Abstract
Elevation of dietary or brain leucine appears to suppress food intake via a mechanism involving mTOR, AMPK and/or branched chain amino acid (BCAA) metabolism. Mice bearing a deletion of mitochondrial branched chain amino transferase (BCATm), which is expressed in peripheral tissues (muscle) and brain glia, exhibit marked increases in circulating BCAAs. Here we test whether this increase in circulating BCAAs alters feeding behavior and brain neuropeptide expression. Circulating and brain levels of BCAAs were increased 2-4 fold in BCATm-deficient mice (KO). KO mice weighed less than controls (25.9 vs. 20.4g, P < 0.01), but absolute food intake was relatively unchanged. In contrast to wildtype mice, KO mice preferred a low BCAA diet to a control diet (P < 0.05), but exhibited no change in preference for low vs. high protein diets. KO mice also exhibited low leptin levels and increased hypothalamic NPY and AgRP mRNA. Normalization of circulating leptin levels had no effect on either food preference or the increased NPY and AgRP mRNA expression. If BCAAs act as signals of protein status, one would expect reduced food intake, an avoidance of dietary protein, and a reduction in neuropeptide expression in BCATm-KO mice. Instead, these mice exhibit increased expression of orexigenic neuropeptides and an avoidance of BCAAs but not high protein. These data thus suggest either that BCAAs do not act as physiological signals of protein status, or that the loss of BCAA metabolism within brain glia impairs the detection of protein balance.
Keywords: protein intake, amino acid metabolism, hypothalamus, leucine
Introduction
An adequate supply of protein is necessary for life, and a number of observations support the existence of regulatory systems which assess endogenous protein/amino acid demand and alter food intake to meet this demand (Anderson and Moore 2004; Du, et al. 2000; Hannah, et al. 1990; Jean, et al. 2001; Lacroix, et al. 2004; Peters and Harper 1984; Tome 2004; Westerterp-Plantenga 2003; White, et al. 1994). This regulatory drive to consume adequate protein can be extremely powerful, resulting in protein intake being regulated at a higher priority than energy intake (Brooks, et al. 2010; Sorensen, et al. 2008). Yet to date very little is known about the mechanisms underlying “protein balance” and its relationship to energy balance.
To achieve this regulation it seems likely that the brain responds to variations in circulating signals that reflect protein balance. To date some evidence supports alterations in circulating hormones (Batterham, et al. 2006), while other work suggests that individual amino acids may act locally within the brain (Panksepp and Booth 1971; Sandoval, et al. 2008). For instance, the branched-chain amino acid (BCAA) leucine suppresses food intake when locally administered into the brain (Blouet, et al. 2009; Cota, et al. 2006; Morrison, et al. 2007; Ropelle, et al. 2008), and supplementing the diet with excess leucine or BCAAs also suppresses food intake (Newgard, et al. 2009; Ropelle et al. 2008). Together, these data suggest that BCAA, and particularly leucine, may be uniquely suited to serve as signals of protein balance.
To date the majority of experiments have focused on mammalian target of rapamycin (mTOR) as a probable mediator of brain leucine signaling (Cota et al. 2006; Morrison et al. 2007; Ropelle et al. 2008). However, Blouet and colleagues (Blouet et al. 2009) demonstrated that local injection of downstream products of branched-chain amino acid metabolism (alpha-ketoisocaproic acid or alpha-ketoisovaleric acid), or pharmacologic manipulation of BCAA metabolism, also altered food intake. As such, it is possible that BCAA metabolism may also produce signals relevant to food intake or protein detection.
To test the effect of altered BCAA metabolism on feeding behavior and hypothalamic function, we focused on mice bearing a whole body deletion of the mitochondrial form of branched-chain amino-transferase (BCATm) (She, et al. 2007). Because these mice exhibit marked increases in circulating BCAAs, they provide a novel model system in which to test whether elevated circulating BCAAs represent a signal of protein excess. We hypothesized that BCATm KO would exhibit a phenotype consistent with excess protein, including reduced food intake, reduced orexigenic neuropeptides gene expression and an avoidance of high protein diets.
Materials and Methods
Animals
BCATm-deficient mice (KO mice) were developed as previously described by She and colleagues (She et al. 2007), who also described the general phenotype of elevated circulating BCAAs, leanness and increased oxygen consumption. All studies used male KO mice or wildtype littermates generated at Virginia Tech (Experiment 1) or from breeders subsequently sent to Pennington Biomedical Research Center (Experiments 2 and 3). Mice were group housed (2-4/cage) in shoebox cages under 12:12 light/dark cycle and were fed standard chow ad libitum unless otherwise noted. All experiments were approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center.
Experiment 1: Brain Amino Acids And Orexigenic Neuropeptides In Ko Mice
To identify baseline differences in plasma and brain amino acids and hypothalamic neuropeptide expression, 20 week old male homozygous BCATm-deficient mice (KO; n = 5) or wildtype littermates (WT; n=10) were rapidly sacrificed, trunk blood collected and brains isolated. Samples of mediobasal hypothalamus containing principally arcuate nucleus were isolated for RNA extraction, while forebrains were collected to assess brain amino acid concentrations via HPLC (see below).
Experiment 2: Effects of leptin replacement on feeding behavior, body adiposity and hypothalamic neuropeptides in KO mice
To determine whether normalization of circulating leptin levels would normalize hypothalamic neuropeptides and food intake, 8-week old male BCATm KO mice and wildtype littermates were adapted to a two choice diet paradigm in which mice were allowed to self-select between a control diet, and a diet containing BCAAs at only 2.5% of the control (BCAA Low). Mice were group-housed and acclimated to the 2-choice paradigm for 2 weeks, and were then single housed prior to implantation with subcutaneous osmotic mini-pumps (Alzet) delivering either PBS, 3 ug/day leptin, or 10 ug/day leptin (n=9-10/group) (White, et al. 2009). Leptin was procured from the National Hormone Pituitary Program (Dr. A.F. Parlow). Food intake and body weight were measured daily, and after 6 days of infusion mice were rapidly sacrificed, retroperitoneal and epididymal fat pads collected and weighed, and hypothalami isolated for RNA extraction.
