;112(2):270–275. doi: 10.1046/j.1365-2249.1998.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witowski J, Thiel A, Dechend R, et al. Synthesis of C-X-C and C-C chemokines by human peritoneal fibroblasts: induction by macrophage-derived cytokines. Am J Pathol. 2001;158(4):1441–1450. doi: 10.1016/S0002-9440(10)64095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanfrancone L, Boraschi D, Ghiara P, et al. Human peritoneal mesothelial cells produce many cytokines (granulocyte colony-stimulating factor [CSF], granulocyte-monocyte-CSF, macrophage-CSF, interleukin-1 [IL-1], and IL-6) and are activated and stimulated to grow by IL-1. Blood. 1992;80(11):2835–2842. [PubMed] [Google Scholar]
- 30.Colotta F, Borré A, Wang JM, et al. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992;148(3):760–765. [PubMed] [Google Scholar]
- 31.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79(2):319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 33.Perlman H, Pagliari LJ, Georganas C, et al. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999;190(11):1679–1688. doi: 10.1084/jem.190.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Perlman H, Pagliari LJ, et al. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med. 2001;194(2):113–126. doi: 10.1084/jem.194.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel RM. Caspases at the crossroads of immune-cell life and death. Nat Rev Immunol. 2006;6(4):308–317. doi: 10.1038/nri1809. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC, Dimitriadis G, Newsholme P. Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr Opin Clin Nutr Metab Care. 2007;10(4):531–540. doi: 10.1097/MCO.0b013e3281e72ad4. [DOI] [PubMed] [Google Scholar]
- 37.Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22(4-5):189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 38.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 39.El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008;30(3):279–289. doi: 10.1007/s00281-008-0118-3. [DOI] [PubMed] [Google Scholar]
- 40.Wood GW, Hausmann E, Choudhuri R. Relative role of CSF-1, MCP-1/JE, and RANTES in macrophage recruitment during successful pregnancy. Mol Reprod Dev. 1997;46(1):62–69. doi: 10.1002/(SICI)1098-2795(199701)46:1<62::AID-MRD10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Wood GW, Hausmann EH, Kanakaraj K. Expression and regulation of chemokine genes in the mouse uterus during pregnancy. Cytokine. 1999;11(12):1038–1045. doi: 10.1006/cyto.1999.0513. [DOI] [PubMed] [Google Scholar]
- 42.Medina RA, Southworth R, Fuller W, Garlick PB. Lactate-induced translocation of GLUT1 and GLUT4 is not mediated by the phosphatidyl-inositol-3-kinase pathway in the rat heart. Basic Res Cardiol. 2002;97(2):168–176. doi: 10.1007/s003950200008. [DOI] [PubMed] [Google Scholar]
- 43.Kim MS, Lee J, Ha J, et al. ATP stimulates glucose transport through activation of P2 purinergic receptors in C2C12 skeletal muscle cells. Arch Biochem Biophys. 2002;401(2):205–214. doi: 10.1016/S0003-9861(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 44.Salamonsen LA, Dimitriadis E, Robb L. Cytokines in implantation. Semin Reprod Med. 2000;18(3):299–310. doi: 10.1055/s-2000-12567. [DOI] [PubMed] [Google Scholar]
- 45.Bartocci A, Pollard JW, Stanley ER. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, et al. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991;148(1):273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]