Abstract
The kidney is widely regarded as an organ without regenerative abilities. However, in recent years this dogma has been challenged on the basis of observations of kidney recovery following acute injury, and the identification of renal populations that demonstrate stem cell characteristics in various species. It is currently speculated that the human kidney can regenerate in some contexts, but the mechanisms of renal regeneration remain poorly understood. Numerous controversies surround the potency, behaviour and origins of the cell types that are proposed to perform kidney regeneration. The present review explores the current understanding of renal stem cells and kidney regeneration events, and examines the future challenges in using these insights to create new clinical treatments for kidney disease.
Keywords: kidney, kidney development, kidney disease, neonephrogenesis, regeneration, repair, stem cell
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor(s); AKI, acute kidney injury; ALDH, aldehyde dehydrogenase; BMSC, bone marrow stem cell; BrdU, bromodeoxyuridine; CAKUT, congenital and acquired diseases of the kidney and urinary tract; CM, cap mesenchyme; eGFP, enhanced green fluorescent protein; EMT, epithelial–mesenchymal transition; ES, embryonic stem; ESRD, end-stage renal disease; GBM, glomerular basement membrane; GFP, green fluorescent protein; Lhx1, Lim homeobox 1; LRC, label-retaining cell; MET, mesenchymal–epithelial transition; MKPC, mouse kidney progenitor cell; MM, metanephric mesenchyme; MRPC, multipotent renal progenitor cell; MSC, mesenchymal stem cell; Myh9, myosin heavy chain 9; NFATc1, nuclear factor of activated T-cells cytoplasmic 1; Oct4, octamer-binding transcription factor 4; Osr1, odd-skipped-related 1; PA, pretubular aggregate; Pax2, paired box gene 2; PDX, podocalyxin; PEC, parietal epithelial cell; RV, renal vesicle; Sca-1, stem cell antigen-1; SM, stromal mesenchyme; SP, side population; UB, ureteric bud; VIM, vimentin; Wt1, wilms tumour 1
INTRODUCTION
The kidney has several essential roles that include metabolic waste excretion and the maintenance of fluid and electrolyte balance. Kidney diseases originate from congenital, acute and chronic causes that eliminate renal function. Kidney diseases affect epidemic numbers worldwide and have risen in incidence over recent years, thus representing a burgeoning global healthcare burden [1,2]. Cellular damage to the functional units of the kidney, known as nephrons, is a common attribute among diverse kidney diseases. Progressive destruction of nephrons culminates in kidney failure. Fortunately, dialysis treatments provide life-saving renal replacement therapy to patients with abrogated kidney function. Although effective, dialysis is grueling, expensive and the only medical option for patients during the lengthy wait (sometimes >10 years) to obtain an organ transplant. Patients that receive a kidney transplant can face numerous health complications that require ongoing medical care. Thus the limitations to current treatments for kidney disease are not trivial and pose challenges in terms of managing medical resources and high economic costs [1,2]. There is an urgent need to find new therapies to promote kidney health in the wake of continued escalations in renal disease. Research aimed at finding ways to facilitate renal regeneration has recently gained significant interest [3].
Historically, the kidney numbers among those body parts thought to lack regenerative powers. This notion is certainly supported by the prevalence and dire outcomes of kidney diseases. Scientific observations about the events of kidney development and rates of cellular turnover in the adult organ have supported the idea that the mammalian kidney is deficient in regenerative properties. For example, nephrogenesis (nephron production) ceases during human gestation (at approximately week 36), whereas mouse nephrogenesis continues until birth and then rapidly attenuates [4,5]. Examinations of uninjured adult mouse kidneys hinted at the presence of some endogenous cell proliferation [6], but these findings were interpreted to represent a negligible contribution to organ homoeostasis. Reports that mammalian nephrons exhibited extensive cell regeneration after injury were published at the turn of the 20th century [7]. These findings were not integrated into mainstream knowledge about the kidney, and surprisingly little attention was given to this phenomenon for another century [7]. As a result, the long-standing dogma has been that kidney organs are endowed with a set number of nephrons that can only decline in activity from injury/disease and cannot be repaired during the lifespan of an individual.
The phenomenon of kidney regeneration has been increasingly re-evaluated over the past two decades for several reasons. First, a number of studies observed cell proliferation and restoration of kidney function in mouse and rat models of renal injury following ischaemia or the exposure to a chemical toxin [8–11]. Secondly, both the scientific and medical communities have come to a new appreciation for the role of adult stem cells in human body homoeostasis, heralded by the ongoing identification of resident stem cells in organs that were long believed to lack substantial cell production during adulthood. Taken together, these findings have spurred a search for endogenous renal stem cells, igniting an intense reappraisal of adult kidney cells and their properties. In addition, increased attention has been paid to delineating the molecular programme of kidney development, with the connection being that knowledge about how renal lineages arise from mesoderm progenitors may provide clues about the characteristics of renal stem cells that will facilitate their identification. There is pervading excitement about these recent trends in kidney research, founded in the hope that such studies will eventually lead to the creation of innovative treatments for kidney diseases [12].
The search for renal stem cells has produced controversial findings in many regards [13–18]. To date, several intrarenal cell populations have been found that demonstrate stem cell-like characteristics. There is also evidence that differentiated tubular epithelial cells in the nephron undergo a dedifferentiation process and then proliferate to replace damaged neighbouring cells. The potency and activities of these different renal regeneration cell sources remain debatable. Interestingly, the adult kidney in several lower vertebrates houses renal stem cells that produce new nephrons in response to damage [19,20], which further begs the question of whether analogous cells (or residual stem cell properties) are conserved in humans. The present review examines the current state of knowledge about the renal stem cell populations that exist during kidney organogenesis and adulthood, and the mechanisms of kidney regeneration in various damage settings. Finally, we address the future outlook and challenges in the search for reparative treatments for kidney disease.
KIDNEY COMPOSITION AND DISEASE
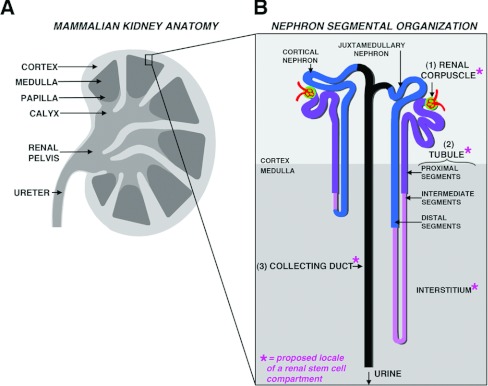
The mammalian kidney performs numerous physiological functions: it collects metabolic waste for excretion by filtering the circulation, maintains fluid homoeostasis by co-ordinating salt and water levels, regulates acid/base balance and secretes hormones that serve numerous endocrine functions [21,22]. As such, it is unsurprising that this organ is architecturally complex. The human kidney is organized into an outer cortex and inner medullary pyramids that culminate in renal papilla which drain to the bladder (Figure 1A) [23]. The nephron functional units are specialized epithelial tubes located in the cortex and medulla, packed in tiered arrays that enable them to interface at opposing ends to a capillary and the central drainage system (Figure 1B) [23]. The number of nephrons varies between mammals, ranging from many thousands (in rodents such as the mouse) to millions (in humans). Within a species, the number of nephrons varies widely among individuals; for example, humans can possess from 200000 to upwards of 1.8 million nephrons in a given kidney [24–26].
Figure 1. Composition of the adult mammalian kidney.
(A) The mammalian kidney is comprised of an outer cortex and inner medulla, and urinary waste from the collecting ducts is drained into the respective calyxes and then funneled through the renal pelvis to the ureter. (B) The functional units of the kidney are situated throughout various strata in the cortex, and many tubules elongate throughout the medulla region. Each nephron consist of three main components: a renal corpuscle (1), a tubule with many discrete functional segments in respective proximal, intermediate and distal regions (2), and lastly a collecting duct (3). Sites that have been proposed to house adult renal stem cells are indicated with an asterisk (*).
Kidney health depends on the net functionality of the nephrons and their component parts. Nephrons are organized into three major segments, a renal corpuscle, a tubule and a collecting duct, which are conserved among vertebrates [27]. The renal corpuscle is the site of blood filtration and consists of a glomerulus that filters the blood, and the Bowman's capsule that collects the filtrate. The filtrate passes from the capsule into the tubule and later into the collecting duct. The tubule is comprised of multiple segments that are specialized for different secretion and/or reabsorption tasks: for example, the proximal segments reabsorb amino acids and electrolytes, whereas distal segments make fine adjustments in urinary salt content [21]. Overall, the daily volume of filtration and fluid regulation performed by the kidney is immense: the kidneys in a healthy adult human filter on the order of 170 litres of blood each day, typically excreting between 1 and 2 litres of fluid [28]. Because of this high workload, the abrogation of nephron activity has dramatic consequences on body homoeostasis.
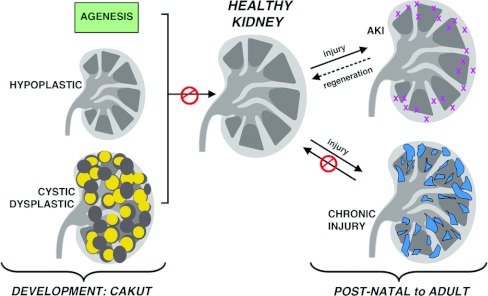
Kidney diseases affect millions of individuals worldwide, and arise from conditions that alter nephron development or trigger nephron damage during neonatal, juvenile and adult stages (Figure 2). CAKUT (congenital and acquired diseases of the kidney and urinary tract) conditions are anomalies that lead to absent kidneys (agenesis), reduced kidney size (hypoplastic) or malformed nephrons [29–32]. The nature of the developmental defect dictates the physiological deficiency, with the most severe being renal failure and premature death [33]. Increasing evidence has also supported a link between nephron endowment and long-term health: reduced nephron numbers correlate with the development of hypertension, chronic renal failure and predisposition to heart disease [34]. In addition, AKI (acute kidney injury) or chronic disease can disrupt nephron function. Particular nephron cell types tend to incur the highest rates of damage and are a common site of primary insult that can lead to nephron degeneration. These include the podocytes, which are subject to strain from the sheer rate of high-pressure fluid forces at the glomerulus and toxic pharmacological compounds in the filtrate [35]. The proximal tubule epithelial cells also incur a high rate of damage, as they similarly receive intense exposure to filtrate compounds and are also very sensitive to ischaemia. Although some patients can recover from AKI, suggesting that renal regeneration occurs, chronic kidney diseases involve irreversible accumulation of scar tissue [36–38]. With progressive fibrosis, renal function declines and culminates in ESRD (end-stage renal disease) where life must be maintained by dialysis or organ transplantation.
Figure 2. Kidney dysfunction: disruptions to the embryonic and adult kidney.
Kidney disorders that interfere with healthy kidney functions can be generally characterized into CAKUT conditions with affect renal development (left) and conditions that injure the normally developed organ (right). Left: kidney development defects can lead to the absence of one or both kidneys, termed agenesis (top), a significantly smaller kidney termed hypoplastic (middle), or a kidney with malformed or cystic (fluid-filled and enlarged) kidneys (bottom). Right: post-natal to adult disruptions in kidney function arise from acute injuries (top), from which complete or partial function can be restored through to regeneration and chronic injuries (bottom) which progressively scar the organ and are thought to be irreparable.
STEM CELLS AND REGENERATIVE MEDICINE
Regenerative medicine using stem cells has received a flurry of attention. Many have proposed that stem-cell-based therapies could be a revolutionary solution to combat numerous human diseases, including those facing nephrology today [39]. There has been an incredible interest in identifying stem cells and their characteristics in embryonic and adult contexts partly for this reason. Much of the focus has revolved around the idea of using knowledge about stem cell biology to generate replacement tissues in vitro or in vivo through the use of various patient- or donor-derived cell sources [40].