To test whether KO mice also exhibited an altered preference for low vs. high protein diet, wildtype (n=9) and KO mice (n=13) were offered a choice between isocaloric diets providing casein protein at 10% of energy (low protein) or 35% of energy (high protein; Table 1). Food intake was measured daily for 10 days, but mice were otherwise not manipulated.
Table 1. Experimental Diets.
| Ingredient (gram) | Control | BCAA Low | Low Protein | High Protein |
|---|---|---|---|---|
| Casein | 0 | 0 | 100 | 350 |
| Corn Starch | 300 | 300 | 440.3 | 291.7 |
| Maltodextrin | 125 | 125 | 150 | 75 |
| Sucrose | 250 | 250 | 107.077 | 107.077 |
| Cellulose | 50 | 50 | 50 | 50 |
| Soybean Oil | 50 | 50 | 25 | 25 |
| Lard | 0 | 0 | 75 | 75 |
| Mineral Mix S10001 | 35 | 35 | 0 | 0 |
| Mineral Mix S10022C | 0 | 0 | 3.5 | 3.5 |
| Sodium Bicarbonate | 7.5 | 7.5 | 0 | 0 |
| Vitamin Mix | 10 | 10 | 10 | 10 |
| Choline Bitrartrate | 2 | 2 | 2.5 | 0 |
| Diammonium citrate | 0 | 24 | 0 | 0 |
| Calcium Carbonate | 0 | 0 | 10 | 12.38 |
| Potassium Citrate | 0 | 0 | 2.48 | 6.58 |
| Potassium Phosphate | 0 | 0 | 6.86 | 1.6 |
| Sodium Chloride | 0 | 0 | 2.59 | 2.59 |
| L-Arginine | 8.27 | 8.27 | ||
| L-Histidine-HCl-H2O | 6 | 6 | ||
| L-Isoleucine | 8 | 0.205 | ||
| L-Leucine | 12 | 0.278 | ||
| L-Lysine-HCl | 14 | 14 | ||
| DL-Methionine | 6 | 6 | ||
| L-Phenylalanine | 8 | 8 | ||
| L-Threonine | 8 | 8 | ||
| L-Tryptophan | 2 | 2 | ||
| L-Valine | 8 | 0.205 | ||
| L-Alanine | 10 | 10 | ||
| L-Asparagine-H2O | 10 | 10 | ||
| L-Cystine | 4 | 4 | 1.5 | 5.25 |
| L-Glutamic Acid | 10 | 10 | ||
| L-Glutamine | 10 | 10 | ||
| Glycine | 10 | 10 | ||
| L-Proline | 10 | 10 | ||
| L-Serine | 10 | 10 | ||
| L-Tyrosine | 4 | 4 | ||
| Total | 987.8 | 984.5 | 990 | 1018 |
| kcal/gm | 3.87 | 3.77 | 4.13 | 4.02 |
Experiment 3: Effects of acute, high-dose leptin treatment on hypothalamic neuropeptides in control and BCATm KO mice
To determine if KO mice were unresponsive to leptin-dependent regulation of mRNA expression, BCATm-KO mice and wildtype littermates received IP injections of leptin in the fed or fasted state (n=8 per group). Mice received an IP injection of either leptin (5ug/g body weight) or saline, at the start of the fast (Time 0), and then after 12 and 24hrs. 4 hours after the final injection, mice were sacrificed and hypothalami isolated for RNA extraction.
Amino Acid Analysis
Brain and plasma amino acids were measured using fluorometric HPLC via methods described previously (Wu and Knabe 1994), except that a methanol extraction was used for brain tissue rather than acid precipitation. Separation of the o-phthaldialdehyde amino acid derivatives was made by gradient elution from a Supelcosil LC-18 column (15cm × 4.6 mm, 3μm, Sigma).
Hormone Analysis
Trunk blood was collected at sacrifice and allowed to clot at 4°C overnight, centrifuged at 3000xg for 30 minutes, and serum collected and stored at −80°C. Serum levels of leptin were measured via radioimmunoassay kit (Millipore, St. Charles, MO), according to the manufacturer’s instructions.
RNA extraction and real-time PCR
RNA extraction and real-time PCR was conducted as described previously (Morrison et al. 2007; White, et al. 2010). Total RNA was extracted from mediobasal hypothalamus using Tri-Reagent (Molecular Research Center, Cincinnati, OH), according to the manufacturer’s instructions. Confirmation of intact 18S and 28S RNA bands was achieved by ethidium bromide staining after electrophoresis of samples in 1% agarose gels. Samples were quantified by spectrophotometry then mRNA was reversed transcribed into cDNA and mRNA expression determined using the SYBR green methodology in optical 384-well plates in an ABI PRISM 7900 sequence detector (Applied Biosystems, Branchburg, NJ). All expression data were normalized to cyclophilin mRNA levels.
Statistical Analysis
Data were analyzed using the SAS software package (SAS V9, SAS Institute, Cary NC) using a two-tailed t-test or ANOVA using the general linear model (GLM) procedure. When experiment wide tests were significant, post-hoc comparisons were made using the LSMEANS statement with the PDIFF option, and thus represent least significant differences tests for pre-planned comparisons. For real-time PCR, expression levels were normalized to cyclophilin prior to analysis. All data are expressed as mean ± SEM, with a probability value of 0.05 considered statistically significant.
Results
Experiment 1
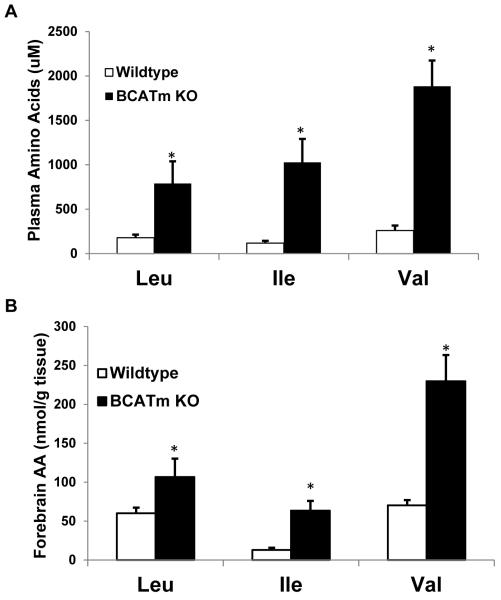
Impact of BCATm deletion on brain amino acids and orexigenic neuropeptides. Consistent with previous experiments (She et al. 2007), BCATm KO mice exhibited a marked increase in circulating BCAA levels (P < 0.01; Figure 1A), with plasma leucine, isoleucine, and valine concentrations increased 4, 8 and 7 fold, respectively. Similarly, brain BCAA levels were increased by 2, 5 and 3 fold, respectively (P < 0.01; Figure 1B). This ratio of blood to brain levels of BCAAs is consistent with the kinetic properties of the system L amino acid transporters (LAT1) expressed in tissues forming the blood brain barrier (Kanai, et al. 1998). The elevated BCAA concentrations had a variable effect on brain levels of non-BCAAs (Table 2). For example, Glu and Gln concentrations were statistically higher in the BCATm KOs compared to wildtype controls and GABA was unchanged. Amino acids that are precursors of the monoamine neurotransmitters and/or substrates for LAT1 were either unchanged (Phe, Trp, Met) or higher (Tyr). These data do not support the possibility that elevations in BCAAs interfered with brain uptake of other large, neutral amino acids (Tews, et al. 1979).
