Abstract
The human BCR gene on chromosome 22 is specifically involved in the Philadelphia translocation, t(9;22), a chromosomal rearrangement present in the leukemic cells of patients with chronic myeloid leukemia or acute lymphoblastic leukemia. In most cases, the breakpoints on chromosome 22 are found within a 5.8 kb region of DNA designated the major breakpoint cluster region (Mbcr) of the BCR gene. Hybridization experiments have indicated that the human genome contains BCR gene-related sequences. Here we report the molecular cloning of one of these loci, for which we propose the name ABR. In contrast with the other BCR-related genes studied to date, ABR represents a functionally active gene and contains exons very similar to those found within the Mbcr. Unlike the BCR gene, the ABR gene exhibits great genomic variability caused by two different variable tandem repeat regions located in two introns. All other BCR gene-related sequences isolated so far and the BCR gene itself are located on chromosome 22. In contrast, the ABR gene is located on chromosome 17p.
Full text
PDF

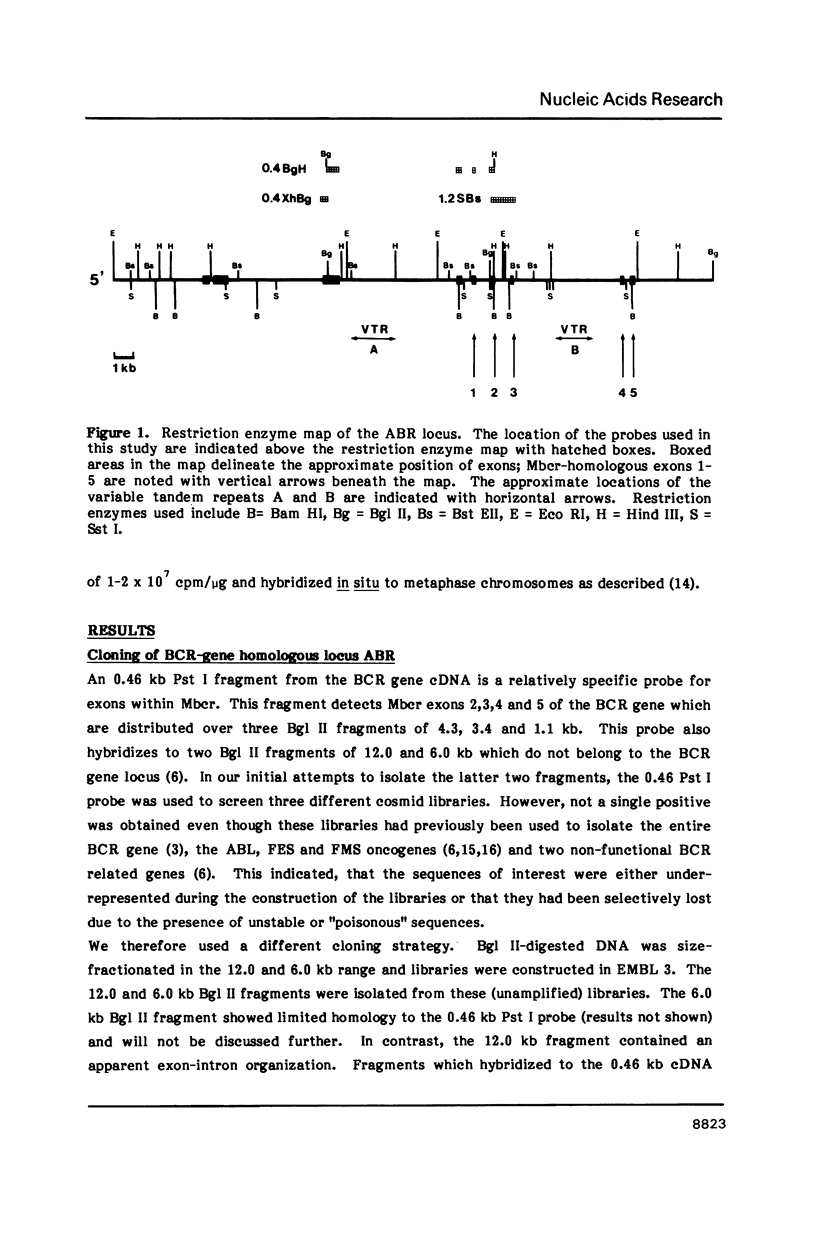



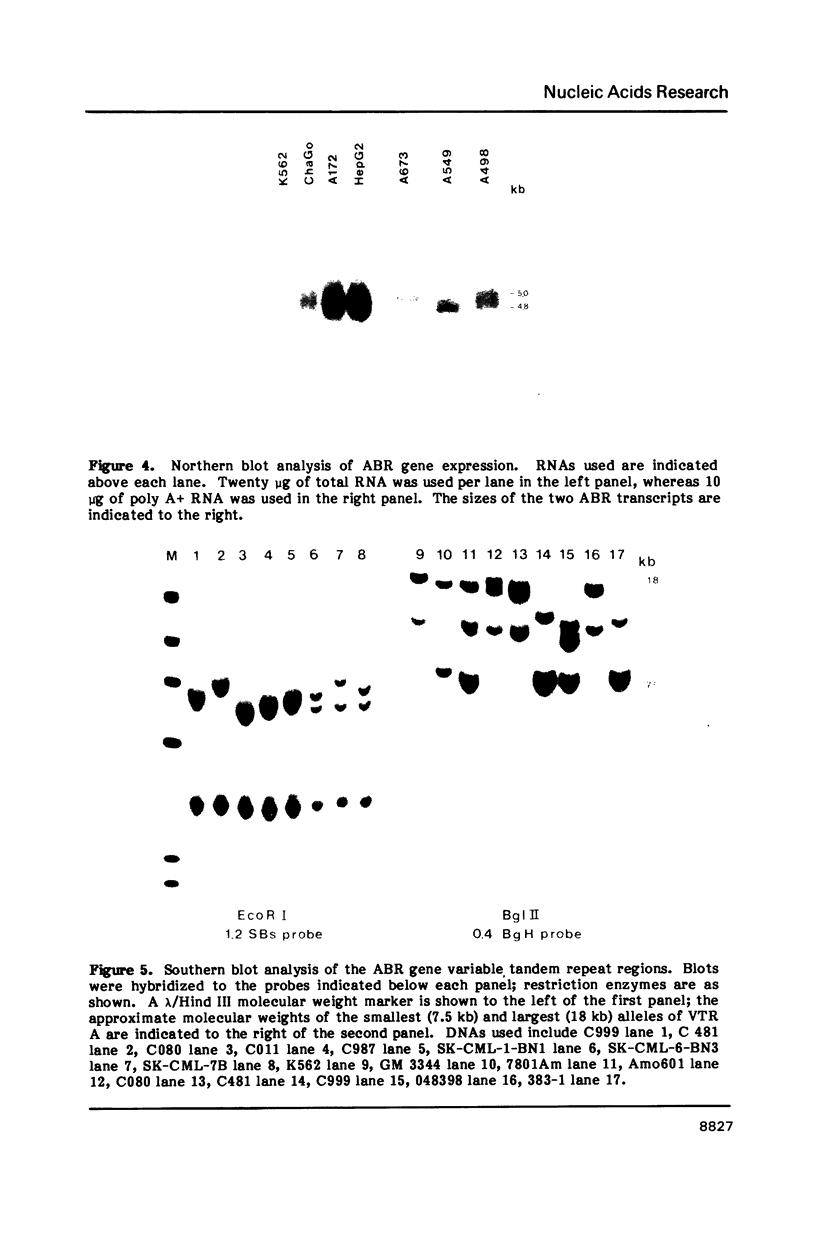
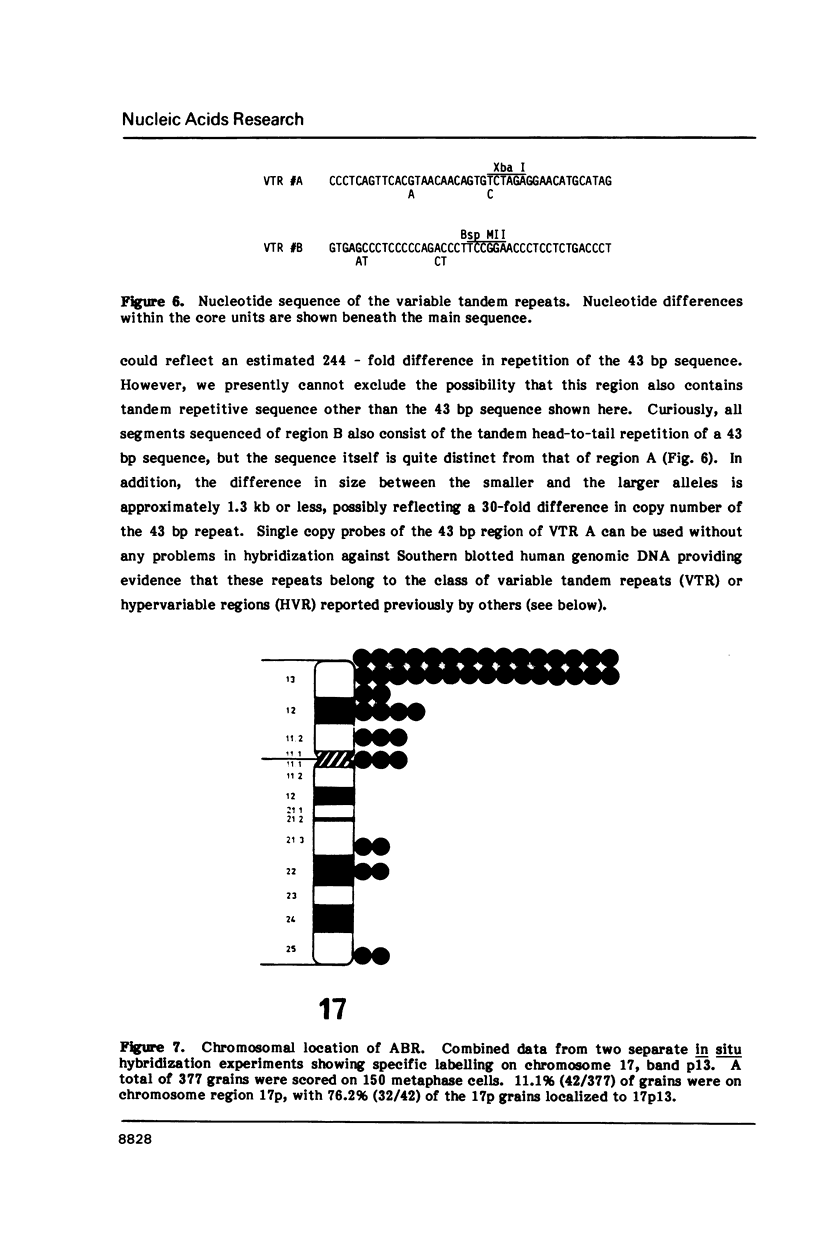



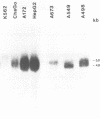
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Selby M. J., Rutter W. J. The highly polymorphic region near the human insulin gene is composed of simple tandemly repeating sequences. Nature. 1982 Jan 7;295(5844):31–35. doi: 10.1038/295031a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins S., Coleman H., Groudine M. Expression of bcr and bcr-abl fusion transcripts in normal and leukemic cells. Mol Cell Biol. 1987 Aug;7(8):2870–2876. doi: 10.1128/mcb.7.8.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Huebner K., Isobe M., Fainstain E., Lifshitz B., Shtivelman E., Canaani E. Mapping of four distinct BCR-related loci to chromosome region 22q11: order of BCR loci relative to chronic myelogenous leukemia and acute lymphoblastic leukemia breakpoints. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7174–7178. doi: 10.1073/pnas.84.20.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Braekeleer M. Breakpoint distribution in variant Philadelphia translocations in chronic myeloid leukemia. Cancer Genet Cytogenet. 1986 Oct;23(2):167–170. doi: 10.1016/0165-4608(86)90414-0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Goodbourn S. E., Higgs D. R., Clegg J. B., Weatherall D. J. Molecular basis of length polymorphism in the human zeta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5022–5026. doi: 10.1073/pnas.80.16.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J., Heisterkamp N., Grosveld F., Van de Ven W., Stephenson J. R. Isolation of human oncogene sequences (v-fes homolog) from a cosmid library. Science. 1982 Jun 4;216(4550):1136–1138. doi: 10.1126/science.6281890. [DOI] [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Adams J. M. cDNA sequence for human bcr, the gene that translocates to the abl oncogene in chronic myeloid leukaemia. EMBO J. 1987 Jan;6(1):115–119. doi: 10.1002/j.1460-2075.1987.tb04727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Billström R., Kristoffersson U., Mandahl N., Strömbeck B., Mitelman F. Variant Ph translocations in chronic myeloid leukemia. Cancer Genet Cytogenet. 1985 Nov;18(3):215–227. doi: 10.1016/0165-4608(85)90086-x. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J. Duplication of the bcr and gamma-glutamyl transpeptidase genes. Nucleic Acids Res. 1988 Aug 25;16(16):8045–8056. doi: 10.1093/nar/16.16.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R. Isolation of v-fms and its human cellular homolog. Virology. 1983 Apr 15;126(1):248–258. doi: 10.1016/0042-6822(83)90476-2. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R. The human v-abl cellular homologue. J Mol Appl Genet. 1983;2(1):57–68. [PubMed] [Google Scholar]