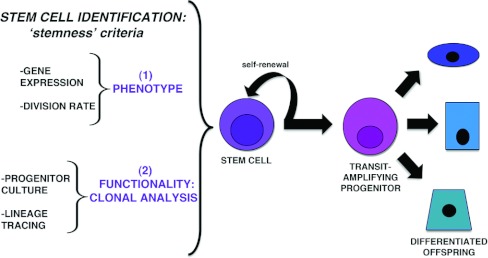
A stem cell is defined as a cell that, upon division, can self-renew and give rise to differentiated cell types or their precursors (Figure 3) [41]. The earliest cells present during mammalian development are totipotent, with the capacity to make all cell types of the fetus, as well as contribute to the extra-embryonic tissues associated with the maternal placenta; this potential is progressively restricted as development proceeds [41,42]. Cells isolated and cultured from the early embryo can retain pluripotent abilities, such that they possess the ability to produce cell lineages from all three germ layers, and are termed ES (embryonic stem) cells [41,42].
Figure 3. Definition and identification of the stem cells.
Middle and right: stem cells are operationally defined by having the ability to self-renew upon division and produce more differentiated offspring. Often, the immediate offspring are transit-amplifying cells that have great proliferative capacity and exhibit the ability to make multiple differentiated cell types. Left: stem cells can be assessed by multiple criteria that gauge stemness, or stem cell-like qualities of a cell. Stemness attributes fall into two broad categories: (1) their phenotype on the basis of gene expression, epigenetic signature and a label-retaining ability indicating rare division events, and (2) their functionality on the basis of analysis of their ability to give rise to cells in culture and in vivo, such as measured by lineage tracing.
After development is complete, many adult tissues contain stem cells that remain immature and multipotent, harbouring the capacity to self-renew and produce progeny with several distinct differentiated phenotypes [43]. These tissue-specific or adult stem cells have markedly less capacity for self-renewal and potency compared with early embryonic or pluripotent ES cells. Upon division, adult stem cells typically produce a transit-amplifying progenitor, a cell that acquires a more differentiated state, but exhibits a high capacity for growth. Adult stem cells support homoeostasis in tissues with high rates of turnover, including the blood, skin and intestine, as well as tissues with low rates of turnover such as the lung and brain [43,44]. They reside in unique anatomical microenvironments, or niches, which provide trophic support and also modulate their behaviour [43,44]. Adult stem cells can often respond dynamically to regenerate damaged tissue [43,44]. For example, haemopoietic stem cells undergo migratory behaviour in response to blood loss or congenital anaemia, exiting from their bone marrow niche and colonizing the spleen where additional blood is made [45].
Medical treatments using stem cells are in their infancy. Currently, only adult haemopoietic stem cells are used routinely in the clinic for bone marrow transplantation, although clinical trials are underway to test other stem cell therapeutics. There are significant challenges to be faced in devising regenerative medicine strategies to treat the kidney, but nonetheless there is a great impetus to tackle these hurdles. Many have envisioned that the current void of therapies to ameliorate kidney disease could be filled at the level of restoring functionality to individual nephrons if endogenous renal stem cells were identified or if renal stem cells could be grown and manipulated in vitro. Thus some major questions in the nephrology field include whether endogenous renal stem cells or some other cell type exists that can regenerate the kidney, whether renal regenerative powers could be exacerbated with the right signals, and whether exogenous cell therapies could be successful by producing and delivering replacement cells to the kidney of a patient. To explore these and other questions, researchers have undertaken strategies to identify renal stem cells on the basis of the criteria that are used to define adult stem cells.
IDENTIFICATION OF ADULT STEM CELLS
Across diverse tissues, adult stem cells are defined by hallmarks of so-called ‘stemness’, a constellation of traits that typify the state of being a stem cell (Figure 3) [46,47]. The working definition of stemness includes the properties of cellular immaturity, multi-lineage potency and self-renewal capacity, and also encompasses characteristics such as gene expression profiles and cell behaviours which correlate with these traits. In theory, the intersection of stemness attributes is analogous to a molecular fingerprint that can readily identify stem cells in vivo; in actual practice, however, the biometrics of fingerprint matching for stem cells has been an enigmatic task and represents a significant obstacle in current stem cell research. Distinguishing the relationships between stem cells and their progeny is not straightforward, and it is further complicated by attempts to appreciate the dynamic flux and heterogeneity present among populations [48].
Stem cell identification studies have relied on an arsenal of cellular and molecular strategies to collect information that would indicate the respective stemness of the cells residing in a tissue of interest. The use of histological characteristics, such as cell ultrastructure, to identify stem cells is relatively rare, although niches have been associated with locales that protect the resident stem cells from environmental damage and provide access to resources such as a vascular supply [49]. Adult stem cells often share gene expression and epigenetic profiles with the stem/progenitor cells that give rise to the organ during development [50,51]. This phenomenon probably represents their shared common ontological origins and the congruous usage of genetic and molecular processes to direct the production of cell types in a specific tissue or organ. Interestingly, there are many markers common to diverse lineages that approximate a category of universal stem cell traits. For example, adult stem cells express similar cohorts of cell surface proteins, such as c-Kit, Sca-1 (stem cell antigen-1), CD34 and CD133; the presence of these antigens is used to isolate stem cell fractions with flow cytometry [52]. Stem cells also exhibit low vital dye staining due to the expression of membrane pumps of the ABC (ATP-binding cassette) transporter family [52]. Because of these transporter proteins, stem cells actively efflux high levels of the fluorescent DNA-staining dyes Hoeschst 33342 and Rhodamine 123 compared with other cells, and distribute in a side population upon flow cytometry [52]. These efflux properties are hypothesized to be a mechanism to protect stem cell integrity from cytotoxic compounds, thus promoting long-term survival [52].
Adult stem cells share archetypal behaviours that are closely tied to their potency and self-renewal. For example, many adult stem cells are quiescent, sharing the property of infrequent cell division over long periods of time. This may represent a mechanism to limit the accrual of mutations during DNA replication, thus staving off the potentially deleterious consequences of replicative aging [53]. Cell turnover can be gauged by providing a pulse of a nucleotide analogue, such as BrdU (bromodeoxyuridine), which is incorporated into dividing cells. Over time, adult stem cells tend to be LRCs (label-retaining cells) because they do not proliferate (in normal conditions) at a rate that dilutes the label, whereas transit-amplifying progeny rapidly dilute such labels.
Regardless of its gene expression and proliferation dynamics, a stem cell assignation is only indisputably determined by assessing functionality, i.e. by operational tests that can reveal which progeny can be produced and over what period of time [54]. Functionality can be probed through many assays, which can be broadly categorized into in vitro or in vivo tests. Putative stem cells are isolated, typically on the basis of complements of cell-surface markers, then cultured in vitro to observe their activities in different conditions, namely to perform clonal assays to assess self-renewal and find out which other cell types can be produced [43]. The operational activity of a putative stem cell is most stringently evaluated by tracking its progeny in vivo. Some tissues are amenable to transplantation techniques where prospective stem cells are isolated from a donor, re-introduced into a genetically distinct (but compatible) recipient, and then tracked by various methods [55]. Serial transplantation enables the assessment of long-term self-renewal and is necessary to functionally distinguish stem cells from their transit-amplifying offspring. Lineage tracing using genetic fate mapping in the mouse model has been invaluable to assess stem cell progeny production [56]. Genetic fate mapping is performed by creating transgenic animals in which subsets of cells (on the basis of tissue-specific promoter activity) can be marked at a desired time by inducing the stable genetic expression of a reporter such as GFP (green fluorescent protein). The offspring of the labelled cells inherit the reporter expression, enabling their fate to be tracked over time.
To date, the search to find renal stem cells and discover if/how kidney regeneration works has occurred on two related fronts that have implemented the scientific tools that gauge stemness parameters. Research to identify renal stem cells during development has provided a new understanding of lineage relationships in the kidney, and delineated a multipotent renal stem cell that generates the nephrons. Meanwhile, adult kidney populations have received intense scrutiny to re-evaluate their properties in the context of normal homoeostasis and disease, identifying a cast of stem and progenitor cells that are the subject of ongoing research and debate. Putative adult renal stem cells have been proposed to exist in several adult kidney sites (shown by an asterisk in Figure 1B, and see Table 1). We first consider renal stem cells in development and then discuss the evidence for adult renal stem cells.
Table 1. Proposed renal stem, progenitor and regenerative cell types in the mammalian kidney.
Cells exhibiting one or more stemness attributes have been characterized in both the developing and adult mammalian kidney. The cells have been found in mesenchymal and epithelial compartments, and the origins of several isolated by fractionation techniques have yet to be precisely determined.
| Location | Molecular and cell characteristics | Potency in vitro | Potency in vivo | Contribution in kidney injury or disease model | Reference(s) |
|---|---|---|---|---|---|
| Embryonic kidney | |||||
| CM | Six2+, self-renewing | Nephron | [74,75] | ||
| SM | FoxD1+ | Interstitium | [72,73] | ||
| UB | Collecting duct | [62–64] | |||
| Adult kidney | |||||
| Nephron | |||||
| PEC stem cell | CD24+CD133+, PDX− | Proximal and distal tubule | Podocyte | Yes; gave rise to both podocytes and tubule cells | [96,97,103,104] |
| PEC progenitor | CD24+CD133+, PDX+ | Podocyte | Limited; rare podocyte contribution | [103] | |
| Tubule differentiated epithelia | Tubule cells marked by eGFP or Six2-reporter+ | Tubule | [127,128] | ||
| Tubular stem cell-proximal | VIM+, from proximal tubule (S3 segment) | [133] | |||
| Tubular stem cell-proximal | NFATc1−lacZ+, proximal tubule | [134] | |||
| Tubular stem cell | CD24+CD133+ | Multipotent | [135] | ||
| Tubular stem cell | CD24+CD133+Aldhhigh from renal cortex | Multipotent | [136] | ||
| Tubular stem cell | CD133+, heterogenous | Multipotent | Tubule | Yes | [138] |
| Renal papilla | |||||
| Papilla stem cell | BrdU label retention in tubules and interstitium | [129,133,139] | |||
| Papilla stem cell | Nestin+CD133+ | Multipotent | Multipotent | [142] | |
| Whole kidney fractionation | |||||
| Fractionation by antigens, behaviour | Sca-1+Lin− | Multipotent | Tubule | [143] | |
| Fractionation by side population | Sca-1+, musculin+, noted to reside in interstitium | Yes | [144] | ||
| Fractionation by side population | Sca-1+ | Multipotent | Yes | [145,146] | |
| Long-term proliferation in culture | Oct4, Pax2 | Multipotent | Tubule | Yes | [148] |
| Long-term proliferation in culture | Myh9−GFP+, Pax2, Oct4, Wt1 | Multipotent | Tubule | Yes | [149] |
RENAL STEM CELLS AND MAMMALIAN KIDNEY ONTOGENY
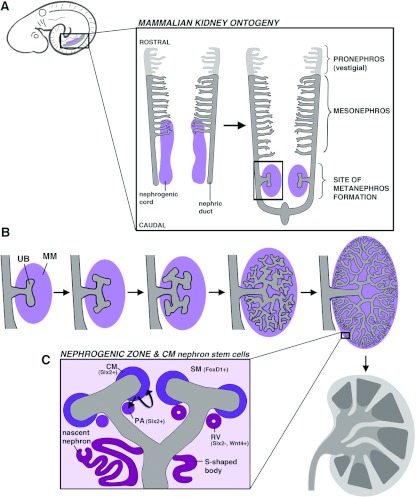
Kidney organogenesis is a unique developmental phenomenon that progresses through a sequence of stages wherein a renal structure is formed and later replaced with a more intricate organ configuration [57,58]. Each kidney derives from the mesoderm and is comprised of nephron tubules, although whether the nephrons become functional and how long they persist varies by species. Mammals form a pronephros, then a mesonephros and finally a metanephros that becomes the adult kidney (Figure 4A). The mammalian pronephros is a rudimentary vestigial structure. The mesonephros exists for a short time and has limited functionality, degenerating with the onset of metanephros formation. Nephron arrangements in pro- and meso-nephric kidneys are simple, with nephrons few in number. These early kidneys have a pair of nephric (or Wolffian) ducts, derived from mesoderm that coalesces to form an epithelial tube, and form nephrons from surrounding mesenchyme called the nephrogenic cord. The pronephros forms anteriorly, with the mesonephric tubules located more posteriorly. The nephric ducts later grow caudally along the trunk, where the bilateral metanephric kidneys will be induced.