Figure 1. Plasma and brain BCAAs in KO and wildtype mice.
Plasma (A) and brain (B) BCAAs were measured in BCATm KO mice (n=5) and wildtype littermates (n=10; 20 wks of age) using fluorometric HPLC methods. For brain measures, forebrains were utilized, as hypothalami were used to assess neuropeptide expression. Levels of brain and plasma BCAA were elevated in KO relative to control mice. *P<0.05 compared to wildtype.
Table 2. Forebrain Amino Acids (uM) in BCATm KO mice compared to wildtype littermates.
| Amino Acid | WildType | BCATm KO | p |
|---|---|---|---|
| TYR | 37 ± 9 | 127 ± 12 | 0.001* |
| ORN | 314 ± 29 | 460 ± 1 | 0.001* |
| GLN | 4116 ± 497 | 6388 ± 595 | 0.002* |
| ASN | 144 ± 8 | 187 ± 14 | 0.01* |
| THR | 452 ± 54 | 601 ± 73 | 0.02* |
| ALA | 823 ± 193 | 724 ± 31 | 0.02* |
| LYS | 81 ± 5 | 99 ± 12 | 0.02* |
| GLU | 9509 ± 603 | 10532 ± 563 | 0.03* |
| SER | 840 ± 50 | 916 ± 61 | 0.03* |
| ARG | 287 ± 29 | 365 ± 32 | 0.07 |
| ASP | 1993 ± 214 | 2264 ± 199 | 0.13 |
| MET | 75 ± 6 | 71 ± 18 | 0.21 |
| TRP | 22 ± 4.8 | 14 ± 6.6 | 0.22 |
| CIT | 16 ± 1.1 | 22 ± 1.5 | 0.24 |
| GLY | 983 ± 95 | 1178 ± 83 | 0.5 |
| TAU | 9357 ± 225 | 9125 ± 125 | 0.67 |
| PHE | 51 ± 3.1 | 50 ± 11 | 0.93 |
| GABA | 2859 ± 280 | 3020 ± 126 | 0.97 |
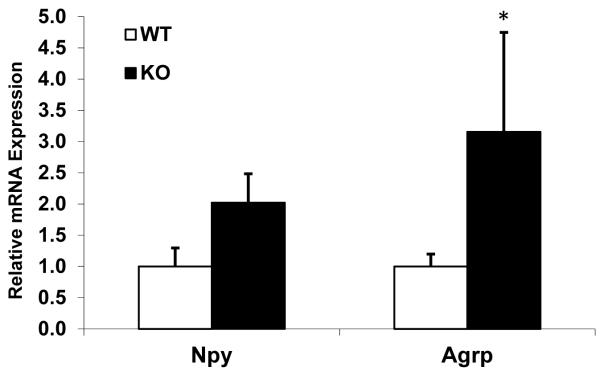
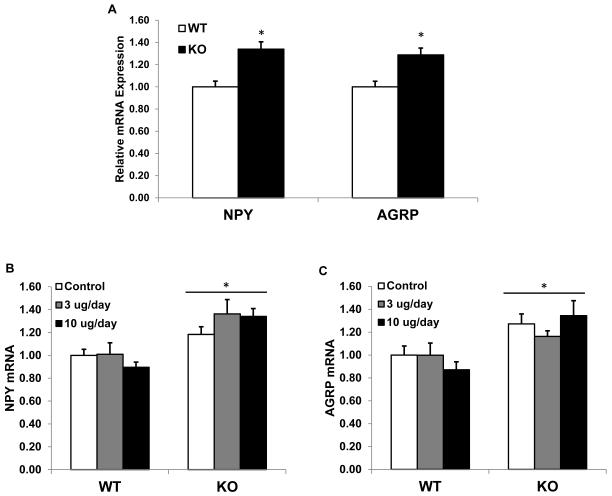
Agrp mRNA levels were significantly increased within BCATm-deficient mice as compared to controls (P < 0.05; Figure 2). NPY mRNA levels were roughly doubled, but this difference did not reach statistical significance (P = 0.14). POMC mRNA expression was also measured, but the results were inconsistent between experiments and as such inconclusive (data not shown). Interestingly, the changes in hypothalamic NPY and AgRP mRNA expression are consistent with the profile of animals on low protein diets (Morrison et al. 2007; White et al. 1994).
Figure 2. Hypothalamic NPY and AGRP mRNA expression in KO and wildtype mice.
Total RNA was extracted from hypothalami from 20 week old BCATm KO mice (n=5) and wildtype littermate (n=10), and expression of NPY and AGRP mRNA was measured via real-time PCR. NPY mRNA levels were numerically but not statistically increased, while AGRP mRNA expression was significantly increased in KO mice. *P<0.05 compared to wildtype.
