Connective tissue growth factor (CTGF/CNN2) is a novel APF target gene. A novel mechanism is described by which the APF cellular receptor, cytoskeleton-associated protein 4 (CKAP4), mediates APF-induced CTGF transcription.
Abstract
Antiproliferative factor (APF) is a sialoglycopeptide elevated in the urine of patients with interstitial cystitis (IC)—a chronic, painful bladder disease of unknown etiology. APF inhibits the proliferation of normal bladder epithelial and T24 bladder carcinoma cells in vitro by binding to cytoskeleton-associated protein 4 (CKAP4) and altering the transcription of genes involved in proliferation, cellular adhesion, and tumorigenesis; however, specific molecular mechanisms and effector genes that control APF's antiproliferative effects are unknown. In this study, we found that there was a 7.5-fold up-regulation of connective tissue growth factor (CTGF/CCN2) expression in T24 bladder carcinoma cells treated with APF. Western blot revealed a dose-dependent increase in CCN2 protein levels, with secretion into the culture medium after APF treatment. CCN2 overexpression enhanced APF's antiproliferative activity, whereas CCN2 knockdown diminished APF-induced p53 expression. Using a luciferase reporter construct, we found that APF treatment resulted in fivefold activation of the CCN2 proximal promoter and, of importance, that small interfering RNA–mediated knockdown of CKAP4 inhibited CCN2 upregulation. In addition, we demonstrate that CKAP4 translocates to the nucleus and binds to the CCN2 proximal promoter in an APF-dependent manner, providing evidence that CCN2 regulation by APF involves CKAP4 nuclear translocation and binding to the CCN2 promoter.
INTRODUCTION
Antiproliferative factor (APF) is a low–molecular weight, Frizzled 8–related, sialoglycopeptide elevated in the urine of patients with interstitial cystitis (IC)—a chronic, painful bladder disease of unknown etiology (Keay et al., 2000, 2004b). APF inhibits normal bladder epithelial cell growth (Keay et al., 1996, 2000, 2004a), as well as the proliferation of T24 bladder carcinoma cells and HeLa cells in vitro, with an IC50 of ∼1 nM (Keay et al., 2004a; Conrads et al., 2006; Kim et al., 2007; Planey et al., 2009; Shahjee et al., 2010). Although the specific molecular mechanisms and effector genes that control the antiproliferative effects of APF are not known, APF signal transduction and gene expression requires binding to its palmitoylated, cellular receptor, cytoskeleton-associated protein 4 (CKAP4; Keay et al., 2003a; Conrads et al., 2006; Kim et al., 2007; Planey et al., 2009; Shahjee et al., 2010). Reducing CKAP4 expression with either small interfering RNA (siRNA) or anti-CKAP4 antibodies in competition experiments or inhibition of CKAP4 palmitoylation inhibits APF effects on cell proliferation and gene expression (Conrads et al., 2006; Planey and Zacharias, 2009). More recently, it was shown that changes in MMP2/p53 protein expression and Akt/GSK3β/β-catenin phosphorylation in response to APF treatment were specifically abrogated after CKAP4 siRNA knockdown in T24 bladder carcinoma cells (Shahjee et al., 2010). These data indicate that CKAP4 is essential for mediating the antiproliferative activity of APF, although its function in this regard remains undefined.
CKAP4 (also known as p63, CLIMP-63, or ERGIC-63) is a 63-kDa, nonglycosylated, reversibly palmitoylated, type II transmembrane protein. Original studies described CKAP4 as an endoplasmic reticulum (ER)–resident protein that anchors the rough ER to microtubules in epithelial cells (i.e., COS and HeLa; Schweizer et al., 1993, 1994) and plays an essential role in the maintenance of ER structure (Klopfenstein et al., 1998; Vedrenne et al., 2005; Vedrenne and Hauri, 2006); however, recent studies provide growing evidence that CKAP4 also localizes to the cell surface, where it functions as a receptor (Razzaq et al., 2003; Bates, 2010; Kazi et al., 2010) and translocates to the nucleus in response to specific ligands such as APF (Zhang et al., 2008; Planey et al., 2009). CKAP4 has a 106–amino acid–long cytosolic tail, a single, 21–amino acid transmembrane (TM) domain, and a large extracellular domain of 474 amino acids. The luminal/extracellular domain is predicted to fold into an extensive coiled-coil containing five heptad repeats (residues 468–503) that is predicted to be a leucine zipper (www.predictprotein.org). This coiled-coil domain is required for the formation of CKAP4 oligomers, as oligomerization is lost when this region is deleted (Klopfenstein et al., 2001). The sequence of residues that follows (503–602) is predicted to be helical as well (www.ebi.ac.uk/Tools/InterProScan/) and is relatively rich in the positively charged, basic amino acids. Examination of this sequence in helical wheel programs (e.g., http://cti.itc.virginia.edu/~cmg/Demo/wheel/wheelApp.html) shows that the helix is amphipathic, suggesting that this region may also be involved in intermolecular interactions. This arrangement of a leucine zipper domain and an adjacent amphipathic helix rich in basic residues is the hallmark of the bZIP DNA-binding transcription factor proteins (Vinson et al., 2006). CKAP4 is also regulated by palmitoylation of a cysteine residue adjacent to the TM domain at position 100 (C100). Of importance, DHHC2 was identified as the palmitoyl acyltransferase that palmitoylates CKAP4 on C100, and siRNA-mediated knockdown of DHHC2 was shown to inhibit plasma membrane localization of CKAP4 and, consequently, the ability of APF to inhibit proliferation (Zhang et al., 2008; Planey et al., 2009).