Figure 4. The mammalian kidney develops from distinct pools of renal progenitors.
(A) Top left: the mammalian embryo will form a series of three kidney structures from the intermediate mesoderm (indicated in grey and purple) in a caudal region of the trunk. Enlargement: the three kidneys from along the rostral–caudal axis in an archetypal order, with the pronephros first, followed by the mesonephros and finally the metanephros. The pronephros and mesonephros consist of simple tiered arrays of nephrons. The pronephros is vestigial, whereas the mesonephros functions for a short time and then degenerates upon metanephros formation. (B) The metanephros develops when the nephric duct (grey) is induced to form the UB outgrowth by the MM (purple). Branching morphogenesis of the UB generates a highly branched collecting duct system. (C) The MM gives rise to two mesenchymal compartments, the CM which caps the UB branch points, and the SM, which is loosely distributed in the vicinity. The CM is a Six2+ self-renewing stem cell compartment, and adjacent to each UB branch points forms a PA, which initially maintains Six2+ and will become a nephron. The PA progresses to form an epithelial RV that is Six2−Wnt4+, and grows to make various shapes, including an S-shaped body that will eventually proliferate and elongate to make nascent nephrons that connect to the UB ductal network.
The characterization of renal stem cells during mammalian development has been focused on the metanephros because it becomes the permanent organ and is much more substantial in size. Many questions remain about the genetic programmes and signalling pathways that direct pro- and meso-nephros specification, and the molecular relationships between renal progenitors of these and metanephric kidneys. During metanephros formation, discrete populations of mesodermal derivatives interact to generate the arborized arrangement of nephrons and collecting system [59,60]. The collecting duct system originates from the nephric duct, which in the course of its caudal growth will be induced to evaginate by a specialized MM (metanephric mesenchyme) and form an outgrowth known as the UB (ureteric bud) (Figure 4B) [61]. Classic cell association and retroviral labelling studies demonstrated that the MM could generate nephrons, whereas the UB epithelium could make collecting ducts [62–64]. The UB undergoes a reiterative process of branching morphogenesis during which nephrons are induced from the MM located adjacent to the UB branch points, generating an arborized nephron array. Over time, these events beget a dense periphery of nephrogenesis activity, called the nephrogenic zone. Elaborate reciprocal signalling between the MM and UB underlies the repeated cycles of UB branching and growth.
As the MM and UB interact, the MM is subdivided into the CM (cap mesenchyme) and SM (stromal mesenchyme), representing a bifurcation in kidney lineages (Figure 4C and Table 1) [65,66]. The CM is a condensed mesenchyme that encapsulates the UB and is broadly defined by expression of the homeobox transcription factor Six2 (sine oculis-related homeobox 2 homologue) after UB invasion [67,68]. A subset of the CM that flanks each branch point will become a PA (pretubular aggregate) that maintains Six2 expression and is induced to undergo an epithelial transition, forming a circular RV (renal vesicle) in which Six2 expression is lost (Figure 4C). Each RV proliferates to make a nephron, expanding into a comma-shape, next an S-shape and then elongates further to make convolutions and fuse with the collecting duct system [69–71]. The elongating nephron is polarized along its proximal–distal axis such that podocytes always arise from the nephron progenitors furthest from the point of fusion, and the intervening progenitors develop into the tubule [69–71]. In stark contrast with the CM, the SM is a loose cellular array situated between the UB branches and growing nephrons. The SM is defined by expression of the forkhead transcription factor FoxD1 (forkhead box D1) and these cells will contribute to the interstitial population [72,73].
Previous studies have demonstrated that the CM contains a self-renewing group of nephrogenic stem cells. Genetic fate mapping has shown that the CM is specified early, when the UB first enters the MM [74,75]. On the basis of gene expression and functional studies, CM specification probably involves a series of cues originating from the signalling of BMPs (bone morphogenetic protein) acting through the Alk3 (activin-like kinase 3) receptor, and transcriptional activities mediated by the Hox11 paralogues, Osr1 (odd-skipped-related 1) and Pax2 (paired box gene 2), all of which guide MM patterning [65,76–78]. CM cells are defined by the continued expression of the Hox11 paralogues, Osr1 and Pax2, as well as the transcription factors Cited1 {CBP [CREB (cAMP-response-element-binding protein)-binding protein]/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain 1}, Eya1 (eyes absent homologue 1), Sall1 (sal-like 1), Six2 and Wt1 (Wilms tumour 1) [59,60,69–71]. Following its specification, the CM is not believed to receive additional cell contributions, but rather self-renews to perpetuate the expanding nephrogenic zone [74,75]. Among the genes associated with CM identity, Six2 activity is essential to propagate the stem cell population. In the absence of Six2, nephrogenesis terminates rapidly after it is initiated because the CM compartment is not maintained [75]. Clonal analysis of Six2-expressing CM has demonstrated that at least a subset of these cells can contribute to multiple nephron segments, suggesting that the CM contains a multipotent population [75]. During the progressive rounds of nephrogenesis, the CM is heterogenous, containing a self-renewing pool of Six2+ cells and a subgroup in transit to the nephron fate that is marked by expression of Wnt4 (wingless-type MMTV integrator site family, member 4) [75]. Recent findings suggest that Wnt9b (winglesstype MMTV integrator site family, member 9b)/β-catenin signals positively regulate Six2+ CM self-renewal, although the precise molecular relationship of these factors has yet to be resolved [79]. The phenomenon of CM self-renewal is transient, and the cessation of nephrogenesis is associated with CM disappearance. Further work is needed to address how the CM compartment is terminated, as it is unclear whether this is due to the active commitment of cells to nephrogenesis and/or an exhaustion of self-renewal properties [75]. Elucidation of how the CM is regulated could provide valuable insights into the cell-autonomous and environmental signalling pathways that influence kidney lineage self-renewal.
OVERVIEW: THE HUNT FOR ADULT RENAL STEM CELLS
Searches for renal stem cells in the adult mammalian kidney have not identified a multipotent cell that can self-renew and make the >20 specialized renal cell types. However, distinct locations within the nephron and elsewhere in the kidney appear to house cells that exhibit various degrees of potential to make differentiated cell types during normal turnover and following injury. These sites include the renal corpuscle, the nephron tubule and the renal papilla. The spectrum of regenerative cells includes immature stem-like cells and mature differentiated cells. In the following sections, we discuss renal regeneration at these distinct sites and the features of the cells that are purportedly involved.
EVIDENCE FOR A PODOCYTE STEM CELL IN THE RENAL CORPUSCLE
The renal corpuscle is made up of several cell types: fenestrated endothelial cells that form the capillary network, mesangial cells interspersed between the capillary loops, glomerular podocytes (or visceral epithelial cells) and the PECs (parietal epithelial cells) of Bowman's capsule (Figure 5) [80]. Of these cell types, the latter three derive from kidney mesoderm during development, with the origin of angioblasts not yet resolved [80]. The endothelial cells and podocytes sit in apposition, attaching on either side of the GBM (glomerular basement membrane) to form the blood filter. The podocytes are octopus-shaped cells with long axonal-like extensions. They connect to neighbouring podocytes with interdigitating projections (called foot processes) that create small spaces for fluid to pass through. Foot processes are joined by the slit diaphragm, a dense velcro of elaborate protein complexes at the cell membrane that are harnessed to the interior cytoskeleton [81]. As such, podocytes are highly specialized differentiated cells, and are thought to be mitotically quiescent, except in certain pathological conditions [82–84]. The integrity of the blood filter depends on the ability of podocytes to retain their connections with the GBM and adjacent podocytes. This intact barrier prevents the leakage of high-molecular-mass proteins and cells into the tubule.
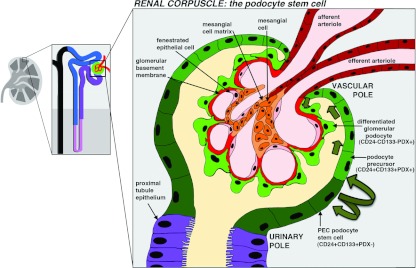
Figure 5. Glomerular injury and source(s) of replacement cells.
The renal corpuscle is composed of (1) a glomerulus, which is a ball of capillaries made of fenestrated epithelial cells that are surrounded by a GBM and podocytes, and (2) the Bowman's capsule, which is an epithelium that encapsulates the glomerulus and serves as a collecting space for fluid filtered through the glomerulus. Current evidence is consistent with the epithelium at the urinary pole, the so-called PECs, containing a podocyte stem cell that is CD24+CD133+PDX−. The PEC stem cell gives rise to transitional offspring that are podocyte progenitor cells; these transitional cells express CD24+CD133+PDX+ and are speculated to migrate around the capsule and later enter the glomerulus (pathway indicated by green arrows), where they differentiate into CD24−CD133−PDX+ podocytes.
Proper development and survival of podocytes is essential for kidney health [35,82]. Podocyte disruption has catastrophic consequences, for the majority of patients who develop ESRD, the primary pathological insult is glomerular disruption that causes proteinuria and progresses to scarring and loss of nephron function [35,82]. Surprisingly, podocytes are shed at a low frequency in the urine of healthy individuals [85]. They can be recovered from the urine and even cultured, where they display hallmarks typical of normal podocytes [85]. It is thought that the mechanical forces of fluid flow disrupt podocyte attachments, leading to their effacement even though they are otherwise viable. Podocyte excretion is dramatically elevated in the setting of many renal diseases, probably reflecting a harmful environment [85]. There are some data suggesting that compensatory podocyte hypertrophy can occur to counteract shedding, and that podocytes hypertrophy in a number of disease states [86–88]; in vitro, podocytes have been observed to undergo hypertrophy in response to glucose and mechanical stretch [89,90]. The observations of podocyte loss and absence of proliferation are at odds with the ability to maintain sufficient podocyte numbers and renal function with age. In addition, a reversal of glomerular scarring and regression of renal disease was reported in patients with diabetes that had pancreatic transplants and in patients with chronic nephropathy that received ACEi (angiotensin-converting enzyme inhibitors), suggestive of podocyte regeneration [90–94].
A re-evaluation of the cells at the renal corpuscle revealed that some PECs showed stem cell characteristics [95–97]. In biopsies from normal adult human kidneys, researchers detected a subset of PECs that express the surface antigens CD24 and CD133, which mark several adult stem cell types [96]. The PEC subset was purified and grown in culture, where individual cells exhibited clonogenic self-renewal and could generate multiple progeny with characteristics of proximal and distal tubule epithelia [96]. In a mouse model of AKI, tubular regeneration occurred in animals that received an injection of the human CD24+CD133+ PEC fraction, but not with CD24−CD133− PECs [96]. Interestingly, in developing human kidneys CD24+CD133+ cells were identified in the renal vesicles and S-shaped bodies, and were later restricted to the urinary pole of Bowman's capsule [97]. The embryonic CD24+CD133+ PECs gave rise to cells with phenotypes comparable with the adult PEC source when grown in culture or administered to the same mouse AKI model [97]. Taken together, these results suggested that the CD24+CD133+ PECs could be multipotent nephron progenitor descendents.
Interestingly, previous studies had reported morphological heterogeneity in the PEC of Bowman's capsule on the basis of histological analyses. In human glomeruli, cells close to the urinary pole have a flat epithelial shape, whereas cells close to the vascular pole exhibit a podocyte-like appearance with long cell processes, and were termed parietal podocytes [98,99]. Examinations of Bowman's capsule in sheep described heterogeneity as well, reporting a unique cell type located near the vascular pole on the basis of prominent cytoplasmic granules, and these were called peripolar cells [100,101]. The mixture of morphological phenotypes in Bowmans' capsule, along with multipotency of CD24+CD133+ PECs, are suggestive that the PEC population is comprised of several cell types with various roles, some of which might serve to replace podocytes. This hypothesis has been supported by clonogenic analysis and lineage studies [102–104].