- Heisterkamp N., Knoppel E., Groffen J. The first BCR gene intron contains breakpoints in Philadelphia chromosome positive leukemia. Nucleic Acids Res. 1988 Nov 11;16(21):10069–10081. doi: 10.1093/nar/16.21.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Huret J. L., Tanzer J., Henry-Amar M. Aberrant breakpoints in chronic myelogenous leukaemia; oncogenes and fragile sites. Hum Genet. 1986 Dec;74(4):447–448. doi: 10.1007/BF00280504. [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Nicholls R. D., Weatherall D. J., Clegg J. B., Higgs D. R. Molecular characterisation of a hypervariable region downstream of the human alpha-globin gene cluster. EMBO J. 1986 Aug;5(8):1857–1863. doi: 10.1002/j.1460-2075.1986.tb04437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Lifshitz B., Fainstein E., Marcelle C., Shtivelman E., Amson R., Gale R. P., Canaani E. bcr genes and transcripts. Oncogene. 1988 Feb;2(2):113–117. [PubMed] [Google Scholar]
- McBride O. W., Merry D., Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13). Proc Natl Acad Sci U S A. 1986 Jan;83(1):130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes-Masson A. M., McLaughlin J., Daley G. Q., Paskind M., Witte O. N. Overlapping cDNA clones define the complete coding region for the P210c-abl gene product associated with chronic myelogenous leukemia cells containing the Philadelphia chromosome. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9768–9772. doi: 10.1073/pnas.83.24.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. M., Fitzgerald P. H. Complexity of an apparently simple variant Ph translocation in chronic myeloid leukemia. Leuk Res. 1987;11(2):163–169. doi: 10.1016/0145-2126(87)90022-1. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rubin C. M., Blazar B. R., Hooberman A. L., Dickler M. N., Miller B. A., Westbrook C. A. A deletion/insertion polymorphism in the human BCR gene on chromosome 22. Nucleic Acids Res. 1988 Sep 12;16(17):8741–8741. doi: 10.1093/nar/16.17.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. E., Johnson J. P., Holycross B., Mandeville T. M., Sears T. S., Graul E. A., Carey J. C., Schroer R. J., Phelan M. C., Szollar J. Detection of submicroscopic deletions in band 17p13 in patients with the Miller-Dieker syndrome. Am J Hum Genet. 1988 Nov;43(5):597–604. [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stam K., Heisterkamp N., Grosveld G., de Klein A., Verma R. S., Coleman M., Dosik H., Groffen J. Evidence of a new chimeric bcr/c-abl mRNA in patients with chronic myelocytic leukemia and the Philadelphia chromosome. N Engl J Med. 1985 Dec 5;313(23):1429–1433. doi: 10.1056/NEJM198512053132301. [DOI] [PubMed] [Google Scholar]
- Stratton R. F., Dobyns W. B., Airhart S. D., Ledbetter D. H. New chromosomal syndrome: Miller-Dieker syndrome and monosomy 17p13. Hum Genet. 1984;67(2):193–200. doi: 10.1007/BF00273000. [DOI] [PubMed] [Google Scholar]
- van der Feltz M. J., Shivji M. K., Grosveld G., Wiedemann L. M. Characterization of the translocation breakpoint in a patient with Philadelphia positive, bcr negative acute lymphoblastic leukaemia. Oncogene. 1988 Aug;3(2):215–219. [PubMed] [Google Scholar]
- vanTuinen P., Dobyns W. B., Rich D. C., Summers K. M., Robinson T. J., Nakamura Y., Ledbetter D. H. Molecular detection of microscopic and submicroscopic deletions associated with Miller-Dieker syndrome. Am J Hum Genet. 1988 Nov;43(5):587–596. [PMC free article] [PubMed] [Google Scholar]
- vanTuinen P., Dobyns W. B., Rich D. C., Summers K. M., Robinson T. J., Nakamura Y., Ledbetter D. H. Molecular detection of microscopic and submicroscopic deletions associated with Miller-Dieker syndrome. Am J Hum Genet. 1988 Nov;43(5):587–596. [PMC free article] [PubMed] [Google Scholar]