Little is known about the signaling mechanism by which APF alters gene expression and induces its antiproliferative activity. At least 14 genes have been shown to be altered in response to APF in bladder epithelial cells, including E-cadherin, cyclin D1, vimentin, α-1–catenin, heparin-binding epidermal growth factor–like growth factor, α-2–integrin, p53, occludin, zonula occludens-1, matrix metalloproteinase-2 (MMP2), β-catenin, and cyclooxygenase-2 (COX-2). However, the molecular mechanism(s) through which APF signaling acts and/or the molecular role of CKAP4 in modulating these genes has not been characterized (Keay et al., 2003a; Conrads et al., 2006; Kim et al., 2007; Shahjee et al., 2010; Yang et al., 2011). In this study, we sought to identify and characterize genes regulated by APF in T24 bladder carcinoma cells. We identified the matricellular protein CCN2 (formerly known as connective tissue growth factor [CTGF]) as a novel downstream target of APF signaling (Brigstock et al., 2003). CCN2 is a member of the CCN family, which consists of six secreted proteins that associate with the extracellular matrix primarily through direct binding to specific integrin receptors and heparin sulfate proteoglycans, thereby triggering signal transduction events that culminate in the regulation of cell adhesion, migration, proliferation, gene expression, differentiation, and survival (Chen and Lau, 2009). CCN proteins are induced by and have been shown to regulate the activity of various cytokines and growth factors, including transforming growth factor-β (TGF-β), tumor necrosis factor-α, vascular endothelial growth factor, bone morphogenetic proteins (BMPs), and Wingless-int (Wnt) proteins. They are also overexpressed in pathological conditions that affect connective tissues, including scarring, fibrosis, and cancer (Leask and Abraham, 2006). CCN2 appears to play a role in the extracellular matrix remodeling that occurs in normal physiological processes such as embryogenesis, implantation, and wound healing; however, in some contexts, it has been shown to have negative effects on cell growth, in that it can be antimitotic and apoptotic (Moussad and Brigstock, 2000; Perbal, 2004). In this study, we demonstrate a novel role for CKAP4 as a required downstream mediator for APF-induced CCN2 transcription.
RESULTS
APF induces CCN2 expression and promoter activation in T24 bladder carcinoma cells
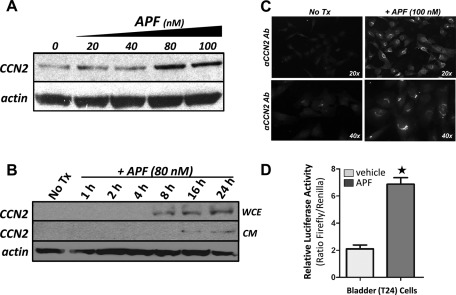
APF inhibits the proliferation of normal bladder epithelial, T24 bladder carcinoma, and HeLa cervical adenocarcinoma cells in vitro; however, the molecular mechanisms or downstream effector genes responsible for the antiproliferative effects of APF are not known (Keay et al., 2004a, 2004b; Rashid et al., 2004; Zhang et al., 2005; Planey and Zacharias, 2009; Shahjee et al., 2010). When we analyzed T24 bladder carcinoma cells treated with APF using a highly sensitive commercial PCR-based array system, we found that there was a 7.5-fold up-regulation of CCN2 expression, suggesting that CCN2 was a potential downstream effector gene for APF in T24 bladder carcinoma cells. To confirm that up-regulation of CCN2 mRNA coincided with an increase in CCN2 protein levels, we performed PAGE, followed by Western blot analysis on whole-cell lysates from T24 cells treated with or without APF. Western blot analysis revealed that APF induced CCN2 protein levels in a dose-dependent manner, and expression of CCN2 continued to increase up to 24 h after addition of APF to the medium, which was the latest time point examined (Figure 1, A and B, respectively). This increase in CCN2 protein expression was associated with an increase in secretion of CCN2 into the cell culture medium at 16 h and did not appear to be increased by 24 h (Figure 1B). Using immunocytochemistry, we also confirmed increased expression of CCN2 protein in T24 cells using a primary antibody against CCN2 and DyLight 488–labeled secondary antibody (Figure 1C). In addition, we tested whether the CCN2 promoter was responsive to APF treatment using a luciferase reporter containing the CCN2 proximal promoter transfected into T24 cells. APF treatment resulted in a fivefold activation of the CCN2 proximal promoter (Figure 1D), demonstrating that the CCN2 proximal promoter is responsive to APF and suggesting that there are APF-responsive elements in the CCN2 promoter.
FIGURE 1:
APF induces CCN2 expression in a dose-dependent and specific manner. (A) Whole-cell lysates from T24 bladder carcinoma cells exposed to APF (at indicated doses) for 24 h or vehicle treated (0) were examined for CCN2 expression (α-CCN2; diluted 1:200). (B) Western blot analysis of whole-cell lysates from T24 cells treated with APF for 0–24 h as indicated. Because CCN2 is known to be secreted, conditioned media from cells harvested at each time point were also analyzed by Western blot. Membranes were stripped and reprobed with an actin antibody as a control for loading. Cells were serum starved 24 h before APF exposure. We observed that T24 cells do express a basal amount of CCN2 and that expression is greatly enhanced upon treatment with APF at doses of 80–100 nM (A); however, we have not detected basally secreted CCN2 in our cell cultures (B). The apparent discrepancy in CCN2 basal expression in A and B is a matter of exposure of the blot, as showing the difference in expression of the 8-, 16-, and 24-h treatment points required a lighter exposure. (C) T24 cells were grown on LabTek multiwell glass slides, fixed, and immunolabeled with a goat polyclonal antibody against CCN2 (diluted 1:200) followed by a DyLight 488–conjugated rabbit anti-goat secondary antibody (diluted 1:1000). The cells were imaged by epifluorescence microscopy at 20× and 40×. (D) T24 bladder carcinoma cells were plated in 96-well tissue culture plates and transfected with 0.1 μg of pGL3-W787 (CCN2 promoter) and cotransfected with 0.2 μg of a Renilla luciferase expression vector (internal control). The cells were serum starved for 24 h and then treated with APF (100 ng/ml) for 24 h. Luciferase activity was then assessed and expressed as a ratio of firefly/Renilla luciferase (+SEM , n = 3). * p < 0.001.