Further molecular characterization of CD24+CD133+ human PECs revealed that this group is heterogenous, and includes cells that express markers typical of differentiated podocytes, like nestin and PDX (podocalyxin) [103]. Human PECs are spatially organized in a continuum along the capsule, with CD24+CD133+PDX− cells present closest to the urinary pole, CD24+CD133+PDX+ cells concentrated towards the vascular pole, and differentiated podocytes exhibiting a CD24−CE133−PDX+ character (Figure 5) [103]. After sorting of CD24+CD133+ PECs into PDX− and PDX+ fractions, only the individual PDX− cells exhibit multipotency in vitro on the basis of the ability to generate tubular cells and podocytes [103]. Furthermore, injection of the CD24+CD133+PDX− fraction produced podocytes and tubular cells in mice with adriamycin-induced renal injury (a model of the human podocyte disease known as focal segmental glomerulosclerosis), and was associated with reduced proteinuria [103]. A similar infusion with the CD24+CD133+PDX+ fraction, in contrast, only gave rise to rare podocytes [103]. These findings support the notion that CD24+CD133+PDX+ cells are transitional cells with limited proliferative capacity that express progenitor and podocyte markers, and do not replace injured podocytes [103]. Genetic labelling has been used to mark the PECs and irreversibly track their progeny in newborn and adolescent mice, demonstrating that PEC offspring become podocytes during the completion of nephrogenesis in the postnatal period and during rapid kidney growth in juveniles [104].
These findings provide compelling evidence that a podocyte stem cell resides among the PECs. The ability of cultured PECs to contribute to the tubule could indicate a broader potency of PECs, but future experiments are needed to determine whether the PEC is multipotent in vivo. Genetic tracking is also needed to assess the extent to which the PEC progenitor pool can self-renew in adults over the long-term. Furthermore, the mechanisms of progenitor migration are unresolved. The gradient of cell phenotypes along the capsule is consistent with a cycle wherein podocytes gradually shift towards the glomerulus as shedding occurs (an example pathway is indicated by green arrows in Figure 5) [95,96,103,104]. Differentiating podocytes might also take a more direct route to the glomerulus [95,104], on the basis of the observation of cell bridges or so-called tuft-to-capsule adhesions, where chains of cells link the glomerulus and capsule [105]. Finally, the developmental pathways essential to specify the PEC podocyte progenitor are largely obscure. In a recent study, the removal of β-catenin signalling during the late S-shaped body stage of nephrogenesis resulted in the abrogation of PECs and a replacement with parietal podocytes [106]. This phenotype could reflect a number of possibilities, such as a requirement for β-catenin in PEC self-renewal, PEC survival or a lineage conversion event. Understanding the signals that modulate PEC development will be vital in understanding disease pathology, as exemplified by reports that PEC progenitors contribute to cell lesions in several glomerular diseases [107,108] and can be enhanced by activating Notch signalling [109]. Interestingly, a recent report provided evidence that ACEi moderates renal progenitor dynamics using a rat model of progressive glomerular injury [110]. In this setting, treatment with ACEi was associated with improved glomerular architecture and linked to moderated progenitor activation [110]. These findings suggest that moderating the cellular response to renal damage may confer renoprotective effects.
NEPHRON TUBULAR REGENERATION AND STEM CELLS?
Cellular regeneration in the nephron tubules was noted as far back as the late 19th century, and was suggested to arise from surviving cells within injured tubules [7]. Since this time, both intra- and extra-renal cell sources have been proposed to support nephron tubule regeneration (Figure 6). Genetic fate-mapping studies have shown that intratubular cells restore nephron integrity following acute injury, although the origin of the cells is disputed. Putative stem cells have been located in the tubules themselves, and the collecting ducts and interstitium of the renal papilla, but the identities and behaviours of these cells are controversial. Although extrarenal cells such as BMSCs (bone marrow stem cells) and MSCs (mesenchymal stem cells) were once thought to make direct cell contributions to renal regeneration, they are now thought to provide humoral support. In the following sections we address the lines of evidence concerning the proposed contributors to tubule regeneration.
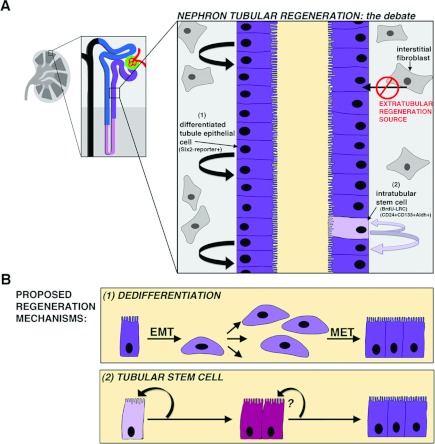
Figure 6. Tubular injury and source(s) of replacement cells.
(A) Injury models that damage the proximal tubule by ischaemia/reperfusion or nephrotoxins have been most extensively used to study tubular regeneration. These analyses have led to a current debate between several cellular sources of regeneration that have converged to focus on (1) differentiated tubule epithelial cells (labelled with Six2-reporter expression) and (2) intratubular stem cells (labeled by BrdU and CD24+CD133+Aldhhigh in various studies). A third cell source, the extratubular compartment, which over the years has been speculated to include kidney-resident interstitial fibroblasts, BMSCs and MSCs, has been negated by evidence of intratubular sources of regeneration. (B) The scenarios of tubular regeneration are proposed to involve either a dedifferentiation (1, top panel) or a stem cell mechanism (2, bottom panel). Dedifferentiation includes events through which the tubular epithelium oscillates between a mesenchymal phenotype (EMT and then MET), with the mesenchyme purported to show migration and division to replace lost tubular cells. A tubular stem cell has been proposed to share hallmarks with its differentiated neighbours, and replace lost cells through division that may or may not involve transit-amplifying progenitors.
Intratubular cells and maintenance of nephron integrity
Nephron tubule epithelia residents are highly specialized and differentiated, with distinct cuboidal or columnar shapes, and are polarized along their apical–basal axis [21]. As such, a reasonable expectation is that these cells are quiescent, similar to podocytes; however, several lines of evidence indicate that tubule cells have a low basal turnover rate. Early cell division studies using a pulse–chase of tritiated thymidine reported incorporation of the label throughout the proximal, distal and collecting tubules of healthy male rats [6]. Furthermore, antibody staining with the cell-division markers PCNA (proliferating-cell nuclear antigen) and Ki67 detected positive tubule cells in adult human kidneys [111]. Later, proliferation was documented using mitotic figures, BrdU pulse–chase experiments, and cell-cycle markers in juvenile rat kidneys (as the rate of cell division was noted to range between 2 and 4%, higher than the 0.4% seen in adults) [112–114]. Proliferating cells were reported in both proximal and distal tubules [111]. This investigation found that mitotic cells in the proximal tubule had differentiated traits, as they co-expressed markers of the brush border and showed proper distribution of several polarity proteins, suggesting that dividing cells were differentiated epithelia [112]. Interestingly, a high incidence of cells in both juvenile and adult tubules were positive for cyclin D1, indicating that many tubular cells were poised in G1-phase [114].
Over the past decades, studies of AKI from ischaemia or nephrotoxin exposure have chronicled a prototypical sequence of cellular changes that precede a full restoration of nephron tubule integrity [113–123]. The descriptions have primarily centred on the proximal tubule because the prominent feature of these particular acute injuries is damage to the proximal epithelial cells. Following injury, damaged and apoptotic tubule cells slough into the lumen, denuding the basement membrane. The exposed basement membrane is then progressively covered by cells with mesenchymal features, including a flattened elongated appearance and the expression of proteins that are characteristic of motile mesenchyme. High rates of intratubular proliferation ensue soon after injury (within 12–36 h), and several studies have found that the dividing cells have features of differentiated cells, as in the cycling cells of healthy kidneys [112,113]. Eventually, tubular integrity is re-established as the mesenchymal cells transition to become epithelial, with regeneration complete in as fast as 2 weeks. The regenerating cells express renal development genes, including Pax2, NCAM (neural cell adhesion molecule) and Lhx1 (Lim homeobox 1), supporting the hypothesis that tubule regeneration may recapitulate some developmental processes [124–126].
The source of the replicating mesenchyme has been the subject of intense debate between multiple hypotheses: first, that differentiated cells regenerate the tubule; secondly, that a rare stem cell population interspersed between the differentiated epithelia facilitates repair; and thirdly, that extrarenal stem cells contribute a proliferating pool (the latter two are discussed in subsequent sections) [127,128]. The model for the first hypothesis is that differentiated tubule cells undergo a dedifferentiation process (Figure 6B). Dedifferentiation includes a transition from the epithelial to mesenchyme state [EMT (epithelial–mesenchymal transition)] and a re-entry into the cell cycle, with the mesenchymal progeny eventually transitioning back to an epithelial state [MET (mesenchymal–epithelial transition)]. The positive correlation between differentiated tubule markers and mitosis markers in healthy kidneys supports the idea that resident tubule epithelia could fuel regeneration. The dedifferentation hypothesis is also attractive due to the precedence set by nephron development, i.e. that the tubule arises from mesenchyme that is induced to become epithelium.
Several strategies have been used to track the origins of regenerated tubule cells by re-examining the events associated with ischaemia/reperfusion injury. In mice where tubule epithelia were mosaically marked with eGFP (enhanced GFP) using the Ksp (kidney-specific)-cadherin promoter, eGFP+ cells incorporated BrdU and co-stained with differentiated markers during regeneration, suggesting an intratubular source of regeneration [127]. Another study used the Six2 promoter to stably mark the entire differentiated tubular epithelium with a reporter, and then determined whether regenerated tubules had a ubiquitous or diluted reporter pattern [128]. In this transgenic system, the Six2 reporter marks the tubule cells that arise from the CM during nephrogenesis. Following kidney repair, no dilution of the Six2 marker was observed, evidence that Six2 descendents were the primary regeneration source. Interpretation of this data does hinge on the transgenic marking strategy: any re-expression of Six2 during regeneration would trigger stable reporter expression in those cells and their descendents that would be indistinguishable from the adult tubule population. By several measures, the authors did not detect Six2 re-expression at the timepoints they examined, although a rapid pulse of Six2 activity could always be possible [128]. Overall, these data provide strong evidence in favour of an intratubular regeneration source.
In a subsequent study, proximal tubule regeneration was tracked in mice with a two-step sequence of nucleotide analogue pulses after ischaemia [first CldU (5-chloro-2-deoxyuridine), and then IdU (5-iodo-2-deoxyuridine)] that were administered close in time to one another, with the hypothesis being that the offspring of a stem cell that divided to make a transit-amplifying progenitor would retain both labels [129]. The researchers observed low co-labelling, concluding that these data illustrate a stochastic process of tubular proliferation among differentiated cell types. These findings provide an intriguing snapshot into tubular cell dynamics during the time window defined by the labelling pulses (between 24 and 45 h post-injury). Additional analysis of earlier time windows could potentially be informative, since they noted a rise in tubular proliferation starting at 12 h post-injury [129], and similar rapid escalations in division were observed in studies of rat ischaemic injury [130]. Future studies tracking individual cells are needed in order to definitively resolve cell dynamics during tubule regeneration. Taken together, the cell-tracking studies performed to date are consistent with the model that an intratubular cell source fuels regeneration, and that this source has differentiated traits. However, the remaining questions revolve around how to interpret this combination of the differentiated traits: are they the sign of mature cells, or are tubular stem cells scattered in nephrons?
