Highly motile fungal early endosomes can be easily distinguished from more static late endosomes and vacuoles, a feature that is exploited to study endosomal maturation. RabA/RabB early endosomes mature into RabSRab7 late endosomes as they move away from the tip where endocytosis predominates, augmenting their size, with concomitant loss of motility.
Abstract
We exploit the ease with which highly motile early endosomes are distinguished from static late endosomes in order to study Aspergillus nidulans endosomal traffic. RabSRab7 mediates homotypic fusion of late endosomes/vacuoles in a homotypic fusion- and vacuole protein sorting/Vps41–dependent manner. Progression across the endocytic pathway involves endosomal maturation because the end products of the pathway in the absence of RabSRab7 are minivacuoles that are competent in multivesicular body sorting and cargo degradation but retain early endosomal features, such as the ability to undergo long-distance movement and propensity to accumulate in the tip region if dynein function is impaired. Without RabSRab7, early endosomal Rab5s—RabA and RabB—reach minivacuoles, in agreement with the view that Rab7 homologues facilitate the release of Rab5 homologues from endosomes. RabSRab7 is recruited to membranes already at the stage of late endosomes still lacking vacuolar morphology, but the transition between early and late endosomes is sharp, as only in a minor proportion of examples are RabA/RabB and RabSRab7 detectable in the same—frequently the less motile—structures. This early-to-late endosome/vacuole transition is coupled to dynein-dependent movement away from the tip, resembling the periphery-to-center traffic of endosomes accompanying mammalian cell endosomal maturation. Genetic studies establish that endosomal maturation is essential, whereas homotypic vacuolar fusion is not.
INTRODUCTION
The mechanisms by which membrane and associated proteins traffic across the endocytic pathway toward degradative organelles (metazoan lysosomes and fungal vacuoles) have been intensively investigated (Huotari and Helenius, 2011). Rather than being vesicle mediated, traffic between early endosomes (EEs) and late endosomes (LEs) occurs by maturation. According to this view, EEs, which receive biosynthetic traffic from the Golgi, progressively undergo changes in lumenal pH and composition as they fuse homotypically to give rise to gradually larger organelles. Simultaneously, portions of endosomal membranes that are sorted into the multivesicular body pathway bud inward, thereby delivering lipids and their associated proteins into the lumen of the organelle. Thus maturation results in organelles that are larger than EEs and display a characteristic multivesicular appearance. These multivesicular, “late” endosomes undergo further fusion between themselves and with the vacuoles/lysosomes, thus making their cargo accessible to digestion by the vacuolar/lysosomal hydrolases.
Rabs are small GTPases that, when switched to their GTP conformation, associate with intracellular membranes, recruiting to them “effector” interacting proteins from the cytosol (Behnia and Munro, 2005). These effectors include tethers, soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) regulators, and lipid-modifying enzymes that are major determinants of membrane identity (Zerial and McBride, 2001; Pfeffer and Aivazian, 2004). Owing to the high specificity of any given Rab for a certain membrane, Rabs have been extensively used in membrane traffic studies, including endosomal maturation itself, as the transition between EEs and LEs appears to be mediated by Rab conversion (Rink et al., 2005; Segev, 2011). In its simplest version, Rab conversion implies that early endosomal membrane domains containing Rab5 are converted into late endosomal domains containing Rab7. As a result, Rab7 effectors substitute for Rab5 effectors, leading to a shift in compartmental identity toward LEs. Replacement of Rab5 by Rab7 on endosomes requires that the positive feedback loops that stabilize Rab5 domains (Zerial and McBride, 2001) need to be inactivated, and the mechanisms facilitating Rab7 recruitment need to be activated coordinately (Rink et al., 2005; Nordmann et al., 2010; Segev, 2011).
Major insight into the mechanisms of endosomal maturation came from work with Saccharomyces cerevisiae (Price et al., 2000; Peplowska et al., 2007; Markgraf et al., 2009; Brocker et al., 2010; Cabrera et al., 2010; Nordmann et al., 2010; Ostrowicz et al., 2010). Endosomal Rab conversion results in substitution of the Vps21p (i.e., Rab5) effector class C core vacuole/endosome tethering (CORVET) complex (Peplowska et al., 2007) by the Ypt7p (i.e., Rab7) effector homotypic fusion and vacuole protein sorting (HOPS) complex (Seals et al., 2000). However, the actual mechanism by which HOPS substitutes for CORVET has not been established. Both HOPS and CORVET are multiprotein complexes that share four class C proteins—Vps11p, Vps16p, Vps18p, and Vps33p (Rieder and Emr, 1997)—and only differ in two specific components each—Vps8p and Vps3p for CORVET and Vps41 and Vps39 for HOPS.
A hurdle hindering correlation between subcellular and mechanistic studies of endosomes is implicit in the maturation concept: by definition, the distinction between EEs and LEs is unavoidably blurred (Huotari and Helenius, 2011). The basidiomycete Ustilago maydis and the ascomycete Aspergillus nidulans are ideally suited for studying endosomal maturation because their EEs can be unequivocally identified by their characteristic long-distance bidirectional movement on microtubule-dependent motors (Wedlich-Soldner et al., 2002; Lenz et al., 2006; Steinberg, 2007; Zekert and Fischer, 2008; Abenza et al., 2009, 2010; Hervás-Aguilar et al., 2010; Peñalva, 2010; Zhang et al., 2010, 2011; Schuster et al., 2011).
A. nidulans has two Rab5 paralogues—RabA and RabB—localizing to EEs (Abenza et al., 2009, 2010) and a single Rab7 homologue (Ohsumi et al., 2002; Sánchez-Ferrero and Peñalva, 2006), which we denoted RabS (Rab seven, RabSRab7). The CORVET complex is an effector of RabB and, to a lesser extent, of RabA (Abenza et al., 2010). Whether HOPS is a RabSRab7 effector was unknown. Because maturation involves the CORVET-mediated coalescence of EEs into progressively larger endosomes (Markgraf et al., 2009), it seems plausible that the resulting increase in size inevitably results in decreased motility. Such decreased motility would be a bona fide feature of LEs. Indeed, overexpression of the CORVET recruiters RabA/RabB leads to formation of endosomal aggregates with reduced motility and multivesicular appearance (a landmark of LEs; Abenza et al., 2009, 2010; Griffith et al., 2011). However, as discussed by Huotari and Helenius (2011), the presence of internal vesicles cannot be the sole criterion to define LEs. Indeed ESCRT-III appears to operate in A. nidulans EEs (Galindo et al., 2007; Hervás-Aguilar et al., 2010). LEs and vacuoles can be visualized with 7-amino-4-chloromethyl-coumarin (CMAC), a fluorescent compound that labels the lumen of hydrolase-containing acidic organelles, including vacuoles and smaller structures. Here we use the term “vacuole” to refer to spherical CMAC-positive organelles whose limiting membrane is stainable with FM4-64 (Peñalva, 2005). However, the limiting membrane/shape of the smaller CMAC-positive structures cannot be resolved by optical microscopy. Thus we will use the generic term “LE/vacuole” to collectively refer to all “mature” structures that are CMAC positive, irrespective of their size.
Here we characterize in detail RabSRab7 and demonstrate that it localizes to vacuoles and to smaller structures, that HOPS is a RabSRab7 effector, and that deletion of rabS leads to the formation of small yet proteolytically competent CMAC-positive structures that, unlike normal vacuoles, undergo long-distance movement and contain early endosomal Rabs in their membranes. Time-lapse analyses showed that the transition between RabA/B-containing EEs and RabSRab7 structures is sharp, although colocalization can be detected on large (and thus less motile) endosomal aggregates. Dynein impairment results in the accumulation of vacuoles in a region near the tip that is normally devoid of them. Taken together, our data support a model in which endosomal maturation progresses as endosomes move away from the tip, losing motility as they enlarge through homotypic fusion.
RESULTS
RabSRab7 localizes to the vacuolar membranes
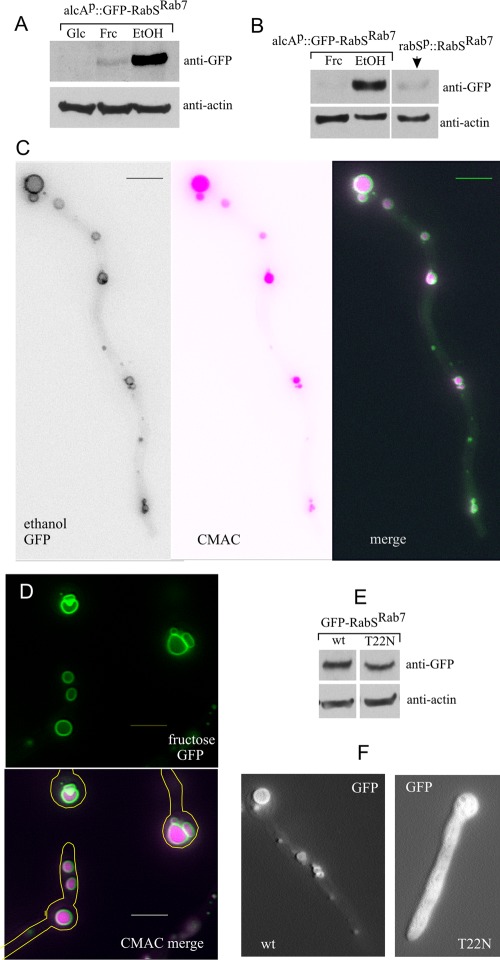
We studied the localization of green fluorescent protein (GFP)–tagged or mCherry-tagged versions of RabSRab7 with constructs containing the rabS promoter or the regulatable promoter alcAp. All constructs were single-copy integrated into the genome by targeted recombination (Calcagno-Pizarelli et al., 2007). Anti-GFP Western analysis, used to determine the levels of GFP-RabSRab7 expression obtained with the alcAp driver, showed that these were below detection on glucose (alcAp-repressing conditions), very high on ethanol (alcAp-inducing conditions), and low on 0.1% fructose (noninduced, nonrepressed alcAp conditions; Figure 1A). alcAp-driven fructose levels were similar or slightly below those driven by the rabSp (Figure 1B). Whether GFP or mCherry tagged, overexpressed (ethanol; Figure 1C) or expressed to approximately physiological levels (fructose; Figure 1D), RabSRab7 robustly localizes to the membrane or spherical vacuoles whose lumen is stainable with the vacuolar tracer CMAC.
