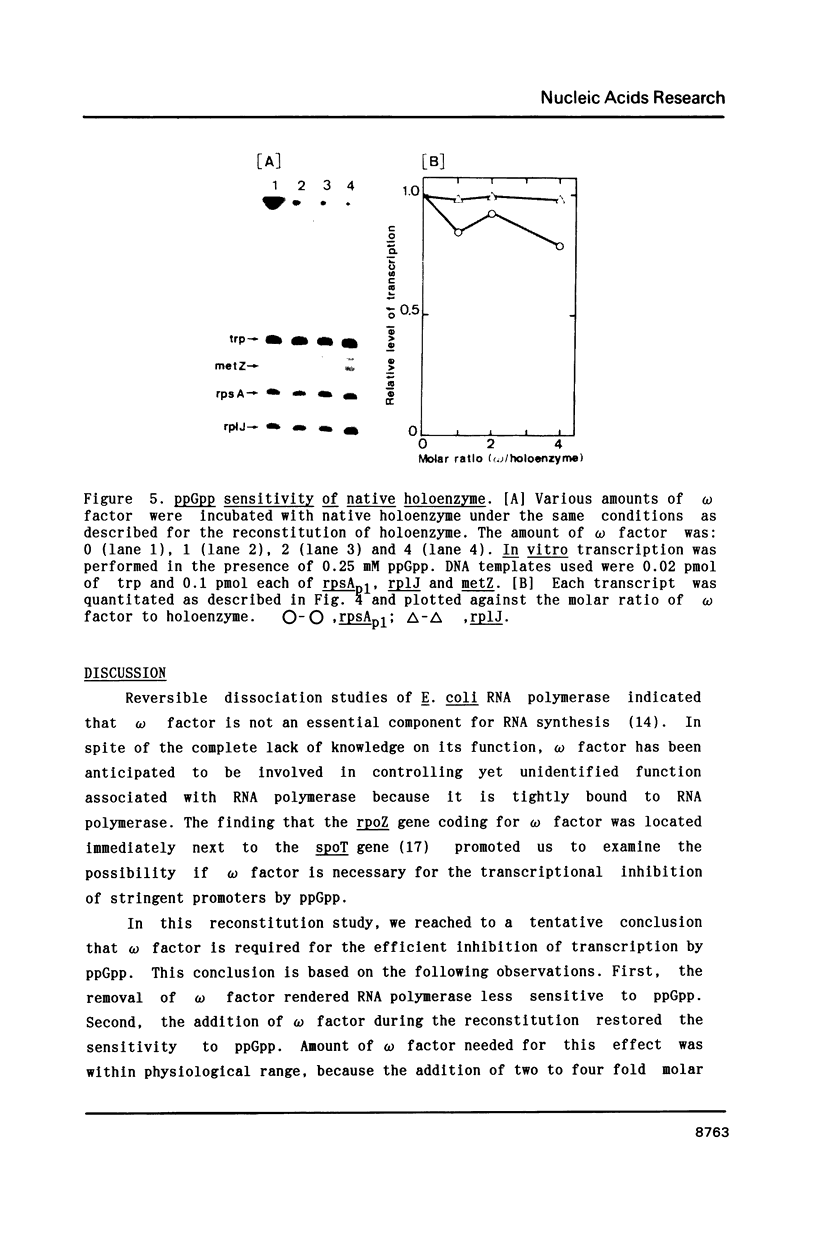
Abstract
Transcription in vitro of stringently controlled Escherichia coli genes by purified RNA polymerase holoenzyme is inhibited by guanosine tetraphosphate (ppGpp). In order to examine possible role of omega factor in this ppGpp sensitivity, RNA polymerases with or without the omega factor were reconstituted and tested for their ppGpp sensitivity using an in vitro mixed transcription system. RNA polymerase lacking the omega factor was found virtually insensitive to ppGpp but the addition of a purified omega factor restored the ppGpp sensitivity of this omega-free RNA polymerase. These results raise a possibility that the omega factor is a regulatory protein of RNA polymerase and is involved in the ppGpp-mediated alteration of the promoter selectivity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- Fukuda R., Iwakura Y., Ishihama A. Heterogeneity of RNA polymerase in Escherichia coli. I. A new holoenzyme containing a new sigma factor. J Mol Biol. 1974 Mar;83(3):353–367. doi: 10.1016/0022-2836(74)90284-8. [DOI] [PubMed] [Google Scholar]
- Gentry D. R., Burgess R. R. The cloning and sequence of the gene encoding the omega subunit of Escherichia coli RNA polymerase. Gene. 1986;48(1):33–40. doi: 10.1016/0378-1119(86)90349-5. [DOI] [PubMed] [Google Scholar]
- Gentry D. R., Burgess R. R. rpoZ, encoding the omega subunit of Escherichia coli RNA polymerase, is in the same operon as spoT. J Bacteriol. 1989 Mar;171(3):1271–1277. doi: 10.1128/jb.171.3.1271-1277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. F., de Boer H. A., Nomura M. Identification of initiation sites for the in vitro transcription of rRNA operons rrnE and rrnA in E. coli. Cell. 1979 May;17(1):211–224. doi: 10.1016/0092-8674(79)90309-x. [DOI] [PubMed] [Google Scholar]
- Glaser G., Sarmientos P., Cashel M. Functional interrelationship between two tandem E. coli ribosomal RNA promoters. Nature. 1983 Mar 3;302(5903):74–76. doi: 10.1038/302074a0. [DOI] [PubMed] [Google Scholar]
- Glass R. E., Jones S. T., Ishihama A. Genetic studies on the beta subunit of Escherichia coli RNA polymerase. VII. RNA polymerase is a target for ppGpp. Mol Gen Genet. 1986 May;203(2):265–268. doi: 10.1007/BF00333964. [DOI] [PubMed] [Google Scholar]
- Gonzalez N., Wiggs J., Chamberlin M. J. A simple procedure for resolution of Escherichia coli RNA polymerase holoenzyme from core polymerase. Arch Biochem Biophys. 1977 Aug;182(2):404–408. doi: 10.1016/0003-9861(77)90521-5. [DOI] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Ishihama A. Promoter selectivity of prokaryotic RNA polymerases. Trends Genet. 1988 Oct;4(10):282–286. doi: 10.1016/0168-9525(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Ishihama A. Subunit of assembly of Escherichia coli RNA polymerase. Adv Biophys. 