Evidence for tubular stem cells
Several groups have proposed the existence of rare tubular stem cells on the basis of the observation of stemness attributes in a minor subset of the resident tubule population. For example, cell-cycling differences among tubular cells have been suggested by a cohort of pulse–chase experiments. Individual rat kidney tubule cells were shown to incorporate BrdU and retained the label during a short (2 week) chase [130]. When a viable subset of the BrdU+ cells was isolated, they retained low Hoeschst staining; in addition, they could be cultured in vitro to generate tubule-like structures, and contributed to nephrons and collecting ducts when transplanted to a growing metanephros [131]. These studies characterized cells that retained BrdU over just a short chase, and thus may represent a pool of transit-amplifying cells and/or stem cells. In a much longer pulse–chase study, infrequent BrdU+ proximal tubule cells were detected after 35 weeks following BrdU administration in normal newborn rats [112]. Rare cortical nephron labelling was reported in a similar chase, but with 3-day-old rats examined after 2 months [132]. The low number of BrdU+ cells observed after these much longer chases is consistent with the existence of a tubule stem cell that divides relatively infrequently.
Prospective tubule stem cells in the rodent kidney have also been identified from gene expression and functional assays. One group microdissected a single nephron from an adult rat kidney and established a cell line from the proximal segment with expansive growth potential, designated rKS56 [133]. rKS56 cells co-expressed mesenchymal and epithelial markers, such as the intermediate filament VIM (vimentin) and the water channel aquaporin respectively, a phenotype likening them to immature tubule cells. rKS56 cells also expressed c-Kit and Sca-1, markers associated with an immature progenitor state. Consistent with this comparison, rKS56 differentiated into mature epithelium in vitro and after transplant into the post-ischaemic adult kidney. In the mouse, a proximal tubule progenitor-like population was identified through studies of NFATc1 (nuclear factor of activated T-cells cytoplasmic 1), a transcription factor expressed in cortical nephron tubules [134]. An NFATc1–LacZ reporter labelled a subset of proximal tubule epithelia that expanded in the day following toxin exposure, and then contracted in number within several days. NFATc1-labelled cells were resistant to apoptosis, suggesting that they were fated to survive the renal injury. Lineage analysis of these cells using an NFATc1–Cre reporter revealed a subpopulation of proximal tubule that divided after renal injury, with the progeny reconstituting large tubule stretches [134].
Corroborating findings from several reports have suggested that a multipotent CD24+CD133+ tubule cell is present in the human kidney. This nephron population was first identified by searching for CD133+ cells in tubular fractions on the basis of the correlation between this antigen and the PEC glomerular stem cell [135]. A subset of the CD133+ tubular cells was found to co-express CD24 and, by clonogenic analysis, the CD24+CD133+ tubule population could differentiate into multiple cell types in vivo. This tubule population also had a similar gene signature to CD24+CD133+ glomerular cells by microarray. Polarized tubule cells expressing both CD24 and CD133 were found in proximal and distal segments, although the frequency was not quantified. More recently, another research group discovered and characterized a human CD24+CD133+ multipotent tubule cell using an entirely different regime of stem cell traits [136]. The initial isolation strategy in this case was on the basis of the precedence that high ALDH (aldehyde dehydrogenase) activity is associated with stemness [137]. Kidney cells with ALDHhigh activity were isolated from the renal cortex and had an increased capacity to form sphere-like clusters of epithelial cells in culture, and were capable of anchorage-independent growth features seen in several types of multipotent stem cells. By whole-genome expression profiling, the ALDHhigh cells showed high levels of CD24, CD133, VIM, and cytokeratins 7 and 19. Localization studies showed that CD24+CD133+ cells were rare tubule cells (only occasionally found in pairs), and cells in the parietal region of the renal corpuscle, the latter in keeping with previous studies [96,103,104]. CD133+VIM+ co-expressing cells were interspersed in a similar fashion throughout the healthy tubules; in human acute renal damage biopsies, these were expanded into long stretches of CD133+VIM+ cells, suggesting that they expanded during regeneration. Interestingly, a previous study isolated CD133+ cells from the adult human kidney cortex that also expressed Pax2 [138]. The CD133+ cells produced both renal epithelial and endothelial cells in culture, and integrated into nephron tubules in mice with AKI. The expression of CD133 is a characteristic of haemopoietic stem cells and endothelial cells. Thus the isolated CD133+ fraction in this case may represent a heterogenous population that has been suggested by some to include interstitial cells [14].
Taken together, these findings support the hypothesis of a tubular stem cell, but the evidence remains incomplete and requires much more investigation. The very idea of the tubular stem cell hypothesis is at odds with a long history of studies that examined tubule cells by ultrastructural and molecular methods, and never reported a minor population of ‘different’ cells in tubular segments. To reconcile all of these findings, one must reason that tubular stem cells are very rare, and have been overlooked by many methods. Tubule stem cells could also look like differentiated cells, possessing traits of apical–basal polarity that are actually needed for them to physically reside in the tubule, but which make them hard to discriminate from bona fide differentiated neighbours with the typically used repertoire of markers. The analysis of NFATc1+ cells in the mouse and CD24+CD133+ cells in humans has now delineated several unique markers that set some tubule cells apart from their neighbours. Future comparative studies will be valuable to learn more about the identity and correspondence between these cell types. Moving forward, more markers are absolutely essential. Genetic tracking using validated indicators of differentiated epithelium or tubule subtypes is vital to unequivocally distinguish the mechanisms of tubular regeneration. Finally, it will be interesting to determine whether a generic tubular stem cell type possesses ‘tubule-wide’ multipotency and can make epithelial cells of multiple segments, or whether different nephron segments house more specialized tubular stem cell residents.
STEM CELLS OUTSIDE THE NEPHRON PROPER: STUDIES OF THE RENAL PAPILLA
The renal papilla, or inner medulla, is the apex of the medullary zones and contains tracks of collecting ducts, intermediate nephron segments (the loops of Henle) and intervening interstitial cells (Figure 7) [21]. The renal papilla is a hypoxic hyperosmotic tissue where the business of water conservation is transacted through a countercurrent exchange system made possible by this special local environment [21]. The existence of stem cells in the renal papilla was first proposed after the discovery of label-retaining populations in this region. Pulse–chase studies using BrdU in the healthy rat kidney showed that LRCs were present in the papilla after 2 months, mostly in the interstitium, but also in tubules [132]. Independent pulse–chase experiments have shown that the tubular fraction includes the collecting duct and loop of Henle cells [129]. Following ischaemia, proliferating cells were found concentrated in the upper-most papillary regions, and then, several months later, these regions were largely devoid of LRCs [132,139]. These findings were surprising because transient ischaemia was not damaging to the cells within the renal papilla, although of course is well-established to trigger destruction of the nephron proximal tubule cells. On the basis of these observations, the renal papilla was hypothesized to be a source for tubular regeneration, and cells generated from the papilla region were proposed to migrate into damaged nephrons throughout the medulla and cortex [132,139].
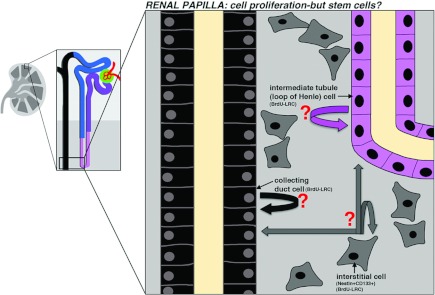
Figure 7. The renal papilla and its proliferative compartments.
The renal papilla is a zone of the kidney that contains collecting ducts, nephron tubule portions that include the intermediate and distal segments, and interstitial fibroblast cells. BrdU-labelled LRCs have been found in all three cellular compartments, although whether these represent self-renewing stem or progenitor cells is not known. In addition, interstitial cells that are Nestin+CD133+ have been detected and are speculated to represent a potential stem cell compartment.
Currently, there are several conflicting reports surrounding the idea of papillary migration. Evidence in favour of renal papilla migration was provided by labelling mouse papillary cells with a pulse–chase of a vital dye and then inducing an ischaemic injury; post-ischaemia the labelled cells were found in a redistributed pattern, and some were even associated with unlabelled tubules, suggesting that local movement and nephron integration occurred over time [139]. Cell redistributions toward the cortex following ischaemia were also observed in a Nestin−GFP+ transgenic mouse, although Nestin+ cells probably included a widely heterogenous population of labelled cell types in the papilla [140]. In contrast, other time-course studies have failed to detect a change in the distribution of nucleotide analogue label-retaining papillary cells after injury [128]. Genetic tracing of papilla epithelial cells marked using mTert (mouse telomerase reverse transcriptase), an enzyme expressed by ES cells and several adult stem cells, also failed to detect cells of papillary origin moving to the outer medulla or cortex following ischaemia [141]. In this study the label-retaining papilla interstitial cells were not marked, therefore migrating interstitial cells could not be evaluated [141].
Given these sets of conflicting data and the strong genetic evidence that intratubular cells regenerate nephrons, it seems unlikely that the renal papilla serves as the primary source for the bulk of cortical nephron regeneration. However, this does not preclude the existence of a renal papilla stem cell pool, especially when one considers the label-retention capacity and the in vitro behaviours attributed to subsets of papillary cells. In particular, more research is needed to resolve the identity and true potency of the papillary interstitial cells. For example, a recent study found a fraction of Nestin+CD133+ co-expressing interstitial cells in the human renal papilla, which is intriguing given the traits associated with CD133+ cells in the renal corpuscle [142]. These Nestin+CD133+ interstitial cells showed expression of mesenchymal and ES cell markers, and contributed to tubulogenesis in a three-dimensional culture assay and when transplanted into developing mouse kidneys. Future genetic-tracking studies will be critical to determine the fates of this interstitial compartment after renal injury, and additional work is needed to delineate the identity and phenotypes of the heterogenous interstitial population.
ISOLATION OF OTHER ENDOGENOUS PUTATIVE RENAL STEM CELLS
Several groups have sought to identify renal stem cells ‘at large’ without focusing on any one area of the kidney. A common approach has been to isolate particular cell fractions using a stemness marker(s) and then ascertain the multipotency of the isolated fractions in vitro. On the basis of the precedence that Sca-1 marks adult stem cells across several mesodermal derivatives, one group isolated and characterized a Sca-1+Lin− fraction from the adult mouse kidney [143]. Clonally derived lines of Sca-1+Lin− cells were capable of differentiating into myogenic, osteogenic, adipogenic and neural lineages in vitro, and adopted a tubular phenotype when injected into the renal parenchyma following ischaemic injury. Immunohistochemistry studies detected Sca-1 on numerous cell types in the kidney, which included tubule and renal papilla interstitium, suggesting that the clonal lines could represent heterogenous origins within the kidney.
A second stemness trait used to fractionate the kidney has been the SP (side population) assay. Several groups have reported the isolation and characterization of SP cells from mouse and human kidneys [144–147]. The SP isolated from adult mouse kidneys was initially reported to be Sca-1+ with >95% of the SP expressing the basic helix–loop–helix transcription factor musculin, which has been linked to promoting a dedifferentiated fate [144]. When mice with AKI from the nephrotoxin cisplatin were given an injection of the kidney SP, they displayed renal function improvement on the basis of creatinine excretion levels. Interestingly, musculin+ cells were shown to reside in the interstitial space of the kidney. Subsequent expression profiling of kidney SP from embryonic and adult mouse kidneys reported similar Sca-1, but not musculin, positivity [145,146]. Functional assays have found that the kidney SP can exhibit multipotency in vitro, contribute to UB and MM structures in metanephric organ culture, and engraft into adriamycin-damaged kidneys at a low frequency [145]. Molecular and functional analyses of the human kidney SP have not yet been reported.