FIGURE 1:
Subcellular localization of RabSRab7. (A) Anti-GFP Western blot analysis showing the different steady-state levels of GFP-RabSRab7 achieved under repressing (1% glucose, Glc), noninducing, nonrepressing (0.1% fructose, Frc) and inducing (1% ethanol, EtOH) conditions for the alcAp driver. Actin was used as loading control. (B) GFP-RabSRab7 levels driven by the physiological promoter compared with those obtained with alcAp. (C) Vacuolar localization of GFP-RabSRab7 (alcAp driver, ethanol conditions). (D) Vacuolar localization of GFP-RabSRab7 (alcAp driver, fructose conditions). (E) Western blot demonstrating that Thr22Asn substitution does not affect the steady-state level of GFP-RabSRab7 (T22N). (F) GFP-RabSRab7 (T22N) locked in the GDP conformation is cytosolic. Bars, 5 μm.
Rabs associate with membranes in their GTP conformation (Behnia and Munro, 2005). On GTP hydrolysis, GDP-Rabs are extracted to the cytosol by the GDP dissociation inhibitor (GDI; encoded by A. nidulans gdiA). Figure 1F shows that the vacuolar membrane localization of RabSRab7 is strictly dependent on the nucleotide switch of the GTPase, as mutant Thr22Asn GFP-RabSRab7, locked in the GDP conformation, is cytosolic (Figure 1F), even though the mutation does not affect the steady-state protein levels (Figure 1E). This shows that GFP-RabSRab7 reflects the physiological localization of RabS.
The size and relative abundance of vacuoles increase with the distance to the tip
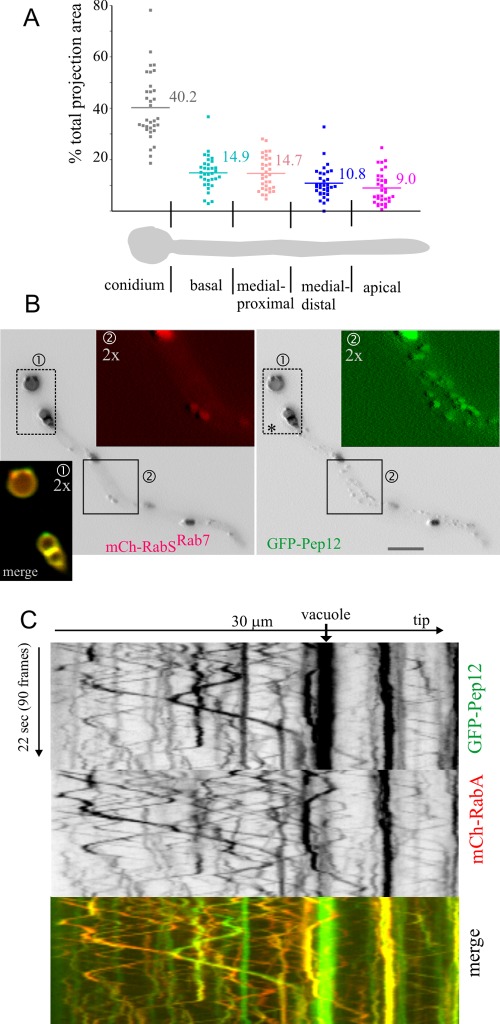
We exploited the fact that RabSRab7 specifically labels vacuoles to measure their distribution along hyphae. We determined the percentage of cell projection surface occupied by vacuoles in 30- to 60-μm-long germlings (n = 33), which were segmented into five zones (Figure 2A). Vacuoles occupied, on average, 40% of the conidiospore projection but only 9% of the region nearest to the tip. The regions between the tip and the base showed intermediate scores, the overall tendency being that vacuole-occupied surface increases with the distance to the tip. This increase correlates with a parallel increase in vacuolar diameter (Figure 1C; Peñalva, 2005; Findon et al., 2010). Thus the biogenesis of vacuoles is connected to the relative position within hyphae, such that tip-proximal regions are relatively devoid of these organelles. Vacuoles rarely underwent long-distance movements (Figure 3; see later discussion for a detailed consideration), making unlikely that the mechanism underlying their asymmetrical distribution involves basipetal transport. The strict and robust GFP-RabSRab7 localization to vacuolar membranes facilitated time-lapse studies permitting visualization of vacuoles undergoing fusion (Supplemental Figure S1).
FIGURE 2:
A. nidulans vacuolar distribution. Pep12 is present in EEs and LEs/vacuoles. (A) Vacuolar distribution: 30- to 60-μm germlings were arbitrarily divided into the “conidium” and four “rectangular” sections of approximately equivalent length, as indicated. The percentage of the total projection area occupied by vacuoles in each section was plotted in n = 33 germlings. (B) mCherry-RabSRab7 colocalizes with GFP-Pep12 on vacuoles. However, GFP-Pep12 additionally localizes to punctate structures that are not labeled by mCherry-RabSRab7. Regions of interest are shown at double magnification in the indicated red, green, and merge channels. (C) Kymograph of a 4-frame/s time-lapse sequence of a cell coexpressing GFP-Pep12 and the EE marker mCherry-RabA. Pep12 and RabA colocalize in the population of rapidly moving EEs and in less abundant larger and static RabA-containing structures but not on vacuoles, which do not contain RabA.
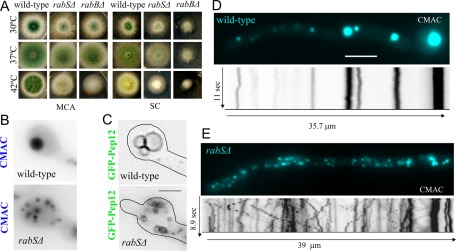
FIGURE 3:
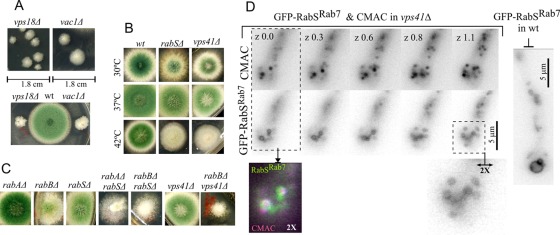
rabS∆ results in abnormally motile minivacuoles. (A) Growth phenotypes of the indicated strains at the indicated media and temperatures. MCA, complete medium. SC, synthetic complete medium. (B) CMAC and (C) GFP-Pep12 visualization of rabS∆ minivacuoles. All images are depicted at the same magnification. Bar, 2 μm. (D) Still frames (blue) and kymographs (inverted contrast, shown at the same scale as still images) correspond to 10-s time-lapse series of CMAC-stained vacuoles. Note the multiple diagonal lines seen in the rabS∆ example. Both images are at the same magnification. Bar, 5 μm.
Partial overlap of the target-SNARE Pep12 with RabSRab7
The A. nidulans orthologue of S. cerevisiae Pep12p is the only syntaxin across the endovacuolar system (Sánchez-Ferrero and Peñalva, 2006; Findon et al., 2010; S. cerevisiae has two—Pep12p for prevacuolar endosomes and Vam3p for vacuoles). mCherry-RabSRab7 strictly colocalizes with GFP-Pep12, but the reverse is not true, as GFP-Pep12 labels punctate structures that do not contain RabSRab7 (Figure 2B). These punctate structures undergo long-distance bidirectional movements, suggesting that they are EEs. Thus we constructed a strain coexpressing GFP-Pep12 and the EE marker mCherry-RabA and recorded time-lapse sequences (∼4 frames/s) simultaneously in the GFP and mCherry channels. These sequences were analyzed with kymographs in which the y-axis represents the time scale. Thus static and short-range moving structures appear as vertical lines, whereas fast-moving structures appear as diagonal lines whose slope reflects the speed/direction and length reflects the range of movement of any given particle. Figure 2C shows that GFP-Pep12 localizes to static vacuoles (bright vertical lines) that do not contain RabA, to static structures containing RabA (LEs; see later discussion), and, in addition, to a population of small, bidirectionally motile structures (thus EEs; Figure 2C diagonal lines; Supplemental Movie S1) in which it colocalizes with RabA. This important finding indicates that Pep12 plays roles at the levels of the A. nidulans EEs, LEs, and vacuoles.
rabS∆ slightly impairs vegetative growth and results in a temperature-dependent defect in conidiation
We constructed a null rabS∆ allele, which results in a minor vegetative growth defect at any of the three temperatures tested (30, 37, and 42°C), more noticeable on synthetic than on rich medium (Figure 3A). Its most conspicuous phenotype was the nearly complete absence of conidiation at 42°C (Figure 3A). These phenotypic aspects of rabS∆ somewhat resemble those of rabBΔ (RabB is the major Rab5), but they are markedly less pronounced (Figure 3A; compare middle and right columns on SC). This indicates that RabSRab7 plays a less important physiological role than RabB. The findings that rabSp::gfp-rabS and alcAp::gfp-rabS transgenes complement the 42°C rabSΔ growth and conidiation defects (demonstrating that GFP-RabSRab7 is functional; Supplemental Figure S2) and that rabSΔ strictly cosegregates with the growth/conidiation defects in the progeny of crosses established that these phenotypic features are solely attributable to the rabSΔ allele.