CCN2 overexpression enhances APF antiproliferative activity in T24 bladder carcinoma cells
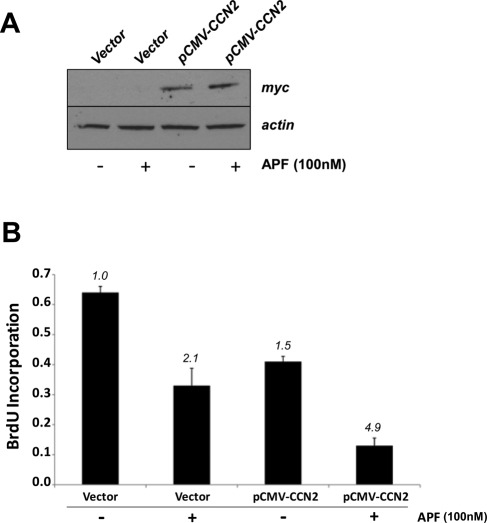
The inhibitory effect of APF on the growth of T24 bladder carcinoma cells has been demonstrated (Kim et al., 2007; Shahjee et al., 2010). To assess whether a high CCN2 protein level is associated with the inhibitory effect of APF on T24 cell proliferation, we transiently transfected cells with either pCMV-Tag1 vector containing full-length CCN2 fused to a c-myc epitope tag at its C-terminus or empty vector as a control and then treated the cells with 100 nM APF for 48 h. CCN2 overexpression was confirmed by Western blot using an anti-myc antibody (Figure 2A), and the effect of CCN2 on cell growth was evaluated using the bromodeoxyuridine (BrdU) proliferation assay. As demonstrated in Figure 2B, the rate of growth of T24 cells overexpressing CCN2 was reduced 1.5-fold relative to control cells. This reduction was enhanced in CCN2-overexpressing cells that had been treated with APF (4.9-fold reduction relative to control). APF treatment alone reduced T24 bladder carcinoma cell proliferation 2.0-fold relative to control cells.
FIGURE 2:
Overexpression of CCN2 inhibits cell proliferation of T24 bladder carcinoma cells. (A) Cells were transfected with either a CCN2 expression vector (pCMV-CCN2-myc) or an empty vector (pCMV-Tag 1) as described in Materials and Methods. On the third day, cells were serum starved for 8 h and then treated with 100 nM APF (Peptides International) diluted in serum-free McCoy's medium for 24 h. Cells were then harvested and total protein extracts separated by SDS–PAGE for analysis of CCN2 overexpression by Western blot using an anti-myc monoclonal antibody against the c-myc epitope tag fused to the 3′ end of CCN2. (B) The effect of CCN2 on cell growth was evaluated using the BrdU proliferation assay as described in Materials and Methods. Briefly, T24 bladder carcinoma cells were seeded at 1 × 103 per well in a 96-well cell culture plate, and the next day, designated wells were transfected with pCMV-CCN2-myc plasmid or pCMV-Tag 1 control plasmid. On the second day, cells were serum starved for 8 h and then treated with 100 nM APF diluted in serum-free McCoy's medium or vehicle (serum-free medium only). Twenty-four hours later, BrdU-labeling solution was added to each well, and cells were incubated at 37°C in a 5% CO2 atmosphere for an additional 24 h. BrdU incorporation into newly synthesized DNA was detected immunochemically. The results represent the mean of two independent experiments done in triplicate; bars, SE.
CCN2 mediates APF-induced p53 expression in T24 bladder carcinoma cells
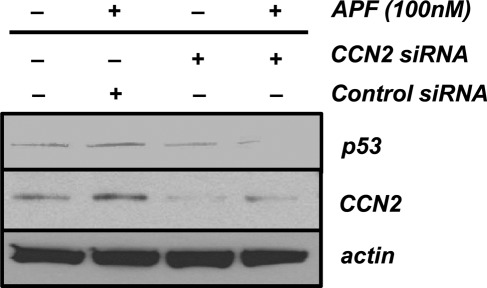
APF increases p53 expression in T24 bladder carcinoma cells in vitro, whereas siRNA-mediated knockdown of p53 decreases the antiproliferative effects of APF (Kim et al., 2007; Shahjee et al., 2010). Increased p53 expression and cellular growth suppression have also been observed in CCN2-overexpressing cancer cell lines, including non–small cell lung cancer (NSCLC) and breast cancer cells (Chang et al., 2004; Jiang et al., 2004; Lin et al., 2005; Chien et al., 2006). To determine whether CCN2 mediates APF-induced p53 expression, we transiently transfected T24 cells with control or CCN2-specific siRNA for 48 h before APF exposure. As demonstrated in Figure 3, transfection of T24 cells with CCN2 siRNA resulted in decreased protein expression of CCN2 relative to β-actin, as well as a significant reduction in p53 expression after APF exposure. In comparison, APF-treated cells transfected with control siRNA had increased p53 expression relative to β-actin and no decrease in CCN2 expression, indicating that CCN2 mediates APF-induced p53 expression in these cells and that APF and CCN2 may work in concert to affect similar signal transduction pathways.
FIGURE 3:
CCN2 mediates APF-induced p53 expression in T24 bladder carcinoma cells. T24 cells were transfected with control or CCN2-specific siRNA for 48 h before treatment with 100 nM APF or vehicle (serum-free medium only) for 24 h. p53 and CCN2 protein expression was analyzed by SDS–PAGE, followed by Western blotting with antibodies to p53 and CCN2 as described in Materials and Methods. To assess equal loading of protein, membranes were stripped and reprobed with an actin antibody. Proteins were visualized by enhanced chemiluminescence and subsequent exposure to film.
CKAP4 is required for APF-induced CCN2 expression
The antiproliferative activity of APF is blocked by preincubation with anti–CKAP4-specific antibodies, siRNA-mediated knockdown of CKAP4, and siRNA-mediated knockdown of DHHC2, the enzyme that palmitoylates CKAP4 (Conrads et al., 2006; Planey et al., 2009). To determine whether CKAP4 is required for APF induction of CCN2, we preincubated T24 cells with an anti-CKAP4 antibody and measured CCN2 protein levels after APF treatment by Western blot. As shown in Figure 4A, preincubation of T24 cells with an anti-CKAP4 antibody blocked the ability of APF to up-regulate CCN2 protein expression, suggesting that CKAP4 is an important downstream mediator of CCN2 induction by APF. To confirm this result, we transiently transfected T24 cells with control or CKAP4-specific siRNA for 72 h (maximal reduction as determined in Figure 4B) before APF exposure. As demonstrated in Figure 4C, transfection of T24 cells with siRNA for CKAP4 resulted in decreased protein expression of CKAP4 relative to β-actin (Figure 4C), as well as a significant reduction in CCN2 expression after APF exposure. In comparison, cells transfected with control siRNA had little or no change in CKAP4 expression relative to β-actin, and there was no decrease in CCN2 expression, indicating that CKAP4 is required for APF-induced CCN2 expression in these cells. CKAP4 siRNA did not affect CCN2 basal expression (unpublished data). Of importance, pretreatment of cells with CKAP4-specific siRNA also inhibited APF activation of the CCN2 promoter, as shown in Figure 4D.