Yet another strategy used to locate renal stem cells has been to select them on the basis of their ability to propagate in long-term culture. One group dissociated adult rat kidneys and grew the cells for several weeks, mimicking the culture conditions used to previously isolate adult stem cells from the bone marrow [148]. The surviving cells were termed MRPCs (multipotent renal progenitor cells), and were defined by co-expression of the transcription factors Pax2 and Oct4 (octamer-binding transcription factor 4). The MRPCs could produce multiple lineages in vitro, ranging from hepatocytes to endothelial cells and neurons, and contributed to tubular cells when injected into normal and ischaemically injured kidneys. The researchers also identified rare Oct4+ cells in tubules of the adult kidney, suggesting a possible tubular origin of the MRPCs. A recent study examining mouse kidneys took a similar long-term culturing tactic using a transgenic line in which the Myh9 (myosin heavy chain 9) gene was disrupted with a GFP cassette, marking several differentiated cell types in nephrons, as well as the interstitium [149]. After culturing the GFP+ cells for 8 weeks, the surviving cells adopted a similar morphology and showed self-renewal characteristics, and were designated MKPCs (mouse kidney progenitor cells). Traits shared by the MKPCs included expression of Pax2, Oct4, Wt1 and VIM, suggesting parallels with pluripotency and renal progenitors during development.
Taken together, these studies could indicate that the kidney could house one or more multipotent cell types. However, there are several caveats and future questions to consider with regard to these findings. One consideration is whether an isolated cell type/line is representative of a cell that normally resides in the kidney. Some of the procedures used to isolate renal cells could capture rare (stem) cells from other tissues that are able to survive in culture. Some procedures could also favour the selection and expansion of genetically altered cells that are not representative of the normal kidney. The relationships between multipotent cells from these various reports have not been defined, nor has their relationship to cells such as the PEC-podocyte stem cell. For example, a multipotent cell line might actually be the derivative of PECs. Another consideration is whether the cells underwent genetic or epigenetic changes in the course of their manipulation. Thus althogh these findings are intriguing, the contribution of these cells to renal homoeostasis and their impact on renal disease remains unclear.
CONCLUSIONS AND FUTURE PERSPECTIVES
Research to find stem cells in the kidney suggests that a number of previously unappreciated renal cell types exist, thereby raising a multitude of questions for future work. Ongoing research is needed to further delineate the respective activities and relationships between the PEC-podocyte stem cell, the tubular regeneration source(s), papillary cells and the growing category of ‘other’ renal stem cell-like types. It is particularly intriguing that the cells for which there is the strongest evidence of regeneration, the podocytes and the proximal tubule, are the most susceptible and most frequently damaged in human kidneys. The observation of regeneration in these renal compartments begs the question as to why such mechanisms are remiss in obviating injuries to these cell types. To begin to understand this puzzle, we will need to know more about the long-term capacities for regeneration of the various renal stem cell compartments, as there is very little data on the mechanisms that control self-renewal properties of each cell type. Knowledge about these mechanisms could provide insights into understanding the parameters of stem cell exhaustion, such as how the aged environment might effect stem cell maintenance and behaviour. Tools for genetic fate mapping will be essential to evaluate the progeny of these cells and the long-term homoeostasis of the stem cell compartment(s). For example, an explanation for different AKI outcomes in patients may reside with their past history of renal damage (over a lifetime of damage/regeneration cycles) and its cumulative effect on the replicative capacity of the resident kidney stem cells. In addition, there is a poor understanding of the niches that are inhabited by these cells, and an increased understanding of their various microenvironments would provide valuable information.
One additional source of insight into renal stem cell properties may come from research in non-mammalian species [150,151]. Interestingly, multipotent renal stem cells with seemingly high replicative potential throughout adult life have been described in several vertebrate species, including the elasmobranchs and teleost fish [152,153]. In these species, the adult renal stem cells function in a unique regard: they make entirely new nephrons in the adult through a process termed neonephrogenesis [152,153]. For example, in a model of AKI in the adult zebrafish, widespread proximal tubule injury was rapidly followed by the generation of new nephrons [153]. Neonephrogenesis was induced from renal stem cell clusters that were defined by the expression of the transcription factors Pax2, Lhx1 and Wt1, genes that mark the kidney lineage during development [153]. Renal stem cell functionality was assessed using serial transplantation, which revealed that these clusters could sequentially generate new nephrons up to three recipients, suggesting that they possessed self-renewal properties [153]. While the adult fish kidney is a multi-nephron mesonephros that differs from mammals in its architectural arrangement of nephrons and collecting ducts, these findings add further weight to the notion that vertebrate kidney cells can possess striking regenerative powers. There are fundamental differences, however, between the physiology of fish and humans: species such as the zebrafish are characterized by continual growth in adulthood, thus the lifelong growth of the kidney probably enables renal functions to keep pace with the increasing demands of the biomass. Nonetheless, future studies that uncover the workings of the renal stem cell properties in other species could provide clues for how to enhance stem cell activity in the human kidney.
There are clearly numerous challenges to surmount in order to apply knowledge about renal stem cells towards clinical therapies. The renal disease(s) amenable to stem cell therapeutics probably depend on the nature of the disease and its stage/severity. Patients with AKI may be more amenable to treatment compared with those with chronic kidney disease, who may be refractory to cellular interventions due to long-term inflammation, the deposition of widespread fibrotic lesions and other tissue pathologies. To circumvent this issue, the identification of biomarkers that can diagnose early stages of chronic conditions is crucial to better facilitate early diagnosis and enable successful intervention(s). In treating these kidney conditions, the ability to trigger reparative behaviours with endogenous renal cells (stem or other) using small molecules would be ideal, as this eliminates the complications in generating and delivering cells. Exogenous cell sources, such as those derived from pluri-or multi-potent embryonic or adult cell lines, may prove viable if safety concerns can be met, such as ensuring the quality and identity of the cells. Interestingly, there are data to suggest that the adult kidney will be a permissible environment to receive exogenous renal cells. Experiments in mice showed that the metanephros could continue to grow when transplanted into the renal cortex post-development, suggesting that the adult kidney may be able to receive and/or support cellular growth [154].
In conclusion, the discovery of renal cells with stemness attributes has heralded a new and exciting chapter in kidney biology. The continued study of these renal populations may one day lead to the creation of regenerative medicine treatments for the kidney.
ACKNOWLEDGEMENTS
We thank Gary F. Gerlach and Yue Li for their critical review of the paper prior to submission, and the other members of our laboratory for providing helpful discussions and support.
FUNDING
The work of our laboratory is supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Disease [grant number K01DK083512], a National Institutes of Health Director's New Innovator award [grant number DP2OD008470], a March of Dimes Basil O'Connor Starter Scholar award, and funds from the University of Notre Dame College of Science and Department of Biological Sciences.
References
- 1.Levey A. S., Atkins R., Coresh J., Cohen E. P., Collins A. J., Eckardt K. U., Nahas M. E., Jaber B. L., Jadoul M., Levin A., et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.Meguid El Nahas A., Bello A. K. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 3.Little M. H. Regrow or repair: potential regenerative therapies for the kidney. J. Am. Soc. Nephrol. 2006;17:2390–2401. doi: 10.1681/ASN.2006030218. [DOI] [PubMed] [Google Scholar]
- 4.Potter E. L., Thierstein S. T. Glomerular development in the kidney as an index of fetal maturity. J. Pediatr. 1943;22:695–706. [Google Scholar]
- 5.Hartman H. A., Lai H. L., Patterson L. T. Cessation of renal morphogenesis in mice. Dev. Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messier B., Leblond C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am. J. Anat. 1960;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- 7.Romagnani P. Family portrait: renal progenitor of Bowman's capsule and its tubular brothers. Am. J. Pathol. 2011;178:490–493. doi: 10.1016/j.ajpath.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toback F. G. Regeneration after acute tubular necrosis. Kidney Int. 1992;41:226–246. doi: 10.1038/ki.1992.32. [DOI] [PubMed] [Google Scholar]
- 9.Thadhani R., Pascual M., Bonventre J. V. Acute renal failure. N. Engl. J. Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 10.Bonventre J. V. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003;14:S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 11.Romagnani P., Kalluri R. Possible mechanisms of kidney repair. Fibrog. Tissue Repair. 2009;2:3. doi: 10.1186/1755-1536-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhabra P., Brayman K. L. The use of stem cells in kidney disease. Curr. Opin. Organ Transplant. 2009;14:72–78. doi: 10.1097/MOT.0b013e328320d2f5. [DOI] [PubMed] [Google Scholar]
- 13.Guo J. K., Cantley L. G. Cellular maintenance and repair of the kidney. Annu. Rev. Physiol. 2010;72:357–376. doi: 10.1146/annurev.physiol.010908.163245. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins C., Li J., Rae F., Little M. H. Stem cell options for kidney disease. J. Pathol. 2009;217:265–281. doi: 10.1002/path.2477. [DOI] [PubMed] [Google Scholar]
- 15.Little M. H., Bertram J. F. Is there such a thing as a renal stem cell? J. Am. Soc. Nephrol. 2009;20:2112–2117. doi: 10.1681/ASN.2009010066. [DOI] [PubMed] [Google Scholar]
- 16.Pleniceanu O., Harari-Steinberg O., Dekel B. Concise review: kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells. 2010;28:1649–1660. doi: 10.1002/stem.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagrinati C., Ronconi E., Lazzeri E., Lasagni L., Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol. Med. 2008;14:277–285. doi: 10.1016/j.molmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Zubko R., Frishman W. Stem cell therapy for the kidney? Am. J. Ther. 2009;16:247–256. doi: 10.1097/MJT.0b013e3181800591. [DOI] [PubMed] [Google Scholar]
- 19.Benigni A., Morigi M., Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 20.Davidson A. J. Uncharted waters: nephrogenesis and renal regeneration in fish and mammals. Pediatr. Nephrol. 2011;26:1435–1443. doi: 10.1007/s00467-011-1795-z. [DOI] [PubMed] [Google Scholar]
- 21.Reilly R. F., Bulger R. E., Kriz W. Structural-functional relationships in the kidney. In: Schrier R. W., editor. Diseases of the Kidney and Urinary Tract. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 2–53. [Google Scholar]
- 22.Khairallah W., Merheb M., Chaiban J., Badr K. F. Hormones and the kidney. In: Schrier R. W., editor. Diseases of the Kidney and Urinary Tract. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 234–284. [Google Scholar]
- 23.Hallgrimsson B., Benediktsson H., Vize P. D. Anatomy and histology of the human urinary system. In: Vize P. D., Woolf A. S., Bard J. B. L., editors. The Kidney, From Normal Development to Congenital Disease. London: Academic Press; 2003. pp. 149–164. [Google Scholar]