rabS∆ results in proteolytically competent minivacuoles capable of undergoing movement
rabS disruption results in small vacuoles (Ohsumi et al., 2002). Strains carrying rabS∆ indeed showed minute vacuoles (CMAC staining; Figure 3B) whose small lumen is hardly resolvable by optical microscopy (using GFP-Pep12 to label their membranes; Figure 3C). Thus, in agreement with previous data and with work in S. cerevisiae Ypt7p, RabSRab7 is involved in the biogenesis of “normal-sized” vacuoles. rabSΔ “minivacuoles” show two noteworthy features: 1) They are evenly distributed across hyphae, unlike wild-type vacuoles (Figure 3, D and E), and 2) a proportion of mutant minivacuoles undergoes long-range movements similar to those of EEs (reflected by diagonal lines in kymographs), contrasting with the essentially immotile wild-type vacuoles (vertical lines in kymographs; Figure 3, D and E, and Supplemental Movies S2 [wild-type] and S3 [rabS∆]). Given that the motility of EEs is RabB dependent (Abenza et al., 2010), we hypothesized that the inability of endosomes to exchange RabSRab7 for RabB might result in RabB persisting on minivacuoles (see later discussion).
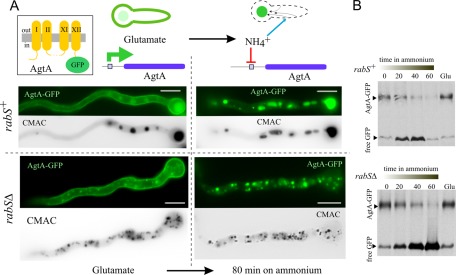
We next determined whether rabS∆ minivacuoles are competent in proteolysis of an endocytic cargo, the plasma membrane amino acid permease AgtA. AgtA is expressed on glutamate medium, localizing to the plasma membrane and vacuoles (Figure 4A). If glutamate-cultured cells are shifted to ammonium, AgtA synthesis is strongly repressed, and the plasma membrane-resident pool of the transporter is delivered to the vacuole via endocytosis (Apostolaki et al., 2009). In rabS∆ cells, AgtA-GFP is also delivered to the lumen of (mini-) vacuoles (Figure 4A). Western blots revealed similar kinetics of AgtA-GFP degradation in rabSΔ and rabS+ cells (Figure 4B). Thus rabSΔ does not affect the sorting of cargoes into the MVB pathway, and minivacuoles are proteolytically competent.
FIGURE 4:
rabS∆ minivacuoles are proteolytically competent. (A) Normal traffic to the vacuoles of the 12-TMD protein AgtA, which mediates the uptake of dicarboxylic amino acids. In these assays, AgtA is endogenously tagged with GFP in its (cytosolic) C-terminus. Wild-type and rabS∆ cells expressing AgtA-GFP were initially cultured on glutamate. Under these conditions, AgtA localizes to the plasma membrane and, to a lesser extent, to the vacuoles. These cells were then shifted to ammonium, which shuts the agtA promoter off and promotes the endocytic delivery of plasma membrane–localizing AgtA-GFP to the vacuolar lumen. (B) Western blots showing the proteolytic vacuolar degradation of AgtA-GFP following transfer of cells to ammonium and further incubation for the indicated times. GFP is recalcitrant to degradation by vacuolar proteases but more so in the rabS∆ mutant.
RabSRab7 recruits HOPS
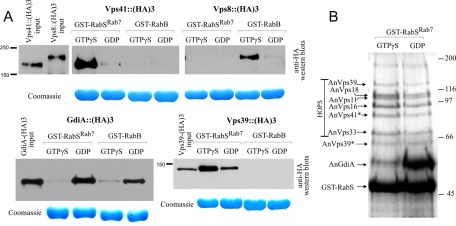
We next used glutathione S-transferase (GST)–RabSRab7, loaded with GDP or GTP-γ-S, and cell extracts containing endogenously 3× hemagglutinin (HA)-tagged interactors in pull-down experiments (Figure 5A). GdiA was specifically and efficiently pulled down by GDP-loaded RabSRab7, indicating that GTP-γ-S shifts RabSRab7 toward a conformation that prevents its interaction with the Rab-GDP effector GdiA (Figure 5A). The fusogenic activity of Ypt7p involves HOPS. Vps41p is the Ypt7p effector subunit in HOPS linking the complex to the GTP-loaded Rab (Cabrera et al., 2010; Ostrowicz et al., 2010). In agreement, A. nidulans Vps41 was specifically pulled down by GTP-γ-S RabSRab7 but not at all by RabB, which instead pulled down Vps8 efficiently (Vps8 is the CORVET equivalent of Vps41; Peplowska et al., 2007; Markgraf et al., 2009). In contrast, Vps39, which also showed strict RabSRab7 specificity, was preferentially retained by GTP-γ-S RabSRab7 but interacted substantially with GDP-RabSRab7 (Figure 5A). Given that we used unfractionated “prey extracts,” this finding is consistent with the finding that whereas the Vps39-containing HOPS complex is a Ypt7p (GTP) effector, “free” Vps39p binds Ypt7p irrespective of the nucleotide status (Ostrowicz et al., 2010).
FIGURE 5:
RabSRab7 recruits HOPS. (A) Glutathione–Sepharose beads containing GST-RabB or GST-RabSRab7 loaded with the indicated nucleotides were used as bait in pull-downs with whole-cell protein extracts, which were obtained from strains expressing, at physiological levels, GdiA (the only A. nidulans guanine nucleotide dissociation inhibitor), the CORVET component Vps8, or the HOPS components Vps39 and Vps41, endogenously tagged with 3× HA. The 3× HA baits were detected by Western blotting. (B) A. nidulans whole-cell extracts were passed through GST-RabSRab7 affinity columns loaded with the indicated nucleotides, and the bound proteins were eluted, separated in an SDS–polyacrylamide gel, and stained. The identity of the bands was determined by MS/MS (Supplemental Table S1). Vps41* and Vps39** indicate that these bands are degradation products. Full-length Vps39 is also detectable in the gel.
We passed cell-free extracts through GDP- or GTP-γ-S–loaded GST-RabSRab7 affinity columns (Figure 5B). SDS–PAGE and tandem mass spectrometry (MS/MS) analyses showed that GTP-γ-S eluates contained all six components of HOPS—four components shared with CORVET (Vps11, Vps18, Vps16, and Vps33) and the HOPS-specific components Vps39 and Vps41 (Figure 5B and Supplemental Table S1). HOPS components were only slightly less efficiently retained by the GDP column, suggesting that GST-RabSRab7 immobilized in the Sepharose support does not completely undergo the nucleotide-induced conformational changes mediating RabSRab7 function in vivo. (Work with S. cerevisiae Ypt7p showed that purified HOPS shows selectivity for the GTP-γ-S–loaded Ypt7p form; Ostrowicz et al., 2010.) In summary, these experiments demonstrate that HOPS is an effector of RabSRab7.
Endosomal maturation is essential but vacuolar fusion is not
The two Rab5s (RabA and RabB) and their effectors mediate the coalescence of EEs and their maturation into LEs (Abenza et al., 2010). Vps45 and its binding partner Vac1Vps19 are specific RabB effectors, whereas the CORVET complex (containing Vps8) is a RabB and RabA effector (Abenza et al., 2010). A double rabA∆ rabB∆ deletion is nearly lethal, and single vps45∆ and vps8∆ mutations are severely debilitating (Abenza et al., 2010), as are deletions involving ESCRT-0, -I, -II, and -III components mediating the biogenesis of multivesicular endosomes (Calcagno-Pizarelli et al., 2011). These data indicate that maturation and coalescence of EEs into larger structures (LEs) is essential. In contrast, rabS∆ mutants are perfectly viable, and thus the RabSRab7-mediated fusion of LEs with vacuoles, of LEs with LEs to form vacuoles, and the homotypic fusion of vacuoles is largely dispensable (Figure 3A).
These conclusions led to three predictions: 1) vac1∆ should be as severely debilitating as vps45∆; 2) deletion of any one gene encoding a HOPS class C component shared with CORVET should lead to near lethality due to its role in CORVET; and 3) deletion of the gene encoding the HOPS-specific subunit Vps41 should be viable, phenocopying rabS∆ mutations. We deleted vac1 and vps18 (Vps18 is a class C CORVET and HOPS subunit). Figure 6A shows the corresponding growth phenotypes. vac1∆ colonies show severely reduced growth, similar to that of vps45∆ colonies. The growth defect of vps18∆ colonies is even more marked, indicating that this class C protein is virtually essential.
FIGURE 6:
Genetic studies of endosomal maturation. (A) Growth phenotypes of vps18∆ (encoding a class C subunit shared by the CORVET and HOPS complexes) and vac1Δ (Vac1, also denoted Vps19, is a specific RabB effector). vps18∆ and vac1Δ are very severely debilitating. Note that vps18 was previously called digA. A digA1 early-truncating mutation removing the Vps18 C-terminal RING finger leads to a temperature-dependent growth defect (Geissenhoner et al., 2001). (B) Growth phenotypes at the indicated temperatures of vps41Δ compared with rabSΔ. The two mutations are phenotypically indistinguishable. (C) Both rabAΔ and rabBΔ result in a synthetic growth defect in double mutants with rabSΔ. rabBΔ also results in a similar synthetic growth defect in double mutants with vps41Δ (rabAΔ was not tested in combination with vps41∆). (D) Like rabSΔ, vps41Δ results in minivacuoles. In the absence of Vps41, the limiting membranes of CMAC-containing minivacuoles are labeled with GFP-RabSRab7. Images of a z-series are shown, with numbers indicating relative z-position in micrometers. For comparison, GFP-RabSRab7 wild-type vacuoles are shown on the right.