FIGURE 4:
siRNA-mediated knockdown of CKAP4 inhibits APF-induced CCN2 expression. Western blot analysis of CCN2 expression in whole-cell lysates from T24 bladder carcinoma cells. (A) Cells were pretreated with anti-CKAP4 antibody (1 μg/ml) for 1.5 h and then treated with APF (100 nM) for 24 h. (B) Cells were transfected with CKAP4-specific siRNA or control siRNA for 24–72 h to determine the optimal time for CKAP4 knockdown. (C) At 72 h after transfection with CKAP4-specific siRNA or control siRNA, T24 cells were exposed to 100 nM APF for 24 h. Membranes were stripped and reprobed with an actin antibody as a control for loading. Cells were serum starved 24 h before APF exposure. (D) T24 bladder carcinoma cells were plated in a 96-well tissue culture plates and transfected with CKAP4-specific siRNA. At 24 h later, cells were cotransfected with 0.1 μg of pSGG-CCN2 promoter construct and 0.2 μg of a Renilla luciferase expression vector (internal control) and serum starved overnight. The cells were then treated with APF (100 ng/ml) or vehicle (serum-free medium) for 24 h, and luciferase activity was measured and expressed as a ratio of firefly/Renilla luciferase (+SEM , n = 3); *p < 0.001.
CKAP4 translocates to the nucleus in response to APF
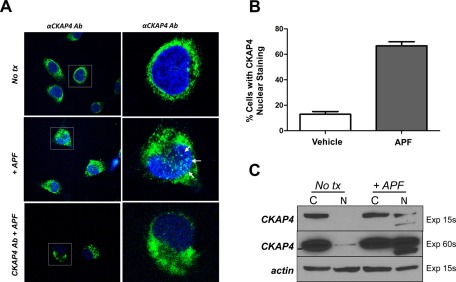
Previously we showed that CKAP4 became more abundant in the nucleus of HeLa cells after APF exposure (Planey et al., 2009). To determine whether CKAP4 localizes to the nucleus of T24 bladder carcinoma cells in response to APF treatment, we performed immunocytochemistry. Vehicle- or APF-treated T24 cells were fixed and incubated with an anti-CKAP4 monoclonal antibody, followed by a DyLight 488–labeled secondary antibody and Hoechst dye to counterstain nuclei and then visualized using confocal microscopy. As shown in Figure 5A, APF treatment resulted in a redistribution of CKAP4 to the nucleus of T24 cells. However, in APF-treated cells that were preincubated with anti-CKAP4 antibody, this increase in nuclear accumulation of CKAP4 was inhibited. Quantitative analysis of APF versus vehicle-treated cells showed that 67% of cells treated with APF contained CKAP4 nuclear staining versus 12.9% of vehicle-treated cells (Figure 5B). In cells pretreated with CKAP4 antibody, CKAP4 nuclear staining was similar to that in vehicle-treated cells (unpublished data). Furthermore, Western blot analysis performed on cytosolic and nuclear protein fractions isolated from APF-treated and untreated cells demonstrated an increase in the relative abundance of CKAP4 in the nucleus (Figure 5C). Prolonged exposure detected a relatively small amount of CKAP4 in the nucleus of untreated cells in this experiment, which is consistent with data from other experiments (Figure 6D), suggesting that CKAP4 may have some inherent nuclear function not related to APF-induced signal transduction. Of importance, the detection of a second, smaller, CKAP4-specific band in the nuclear fraction of APF-treated cells suggests that CKAP4 may be proteolyzed before entering the nucleus. Collectively, these data support our prior observations in HeLa cells (Planey et al., 2009) and demonstrate that APF can stimulate the nuclear translocation of CKAP4 in T24 bladder carcinoma cells.
FIGURE 5:
Endogenous CKAP4 localizes to the nucleus of T24 cells after APF treatment. (A) Confocal microscopy of anti-CKAP4–labeled T24 cells treated with APF (100 nM), APF (100 nM) plus anti-CKAP4 antibody (2 μg), or vehicle (serum-free medium) for 24 h. T24 cells were grown on tissue culture slides, fixed, and incubated with anti-CKAP4 (anti-CLIMP-63, diluted 1:1000), followed by DyLight 488–labeled secondary antibodies and Hoechst dye to counterstain nuclei. Images on the right represent zoomed-in area of images marked by boxes on the left. (B) Quantitative analysis of percentage of cells with CKAP4 nuclear staining in APF vs. vehicle-treated cells. One hundred cells from each treatment condition were counted and the average percentage of cells with CKAP4 nuclear staining was determined. Sixty-seven percent of cells treated with APF contained CKAP4 nuclear staining vs. 12.9% of vehicle-treated cells. (C) T24 cells were treated with APF (100 nM) for 24 h, which resulted in a significant increase in the abundance of CKAP4 in the nucleus compared with control samples. Treated cells were harvested, and the nuclear and cytosolic fractions were isolated and separated by SDS–PAGE as described in Materials and Methods. Protein expression was analyzed by Western blotting with antibodies for CKAP4 (diluted 1:1000) and β-actin (diluted 1:1000, loading control) and then with a horseradish peroxidase–conjugated anti-mouse secondary antibody (1:10,000). The proteins were visualized by enhanced chemiluminescence with multiple exposures to film.
FIGURE 6:
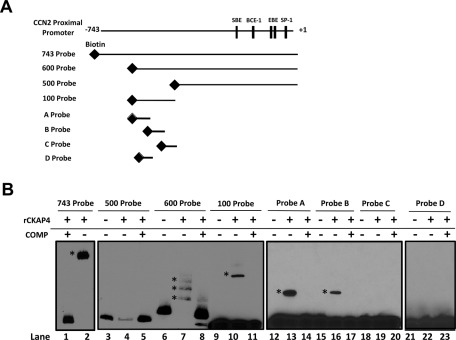
Recombinant CKAP4 binds to specific regions within the CCN2 proximal promoter. (A) 5′-Biotinylated probes spanning various lengths (743 probe, 600 probe, 500 probe, 100 probe) of the CCN2-proximal promoter were created by PCR amplification using biotinylated primers as described in Materials and Methods. Smaller, overlapping (10 base pairs), 40-mer DNA probes spanning the 100–base pair region (probes A–C) and one 20–base pair probe (oligo D) were synthesized courtesy of IDT. (B) The ability of recombinant CKAP4 (rCKAP4) to bind to these probes was assessed by EMSA. rCKAP4 (0.5 μg) was incubated with the indicated probe. Shifts are indicated by an asterisk. As controls, samples were prepared with probe only or no rCKAP4 (lanes 3, 6, 9, 12, 15, 18, and 21), or, in some instances, samples were preincubated with a 50 M excess of unlabeled competitor probe (COMP +) to test for specificity (lanes 1, 5, 8, 14, 17, 20, and 23).