Experiment 2: Role of hypoleptinemia in the phenotype of BCATm deficient mice
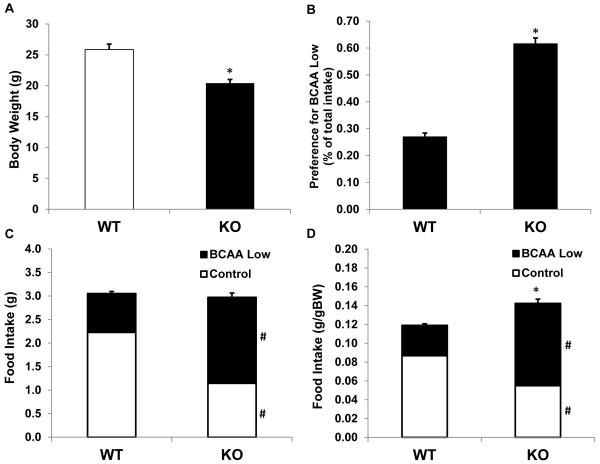
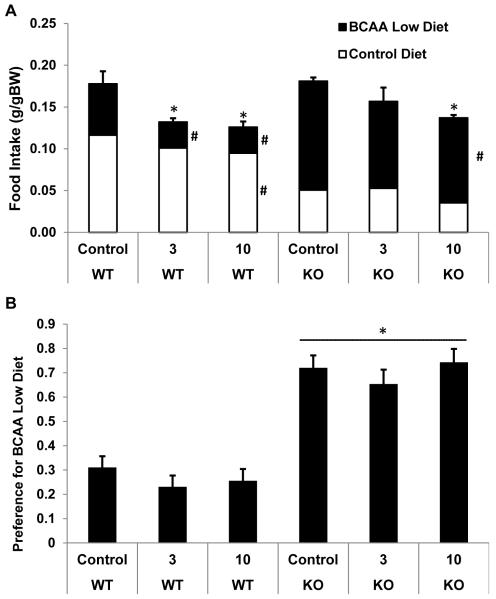
Two weeks prior to minipump implantation, mice were placed on a choice diet paradigm, which offered the choice between a control diet and a diet very low in branched chain amino acids (2.5% BCAAs relative to control; Table 1). KO mice were smaller than wildtype mice (P < 0.001; Figure 3A), and this reduction in body weight was highly consistent with previous work in these mice (She et al. 2007). Baseline food intake (prior to minipump) was not different between KO and wildtype mice on an absolute basis (Figure 3C), but when food intake was adjusted for body weight, KO mice consumed more food than controls (P < 0.001; Figure 3D). While differences in absolute intake were relatively subtle, KO and wildtype mice exhibited marked differences in their preference between the control and low BCAA diet (Figure 3B; P < 0.001). While wildtype mice significantly preferred the control diet, KO mice preferred the diet with very low BCAAs. KO mice therefore appear to avoid dietary BCAAs, presumably in response to their excess endogenous branched chain amino acids.
Figure 3. Body weight, food intake and food preference in BCATm KO mice.
8 week old BCATm KO mice (n=22) and wildtype controls (n=30) were group housed (2-3 per cage) and offered the choice between a control diet and a diet containing only 2.5% of control levels of BCAAs (BCAA-Low). Body weights (A) and per cage food intake (n=9-10 per group) were measured, with food intake being expressed as both total intake (C) and intake adjusted for body weight (D). Food intake is also presented as a preference ratio for the BCAA low diet (BCAA-Low divided by Total intake, B). KO mice were lighter than wildtype mice, and food intake relative to body weight was significantly increased. Most strikingly, KO mice exhibit a marked shift in preference toward the BCAA low diet *P<0.05 compared to wildtype. #P<0.05 for individual diet choices compared to wildtype.
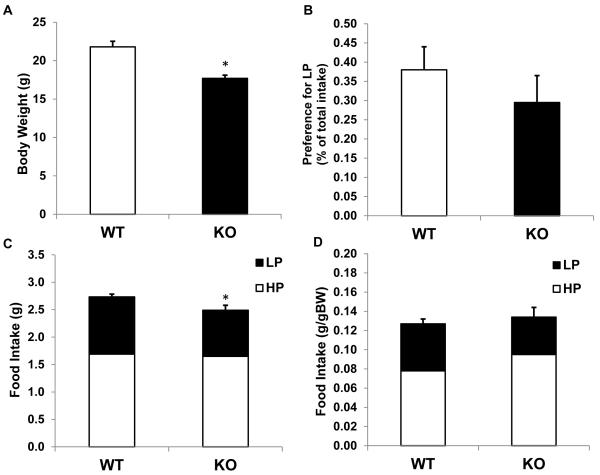
To test whether the elevation of circulating BCAAs represents a signal of excess protein, a separate group of BCATm-deficient mice and control mice were placed on more generic high protein (HP; 35% casein protein, Table 1) and low protein diets (LP; 10% casein protein). BCATm-KO mice exhibited a subtle decrease in absolute food intake compared to controls (Figure 4C) that was accounted for by adjusting to body weight (Figure 4D). Despite their elevation of circulating BCAAs, KO mice did not exhibit altered selection between the LP and HP diets for a LP diet (Figure 4B). Thus the specific avoidance of BCAAs in the KO mice did not translate into a more generalized avoidance of protein.
Figure 4. Food intake and selection between low and high protein diets in BCATm KO mice.
8-week old BCATm KO (n=9) and wildtype littermates (n=13) were single housed and offered a choice between high protein (35% protein energy) and low protein (10% protein energy) diets. Body weight (A), preference ratio for LP diet (B), total food intake (C), body weight adjusted food intake (D) were measured over 10 days. KO mice weighed less and consumed less total food than wildtype mice, but there was no difference between the genotypes regarding preference for low vs high protein diet. *P<0.05 compared to wildtype.