Vps41 is the HOPS equivalent of CORVET Vps8 (Peplowska et al., 2007). In marked contrast with vps8∆ or vps18∆, vps41∆ strains are relatively healthy and indistinguishable from a rabS∆ strains in growth and conidiation (note the slight reduction in colony size and lack of conidiation at 42°C; Figure 6B). CMAC staining (Supplemental Figure S3) showed that vps41∆ mutants also resemble rabS∆ mutants in the “minute vacuole” phenotype and in the loss of the tip-to-base gradient of vacuolar distribution. Thus the rabS∆ minute vacuole phenotype results from inability to recruit HOPS. Vps41, able to bind membranes of LEs via its ALPS domain (Cabrera et al., 2010), might conceivably contribute to the membrane recruitment of its Rab. However, GFP-RabSRab7 is recruited to the membranes of CMAC-stained vps41∆ minivacuoles in the absence of Vps41 (Figure 6D).
The motility of the rabS∆ minivacuoles suggests that they retain endosome features, even though they have the characteristic spherical shape of vacuoles and their lumen is large enough to be optically resolvable. However, in the absence of RabSRab7 (and therefore of HOPS), endosomes can only fuse into minivacuoles by using CORVET (i.e., in a RabA/RabB-dependent manner; Abenza et al., 2010).
rabB∆ and rabA∆ are synthetically sick with rabS∆, the double mutations leading to a stronger impairment of growth and conidiation than either single deletion (Figure 6C). Given that vps41∆ and rabS∆ cause similar synthetic growth defects in combination with rabB∆ (Figure 6C), the debilitating effect of the double rabS∆ rabB∆ mutation involves the inability to recruit HOPS and not another RabSRab7-mediated process. Two non–mutually exclusive interpretations can account for these observations. One is that in the absence of RabSRab7/HOPS, impairing the recruitment of CORVET to endosomes critically compromises the formation of functional rabS∆ minivacuoles. A second is that RabSRab7/HOPS has RabA/RabB-independent functions. For example, rabS∆ could prevent, in addition to LE–vacuole and vacuole–vacuole fusion, the entry of Vps41-dependent traffic into the vacuoles through a pathway akin to the S. cerevisiae AP-3 pathway (Cabrera et al., 2010).
Subcellular distribution of Rab5s and RabSRab7
To study endosomal Rab transitions, we constructed strains expressing mCherry-RabSRab7 and GFP-RabA or GFP-RabB from single-copy alcAp-driven transgenes. Using this regulatable (by the carbon source) promoter allowed us exploit three different levels of expression: low (fructose), intermediate (glycerol), and high (ethanol). None of these carbon source conditions led to any detectable effect on colony growth (Supplemental Figure S4), strongly indicating that expression of the fusion proteins does not interfere with the physiology of the endosomal system.
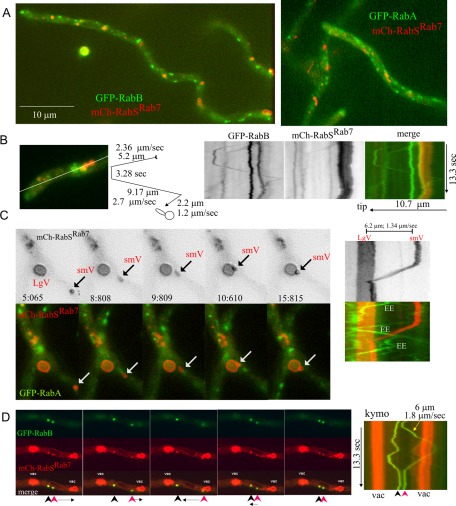
We acquired high-speed time-lapse sequences (10–20 frames/s) of these strains in the red and green channels simultaneously using a Dual-Viewer. RabSRab7 and RabA/RabB showed little colocalization (Figure 7A and Supplemental Movies S4 and S5). RabSRab7 localized to the vacuolar membranes, to relatively static structures, and, occasionally, to moving structures (see later discussion). By contrast, RabA or RabB did not label the vacuolar membranes at all, localizing to moving EEs and to less abundant static structures. As in U. maydis (Schuster et al., 2011), moving RabA/RabB EEs usually traveled for several seconds in one direction before reversing movement in the opposite direction, with (U-shaped tracing in the kymograph; Figure 7B) or without (V-shaped tracings; Figure 8) arresting for some time in between. This behavior reflects the tug-of-war action of dynein and kinesins on the same endosome (Schuster et al., 2011). Time-lapse sequences revealed very unusual examples of vacuolar movement, like the ∼6-μm journey of the small vacuole in Figure 7C to dock at a large vacuole (Supplemental Movie S6). This movement was short ranged and slower than that of EEs (compare slopes of green- and red-channel kymographs).
FIGURE 7:
Coimaging of early endosomal Rabs and RabSRab7. (A) Still frames showing that RabSRab7 and RabB or RabA show little colocalization. (B) Examples of static and moving endosomes: RabB (green) and RabSRab7 (mCherry). A kymograph was plotted across the indicated line. Three static RabB endosomes, one moving EE, and two small vacuoles strongly labeled with RabSRab7are shown. The tip-distal static RabB endosome is docked at the most anterior RabSRab7 vacuole (yellow in the channel merge). The movement of a fourth RabB endosome is schematically represented. (C) One example (arrows) of a basipetally moving small RabSRab7 vacuole (smV) that docks against a large vacuole (LgV). Kymographs on the right depict how the speed (diagonal slope, 1.34 μm/s) of this moving vacuole is markedly lower than that of RabA endosomes. See Supplemental Movie S6. (D) RabB and RabSRab7 can coexist on LEs: two LEs containing RabB and RabSRab7, located between two vacuoles. One of the endosomes moves in one direction before shifting toward the opposite direction, but its speed (1.8 μm/s) is lower than the average speed of EEs. Frames correspond to supplemental Movie S7.
FIGURE 8:
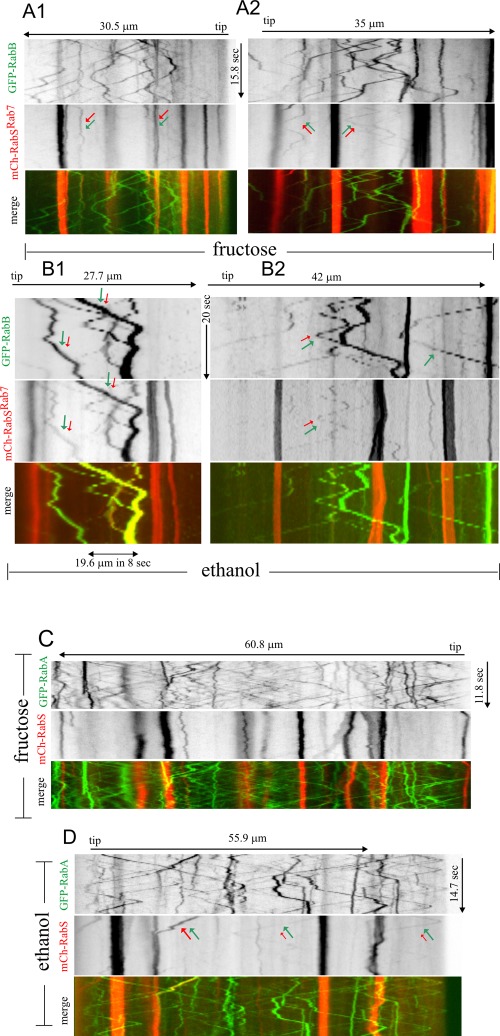
Kymographs of “stream” time-lapse series of GFP-labeled Rab5s and mCherry-RabSRab7. Images in the GFP (Rab5s) and mCherry (RabSRab7) channels were simultaneously acquired using a Dual-View beam splitter with “stream” acquisition, a routine by which images are directly discharged from the camera chip to the computer RAM memory at maximal speed. The resulting time series were used to draw kymographs. All kymographs, whose time and length dimensions are indicated, are displayed to the same scale and are thus directly comparable. (A, B) GFP-RabB and (C, D) GFP-RabA, as indicated. Expression of all three fluorescent fusion proteins was governed by the regulatable alcAp driver and thus can be manipulated by the carbon source in the medium. All transgenes were integrated in single copy into known chromosomal locations. For GFP-RabB: (A1) Arrows show two static endosomes containing RabB and mCherry-RabSRab7. (A2) Arrows show two rare examples of moving endosomes containing RabB and RabSRab7. (B1, B2) Strongly overexpressing conditions. Examples of moving endosomes containing RabB and mCherry-RabSRab7 are indicated with red and green arrows. (B2) One RabB endosome that does not contain mCherry-RabSRab7 is indicated with a single green arrow. GFP-RabA: (C) Virtually no colocalization of GFP-RabA and mCherry-RabSRab7 is seen in the numerous examples of moving endosomes detected across this 60-μm-long region. (D) The endosome on the left (red and green arrows of equal size), containing GFP-RabA and mCherry-RabSRab7, moves toward the tip until it docks at a LE/vacuole. Two examples of GFP-RabA moving endosomes faintly stained with mCherry-RabSRab7 are indicated with green (large) and red (small) arrows. Note that moving RabA structures (diagonals in C and D) are clearly more numerous than moving RabB structures (A and B).