- 24.Hoy W. E., Douglas-Denton R. N., Hughson M. D., Cass A., Johnson K., Bertram J. F. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int. Suppl. 2003;83:S31–S37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughson M., Farris A. B., III, Douglas-Denton R., Hoy W. E., Bertram J. F. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 26.Nyengaard J. R., Bendtsen T. F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 27.Wingert R. A., Davidson A. J. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 28.Arendshorst W. J., Navar L. G. Renal circulation and glomerular hemodynamics. In: Schrier R. W., editor. Diseases of the Kidney and Urinary Tract. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 54–95. [Google Scholar]
- 29.Schedl A. Renal abnormalities and their developmental origin. Nat. Rev. Genet. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 30.Kerecuk L., Schreuder M. F., Woolf A. S. Renal tract malformations: perspectives for nephrologists. Nat. Clin. Pract. Nephrol. 2008;4:312–325. doi: 10.1038/ncpneph0807. [DOI] [PubMed] [Google Scholar]
- 31.Song R., Yosypiv I. V. Genetics of congenital anomalies of the kidney and urinary tract. Pediatr. Nephrol. 2011;26:353–364. doi: 10.1007/s00467-010-1629-4. [DOI] [PubMed] [Google Scholar]
- 32.Woolf A. S. A molecular and genetic view of human renal and urinary tract malformations. Kidney Int. 2000;58:500–512. doi: 10.1046/j.1523-1755.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 33.Renkema K. Y., Winyard P. J., Skovorodkin I. N., Levtchenko E., Hindryckx A., Jeanpierre C., Weber S., Salomon R., Antignac C., Vainio S., et al. Novel perspectives for investigating congenital anomalies of the kidney and urinary tract (CAKUT) Nephrol. Dial. Transplant. 2011;26:3843–3851. doi: 10.1093/ndt/gfr655. [DOI] [PubMed] [Google Scholar]
- 34.Cain J. E., Di Giovanni V., Smeeton J., Rosenblum N. D. Genetics of renal hypoplasia: insights into the mechanisms controlling nephron endowment. Pediatr. Res. 2010;68:91–98. doi: 10.1203/PDR.0b013e3181e35a88. [DOI] [PubMed] [Google Scholar]
- 35.Wiggins R. C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 36.Murugan R., Kellum J. A. Acute kidney injury: what's the prognosis? Nat. Rev. Nephrol. 2011;7:209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricci Z., Cruz D. N., Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat. Rev. Nephrol. 2011;7:201–208. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teo A. K., Vallier L. Emerging use of stem cells in regenerative medicine. Biochem. J. 2010;428:11–23. doi: 10.1042/BJ20100102. [DOI] [PubMed] [Google Scholar]
- 40.Hipp J., Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4:3–11. doi: 10.1007/s12015-008-9010-8. [DOI] [PubMed] [Google Scholar]
- 41.Jaenisch R., Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna J. H., Saha K., Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison S. J., Spradling A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simons B. D., Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 45.Trumpp A., Essers M., Wilson A. Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 2010;10:201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 46.Eckfeldt C. E., Mendenhall E. M., Verfaillie C. M. The molecular repertoire of the ‘almighty’ stem cell. Nat. Rev. Mol. Cell Biol. 2005;6:726–737. doi: 10.1038/nrm1713. [DOI] [PubMed] [Google Scholar]
- 47.Mikkers H., Frisen J. Deconstructing stemness. EMBO J. 2005;24:2715–2719. doi: 10.1038/sj.emboj.7600749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graf T., Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3:480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Al-Awqati Q., Oliver J. A. Stem cells in the kidney. Kidney Int. 2002;61:387–395. doi: 10.1046/j.1523-1755.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 50.He S., Nakada D., Morrison S. J. Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 51.Bloushtain-Qimron N., Yao J., Shipitsin M., Maruyama R., Polyak K. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–817. doi: 10.4161/cc.8.6.7938. [DOI] [PubMed] [Google Scholar]
- 52.Challen G. A., Little M. H. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 53.Orford K. W., Scadden D. S. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 54.Orkin S. H., Zon L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee Z., Dennis J. E., Gerson S. L. Imaging stem cell implant for cellular-based therapies. Exp. Biol. Med. 2008;233:930–940. doi: 10.3181/0709-MR-234. [DOI] [PubMed] [Google Scholar]
- 56.Duffield J. S., Humphreys B. D. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 2011;79:494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- 57.Dressler G. R. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 58.Dressler G. R. Advances in early kidney specification, development, and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costantini F., Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hendry C., Rumballe B., Moritz K., Little M. H. Defining and redefining the nephron progenitor population. Pediatr. Nephrol. 2010;26:1395–1406. doi: 10.1007/s00467-010-1750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saxen L., Sariola H. Early organogenesis of the kidney. Pediatr. Nephrol. 1987;1:385–392. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 62.Auerbach R., Grobstein C. Inductive interaction of embryonic tissues after dissociation and reaggregation. Exp. Cell Res. 1958;15:384–397. doi: 10.1016/0014-4827(58)90039-9. [DOI] [PubMed] [Google Scholar]
- 63.Herzlinger D., Koseki C., Mikawa T., Al-Awqati Q. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development. 1992;114:565–572. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- 64.Qiao J., Cohen D., Herzlinger D. The metanephric blastema differentiates into collecting system and nephron epithelia in vitro. Development. 1995;121:3207–3214. doi: 10.1242/dev.121.10.3207. [DOI] [PubMed] [Google Scholar]
- 65.Mugford J. W., Sipila P., McMahon J. A., McMahon A. P. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev. Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mugford J. W., Yu J., Kobayashi A., McMahon A. P. High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev. Biol. 2009;333:312–323. doi: 10.1016/j.ydbio.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliver G., Wehr R., Jenkins N. A., Copeland N. G., Cheyette B. N. R., Hartenstein V., Zipursky S. L., Gruss P. Homeobox genes and connective tissue patterning. Development. 1995;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- 68.Self M., Lagutin O. V., Bowling B., Hendrix J., Cai Y., Dressler G. R., Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–5228. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgas K., Rumballe B., Valerius M. T., Chiu H. S., Thiagarajan R. D., Lesieur E., Aronow B. J., Brunskill E. W., Combes A. N., Tang D., et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev. Biol. 2009;332:273–286. doi: 10.1016/j.ydbio.2009.05.578. [DOI] [PubMed] [Google Scholar]
- 70.Rumballe B. A., Georgas K. M., Combes A. N., Ju A. L., Gilbert T., Little M. H. Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. Dev. Biol. 2011;360:110–122. doi: 10.1016/j.ydbio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thiagarajan R. D., Georgas K. M., Rumballe B. A., Lesieur E., Chiu H. S., Taylor D., Tang D.T.P., Grimmond S. M., Little M. H. Identification of anchor genes during kidney development defines ontological relationships, molecular subcompartments and regulatory pathways. PLoS ONE. 2011;6:e17286. doi: 10.1371/journal.pone.0017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hatini V., Huh S. O., Herzlinger D., Soares V. C., Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 73.Levinson R. S., Batourina E., Choi C., Vorontchikhina M., Kitajewski J., Mendelsohn C. L. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- 74.Boyle S., Misfeldt A., Chandler K. J., Deal K. K., Southard-Smith E. M., Mortlock D. P., Baldwin H. S., de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kobayashi A., Valerius M. T., Mugford J. W., Carroll T. J., Self M., Oliver G., McMahon A. P. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Giovanni V., Alday A., Chi L., Mishina Y., Rosenblum N. D. Alk3 controls nephron number and androgen production via lineage-specific effects in intermediate mesoderm. Development. 2011;138:2717–2727. doi: 10.1242/dev.059030. [DOI] [PubMed] [Google Scholar]
- 77.Mugford J. W., Sipila P., Kobayashi A., Behringer R. R., McMahon A. P. Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev. Biol. 2008;319:396–405. doi: 10.1016/j.ydbio.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dressler G. R., Deutsch U., Chowdhury K., Nornes H. O., Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- 79.Karner C. M., Das A., Ma Z., Self M., Chen C., Lum L., Oliver G., Carroll T. J. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaughan M. R., Quaggin S. E. How do mesangial and endothelial cells form the glomerular tuft? J. Am. Soc. Nephrol. 2008;19:24–33. doi: 10.1681/ASN.2007040471. [DOI] [PubMed] [Google Scholar]
- 81.Quaggin S. E., Kreidberg J. A. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- 82.Shankland S. J. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- 83.He J. C., Husain M., Sunamoto M., D'Agati V. D., Klotman M. E., Iyengar R., Klotman P. E. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J. Clin. Invest. 2004;114:643–651. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moeller M. J., Soofi A., Hartmann I., Le Hir M., Wiggins R., Kriz W., Holzman L. B. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J. Am. Soc. Nephrol. 2004;15:61–67. doi: 10.1097/01.asn.0000102468.37809.c6. [DOI] [PubMed] [Google Scholar]
- 85.Vogelmann S. U., Nelson W. J., Myers B. D., Lemley K. V. Urinary excretion of viable podocytes in health and renal disease. Am. J. Physiol. Renal Physiol. 2003;285:F40–F48. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhathena D. B. Glomerular basement membrane length to podocyte ratio in human nephronopenia: implications for focal segmental glomerulosclerosis. Am. J. Kidney Dis. 2003;41:1179–1188. doi: 10.1016/s0272-6386(03)00349-4. [DOI] [PubMed] [Google Scholar]
- 87.Gross M. L., Ritz E., Schoof A., Adamczak M., Koch A., Tulp O., Parkman A., El-Shakmak A., Szabo A., Amann K. Comparison of renal morphology in the Streptozotocin and the SHR/N-cp models of diabetes. Lab. Invest. 2004;84:452–464. doi: 10.1038/labinvest.3700052. [DOI] [PubMed] [Google Scholar]
- 88.Gross M. L., Heiss N., Weckbach M., Hansen A., El-Shakmak A., Szabo A., Munter K., Ritz E., Amann K. ACE-inhibition is superior to endothelin A receptor blockade in preventing abnormal capillary supply and fibrosis of the heart in experimental diabetes. Diabetologia. 2004;47:316–324. doi: 10.1007/s00125-003-1309-z. [DOI] [PubMed] [Google Scholar]
- 89.Xu Z. G., Yoo T. H., Ryu D. R., Cheon Park H., Ha S. K., Han D. S., Adler S. G., Natarajan R., Kang S. W. Angiotensin II receptor blocker inhibits p27Kip1 expression in glucose-stimulated podocytes and in diabetic glomeruli. Kidney Int. 2005;67:944–952. doi: 10.1111/j.1523-1755.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 90.Petermann A. T., Pippin J., Durvasula R., Pichler R., Hiromura K., Monkawa T., Couser W. G., Shankland S. J. Mechanical stretch induces podocyte hypertrophy in vitro. Kidney Int. 2005;67:157–166. doi: 10.1111/j.1523-1755.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 91.Fioretto P., Steffes M. W., Sutherland D. E., Goetz F. C., Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N. Engl. J. Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 92.Ruggenenti P., Perna A, Benini R., Bertani T., Zoccali C., Maggiore Q., Salvadori M., Remuzzi G. In chronic nephropathies prolonged ACE inhibition can induce remission: dynamics of time-dependent changes in GFR. J. Am. Soc. Nephrol. 1999;10:997–1006. doi: 10.1681/ASN.V105997. [DOI] [PubMed] [Google Scholar]
- 93.Remuzzi G., Benigni A., Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Macconi D., Sangalli F., Bonomelli M., Conti S., Condorelli L., Gagliardini E., Remuzzi G., Remuzzi A. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am. J. Pathol. 2009;174:797–807. doi: 10.2353/ajpath.2009.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lasagni L., Romagnani P. Glomerular epithelial stem cells: the good, the bad, and the ugly. J. Am. Soc. Nephrol. 2010;21:1612–1619. doi: 10.1681/ASN.2010010048. [DOI] [PubMed] [Google Scholar]
- 96.Sagrinati C., Netti G. S., Mazzinghi B., Lazzeri E., Liotta F., Frosali F., Ronconi E., Meini C., Gacci M., Squecco R., et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 97.Lazzeri E., Crescioli C., Ronconi E., Mazzinghi B., Sagrinati C., Netti G. S., Angelotti M. L., Parente E., Ballerini L., Cosmi L., et al. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J. Am. Soc. Nephrol. 2007;18:3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- 98.Gibson I. W., Downie I., Downie T. T., Han S. W., More I.A.R., Lindop G.B.M. The parietal podocyte: a study of the vascular pole of the human glomerulus. Kidney Int. 1992;41:211–214. doi: 10.1038/ki.1992.29. [DOI] [PubMed] [Google Scholar]