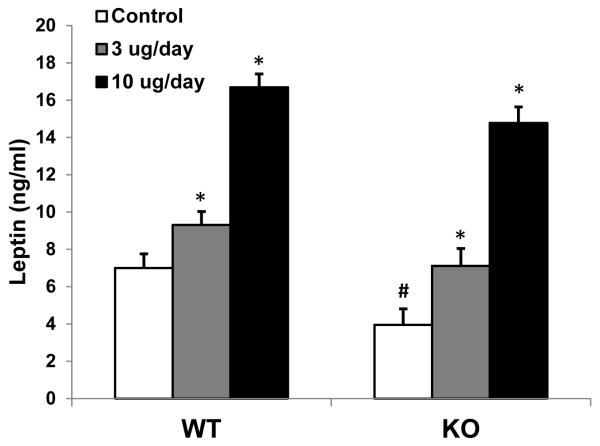
Following this two week dietary acclimation period, male KO mice and their wildtype littermates were implanted with osmotic minipumps designed to a) return plasma leptin levels to normal following administration of leptin at a low dose (3ug/day), and b) increase leptin above normal following administration of leptin at a higher dose (10ug/day). Circulating leptin levels were reduced in KO mice (P < 0.05; Figure 5), although the difference was not as large as previously reported (She et al. 2007). Infusion of 3ug/day of leptin normalized circulating leptin to physiological levels in KO mice, and produced a small but significant increase in the wildtype mice. Contrastingly, the higher 10ug/day dose produced a marked increase in both KO and wildtype mice. Leptin treatment had little effect on body weight gain over the 6 days of infusion, as only KO mice receiving the highest dose exhibited a subtle decrease in weight (P = 0.054; data no shown). Leptin significantly suppressed total daily food intake in both genotypes (P < 0.05; Figure 6A), such that there was a significant main effect of leptin treatment (P < 0.001), but no significant treatment by genotype interaction (P = 0.53). Although it suppressed total intake, leptin treatment had no effect on the preference ratio between the Control and BCAA-low diet in either genotype (Figure 6B), such that KO mice continued to exhibit a marked shift in preference toward the low BCAA diet despite normalization of circulating leptin.
Figure 5. Leptin replacement in BCATm KO mice.
10 week old BCATm KO or wildtype mice were implanted with subcutaneous osmotic minipumps delivering saline, 3ug/day leptin, or 10ug/day leptin (9-10 mice per group). Infusion lasted for 6 days, and at sacrifice trunk blood leptin levels were measured via RIA. Infusion of serum leptin increased leptin at both doses in both genotypes. BCATm KO mice receiving saline exhibited reduced circulating leptin levels, and infusion of the low dose served to fully normalize leptin in these KO animals. *P<0.05 compared to respective saline infused group. #P < 0.05 for WT-Control vs KO-Control.
Figure 6. Food intake and food preference in BCATm KO mice infused with leptin.
BCATm KO or wildtype mice were implanted with subcutaneous osmotic minipumps delivering saline, 3ug/d leptin, or 10ug/d leptin. Mice were offered the choice between a control diet and a diet containing only 2.5% of control levels of BCAAs (BCAA-Low). Food intake normalized to body weight and preference ratio for the BCAA low diet were measured over 6 days of leptin infusion. A) Leptin infusion reduced food intake in both genotypes, such that there was an overall treatment effect, but no genotype effect or treatment*genotype interaction. *P < 0.05 compared to respective saline infused group. #P < 0.05 for individual diet choices compared to saline control. B) KO mice exhibited a marked shift in preference toward the BCAA-low diet, and this shift was unaffected by leptin treatment. *P < 0.05 compared to WT-Control.
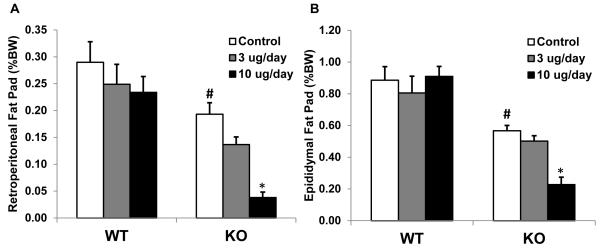
KO mice were lean relative to wildtype controls, exhibiting a significant lower retroperitoneal and epididymal fat pad weights (P < 0.01; Figure 7). While leptin had little effect on adiposity in control mice, the KO mice exhibited significant decreases in body fat, particularly at the high dose (P < 0.05; Figure 7).
Figure 7. Fat pad weights in leptin-treated wildtype and BCATm KO mice.
Retroperitoneal (A) and Epididymal (B) fat pads were collected and weighed at sacrifice. Fat pads in BCATm KO mice were reduced compared to wildtype, while leptin infusion further reduced fat pad weight in KO mice but not wildtype mice. *P<0.05 compared to respective saline infused group; #P < 0.05 for WT-Control vs. KO-Control.
The primary goal of Experiment 2 was to determine whether normalization of circulating leptin levels would reduce hypothalamic NPY and AGRP mRNA expression in BCATm KO mice. Similar to the results from Experiment 1, a significant increase in NPY and AGRP levels was detected within KO mice (P < 0.01; Figure 8A). However, increasing circulating leptin had no effect on either NPY (Figure 8B) or AgRP (Figure 8C) mRNA expression within KO mice. These data suggest the increase in NPY and AGRP mRNA levels in BCATm KO mice cannot be explained by reduced circulating leptin.
Figure 8. Hypothalamic NPY and AGRP gene expression in BCATm KO mice infused with leptin.
Following 6 days of subcutaneous leptin infusion, hypothalami were removed, total RNA extracted, and NPY and AgRP expression measured via real-time PCR. A) Main effect of genotype on mRNA expression, demonstrating that KO mice have increased NPY and AGRP (*P < 0.05). B) NPY expression following leptin treatment. Leptin replacement has no effect on NPY expression. *P < 0.05 compared to WT-Control. C) AGRP expression following leptin treatment. Leptin replacement has no effect on AGRP expression. *P < 0.05 compared to WT-Control.
Experiment 3: Effects of acute, high-dose leptin treatment on hypothalamic neuropeptides in control and BCATm KO mice
It is possible that hypothalamic NPY/AGRP neurons are rendered resistant to the effects of leptin in BCATm KO mice, and that this loss of sensitivity explains the lack of effect of leptin on NPY and AGRP mRNA levels in these mice. To assess this possibility, a separate group of BCATm KO mice were treated with acute doses of leptin every 12 hours during a 28-hour fast, which is a paradigm more typically used to assess the effects of leptin on hypothalamic gene expression (Baskin, et al. 1999; Korner, et al. 2001; Mizuno and Mobbs 1999; Morrison, et al. 2005). While leptin had no effect on either NPY or AGRP in the fed state, fasting increased both NPY and AgRP mRNA expression (P < 0.05; Figure 9), and injection with leptin blocked this fasting induced increase.