The transition between RabA/RabB and RabSRab7 is sharp
RabB is the major determinant of endosomal maturation (Abenza et al., 2010). Fructose kymographs (Figure 8, A1 and A2) revealed occasional yet rare examples of moving GFP-RabB EEs faintly stained with mCherry-RabSRab7 (Figure 8A2, arrows). In contrast, static structures containing RabB and RabSRab7 were clearly more abundant (Figure 8A1, arrows). Similar overall absence of colocalization in moving structures was seen on glycerol, despite the increased fluorescence signal. Again, colocalization predominated in the more static structures or in those motile structures that moved for shorter distances and at slower speeds than EEs (Supplemental Figure S5A). Figure 7D and Supplemental Movies S7 and S8 show structures containing RabB and RabSRab7 moving at 1–1.8 μm/s, more slowly than EEs (∼2.5 μm/s; Abenza et al., 2009). We speculate that these double-labeled structures, whose size would impede/retard movement, represent late or mature endosomes where Rab conversion is taking place.
To confirm that RabB and RabSRab7 can actually coincide on the less motile endosomes, we induced the transgenes with ethanol, which results in strong overexpression of the Rabs. RabB still localized to fast-moving EEs. It also localized to aggregates that, albeit motile, generally moved for shorter distances (Figure 8, B1 and B2). RabSRab7 was clearly present in a proportion of RabB-containing structures, generally corresponding to these large aggregates (Figure 8, B1 and B2, double arrowed; one “control” RabSRab7-negative EE is single arrowed). However, RabB was absent from vacuolar membranes, even though these were strongly positive for RabSRab7. Therefore, although these strongly overexpressing conditions are not physiological, these experiments establish that RabSRab7 can be recruited to endosomal membranes containing high levels of RabB. In contrast, RabB is excluded from vacuolar membranes, where RabSRab7 is abundant. These data are consistent with a maturation model in which endosomes acquire RabSRab7 in their late steps of maturation, losing RabB as they become vacuoles.
Similar experiments using GFP-RabA revealed a previously unnoticed feature of RabA endosomes: even under fructose conditions leading to GFP-RabA levels similar to those attained with the endogenous promoter (Abenza et al., 2009), we detected a background of very abundant small, moving endosomes that we did not see with GFP-RabB; their kymograph tracings formed a net of diagonal lines against which vertical lines of more static aggregates were prominent (Figure 8, C and D, Supplemental Figure S5, and Supplemental Movie S9). As with RabB, we only very occasionally detected EEs labeled with RabSRab7 and RabA. The tracings arrowed in Figure 8D and Supplemental Figure S5B are the only examples among the numerous moving RabA EEs in which RabSRab7 was detectable. We thus conclude that the transition between RabA/RabB and RabSRab7 domains is relatively sharp.
RabB and RabA reach the vacuoles in rabSΔ
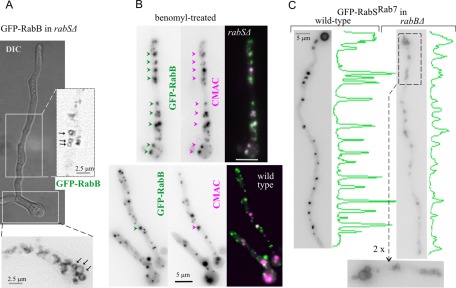
rabS∆ markedly increases the abundance of RabA/RabB structures. In 30-μm-long projections of wild-type tip regions (n = 8 hyphae), GFP-RabB structures accounted for 4.4 ± 0.6% of the area. This figure was markedly higher (sixfold; 23.3 ± 2.5%) in the mutant. This hypertrophy of the rabS∆ endosomal compartment might reflect the inability of LEs to undergo HOPS-mediated fusion or the ability of EE Rabs to invade membranes otherwise restricted to RabSRab7 (or both). One observation lent credence to the “Rab5 invasion” possibility. In the wild type, GFP-RabA/RabB eventually label aggregates of endosomes that are not vacuoles (they are multivesicular, irregularly shaped, and CMAC negative; Abenza et al., 2009, 2010; Griffith et al., 2011). In contrast, in rabS∆ cells, both RabA (Supplemental Movie S10) and RabB (Figure 9A) clearly label the membranes of minivacuoles. To further demonstrate this, cells expressing GFP-RabB were pretreated with benomyl (to abolish the microtubule [MT]-dependent motility of minivacuoles) and loaded with CMAC. Whereas hardly any overlap of RabB with CMAC was detectable in the wild type, most RabB-containing structures in the rabS∆ mutant were CMAC positive (Figure 9B). Because vacuolar membrane labeling by RabA/RabB is never seen in the wild type, these data suggest that displacement of RabA/RabB from EEs once they reach the LE stage requires RabSRab7.
FIGURE 9:
In rabS∆ strains, RabB invades vacuolar membrane domains normally occupied by RabSRab7. (A) GFP-RabB in rabS∆. Inverted contrast images correspond (at double magnification) to the indicated regions of interest in the Nomarski panel. They show how the early endosomal RabB reaches the membrane of rabSΔ minivacuoles. Similar data obtained for GFP-RabA are displayed in Supplemental Movie S10. (B) Microtubules were depolymerized with benomyl. Under such conditions, endosomes form aggregates. In the wild type, endosomal aggregates show little colocalization with CMAC-stained LEs/vacuoles (one colocalizing particle is shown by the arrow). In contrast, colocalization is very conspicuous in the rabSΔ mutant, strongly indicating that CMAC-stained rabS∆ minivacuoles retain early endosomal identity. (C) In rabB∆ cells, GFP-RabSRab7 localizes to the vacuolar membranes but less efficiently than in the wild type. The relative difference of fluorescence is illustrated by the line scans on the right. The inset below the rabB∆ cell shows the faint yet clear labeling of vacuolar membranes.
We next tested whether GFP-RabSRab7 localization to vacuoles necessitates RabB. GFP-RabSRab7 is recruited to the membrane of rabB∆ vacuoles, although clearly less efficiently than in the wild type (Figure 9C). This finding is consistent with the interpretation that the vacuolar RabSRab7 localization involves CORVET, as RabA is also able to recruit CORVET to endosomes, albeit less efficiently than RabB (note that lethality-causing rabA∆ rabB∆ double deletion cannot be tested).
The role of dynein-mediated basipetal movement in endosomal maturation
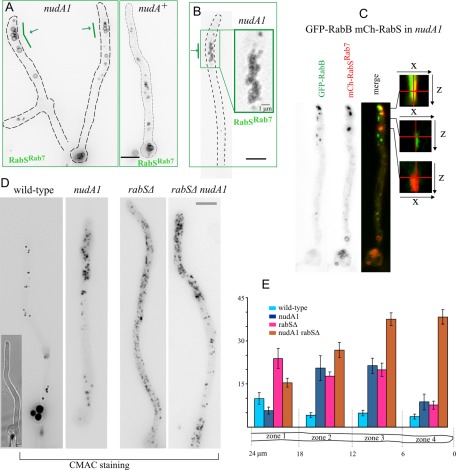
Given that the amount and size of vacuoles increase with the distance to the tip, we hypothesized that maturation of endosomes into vacuoles might be coupled to the dynein-mediated movement of endosomes away from the tip (MTs are oriented with their plus ends toward the apex). Thus, if dynein function is compromised, EEs would be expected to mature in the proximity of the tips. Indeed the dynein (heavy chain) nudA1ts mutation caused a pronounced effect on the apicobasal distribution of GFP-RabSRab7–labeled vacuoles. In nudA1ts germlings (Figure 10A) and hyphae (Figure 10B) there was a marked accumulation of vacuoles near the tip that was never seen in the wild type (wild-type tip-proximal regions are actually devoid of vacuoles). Clusters of nudA1ts vacuoles did not invade the actual tip of hyphae (Figure 10B). These findings strongly support a model according to which endosomes mature to LEs that become engaged in homotypic fusion events to coalesce into larger vacuoles as they move away from the tip (Figure 11). We also constructed nudA1 rabB∆ double-mutant strains expressing GFP-RabSRab7. This double mutation does not prevent the vacuolar localization of RabSRab7 (as noted, RabA is able to recruit CORVET), even though these strains display the characteristic nudA1 accumulation of vacuoles near the tip (Supplemental Figure S6B).
FIGURE 10:
nudA1ts results in the accumulation of vacuoles near the tip. (A) nudA1ts and control wild-type germlings and (B) a nudA1 hyphal tip expressing GFP-RabSRab7 under the control of the alcAp driver. In both cases cells were cultured at 37°C. (C) GFP-RabB and mCherry-RabSRab7 colocalize in tip aggregates of nudA1ts cells cultured on fructose. Shown are x-z orthogonal views of z-stacks in the positions of aggregates containing mCherry-RabSRab7 only (red, bottom), GFP-RabB only (green, middle), or both (top). In the latter, the overlap across several z-planes of red and green signals shows that RabB and RabSRab7 membranes overlap. (D) Accumulation of CMAC-stained vacuoles near the tip of nudA1ts and nudA1ts rabSΔ cells. Note that, in the nudA1ts rabSΔ cell, CMAC minivacuoles invade the hyphal tip. (E) Quantification of the surface occupied by CMAC-stained structures in the different mutant conditions shown in D. Hyphal tip cells were segmented into four 6-μm-long regions as indicated.