CKAP4 binds specific regions of the CCN2 promoter
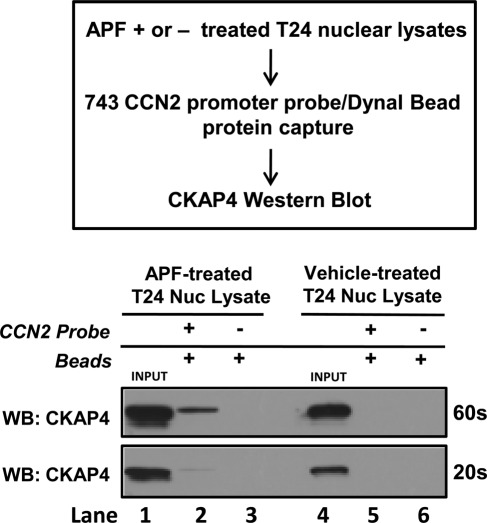
Because CKAP4 localizes to the nucleus in response to APF treatment, is required for APF-induced CCN2 expression, and its predicted structure comprises a region homologous to bZIP transcription factors, we examined whether CKAP4 could bind to the CCN2 proximal promoter. Using electromobility shift assay (EMSA) with multiple, 5′-biotinylated probes spanning various lengths of the CCN2 promoter (Figure 6A), we demonstrated that recombinant CKAP4 (rCKAP4) binds to the CCN2 promoter (Figure 6B). Specifically, we demonstrated that rCKAP4 binds strongly to the 743–base pair probe (spanning +1 to −743) in lane 2 (shift indicated by asterisk) and the 600–base pair probe (spanning bases +1 to −600) in lane 7 (shift indicated by asterisk) but not the 500–base pair probe (spanning bases +1 to −500) in lane 4 in Figure 6B. To confirm that rCKAP4 bound within this 100–base pair region, we made a 100–base pair probe spanning the −500 to −600 region of the CCN2 promoter. Using this probe, we demonstrated specific rCKAP binding (Figure 6B: lane 10; shifts indicated by asterisk), indicating that the potential CKAP4-binding site exists within this 100–base pair region. To more specifically delineate CKAP4-binding regions, additional smaller, overlapping (10 base pairs), 40-mer DNA probes spanning this 100–base pair region (Figure 6A, probes A–C) were constructed. When rCKAP4 binding was assessed using these probes, we detected strong rCKAP4 binding to probe A (indicated by asterisk), weaker binding affinity for probe B, and no rCKAP4 binding to probe C (Figure 6B, lanes 13 and 16). Because probes A and B shared a 10–base pair overlap, we also constructed a 20-mer DNA probe that spanned this region (probe D). However, rCKAP did not bind to this probe (Figure 6C, lane 22). As controls, samples were prepared with probe only (lanes 3, 6, 9, 12, 15, 18, and 21), or in some instances, samples containing rCKAP4 were preincubated with a 50 M excess of unlabeled competitor probe before addition of the labeled probe (COMP +) to test for specificity (lanes 1, 5, 8, 11, 14, 17, 20, and 23). Taken together, these results demonstrate that rCKAP4 binds to specific regions of DNA in the CCN2 promoter. Next, we determined whether endogenous CKAP4 from T24 nuclear lysates could bind to the CCN2 proximal promoter, using a streptavidin-bead pull-down approach (Figure 7; Deng et al., 2003). Briefly, serum-starved, vehicle- or APF-treated T24 nuclear lysates were incubated with the 5′-biotinylated CCN2-proximal promoter probe (Figure 7). Dynal streptavidin-coated magnetic beads were used to capture the probe and any specifically bound proteins, which were resolved by SDS–PAGE. Western blot analysis using an anti-CKAP4 antibody (Figure 7) demonstrated that endogenous CKAP4 from the APF-stimulated, T24 cell nuclear lysate bound the CCN2 promoter, whereas CKAP4 from the vehicle-treated lysate did not (Figure 7; lane 2 vs. lane 5), indicating that CKAP4 binds the CCN2-proximal promoter in an APF-dependent manner. As controls, nuclear lysates from APF- or vehicle-treated T24 cells (20 μg) were loaded alone in lanes 1 and 4, respectively, and as a control for nonspecific bead binding, lysates incubated with beads only (no CCN2 probe) were loaded in lanes 3 and 6, respectively.
FIGURE 7:
Endogenous CKAP4 binds to the CCN2-proximal promoter. To assess whether endogenous CKAP4 could bind to the CCN2 promoter, vehicle- or APF-treated T24 nuclear lysates were prepared as described in Materials and Methods and were subsequently incubated with the biotinylated 743 CCN2-proximal promoter probe (743 probe). Streptavidin-coated magnetic beads were used to capture the biotin-labeled probe and any specifically bound proteins. To test whether endogenous CKAP4 bound to the CCN2 promoter, proteins captured with the 743 probe were subsequently resolved by SDS–PAGE and identified by Western blot analysis using an anti-CKAP4 antibody. These results demonstrate that endogenous CKAP4 from the APF-stimulated, T24 cell nuclear lysates bound the CCN2 promoter (lane 2), whereas CKAP4 from the vehicle-treated lysate did not (lane 5), indicating that CKAP4 binds the CCN2-proximal promoter in an APF-dependent manner. As controls, APF or vehicle-treated nuclear lysates (20 μg) were loaded alone (input) in lanes 1 and 4, respectively, and lysates incubated with beads only and no CCN2 probe in lanes 3 and 6, respectively, as a nonspecific control.
DISCUSSION
In this study, we identified CCN2 as a novel downstream target of APF signaling in T24 bladder carcinoma cells. CCN2 is a 38-kDa, cysteine-rich, extracellular matrix (ECM) protein that belongs to the CCN family of proteins, which includes cysteine-rich 61 (cyr61/CCN1), CTGF/CCN2, nephroblastoma overexpressed (nov/CCN3), and Wnt-induced secreted protein-1 (WISP-1/CCN4), -2 (WISP-2/CCN5), and -3 (WISP-3/CCN6). The CCN2 gene consists of five exons. The first codes for a signal peptide (for secretion), and exons 2–5 code for each of the four different modules. Module 1 is an insulin-like growth factor–binding domain, module 2 is a von Willebrand type C domain, module 3 is a thrombospondin-1 domain, and module 4 is a C-terminal (CT) domain containing a putative cysteine knot (Brigstock, 2003; Perbal, 2004).