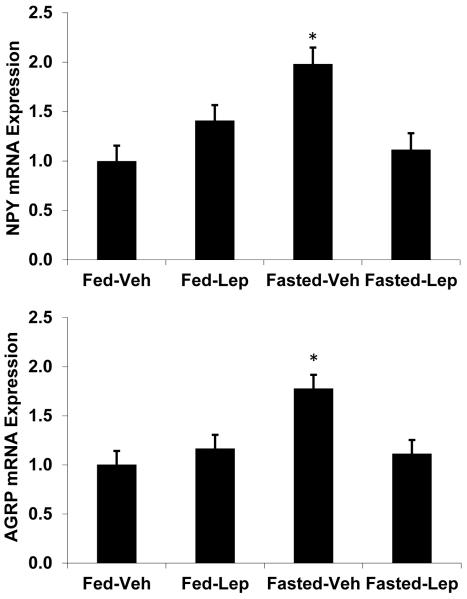
Figure 9. Effect of acute, high dose leptin on NPY and AGRP mRNA.
BCATm KO mice were fed or fasted for 28 hours, and received IP injections of either leptin (5ug/g body weight) or saline at the start of the fast (Time 0), and then after 12 and 24hrs (8 mice per group). 4 hours after the final injection, mice were sacrificed and hypothalami isolated to assess NPY and AGRP expression via real-time PCR. As expected, fasting significantly increased both NPY (A) and AGRP (B). In both cases, leptin treatment blocked the fasting induced increase in neuropeptide expression. *P<0.05 compared Fed-Vehicle
Discussion
Nutrients such as glucose, fatty acids and amino acids can act locally within the brain to regulate neuronal function, food intake and body weight (Blouet and Schwartz 2010; Moran 2010; Sandoval et al. 2008). Dietary protein content or physiological protein demand influences food intake and/or food selection (Anderson and Moore 2004; Tome 2004; Westerterp-Plantenga 2003; White et al. 1994), as well as energy expenditure (Smeets, et al. 2008; Westerterp-Plantenga, et al. 2009; Zhang, et al. 2007). Collectively, these observations suggest that regulatory systems detect variations in protein balance and regulate food intake, food selection and metabolism accordingly (Brooks et al. 2010; Sorensen et al. 2008). Significant emphasis has recently been placed on the branched chain amino acid (BCAA) leucine, based on its ability to suppress food intake when administered directly into the brain or following supplementation in the diet (Blouet et al. 2009; Cota et al. 2006; Morrison et al. 2007; Newgard et al. 2009; Ropelle et al. 2008). Yet some uncertainty exists as to the physiological relevance and specific signaling mechanism(s) underlying brain leucine signaling. Several lines of evidence indicate that mTOR signaling is an important mediator, as central leucine activates mTOR, and rapamycin pretreatment blocks the effects of leucine (Blouet et al. 2009; Cota et al. 2006; Ropelle et al. 2008). However, administration of a downstream product of BCAA metabolism or pharmacological activation of BCAA oxidation was also shown to reduce food intake (Blouet et al. 2009), suggesting that the metabolism of BCAAs might generate regulatory signals.
Considering this evidence, we utilized BCATm-deficient mice as a unique model in which circulating BCAAs are markedly elevated. Branched-chain aminotransferase serves as the initial step in the metabolism of BCAAs. Two individual isozymes of BCAT exist: BCATm, which is expressed in most peripheral tissues and brain glia, and BCATc, which is expressed almost exclusively within neurons (Hutson, et al. 1992; Sweatt, et al. 2004a; Sweatt, et al. 2004b). Because BCATm-deficient mice are unable to metabolize branched-chain amino acids within peripheral tissues, particularly muscle, they exhibit a marked increase in circulating BCAAs (She et al. 2007). Based on previous data (Blouet et al. 2009; Cota et al. 2006; Morrison et al. 2007; Ropelle et al. 2008), one would anticipate that this elevation of circulating BCAAs would signal protein excess, resulting in a decrease in food intake and avoidance of dietary protein. In addition, because our previous work indicates that animals on a low protein diet exhibit hyperphagia and increased NPY and AgRP mRNA expression (Morrison et al. 2007; White et al. 1994), our predication was that the elevated levels of BCAAs within BCATm KO mice would act to suppress food intake and NPY and AgRP mRNA expression. Yet the results are inconsistent with this hypothesis, as BCATm mice do not show a clear suppression of food intake, and NPY and AGRP mRNA expression was actually increased in these mice. It should be noted that while AgRP mRNA expression was increased in both experiments, NPY was only significantly increased in Experiment 2. This lack of statistical significance in Experiment 1 is likely due to the low animal numbers and reduced power in this initial experiment, because the pattern of expression was similar. Lastly, we also measured POMC expression in these samples, but the results were highly inconsistent across experiments. As such the role of POMC in contributing to the phenotype in these mice remains unclear.
At least two possible explanations exist for observed changes in hypothalamic neuropeptide expression: The first possibility is that BCAAs have no effect on hypothalamic neuropeptides in vivo, and that the increases in NPY and AGRP are driven by some secondary effect (i.e., reduced circulating leptin, see below). The second possibility is that, while neuronal BCAA metabolism is intact, the loss of glia BCAA metabolism in some fashion impairs brain amino acid sensing, resulting in the increase in NPY and AGRP despite the increase in BCAAs. Considering that the observed increases in NPY and AgRP are fully consistent with the changes that we observe in animals on a low protein diet, and previous in vivo and in vitro experimentation suggesting that amino acids may regulate these neuropeptides, we propose that altered glial BCAA metabolism contributes at least partly to the altered neuropeptide expression in these mice. However, early work in BCATm KO indicated very low levels of circulating leptin (She et al. 2007), which could also contribute to the observed increases in NPY and AGRP. We therefore wanted to rule out the possibility that low leptin levels contributes to the phenotype of these mice. To test this question, we chronically infused BCATm KO mice and control littermates with two doses of leptin, resulting in a physiological normalization of circulating leptin levels in the low dose, and a physiological increase with the high dose.
Normalization of circulating leptin had several affects in the KO mice, including reduced food intake and body adiposity. Interestingly, the KO mice exhibited a much larger reduction in body adiposity in response to leptin as compared to wildtype mice. Since the KO mice are lean with reduced circulating leptin, it would be logical to speculate that they are more sensitive to exogenous leptin. However, leptin produced a relatively similar decrease in food intake in both genotypes. As such, it is unclear why KO mice responded to leptin with a larger reduction in adiposity, and this difference could be due to direct effects of elevated BCAAs on the adipocyte or to the metabolic consequences of increased BCAAs or deletion of BCATm. Regardless, these data clearly indicate that the doses of leptin infused into the KO mice were physiologically relevant.