FIGURE 11:
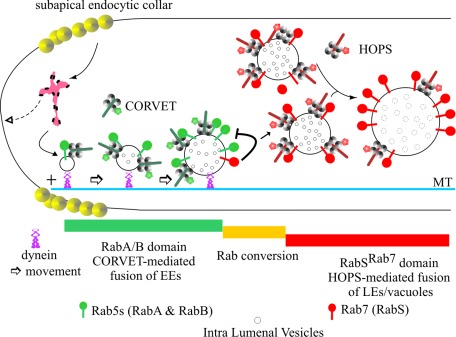
A model for endosomal maturation in A. nidulans. This model for endosomal maturation incorporates the conclusions of this work into the interpretation of the endocytic pathway derived from previous reports (Ohsumi et al., 2002; Peñalva, 2005; Abenza et al., 2009, 2010; Hervás-Aguilar and Peñalva, 2010; Hervás-Aguilar et al., 2010; Zhang et al., 2010, 2011; Griffith et al., 2011; Pantazopoulou and Peñalva, 2011). Endocytosis predominates in the tip. Endocytic vesicles reach a hypothetical endosomal compartment that would act as a sorting endosome, organized as a mosaic of domains (magenta). We speculate that this mosaic includes domains from which membrane and cargo can recycle to the plasma membrane, segregating from other domains in which the two Rab5s—RabA and RabB—determine “degradative” EE identity (i.e., specify the identity of membranes destined to the vacuole). It is generally accepted that efficient endocytic recycling is required for hyphal morphogenesis. Thus the hypothetical existence of a sorting endosome acting upstream of RabB is supported by the fact that rabB∆ has a relatively minor effect on hyphal morphogenesis (Abenza et al., 2010). Degradative EEs become loaded on dynein. RabB is the major player in establishing this “degradative identity,” as it is required for EE movement and mediates recruitment of Vps45 and Vps34 to endosomes (not depicted; Abenza et al., 2010). Vps45 enables endosomes to accept Golgi traffic required for maturation, whereas Vps34 synthesizes phosphatidyl inositol-3-phosphate, the landmark of degradative endosome identity, initiating the MVB pathway at the stage of EEs (Abenza et al., 2010; Hervás-Aguilar et al., 2010). RabB and, less efficiently, RabA recruit CORVET, mediating homotypic fusion between EEs, with key involvement of Vps8 (Markgraf et al., 2009). As endosomes increase their size, they decrease their motility and acquire their final composition (i.e. become LEs). Then RabSRab7 substitutes Rab5s. Finally, LEs undergo further fusion between them and with vacuoles in a HOPS-dependent manner. The negative feedback loop that may help to release Rab5s from endosomes is depicted.
We conducted additional experiments using CMAC to visualize vacuoles in cells that do not express any fluorescent Rab. nudA1 hyphal tip cells indeed accumulate clusters of small vacuoles within the apicalmost 10- to 20-μm region. These clusters were absent in the wild type (Figure 10, D and E), in agreement with data presented earlier. We also noticed that in rabS∆ cells, the otherwise ubiquitous minivacuoles are absent from the tip (Figure 10, D and E), suggesting that they are excluded from this region, predictably by dynein-mediated transport. If this were true, in nudA1ts cells minivacuoles should invade the tip, forming a minivacuolar analogue of the EE cluster characteristic of dynein-deficient hyphal tips (Lenz et al., 2006; Zekert and Fischer, 2008; Abenza et al., 2009). This is indeed the case (Figure 10, D and E). Double-mutant nudA1ts rabS∆ cells accumulate most of their minivacuoles at and near the tip, with the rest of the cell largely devoid of them. Invasion of nudA1ts tip regions by minivacuoles is very conspicuous in young branches (Supplemental Figure S6A).
Finally, we hypothesized that by impairing EE movement, nudA1 would confine maturation of EEs into LEs/vacuoles within the tip. Thus we examined nudA1 cells coexpressing fluorescent RabB and RabSRab7 that had been shifted to the restrictive temperature for 4 h. These cells contained the characteristic nudA1 aggregate of RabB-containing endosomes unable to depart from the tip (Figure 10C) and the also characteristic tip-proximal clusters of RabSRab7 vacuolar structures. RabB aggregates and RabSRab7 structures were very closely associated and showed partial colocalization (Figure 10C and Supplemental Figure S7). However, in every example analyzed (n = 47 tips examined with Dual-Viewer z-stacks), the cluster of LE/vacuolar structures was less apical or, at most, overlapped with the EE aggregate (Supplemental Figure S7), strongly supporting the view that conversion of RabB EEs into RabSRab7 LEs/vacuoles is coupled to basipetal movement of the former.
DISCUSSION
In U. maydis and A. nidulans kinesin-3 and dynein mediate the long-distance movement of EEs (Wedlich-Soldner et al., 2002; Lenz et al., 2006; Zekert and Fischer, 2008; Abenza et al., 2009, 2010). Endocytosis predominates in the tip region (Araujo-Bazán et al., 2008; Taheri-Talesh et al., 2008), which is the major source of EEs (Figure 11). These endosomes move away from the tip using dynein. Thus, in A. nidulans strains deficient in the NudA dynein, in the dynein activator NudF, in the KinA kinesin-1 transporting dynein to MT plus ends, or in the dynactin p25 subunit linking EEs to dynein, EEs aggregate in the tips (Zekert and Fischer, 2008; Abenza et al., 2009; Zhang et al., 2010, 2011).
In time-course experiments, the endocytic tracer FM4-64 arrives first to motile EEs and, in a posterior stage, to a population of larger, static LEs (Peñalva, 2005; Hervás-Aguilar et al., 2010). Thus the transition from EE to LEs takes place with simultaneous loss of motility. In our model, maturation of EEs is coupled to their movement away from the tip (Figure 11), resembling the situation in mammalian cells, in which Rab5 EEs that form in the cell periphery (the hyphal tip equivalent) move centripetally (i.e., basipetally in fungal hyphae) on dynein while they augment their size (Rink et al., 2005). However, in mammalian cells, LEs/lysosomes also undergo dynein-mediated centripetal transport toward the minus ends of MTs. Recruitment of dynein-dynactin to LEs/lysosomes is mediated by the Rab7 effector RILP (Jordens et al., 2001). RILP homologues are absent from fungi. Thus inefficient dynein recruitment might contribute to the reduced motility of A. nidulans LEs enriched in RabSRab7.
Our model in Figure 11 accounts for the observations that tip-proximal regions contain very few vacuoles (the end product of endosomal maturation) and that vacuolar size increases with the distance to the tip. It is strongly supported by three findings: 1) Vacuoles accumulate near the tip region of nudA1ts cells; 2) unlike wild-type vacuoles, rabS∆ minivacuoles, which are abnormally motile, are uniformly dispersed across the cytosol; and 3) In nudA1ts cells, overlap of Rab5 and RabSRab7 membranes is detectable in the tip, suggesting that if retrograde EE movement is prevented, endosomes mature on the spot.
The absence of RabSRab7 prevents the formation of normal-sized vacuoles. The resulting minivacuoles have features of EEs: 1) They are able to eventually undergo long-distance movement; 2) they invade the apicalmost regions of nudA1ts tips, resembling EEs; and 3) they contain EE Rabs on their membranes. The finding that Rab5s invade LE/vacuolar membranes only if RabSRab7 is absent is consistent with the models in which maturation of EEs into LEs/vacuoles occurs by Rab conversion (Rink et al., 2005; Peplowska et al., 2007; Brocker et al., 2010). In a simplified model, this implies that Rab5/CORVET membrane domains are converted into Rab7/HOPS domains (Rink et al., 2005). A key regulator of this conversion is a complex of two proteins formed by Mon1p/Ccz1p in S. cerevisiae (Nordmann et al., 2010) and SAND-1/CCZ-1 in C. elegans (Kinchen and Ravichandran, 2010). The findings that SAND-1/CCZ-1 is itself a Rab5 effector (Kinchen and Ravichandran, 2010), that the localization to endosomes of Mon1p/Ccz1p is Vps21p (Rab5) and CORVET dependent (Nordmann et al., 2010), that the complex interacts with HOPS components (Nordmann et al., 2010; Poteryaev et al., 2010), and, of importance, that Mon1p/Ccz1p is the guanine nucleotide exchange factor (GEF) of Ypt7p (yeast Rab7) provide the mechanistic bases for the Rab5-to-Rab7 conversion: Rab5 recruits Mon1/Ccz1, then Mon1/Ccz1 activates Rab7, and the GEF is further stabilized by Rab7 effectors. Mon1/Ccz1 influences Rab5 localization (Nordmann et al., 2010). Given that C. elegans SAND-1 displaces the Rab5 GEF from membranes (Poteryaev et al., 2010), fungal Mon1/Ccz1 could plausibly act in a similar manner. The relatively sharp Rab transition that we observe is indeed consistent with this possibility. Moreover, the finding that, in the absence of RabSRab7, Rab5s (RabA/RabB) reach the vacuolar membranes suggests that RabSRab7 contributes to destabilizing the association of Rab5s with membranes (Figure 11).
Our data strongly indicate that the transition between Rab5s and RabSRab7 is sharp, as examples of rapidly moving RabA/RabB EEs that also contain RabSRab7 are very rare. Inspection of a large number of movies taken under a variety of conditions revealed that colocalization of RabA/B and RabSRab7 is relatively frequent in static or short-range moving particles or in large particles that move at speeds considerably below the average speed of EEs, most likely representing LEs whose mobility is constrained by their size. Thus RabSRab7 is recruited to membranes already at the level of LEs and it is not confined to vacuoles, in agreement with data in S. cerevisiae (Balderhaar et al., 2010). In contrast, RabA or RabB, both of which reside in EEs, can reach LEs but are normally absent from vacuoles.