CCN2 regulates diverse biological processes, including proliferation, migration, adhesion, survival, differentiation, and synthesis of ECM proteins in various cell types (Perbal, 2001, 2004; Brigstock, 2003). Many of the effects of CCN2 manifest through its ability to bind integrins (Lau and Lam, 1999), whereas others arise through its interaction with TGF-β and BMPs (Abreu et al., 2002). Recent studies demonstrated that CCN2 may have an important role in a variety of human cancers. For example, overexpression of CCN2 is found in prostate cancers (Yang et al., 2005), gliomas (Xie et al., 2004), breast cancers (Xie et al., 2001), and esophageal squamous cell carcinomas (Deng et al., 2007). In contrast, in lung adenocarcinomas and colon cancers, overexpression of CCN2 inhibits invasion and metastasis of the cancer cells both in vitro and in vivo (Chang et al., 2004; Jiang et al., 2004; Lin et al., 2005; Chien et al., 2006). The present study demonstrates that treatment of T24 human bladder carcinoma cells with APF induces CCN2 expression and that experimentally induced CCN2 overexpression enhances APF-induced growth suppression. In certain NSCLC lines, forced overexpression of CCN2 was shown to inhibit cell growth by activation of E2F1, ARF, and p53 and by suppression of cyclin D1 (Chien et al., 2006). APF was also shown to drastically down-regulate cyclin D1 in primary bladder epithelial cells in another microarray study (Keay et al., 2003a). APF has also been shown to inhibit the growth of T24 cells via a mechanism that involves increased p53 expression (Kim et al., 2007; Shahjee et al., 2010). Our results, which showed that CCN2 knockdown by siRNA inhibited APF stimulation of p53 expression, indicate that CCN2 mediates APF-induced p53 expression in these cells and that APF and CCN2 may affect similar signal transduction pathways.
One pathway in which both APF and CCN2 have been implicated is Wnt/Frizzled signaling. Shahjee et al. (2010) demonstrated that APF decreases phosphorylation of AKR-transforming enzyme (Akt), glycogen synthase kinase-3β (GSK3β), β-catenin, and MMP2 in T24 bladder carcinoma cells (Shahjee et al., 2010). Of importance, these enzymes are downstream signal transducers for Wnt signaling. The authors concluded that the specific changes observed in Akt/GSK3β/β-catenin phosphorylation and lack of an effect of APF on total cellular β-catenin levels suggest that APF does not inhibit T24 bladder cell proliferation solely via inhibition of canonical Wnt/Frizzled signaling. Of interest, Yang et al. (2011) recently identified β-catenin as an element of the signaling response to APF. Their work showed that APF down-regulated β-catenin via proteasomal and lysosomal degradation and that this down-modulation of β-catenin elevated COX-2 expression, implying a potential connection between APF and inflammation. CCN2 has also been shown to regulate signaling through the Wnt pathway. In a study by Mercurio et al. (2004), overexpression of CCN2 was shown to mimic the effects of inhibiting components of the Wnt signaling pathway. The authors demonstrated that CCN2 can interfere with the noncanonical Wnt pathway, as well as with the canonical pathway, and showed that the ability of CCN2 to inhibit Wnt signaling resides in the CT domain. Of importance, they demonstrated that CCN2 interacts with the extracellular regions of both low-density-lipoprotein receptor–related protein 6 (LRP6) and Frizzled 8 through its CT domain, suggesting that CCN2 may inhibit Wnt signaling by displacing or competing with Wnt family members for binding to LRP6 (Mercurio et al., 2004). Of interest, CCN2 has also been shown to inhibit the migration of oral cancer cells by regulating COX-2 expression through the αvβ5 integrin receptor (Chuang et al., 2011). In light of these findings, it is reasonable to speculate that inhibition of T24 bladder carcinoma cell proliferation by APF is mediated through Wnt signaling and that CCN2 induction by APF is one mechanism by which APF imparts its antiproliferative effects. Future studies are necessary to investigate the role of Wnt signaling and CCN2 in mediating APF-induced gene expression.
It is well established that APF activity is mediated by binding to CKAP4; however, CKAP4's function in this regard has been elusive (Conrads et al., 2006; Planey et al., 2009; Planey and Zacharias, 2009). In T24 bladder carcinoma cells, siRNA-mediated knockdown of CKAP4 specifically abrogates APF's effects on cell proliferation, MMP2/p53 protein expression, and Akt/GSK3β/β-catenin phosphorylation (Shahjee et al., 2010). Results from the present study show that CKAP4 is also required for APF-induced CCN2 expression, as reducing CKAP4 with siRNA or using anti-CKAP4 antibodies inhibits CCN2 up-regulation in response to APF treatment. These data confirm that CKAP4 is essential for mediating the antiproliferative activity of APF in T24 bladder carcinoma cells. In addition, using confocal microcopy and Western blot analysis, we showed that CKAP4 translocates to the nucleus of T24 cells after APF treatment. These data are consistent with our prior immunolocalization studies demonstrating that CKAP4 is expressed on the surface of HeLa cells and that its nuclear abundance increases following APF treatment (Planey et al., 2009). Of significant importance in this study was the detection of a second, smaller, CKAP4-specific band in the nuclear fraction of APF-treated cells, suggesting that CKAP4 may be proteolyzed before entering the nucleus. Moreover, the detection of a relatively small amount of CKAP4 in the nucleus of untreated cells in this study and in other, unpublished experiments suggests that CKAP4 may have some inherent nuclear function not related to APF-induced signal transduction. Because of its size and TM domain, it would seem unlikely that CKAP4 would have a nuclear function. However, there is precedent for large, TM-domain proteins entering the nucleus. The epidermal growth factor receptor family is comprised of glycosylated, single-pass TM-domain proteins of ∼140 kDa that also translocate from the plasma membrane into the nucleus after ligand binding (Wang, 2009). Within the nucleus, they regulate transcription and participate, enzymatically, in signal transduction pathways. Although initially the idea that these proteins translocated to and had functional activity in the nucleus beyond their tyrosine kinase activity at the plasma membrane was controversial, recent mechanistic details have shed further light on how they make their way into the nucleus and function there (reviewed in Wang, 2009).