While the KO mice clearly responded to leptin, neither dose of leptin had any effect to reduce NPY or AGRP expression in these mice. To confirm that leptin is capable of reducing NPY and AGRP in these mice, we injected a very high dose leptin into fed and fasted BCATm KO mice. At these pharmacological doses, leptin blocked the fasting induced increase in AGRP and NPY. Thus, we conclude that BCATm KO mice are capable of responding to leptin, but that low leptin levels are not responsible for the observed increase in NPY and AGRP expression. Instead, these data are more consistent with innate alterations in brain BCAA sensing or metabolism as contributing to the alterations in hypothalamic neuropeptide gene expression.
While BCATm KO mice exhibit leptin-independent increases in NPY and AGRP, absolute food intake in these mice was only modestly altered. Previous work in these mice demonstrated that KO mice were smaller and leaner than controls, but that they consumed more food when that intake was normalized to body weight (She et al. 2007). In our hands, BCATm KO mice are consistently smaller and leaner than controls, and initial work in Experiment 2 also indicated that they consumed similar amounts of food on an absolute basis and thus more food on a per gram body weight basis. However, subsequent experiments did not detect this difference, with KO mice showing a slight decrease in absolute food intake, but no change when intake was normalized to their reduced body weight. Taken together, we conclude that absolute food intake is relatively unperturbed in these mice, despite the marked elevated BCAAs. Thus these data suggest either that increased BCAAs have no effect on food intake, or that some other defect (loss of glial BCAA metabolism) attenuates the response to elevated BCAAs.
In contrast to their relatively normal absolute food intake, BCATm KO mice exhibit a specific change in their preference for BCAAs. When given the choice between a control diet and a diet that was practically devoid of BCAAs, the BCAA-low diet constituted only 27% of the wildtype intake, but 62% the KO intake. Thus the KO selected significantly more of the BCAA-low diet, suggesting that the BCATm KO mice detect their abnormally high BCAAs and avoid consuming these amino acids. Surprisingly, this altered preference did not translate protein in general. Thus despite the evidence that leucine or BCAAs may signal excess protein, the KO mice chose normal amounts of protein when given the choice between low or high in protein. Because the BCAA-low and control diets were isocaloric and contained normal amounts of all amino acids besides BCAAs, BCATm mice had the luxury of avoiding BCAAs without negatively impacting energy or protein intake. However, when placed on the standard LP and HP diets, avoiding BCAAs would result in a marked reduction in overall protein intake. Taken together, these data suggest that an elevation of BCAAs does not represent an overall signal of excess protein (Anderson, et al. 1990), and instead suggest that the altered preference was limited to the three BCAAs. Although further work is required to define why KO mice avoid BCAAs, these data are consistent with previous work demonstrating that neuronal systems detect imbalances in dietary and/or circulating amino acids and alter food intake and selection to counteract these imbalances (Fromentin and Nicolaidis 1996; Gietzen and Rogers 2006; Rudell, et al. 2011). In addition, this shift in preference was insensitive to leptin treatment, suggesting that the regulation of protein intake and selection is regulated separately from energy intake.
Lastly, these data also highlight the possibility that glia may contribute to amino acid detection within the brain. Astrocytes are classically associated with neuronal support, functioning to maintain the extracellular environment by both regulating nutrient and metabolite balance and contributing to the uptake and break-down of secreted neuropeptides (Volterra and Meldolesi 2005). Yet recent evidence indicates that astrocytes may also act in a regulatory role by detecting and secreting various signaling molecules, influencing the activity of nearby neurons, and having direct effects on synaptic plasticity (Araque, et al. 1999; Haydon and Carmignoto 2006; Hermann, et al. 2009; Newman 2003). In particular, studies of brain glucose sensing have implicated astrocytes as a functional mediator of glucose detection (Guillod-Maximin, et al. 2004; Marty, et al. 2005; Young, et al. 2000), while BCAA metabolism in astrocytes contributes to the glutamate-glutamine cycle and neuronal glutamate turnover (Lieth, et al. 2001; Yudkoff 1997). Because BCATm KO mice exhibit elevated circulating BCAAs but intact neuronal BCAA metabolism, we anticipated a phenotype more consistent with excess protein availability (i.e. reduced food intake and orexigenic neuropeptide expression). Instead, BCATm KO mice exhibit a phenotype that is more representative of animals on low protein diets. Therefore, while the current data do not provide unequivocal proof for the importance of glial BCAA metabolism in amino acid detection, these data together with previous work are consistent with the possibility that impaired glial BCAA metabolism may alter neuronal function and hypothalamic neuropeptide expression, and future experiments utilizing glial and neuronal specific knockouts will be required to directly test this hypothesis.
In summary, we have demonstrated that loss of BCAA transamination results in specific changes in diet selection and hypothalamic neuropeptide gene expression. These changes in neuropeptide expression and protein selection are independent of changes in circulating leptin, indicating not only that mechanisms other than leptin drive the increased NPY and AGRP, but also that protein intake and selection may be unrelated to the regulation of energy intake. Lastly, considering that hypothalamic NPY and AgRP expression is increased despite elevations in circulating amino acids, these data raise the possibility that impaired glial BCAA metabolism results in a loss of amino acid sensing within the brain.
Acknowledgments
Funding
This work was supported by NIH Grants R01-DK081563 and P20-RR021945 to CDM, as well as core facilities supported in part by COBRE (NIH P20-RR021945) and NORC (NIH 1P30-DK072476) center grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is not the definitive version of record of this article. This manuscript has been accepted for publication in Journal of Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the Society for Endocrinology accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at http://dx.doi.org/10.1530/JOE-11-0270.R1.
Declarations of Interest
The authors have nothing to disclose.