1981;14:1–35. [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Determination of the promoter strength in the mixed transcription system. II. Promoters of ribosomal RNA, ribosomal protein S1 and recA protein operons from Escherichia coli. Nucleic Acids Res. 1983 Jun 25;11(12):3873–3888. doi: 10.1093/nar/11.12.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Determination of the promoter strength in the mixed transcription system: promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res. 1983 Feb 11;11(3):671–686. doi: 10.1093/nar/11.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem. 1984 Feb 10;259(3):1951–1957. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- Mizushima-Sugano J., Miyajima A., Kaziro Y. Selective inhibition of transcription of the E. coli tufB operon by guanosine-5'-diphosphate-3'-diphosphate. Mol Gen Genet. 1983;189(2):185–192. doi: 10.1007/BF00337802. [DOI] [PubMed] [Google Scholar]
- Nagase T., Ishii S., Imamoto F. Differential transcriptional control of the two tRNA(fMet) genes of Escherichia coli K-12. Gene. 1988 Jul 15;67(1):49–57. doi: 10.1016/0378-1119(88)90007-8. [DOI] [PubMed] [Google Scholar]
- Nene V., Glass R. E. Relaxed mutants of Escherichia coli RNA polymerase. FEBS Lett. 1983 Mar 21;153(2):307–310. doi: 10.1016/0014-5793(83)80630-9. [DOI] [PubMed] [Google Scholar]
- Oostra B. A., van Ooyen A. J., Gruber M. In vitro transcription of three different ribosomal RNA cistrons of E. coli; heterogeneity of control regions. Mol Gen Genet. 1977 Mar 28;152(1):1–6. doi: 10.1007/BF00264932. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Skouv J., Kajitani M., Ishihama A. Transcriptional organization of the rpsA operon of Escherichia coli. Mol Gen Genet. 1984;196(1):135–140. doi: 10.1007/BF00334105. [DOI] [PubMed] [Google Scholar]
- Tacon W., Carey N., Emtage S. The construction and characterisation of plasmid vectors suitable for the expression of all DNA phases under the control of the E. coli tryptophan promoter. Mol Gen Genet. 1980 Feb;177(3):427–438. doi: 10.1007/BF00271481. [DOI] [PubMed] [Google Scholar]
- Travers A. Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet. 1976 Aug 19;147(2):225–232. doi: 10.1007/BF00267575. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Gruber M., Jorgensen P. The mechanism of action of ppGpp on rRNA synthesis in vitro. Cell. 1976 May;8(1):123–128. doi: 10.1016/0092-8674(76)90193-8. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., de Boer H. A., Ab G., Gruber M. Specific inhibition of ribosomal RNA synthesis in vitro by guanosine 3' diphosphate, 5' diphosphate. Nature. 1975 Apr 10;254(5500):530–531. doi: 10.1038/254530a0. [DOI] [PubMed] [Google Scholar]