Because CKAP4 localizes to the nucleus in response to APF treatment, is required for APF-induced CCN2 expression, and its predicted structure includes a region homologous to bZIP transcription factors that is capable of binding genomic DNA (unpublished data), we investigated whether CKAP4 could bind to the CCN2-proximal promoter, using two different approaches. By EMSA we showed that recombinant CKAP4 (C-terminal residues 126–501, which includes the bZIP-like DNA-binding domain) bound specifically to the CCN2-proximal promoter. Furthermore, using a streptavidin-bead pull-down approach (Deng et al., 2003), we demonstrated that endogenous CKAP4 binds the CCN2-proximal promoter in an APF-dependent manner. These data provide strong evidence that CKAP4 is an essential downstream mediator of APF-induced CCN2 expression and expand our knowledge of CKAP4 cellular function to include a role in regulating transcription, either directly as a DNA-binding protein or indirectly through recruitment of other DNA-binding proteins. Future studies are necessary to determine the specific CKAP4-binding regions in the CCN2 promoter and to determine whether CKAP4 binds the promoters of other potential APF-responsive genes.
Despite multiple hypotheses about the primary cause of IC, the underlying molecular mechanism of this disease remains to be defined. Active theories include increased permeability of the bladder mucosa, abnormal neuronal function, mast cell activation, autoimmunity, infections, and toxic or antiproliferative substances (such as APF) in the urine (Erickson, 1999). However, insufficient data exist to definitively establish their roles in the pathology of IC. IC is characterized by fibrosis of the vesical wall, with consequent loss of bladder capacity. CCN2 is a common matricellular protein involved in fibrosis, as increased CCN2 production has been demonstrated in several fibrotic diseases, including systemic sclerosis (Igarashi et al., 1996), keloids (Goldschmeding et al., 2000), and renal (Lau and Lam, 1999) and lung fibrosis (Oemar and Luscher, 1997). Because APF is detectable in the urine of ∼95–97% of IC patients versus healthy controls (Keay et al., 2001, 2003b; Keay, 2008), one might hypothesize that CCN2 production could be increased in these patients as well. In addition, CCN2 has been shown to be involved in bladder wall remodeling as a result of outlet obstruction (Chaqour et al., 2002). Chaqour et al. (2002) found that two of four 7-d obstructed bladders showed CCN2 immunoreactivity within urothelial cells. Although unexpected, this parallels the observation by Sedlaczek et al. (2001) of a prominent expression of CCN2 in bile duct epithelial cells; however, the biological significance of CCN2 production in epithelial cells in vivo is unknown.
In summary, we identified CCN2 as a novel downstream target of APF signaling in T24 bladder carcinoma cells and showed that experimentally induced changes in CCN2 levels mediate the APF effect on cell growth, indicating that CCN2 is involved in the mechanism of APF-induced growth suppression. Of importance, the induction of CCN2 expression by APF was demonstrated to be specific, as siRNA-mediated knockdown of CKAP4 inhibited this up-regulation. Moreover, CKAP4 was shown to bind specifically to the CCN2-proximal promoter in an APF-dependent manner. To our knowledge, this is the first time that anyone has demonstrated regulation of an APF target gene at the promoter level. Moreover, these data strongly suggest that CKAP4 mediates APF-induced CCN2 transcription by binding either directly or indirectly to the CCN2 promoter via its C-terminal, extracellular domain. Future studies will be necessary to identify specific APF-responsive elements and determine whether CKAP4 plays a similar role in other APF-regulated genes.
MATERIALS AND METHODS
Cell culture and APF treatment
T24 bladder carcinoma cells (HTB-4; American Type Culture Collection [ATCC], Manassas, VA) were maintained in ATCC-formulated McCoy's 5a modified media (30-2007) containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen, Carlsbad, CA). Cells were serum starved for 24 h before treatment with synthetic APF at indicated concentrations (Peptides International, Louisville, KY). Synthetic APF (the triglycosylated ammonium form; Keay et al., 2004a) was purchased lyophilized from the manufacturer and reconstituted in distilled water at 100 μM concentration immediately before use. The APF stock was diluted accordingly in serum-free media and then added to cells to yield the final working concentration as indicated for each experiment. Vehicle-treated cells received serum-free medium alone in all instances.
Quantitative real-time PCR
Total RNA was extracted from untreated or APF-treated (24 h) T24 cells using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. RNA concentration and purity were assessed by UV spectrophotometry using a DU 700 series UV/Vis Spectrophotometer (Beckman Coulter, Brea, CA). Samples with A260:A230 > 1.7 and A260:A280 = 1.8–2.0 were used for analysis of gene expression using a commercial PCR-based array system specific for 84 genes involved in extracellular matrix and cell adhesion (RT2 Profiler PCR Array PAHS-013; SABiosciences, Frederick, MD). Quantitative real-time PCR for gene expression was performed using RT2 First Strand Kit and RT2 PCR SYBR Master Mix Kit reagents (SABiosciences) and an Applied Biosystems (Foster City, CA) 7900HT Fast Real-Time PCR Machine (software version 3.5). Samples were tested in duplicate runs, and the data were analyzed using Web-based PCR Array Data Analysis Software available at http://sabiosciences.com/pcrarraydataanalysis.php.
Western blot analysis
T24 cells were lysed in ice-cold RIPA buffer containing protease inhibitors (ThermoFisher Scientific, Waltham, MA), sonicated, and centrifuged for 15 min at 4°C. The protein concentration of the supernatant was determined using the Micro BCA protein assay kit (ThermoFisher Scientific). For conditioned medium preparation, media was harvested and concentrated to 0.5 ml using a Pierce 9K MWCO media concentrator (ThermoFisher Scientific), which allows for 90% recovery of all proteins >9-kD molecular weight. Proteins were separated by electrophoresis using 4–12% or 10% NuPAGE Novex Bis-Tris polyacrylamide gels in 3-(N-morpholino)propanesulfonic acid running buffer (Invitrogen) and then transferred to nitrocellulose. Membranes were blocked for 1 h at room temperature in TBST buffer (Tris-buffered saline, pH 7.4, with 0.1% Tween 20) containing 5% nonfat milk and incubated with specific antibodies against CCN2 (diluted 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), CKAP4 (diluted 1:2500; Alexis Biochemicals, Plymouth Meeting, PA), p53 (diluted 1:1000, Cell Signaling Technologies, Danvers, MA), or myc (diluted 1:5000; Invitrogen) overnight at 4°C. The membranes were subsequently washed with TBST, incubated for 1 h at room temperature in horseradish peroxidase–conjugated rabbit anti-goat (diluted 1:10,000; Santa Cruz Biotechnology), goat anti-mouse (diluted 1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA), or goat anti-rabbit (diluted 1:10,000; Jackson ImmunoResearch Laboratories) secondary antibodies and developed by enhanced chemiluminescence (ThermoFisher Scientific). To assess equal loading of protein, the membranes were stripped and reprobed for β-actin (diluted 1:1000; Abcam, Cambridge, MA). The membranes were exposed to film (BioMax AR; Kodak, Rochester, NY), and the resulting images were scanned at 300 dpi.