Fusion of A. nidulans LEs with vacuoles and among vacuoles themselves is dispensable, as demonstrated by the relatively mild growth defects resulting from rabS∆, vps41∆ (this work) and vps39∆ (Oka et al., 2004). In contrast, double rabA∆ rabB∆ deletion is lethal (Abenza et al., 2010), and single deletion of each tested gene involved in maturation of EEs into LEs is very severely debilitating. Tested mutations include vps45∆, vps8∆ (CORVET; Abenza et al., 2010), vps18∆ and vac1∆ (this work), ESCRT∆ alleles (single vps27∆, vps23∆, vps20∆, vps24∆, and vps32∆ mutations), and a conditional expression allele of Vps4 (Rodríguez-Galán et al., 2009; Calcagno-Pizarelli et al., 2011; Galindo et al., 2012). Of note, the severely debilitating effect of ESCRT∆ alleles is suppressible by loss-of-function mutations in the filamentous fungal–specific sltA gene regulating cation homeostasis (Findon et al., 2010; Calcagno-Pizarelli et al., 2011), suggesting that the essential role of endosomal maturation might be related to cation homeostasis
rabS∆ minivacuoles are proteolytically competent and contain Pep12. Thus resident proteases or Pep12 must be able to reach vacuoles through endosomes, because the direct AP-3 pathway to the vacuole requires Ypt7p/HOPS/Vps41p (Angers and Merz, 2009; Cabrera et al., 2010). In the absence of RabSRab7, the formation of minivacuoles possibly occurs through the CORVET-mediated homotypic fusion of endosomes (Markgraf et al., 2009), but CORVET alone appears to be insufficient to sustain further enlargement of minivacuoles. Thus an attractive and as yet untested possibility is that CORVET and HOPS complexes mediate homotypic fusion events but that these events involve partners with different degrees of membrane curvature, depending on the Rab/tether combination.
MATERIALS AND METHODS
Aspergillus media and molecular biology
Synthetic complete medium (SC; Cove, 1966) contained 1% glucose and 5 mM ammonium tartrate unless otherwise indicated. Complete medium for Aspergillus (MCA) was used for strain maintenance. Strains are listed in Supplemental Table S2 (vps18Δ, Vac1Δ, and the double mutants rabAΔ-rabSΔ, rabBΔ-rabSΔ, and vps41Δ-rabBΔ strains were impossible to keep beyond the described experiments due to their severely debilitated phenotype). For growth tests involving different carbon sources, we cultivated the strains on SC containing 5 mM ammonium tartrate and 3% glucose, 0.1% fructose, or a combination of 1% ethanol and 0.02% glucose during 72 h at the indicated temperatures.
A. nidulans vps18 (digA), Vac1, vps41, and gdiA were identified as AN2266, AN3144, AN4876, and AN5895, respectively, in the A. nidulans genomic database. Deletion cassettes were constructed by PCR (Szewczyk et al., 2006), using primers listed in Supplemental Table S3 and Aspergillus fumigatus pyrGAf (for vps18Δ, rabSΔ, vac1Δ, and vps41Δ) or pyroAAf (for a second rabSΔ deletion allele) as selection markers. Recipient strains carried nkuAΔ, preventing nonhomologous recombination (Nayak et al., 2006). Constructs for integrative transformation were confirmed by sequencing. They were targeted to the pyroA or argB locus after transformation, using published methodology (Calcagno-Pizarelli et al., 2007; Pantazopoulou and Peñalva, 2009). Single-copy integration was verified by Southern blots.
Western blotting
To determine levels of GFP-RabSRab7 by Western blotting, we extracted proteins as described (Hervás-Aguilar et al., 2007). GFP fusion proteins were detected using mouse anti α-GFP (monoclonal cocktail, 1:5000; Roche, Indianapolis, IN). Actin (detected with mouse anti α-actin from ICN Biomedicals (Irvine, CA) using 1:80,000 dilution of the antibody) was used as loading control. For the extraction of AgtA-GFP we used a reported procedure (Hervás-Aguilar and Peñalva, 2010) involving solubilization of proteins from lyophilized mycelial biomass with NaOH, followed by their precipitation with trichloroacetic acid.
Microscopy
Cells were grown in submerged cultures at 25°C in watch minimal medium (WMM), using Lab-Tek chambers (Nalge Nunc International, Rochester, NY) for 14–16 h before proceeding to microscopy (Pantazopoulou and Peñalva, 2009). To modulate the expression levels of the fluorescent chimeras expressed under the control of alcAp, we used different carbon sources (Abenza et al., 2009): low levels were attained with 0.1% (wt/vol) fructose, and high levels were obtained using 1% ethanol (vol/vol) or by preculturing cells on 0.02–0.05% (wt/vol) glucose and shifting them to 1% (vol/vol) ethanol for 3–4 h. In RabA-RabSRab7 and in RabB-RabSRab7 colocalization experiments, intermediate expression levels were achieved by culturing cells in WMM containing 1% (vol/vol) glycerol as carbon source. Mature endosomes/vacuoles were detected with CMAC as described (Abenza et al., 2009; Pantazopoulou and Peñalva, 2009). To determine the effects of the nudA1ts mutation, cells were cultivated at 37°C, which is a semirestrictive temperature, or overnight at 25°C (permissive temperature) and then shifted to 37°C for a few hours before image acquisition. To impede movement of rabS∆ minivacuoles, benomyl was added to WMM at a final concentration of 4.8 μg/ml as described (Abenza et al., 2009). For experiments involving delivery of AgtA-GFP to the vacuole, cells were cultured at 25°C for 14–16 h in WMM containing 5 mM l-glutamate as nitrogen source and then shifted to the same medium in which 5 mM ammonium tartrate substituted for l-glutamate. AgtA-GFP images were taken at the 0-min time point and 80 min after the shift (Abenza et al., 2009, 2010).
Images were acquired using a Hamamatsu ORCA ER-II camera (Hamamatsu, Hamamatsu, Japan) coupled to a Leica DMI6000B microscope (Leica, Wetzlar, Germany) driven by MetaMorph software (Molecular Dynamics, Sunnyvale, CA) and equipped with an EL6000 external light source for epifluorescence excitation. The microscope was equipped with HCX 63×, 1.4 numerical aperture (NA), and 100×, 1.4 NA, objectives and BrightLine GFP-3035B (Semrock, Rochester, NY), TXRED-4040B (mCherry), and standard 4′,6-diamidino-2-phenylindole (CMAC) filter sets. For colocalization experiments, a strictly simultaneous imaging of GFP and mCherry was carried out using a Dual-View imaging system (Photometrics, Tucson, AZ), using the recommended filter set (Pantazopoulou and Peñalva, 2011). MetaMorph software was used for contrast adjustment, Dual-View channel alignments, color combining, z-stack maximal-intensity projections (contrasted, when indicated, with the MetaMorph “unsharp mask” filter) and for the assembly of kymographs from time-lapse series. Images were converted to 8-bit grayscale (and usually shown in inverted contrast) or to 24-bit RGB and annotated with Corel Draw (Corel, Ottawa, Canada). For some z-stacks we improved image quality by using a blind deconvolution procedure (AutoDeblur software; Media Cybernetics, Bethesda, MD). Time-lapse sequences were converted to QuickTime using ImageJ 1.37 (National Institutes of Health, Bethesda, MD). Statistical analyses were performed using GraphPad Prism 5.00 for Windows (GraphPad Software, La Jolla, CA).
Affinity purification of RabSRab7 effectors
Affinity purification of effectors using GDP- or GTP-γ-S GST-RabSRab7 glutathione-Sepharose beads was made as described for GST-RabB. Glutathione-Sepharose 4B beads containing GST-RabSRab7 were incubated with A. nidulans protein extracts in 50-ml Falcon tubes before eluting the interacting proteins, which were resolved by SDS–PAGE cells, excised, and analyzed by matrix-assisted laser desorption/ionization peptide mass fingerprinting and tandem mass spectrometry (MS/MS), as detailed (Abenza et al., 2010).
Supplementary Material
Acknowledgments
We thank E. Reoyo for technical assistance. This work was supported by the Spanish government through Dirección General de Investigación Científica y Técnica Grant BIO2009-7281 and by Comunidad de Madrid Networking Grant SAL/0246/2006 to M.A.P. J.F.A. was a Consejo Superior de Investigaciones Cientificas I3P predoctoral fellow.
Abbreviations used:
- CMAC
7-amino-4-chloromethyl-coumarin
- CORVET
class C core vacuole/endosome tethering
- EE
early endosome
- GDI
GDP dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- GST
glutathione S-transferase
- HOPS
homotypic fusion and vacuole protein sorting
- MT
microtubule
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-11-0925) on March 28, 2012.