References
- Anderson GH, Moore SE. Dietary proteins in the regulation of food intake and body weight in humans. J Nutr. 2004;134:974S–979S. doi: 10.1093/jn/134.4.974S. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Tews JK, Harper AE. Dietary branched-chain amino acids and protein selection by rats. J Nutr. 1990;120:52–63. doi: 10.1093/jn/120.1.52. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48:828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Brooks RC, Simpson SJ, Raubenheimer D. The price of protein: combining evolutionary and economic analysis to understand excessive energy consumption. Obes Rev. 2010;11:887–894. doi: 10.1111/j.1467-789X.2010.00733.x. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Du F, Higginbotham DA, White BD. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr. 2000;130:514–521. doi: 10.1093/jn/130.3.514. [DOI] [PubMed] [Google Scholar]
- Fromentin G, Nicolaidis S. Rebalancing essential amino acids intake by self-selection in the rat. Br J Nutr. 1996;75:669–682. doi: 10.1079/bjn19960172. [DOI] [PubMed] [Google Scholar]
- Gietzen DW, Rogers QR. Nutritional homeostasis and indispensable amino acid sensing: a new solution to an old puzzle. Trends Neurosci. 2006;29:91–99. doi: 10.1016/j.tins.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Guillod-Maximin E, Lorsignol A, Alquier T, Penicaud L. Acute intracarotid glucose injection towards the brain induces specific c-fos activation in hypothalamic nuclei: involvement of astrocytes in cerebral glucose-sensing in rats. J Neuroendocrinol. 2004;16:464–471. doi: 10.1111/j.1365-2826.2004.01185.x. [DOI] [PubMed] [Google Scholar]
- Hannah JS, Dubey AK, Hansen BC. Postingestional effects of a high-protein diet on the regulation of food intake in monkeys. Am J Clin Nutr. 1990;52:320–325. doi: 10.1093/ajcn/52.2.320. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Van Meter MJ, Rood JC, Rogers RC. Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach. J Neurosci. 2009;29:9292–9300. doi: 10.1523/JNEUROSCI.6063-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J Biol Chem. 1992;267:15681–15686. [PubMed] [Google Scholar]
- Jean C, Rome S, Mathe V, Huneau JF, Aattouri N, Fromentin G, Achagiotis CL, Tome D. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001;131:91–98. doi: 10.1093/jn/131.1.91. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua SC, Jr., Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Gaudichon C, Martin A, Morens C, Mathe V, Tome D, Huneau JF. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R934–942. doi: 10.1152/ajpregu.00100.2004. [DOI] [PubMed] [Google Scholar]
- Lieth E, LaNoue KF, Berkich DA, Xu B, Ratz M, Taylor C, Hutson SM. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J Neurochem. 2001;76:1712–1723. doi: 10.1046/j.1471-4159.2001.00156.x. [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest. 2005;115:3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- Moran TH. Hypothalamic nutrient sensing and energy balance. Forum Nutr. 2010;63:94–101. doi: 10.1159/000264397. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab. 2005;289:E1051–1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–171. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Booth DA. Decreased feeding after injections of amino-acids into the hypothalamus. Nature. 1971;233:341–342. doi: 10.1038/233341a0. [DOI] [PubMed] [Google Scholar]
- Peters JC, Harper AE. Influence of dietary protein level on protein self-selection and plasma and brain amino acid concentrations. Physiol Behav. 1984;33:783–790. doi: 10.1016/0031-9384(84)90048-9. [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Fernandes MF, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JA, et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes. 2008;57:594–605. doi: 10.2337/db07-0573. [DOI] [PubMed] [Google Scholar]
- Rudell JB, Rechs AJ, Kelman TJ, Ross-Inta CM, Hao S, Gietzen DW. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino Acid, showing independent cortical sensory function. J Neurosci. 2011;31:1583–1590. doi: 10.1523/JNEUROSCI.4934-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol. 2008;70:513–535. doi: 10.1146/annurev.physiol.70.120806.095256. [DOI] [PubMed] [Google Scholar]
- She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in Mice Leads to Increased Energy Expenditure Associated with the Activation of a Futile Protein Turnover Cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland O, Westerterp-Plantenga MS. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr. 2008;138:698–702. doi: 10.1093/jn/138.4.698. [DOI] [PubMed] [Google Scholar]
- Sorensen A, Mayntz D, Raubenheimer D, Simpson SJ. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 2008;16:566–571. doi: 10.1038/oby.2007.58. [DOI] [PubMed] [Google Scholar]
- Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM. Branched-chain amino acids and neurotransmitter metabolism: expression of cytosolic branched-chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol. 2004a;477:360–370. doi: 10.1002/cne.20200. [DOI] [PubMed] [Google Scholar]
- Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab. 2004b;286:E64–76. doi: 10.1152/ajpendo.00276.2003. [DOI] [PubMed] [Google Scholar]
- Tews JK, Kim YW, Harper AE. Induction of threonine imbalance by dispensable amino acids: relation to competition for amino acid transport into brain. J Nutr. 1979;109:304–315. doi: 10.1093/jn/109.2.304. [DOI] [PubMed] [Google Scholar]
- Tome D. Protein, amino acids and the control of food intake. Br J Nutr. 2004;92(Suppl 1):S27–30. doi: 10.1079/bjn20041138. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS. The significance of protein in food intake and body weight regulation. Curr Opin Clin Nutr Metab Care. 2003;6:635–638. doi: 10.1097/00075197-200311000-00005. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- White BD, He B, Dean RG, Martin RJ. Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J Nutr. 1994;124:1152–1160. doi: 10.1093/jn/124.8.1152. [DOI] [PubMed] [Google Scholar]
- White CL, Purpera MN, Ballard K, Morrison CD. Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol Behav. 2010;100:408–416. doi: 10.1016/j.physbeh.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Whittington A, Barnes MJ, Wang Z, Bray GA, Morrison CD. HF diets increase hypothalamic PTP1B and induce leptin resistance through both leptin-dependent and -independent mechanisms. Am J Physiol Endocrinol Metab. 2009;296:E291–299. doi: 10.1152/ajpendo.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Knabe DA. Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr. 1994;124:415–424. doi: 10.1093/jn/124.3.415. [DOI] [PubMed] [Google Scholar]
- Young JK, Baker JH, Montes MI. The brain response to 2-deoxy glucose is blocked by a glial drug. Pharmacol Biochem Behav. 2000;67:233–239. doi: 10.1016/s0091-3057(00)00315-4. [DOI] [PubMed] [Google Scholar]
- Yudkoff M. Brain metabolism of branched-chain amino acids. Glia. 1997;21:92–98. doi: 10.1002/(sici)1098-1136(199709)21:1<92::aid-glia10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]