- 99.Bariety J., Mandet C., Hill G. S., Bruneval P. Parietal podocytes in normal human glomeruli. J. Am. Soc. Nephrol. 2006;17:2770–2780. doi: 10.1681/ASN.2006040325. [DOI] [PubMed] [Google Scholar]
- 100.Ryan G. B., Coghlan J. P., Scoggins B. A. The granulated peripolar epithelial cell: a potential secretory component of the renal juxtaglomerular complex. Nature. 1979;277:655–656. doi: 10.1038/277655a0. [DOI] [PubMed] [Google Scholar]
- 101.Kelly G., Downie I., Gardiner D. S., More I.A.R., Lindop G.B.M. The peripolar cell: a distinctive cell type in the mammalian glomerulus. Morphological evidence from a study of sheep. J. Anat. 1990;168:217–227. [PMC free article] [PubMed] [Google Scholar]
- 102.Poulsom R., Little M. H. Parietal epithelial cells regenerate podocytes. J. Am. Soc. Nephrol. 2009;20:231–233. doi: 10.1681/ASN.2008121279. [DOI] [PubMed] [Google Scholar]
- 103.Ronconi E., Sagrinati C., Angelotti M. L., Lazzeri E., Mazzinghi B., Ballerini L., Parente E., Becherucci F., Gacci M., Carini M., et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Appel D., Kershaw D. B., Smeets B., Yuan G., Fuss A., Frye B., Elger M., Kriz W., Floege J., Moeller M. J. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gibson I. W., Downie T. T., More I.A.R., Lindop G.B.M. Tuft-to-capsule adhesions and their precursors: differences between the vascular and tubular poles of the human glomerulus. J. Pathol. 1998;184:430–435. doi: 10.1002/(SICI)1096-9896(199804)184:4<430::AID-PATH1226>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 106.Grouls S., Iglesias D. M., Wentzensen N., Moeller M. J., Bouchard M., Kemler R., Goodyer P., Niggli F., Grone H. J., Kriz W., Koesters R. Lineage specification of parietal epithelial cells requires β-catenin/Wnt signaling. J. Am. Soc. Nephrol. 2012;23:63–72. doi: 10.1681/ASN.2010121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smeets B., Angelotti M. L., Rizzo P., Dijkman H., Lazzeri E., Mooren F., Ballerini L., Parente E., Sagrinati C., Mazzinghi B., et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J. Am. Soc. Nephrol. 2009;20:2593–2603. doi: 10.1681/ASN.2009020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smeets B., Uhlig S., Fuss A., Mooren F., Wetzels J. F. M., Floege J., Moeller M. J. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J. Am. Soc. Nephrol. 2009;20:2604–2615. doi: 10.1681/ASN.2009010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lasagni L., Ballerini L., Angelotti M. L., Parente E., Sagrinati C., Mazzinghi B., Peired A., Ronconi E., Becherucci F., Bani D., et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010;28:1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benigni A., Morigi M., Rizzo P., Gagliardini E., Rota C., Abbate M., Ghezzi S., Remuzzi A., Remuzzi G. Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am. J. Pathol. 2011;179:628–638. doi: 10.1016/j.ajpath.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nadasdy T., Laszik Z., Blick K. E., Johnson L. D., Silva F. G. Proliferative activity of intrinsic cell populations in the normal human kidney. J. Am. Soc. Nephrol. 1994;4:2032–2039. doi: 10.1681/ASN.V4122032. [DOI] [PubMed] [Google Scholar]
- 112.Vogetseder A., Karadeniz A., Kaissling B., Le Hir M. Tubular cell proliferation in the healthy rat kidney. Histochem. Cell Biol. 2005;124:97–104. doi: 10.1007/s00418-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 113.Vogetseder A., Palan T., Bacic D., Kaissling B., Le Hir M. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am. J. Physiol. Cell Physiol. 2007;292:C807–C813. doi: 10.1152/ajpcell.00301.2006. [DOI] [PubMed] [Google Scholar]
- 114.Vogetseder A., Picard N., Gaspert A., Walch M., Kaissling B., Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am. J. Physiol. Cell Physiol. 2008;294:C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 115.Oliver J., MacDowell M., Tracy A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage, and the ischemic episode. J. Clin. Invest. 1951;30:1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cuppage F. E., Tate A. Repair of the nephron following injury with mercuric chloride. Am. J. Pathol. 1967;51:405–429. [PMC free article] [PubMed] [Google Scholar]
- 117.Houghton D. C., Hartnett M., Campbell-Boswell M., Porter G., Bennett W. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am. J. Pathol. 1976;82:589–612. [PMC free article] [PubMed] [Google Scholar]
- 118.Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978;14:31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- 119.Ledda-Columbano G. M., Columbano A., Coni P., Curto M., Faa G., Pani P. Cell proliferation in rat kidney induced by 1, 2-dibromoethane. Toxicol. Lett. 1987;37:85–90. doi: 10.1016/0378-4274(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 120.Grone H. J., Weber K., Grone E., Helmchen U., Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am. J. Pathol. 1987;129:1–8. [PMC free article] [PubMed] [Google Scholar]
- 121.Moll R., Hage C., Thoenes W. Expression of intermediate filament proteins in fetal and adult human kidney: modulations of intermediate filament patterns during development and in damaged tissue. Lab. Invest. 1991;65:74–86. [PubMed] [Google Scholar]
- 122.Witzgall R., Brown D., Schwarz C., Bonventre J. V. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. J. Clin. Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zuk A., Bonventre J. V., Brown D., Matlin K. S. Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am. J. Physiol. 1998;275:C711–C731. doi: 10.1152/ajpcell.1998.275.3.C711. [DOI] [PubMed] [Google Scholar]
- 124.Imgrund M., Grone E., Grone H. J., Kretzler M., Holzman L., Schlondorff D., Rothenpieler U. W. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice. Kidney Int. 1999;56:1423–1431. doi: 10.1046/j.1523-1755.1999.00663.x. [DOI] [PubMed] [Google Scholar]
- 125.Abbate M., Brown D., Bonventre J. V. Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am. J. Physiol. 1999;277:F454–F463. doi: 10.1152/ajprenal.1999.277.3.F454. [DOI] [PubMed] [Google Scholar]
- 126.Villanueva S., Cespedes C., Vio C. P. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 127.Lin F., Moran A., Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J. Clin. Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Humphreys B. D., Valerius M. T., Kobayashi A., Mugford J. W., Soeung S., Duffield J. S., McMahon A. P., Bonventre J. V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 129.Humphreys B. D., Czerniak S., DiRocco D. P., Hasnain W., Cheema R., Bonventre J. V. Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl. Acad. Sci. U.S.A. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maeshima A., Yamashita S., Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J. Am. Soc. Nephrol. 2003;14:3138–3146. doi: 10.1097/01.asn.0000098685.43700.28. [DOI] [PubMed] [Google Scholar]
- 131.Maeshima A., Sakurai H., Nigam S. K. Adult kidney tubular cell population showing phenotypic plasticity, tubulogenic capacity, and integration capability into developing kidney. J. Am. Soc. Nephrol. 2006;17:188–198. doi: 10.1681/ASN.2005040370. [DOI] [PubMed] [Google Scholar]
- 132.Oliver J. A., Maarouf O., Cheema F. H., Martens T. P., Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J. Clin. Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kitamura S., Yamasaki Y., Kinomura M., Sugaya T., Sugiyama H., Maeshima Y., Makino H. Establishment and characterization of renal progenitor like cells from S3 segment of nephron in rat adult kidney. FASEB J. 2005;19:1789–1797. doi: 10.1096/fj.05-3942com. [DOI] [PubMed] [Google Scholar]
- 134.Langworthy M., Zhou B., de Caestecker M., Moeckel G., Baldwin H. S. NFATc1 identifies a population of proximal tubule cell progenitors. J. Am. Soc. Nephrol. 2009;20:311–321. doi: 10.1681/ASN.2008010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sallustio F., De Benedictis L., Castellano G., Zaza G., Loverre A., Costantino V., Grandaliano G., Schena F. P. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J. 2010;24:514–525. doi: 10.1096/fj.09-136481. [DOI] [PubMed] [Google Scholar]
- 136.Lindgren D., Bostrom A. K., Nilsson K., Hansson J., Sjolund J., Moller C., Jirstrom K., Nilsson E., Landberg G., Axelson H., et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am. J. Pathol. 2011;178:828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma I., Allan A. L. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011;7:292–306. doi: 10.1007/s12015-010-9208-4. [DOI] [PubMed] [Google Scholar]
- 138.Bussolati B., Bruno S., Grange C., Buttiglieri S., Deregibus M. C., Cantino D., Camussi G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oliver J. A., Klinakis A., Cheema F. H., Friedlander J., Sampogna R. V., Martens T. P., Liu C., Efstratiadis A., Al-Awqati Q. Proliferation and migration of label-retaining cells of the kidney papilla. J. Am. Soc. Nephrol. 2009;20:2315–2327. doi: 10.1681/ASN.2008111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Patschan D., Michurina T., Shi H. K., Dolff S., Brodsky S. V., Vasilieva T., Cohen-Gould L., Winaver J., Chander P. N., Enikolopov G., Goligorsky M. S. Normal distribution and medullary-to-cortical shift of Nestin-expressing cells in acute renal ischemia. Kidney Int. 2007;71:744–754. doi: 10.1038/sj.ki.5002102. [DOI] [PubMed] [Google Scholar]
- 141.Song J., Czerniak S., Wang T., Ying W., Carlone D. L., Breault D. T., Humphreys B. D. Characterization and fate of telomerase-expressing epithelia during kidney repair. J. Am. Soc. Nephrol. 2011;22:2256–2265. doi: 10.1681/ASN.2011050447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ward H. H., Romero E., Welford A., Pickett G., Bacallao R., Gattone V. H., 2nd, Ness S. A., Wandinger-Ness A., Roitbak T. Adult human CD133/1+ kidney cells isolated from papilla integrate into developing kidney tubules. Biochim. Biophys. Acta. 2011;1812:1344–1357. doi: 10.1016/j.bbadis.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dekel B., Zangi L., Shezen E., Reich-Zeliger S., Eventov-Friedman S., Katchman H., Jacob-Hirsch J., Amariglio N., Rechavi G., Margalit R., Reisner Y. Isolation and characterization of non-tubular Sca-1+Lin− multipotent stem/progenitor cells from adult mouse kidney. J. Am. Soc. Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 144.Hishikawa K., Marumo T., Miura S., Nakanishi A., Matsuzaki Y., Shibata K., Ichiyanagi T., Kohike H., Komori T., Takahashi I., et al. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. J. Cell Biol. 2005;169:921–928. doi: 10.1083/jcb.200412167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Challen G. A., Bertoncello I., Deane J. A., Ricardo S. D., Little M. H. Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J. Am. Soc. Nephrol. 2006;17:1896–1912. doi: 10.1681/ASN.2005111228. [DOI] [PubMed] [Google Scholar]
- 146.Imai N., Hishikawa K., Marumo T., Hirahashi J., Inowa T., Matsuzaki Y., Okano H., Kitamura T., Salant D., Fujita T. Inhibition of histone deacetylase activates side population cells in kidney and partially reverses chronic renal injury. Stem Cells. 2007;25:2469–2475. doi: 10.1634/stemcells.2007-0049. [DOI] [PubMed] [Google Scholar]
- 147.Inowa T., Hishikawa K., Takeuchi T., Kitamura T., Fujita T. Isolation and potential existence of side population cells in adult human kidney. Int. J. Urol. 2008;15:272–274. doi: 10.1111/j.1442-2042.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 148.Gupta S., Verfaillie C., Chmielewski D., Kren S., Eidman K., Connaire J., Heremans Y., Lund T., Blackstad M., Jiang Y., et al. Isolation and characterization of kidney-derived stem cells. J. Am. Soc. Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 149.Lee P. T., Lin H. H., Jiang S. T., Lu P. J., Chou K. J., Fang H. C., Chiou Y. Y., Tang M. J. Mouse kidney progenitor cells accelerate renal regeneration and prolong survival after ischemic injury. Stem Cells. 2010;28:573–584. doi: 10.1002/stem.310. [DOI] [PubMed] [Google Scholar]
- 150.Benigni A., Morigi M., Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 151.Reimschuessel R. A fish model of renal regeneration and development. ILAR J. 2001;42:285–291. doi: 10.1093/ilar.42.4.285. [DOI] [PubMed] [Google Scholar]
- 152.Elger M., Hentschel H., Litteral J., Wellner M., Kirsch T., Luft F. C., Haller H. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J. Am. Soc. Nephrol. 2003;14:1506–1518. doi: 10.1097/01.asn.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- 153.Diep C. Q., Ma D., Deo R. C., Holm T. M., Naylor R. W., Arora N., Wingert R. A., Bollig F., Djordjevic G., Lichman B., et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Woolf A. S., Palmer S. J., Snow M. L., Fine L. G. Creation of a functioning chimeric mammalian kidney. Kidney Int. 1990;38:991–997. doi: 10.1038/ki.1990.303. [DOI] [PubMed] [Google Scholar]