REFERENCES
- Abenza JF, Galindo A, Pantazopoulou A, Gil C, de los Ríos V, Peñalva MA. Aspergillus RabBRab5 integrates acquisition of degradative identity with the long-distance movement of early endosomes. Mol Biol Cell. 2010;21:2756–2769. doi: 10.1091/mbc.E10-02-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenza JF, Pantazopoulou A, Rodríguez JM, Galindo A, Peñalva MA. Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic. 2009;10:57–75. doi: 10.1111/j.1600-0854.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- Angers CG, Merz AJ. HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles. Mol Biol Cell. 2009;20:4563–4574. doi: 10.1091/mbc.E09-04-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolaki A, Erpapazoglou Z, Harispe L, Billini M, Kafasla P, Kizis D, Peñalva MA, Scazzocchio C, Sophianopoulou V. AgtA, the dicarboxylic amino acid transporter of Aspergillus nidulans, is concertedly down-regulated by exquisite sensitivity to nitrogen metabolite repression and ammonium-elicited endocytosis. Eukaryot Cell. 2009;8:339–352. doi: 10.1128/EC.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo-Bazán L, Peñalva MA, Espeso EA. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol Microbiol. 2008;67:891–905. doi: 10.1111/j.1365-2958.2007.06102.x. [DOI] [PubMed] [Google Scholar]
- Balderhaar HJ, Arlt H, Ostrowicz C, Brocker C, Sundermann F, Brandt R, Babst M, Ungermann C. The Rab GTPase Ypt7 is linked to retromer-mediated receptor recycling and fusion at the yeast late endosome. J Cell Sci. 2010;123:4085–4094. doi: 10.1242/jcs.071977. [DOI] [PubMed] [Google Scholar]
- Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Brocker C, Engelbrecht-Vandre S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol. 2010;20:R943–R952. doi: 10.1016/j.cub.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Cabrera M, et al. Phosphorylation of a membrane curvature-sensing motif switches function of the HOPS subunit Vps41 in membrane tethering. J Cell Biol. 2010;191:845–859. doi: 10.1083/jcb.201004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno-Pizarelli AM, Hervás-Aguilar A, Galindo A, Abenza JF, Peñalva MA, Arst HN., Jr Rescue of Aspergillus nidulans severely debilitating null mutations in ESCRT-0, I, II and III genes by inactivation of a salt-tolerance pathway allows examination of ESCRT gene roles in pH signalling. J Cell Sci. 2011;124:1–13. doi: 10.1242/jcs.088344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno-Pizarelli AM, et al. Establishment of the ambient pH signaling complex in Aspergillus nidulans: PalI assists plasma membrane localization of PalH. Eukaryot Cell. 2007;6:2365–2375. doi: 10.1128/EC.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove DJ. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Findon H, Calcagno-Pizarelli AM, Martínez JL, Spielvogel A, Markina-Iñarrairaegui A, Indrakumar T, Ramos J, Peñalva MA, Espeso EA, Arst HN., Jr Analysis of a novel calcium auxotrophy in Aspergillus nidulans. Fungal Genet Biol. 2010;47:647–655. doi: 10.1016/j.fgb.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A, Calcagno-Pizarelli AM, Arst HN, Jr, Peñalva MA. An ordered pathway for the assembly of ESCRT-containing fungal ambient pH signalling complexes at the plasma membrane. J Cell Sci. 2012;125:1784–1795. doi: 10.1242/jcs.098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo A, Hervás-Aguilar A, Rodríguez-Galán O, Vincent O, Arst HN, Jr, Tilburn J, Peñalva MA. PalC, one of two Bro1 domain proteins in the fungal pH signaling pathway, localizes to cortical structures and binds Vps32. Traffic. 2007;8:1346–1364. doi: 10.1111/j.1600-0854.2007.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissenhoner A, Sievers N, Brock M, Fischer R. Aspergillus nidulans DigA, a potential homolog of Saccharomyces cerevisiae Pep3 (Vps18), is required for nuclear migration, mitochondrial morphology and polarized growth. Mol Genet Genomics. 2001;266:672–685. doi: 10.1007/s00438-001-0589-6. [DOI] [PubMed] [Google Scholar]
- Griffith J, Peñalva MA, Reggiori F. Adaptation of the Tokuyasu method for the ultrastructural study and immunogold labelling of filamentous fungi. J Electron Microsc. 2011;60:211–216. doi: 10.1093/jmicro/dfr026. [DOI] [PubMed] [Google Scholar]
- Hervás-Aguilar A, Peñalva MA. Endocytic machinery protein SlaB is dispensable for polarity establishment but necessary for polarity maintenance in hyphal tip cells of Aspergillus nidulans. Eukaryot Cell. 2010;9:1504–1518. doi: 10.1128/EC.00119-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervás-Aguilar A, Rodríguez JM, Tilburn J, Arst HN, Jr, Peñalva MA. Evidence for the direct involvement of the proteasome in the proteolytic processing of the Aspergillus nidulans zinc finger transcription factor PacC. J Biol Chem. 2007;282:34735–34747. doi: 10.1074/jbc.M706723200. [DOI] [PubMed] [Google Scholar]
- Hervás-Aguilar A, Rodríguez-Galán O, Galindo A, Abenza JF, Arst HN, Jr, Peñalva MA. Characterization of Aspergillus nidulans DidBDid2, a non-essential component of the multivesicular body pathway. Fungal Genet Biol. 2010;47:636–646. doi: 10.1016/j.fgb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I, Fernández-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Ravichandran KS. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 2010;464:778–782. doi: 10.1038/nature08853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JH, Schuchardt I, Straube A, Steinberg G. A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J. 2006;25:2275–2286. doi: 10.1038/sj.emboj.7601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markgraf DF, Ahnert F, Arlt H, Mari M, Peplowska K, Epp N, Griffith J, Reggiori F, Ungermann C. The CORVET subunit Vps8 cooperates with the Rab5 homolog Vps21 to induce clustering of late endosomal compartments. Mol Biol Cell. 2009;20:5276–5289. doi: 10.1091/mbc.E09-06-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. A versatile and efficient gene targeting system for Aspergillus nidulans. Genetics. 2006;172:1557–1566. doi: 10.1534/genetics.105.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, Ungermann C. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol. 2010;20:1654–1659. doi: 10.1016/j.cub.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Ohsumi K, Arioka M, Nakajima H, Kitamoto K. Cloning and characterization of a gene (avaA) from Aspergillus nidulans encoding a small GTPase involved in vacuolar biogenesis. Gene. 2002;291:77–84. doi: 10.1016/s0378-1119(02)00626-1. [DOI] [PubMed] [Google Scholar]
- Oka M, Maruyama J, Arioka M, Nakajima H, Kitamoto K. Molecular cloning and functional characterization of avaB, a gene encoding Vam6p/Vps39p-like protein in Aspergillus nidulans. FEMS Microbiol Lett. 2004;232:113–121. doi: 10.1016/S0378-1097(04)00039-4. [DOI] [PubMed] [Google Scholar]
- Ostrowicz CW, Brocker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, Perz A, Auffarth K, Engelbrecht-Vandre S, Ungermann C. Defined subunit arrangement and Rab interactions are required for functionality of the HOPS tethering complex. Traffic. 2010;11:1334–1346. doi: 10.1111/j.1600-0854.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- Pantazopoulou A, Peñalva MA. Organization and dynamics of the Aspergillus nidulans Golgi during apical extension and mitosis. Mol Biol Cell. 2009;20:4335–4347. doi: 10.1091/mbc.E09-03-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulou A, Peñalva MA. Characterization of Aspergillus nidulans RabCRab6. Traffic. 2011;12:386–406. doi: 10.1111/j.1600-0854.2011.01164.x. [DOI] [PubMed] [Google Scholar]
- Peñalva MA. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet Biol. 2005;42:963–975. doi: 10.1016/j.fgb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Peñalva MA. Endocytosis in filamentous fungi: Cinderella gets her reward. Curr Opin Microbiol. 2010;13:684–689. doi: 10.1016/j.mib.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell. 2007;12:739–750. doi: 10.1016/j.devcel.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol. 2000;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Emr SD. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Galán O, Galindo A, Hervás-Aguilar A, Arst HN, Jr, Peñalva MA. Physiological involvement in pH signalling of Vps24-mediated recruitment of Aspergillus PalB cysteine protease to ESCRT-III. J Biol Chem. 2009;284:4404–4412. doi: 10.1074/jbc.M808645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Ferrero JC, Peñalva MA. Endocytosis. In: Goldman GH, Osmani SA, editors. In: The Aspergilli: Genomics, Medical Aspects, Biotechnology, and Research Methods. Boca Raton, FL: CRC Press; 2006. pp. 177–195. [Google Scholar]
- Schuster M, Lipowsky R, Assmann MA, Lenz P, Steinberg G. Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc Natl Acad Sci USA. 2011;108:3618–3623. doi: 10.1073/pnas.1015839108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N. Coordination of intracellular transport steps by GTPases. Semin Cell Dev Biol. 2011;22:33–38. doi: 10.1016/j.semcdb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G. On the move: endosomes in fungal growth and pathogenicity. Nat Rev Microbiol. 2007;5:309–316. doi: 10.1038/nrmicro1618. [DOI] [PubMed] [Google Scholar]
- Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc. 2006;1:3111–3120. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- Taheri-Talesh N, Horio T, Araujo-Bazán L, Doux X, Espeso EA, Peñalva MA, Osmani SA, Oakley BR. The tip growth apparatus of Aspergillus nidulans. Mol Biol Cell. 2008;19:1439–1449. doi: 10.1091/mbc.E07-05-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Straube A, Friedrich MW, Steinberg G. A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis. EMBO J. 2002;21:2946–2957. doi: 10.1093/emboj/cdf296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekert N, Fischer R. The Aspergillus nidulans kinesin-3 UncA motor moves vesicles along a subpopulation of microtubules. Mol Biol Cell. 2008;20:673–684. doi: 10.1091/mbc.E08-07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yao X, Fischer L, Abenza JF, Penalva MA, Xiang X. The p25 subunit of the dynactin complex is required for dynein-early endosome interaction. J Cell Biol. 2011;193:1245–1255. doi: 10.1083/jcb.201011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhuang L, Lee Y, Abenza JF, Peñalva MA, Xiang X. The microtubule plus-end localization of Aspergillus dynein is important for dynein-early-endosome interaction but not for dynein ATPase activation. J Cell Sci. 2010;123:3596–3604. doi: 10.1242/jcs.075259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.