siRNA transfection
Double-stranded siRNA (ON-TARGETplus) targeting CKAP4, CCN2, and nonsense siRNA (ON-TARGETplus Control siRNA) were purchased from Dharmacon (ThermoFisher Scientific). T24 cells were seeded at a density of 1 × 105 cells/well in a 96-well plate and grown to semiconfluence in McCoy's 5a modified medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen). CKAP4- or CCN2-specific siRNA and control siRNA were transfected into T24 cells using Lipofectamine RNAiMAX according to the manufacturer's protocol (Invitrogen). To determine the effectiveness of siRNA-mediated knockdown, Western blot analysis was used to measure the abundance of CKAP4 at times 0, 24, 48, and 72 h after transfection of the siRNA. These experiments were run in triplicate.
Immunocytochemistry
T24 cells were seeded at a density of 2 × 104 cells/well in eight-well LabTek chamber slides (Nalge Nunc, Rochester, NY) and grown to semiconfluence in McCoy's 5a modified medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen). Cells were treated with APF (100 nM) alone, preincubated with anti-CKAP4 antibody (2 μg), and then treated with APF (100 nM) or treated with vehicle (serum-free medium) for 24 h. Cells were fixed for 20 min with 4% paraformaldehyde in phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100 in PBS, and blocked in PBS/5% normal goat serum. The following primary antibodies were used: mouse monoclonal antibody G1/296 against CKAP4 (anti-CLIMP-63, diluted 1:1000; Alexis Biochemicals) and goat polyclonal antibody against CCN2 (diluted 1:200; Santa Cruz Biotechnology). Secondary antibodies were DyLight 488–labeled rabbit anti-goat or goat anti-mouse (diluted 1:1000; Jackson ImmunoResearch Laboratories). Nuclei were counterstained using Hoechst dye according to the manufacturer's protocol (Invitrogen). Slides were mounted in SlowFade Antifade reagent (Invitrogen) and imaged using either a Zeiss Observer.Z1 epifluorescent microscope (Carl Zeiss, Jena, Germany) and a 20× or 40× objective or a Nikon A1 Confocal microscope and a 60× objective where indicated.
Bromodeoxyuridine incorporation assay
Cell proliferation was measured using the BrdU Cell Proliferation Assay Kit (Millipore, Temecula, CA). Briefly, T24 bladder carcinoma cells were seeded at 1 × 103 per well in a 96-well cell culture plate in 100 μl/well McCoy's 5A modified medium containing 10% heat-inactivated FBS and 1% penicillin–streptomycin antibiotic mix. The next day, designated wells were transfected with pCMV-CCN2-myc plasmid or pCMV-Tag 1 control plasmid (Stratagene, La Jolla, CA) using X-tremeGENE HP DNA Transfection Reagent (Roche, Indianapolis, IN). cDNA encoding full-length CCN2 was generated by PCR (forward primer, 5′-GCGAGATCTATACCGCCGCCAGTATGGGC-3′; reverse primer, 5′-CGCCTCGAGTGCCATGTCTCCGTACAT-3′) using pCMV-SPORT6-CTGF (Open Biosystems, ThermoFisher Scientific) as a template and cloned in-frame with the c-myc epitope tag of pCMV-Tag1 (Stratagene) at the 3′ end. On the third day, cells were serum-starved for 8 h and then treated with 100 nM APF (Peptides International) diluted in serum-free McCoy's medium. Cells were then incubated at 37°C in a 5% CO2 atmosphere for an additional 24 h, after which 20 μl of BrdU labeling solution was added to each well. Cells were then incubated at 37°C in a 5% CO2 atmosphere for an additional 24 h. BrdU incorporation into newly synthesized DNA was detected immunochemically using an anti-BrdU monoclonal antibody followed by incubation with goat, anti–mouse immunoglobulin G, peroxidase conjugate, and TMB peroxidase substrate as described in the product manual from Millipore (catalogue #2750). The reaction was stopped after a 30-min incubation, and the absorbance of the assay wells containing treated cells was measured at a wavelength of 450 nm using an M5 microplate reader (Molecular Devices, Sunnyvale, CA). The higher the OD reading, the higher is the BrdU concentration in the sample.
Transfections and reporter assays
T24 cells were transfected with a reporter plasmid containing the full-length CCN2 proximal promoter (nucleotides −744 to +1 cloned into the pSGG luciferase reporter vector (pSGG-CCN2) [Switchgear Genomics, Menlo Park, CA]) together with Renilla luciferase plasmid in quadruplicate using DharmaFECT Duo reagent (ThermoFisher Scientific). The cells were then serum starved for 24 h and treated with or without APF (100 nM) for 24 h. For siRNA experiments, cells were pretreated with CKAP4-specific siRNA 24 h before transfection with the luciferase reporter constructs. Luciferase activities were measured by a Dual Glo Luciferase Reporter Assay System (Promega, Madison, WI) and normalized to Renilla luciferase activity to control for transfection efficiency. All assays were repeated at least three independent times.
Purification of recombinant CKAP4
cDNA encoding the extracellular domain of CKAP4 (C-terminal residues 126–501) was generated by PCR and cloned in-frame with the hexahistidine-epitope tag of pRSET-B (Invitrogen) at the 5′ end. The rCKAP4 was expressed in BL21 Star (DE3)pLysS bacteria (Invitrogen) and purified under native conditions using ProBond Nickel-Chelating Resin (Invitrogen). The recombinant CKAP4 protein was then buffer exchanged into PBS, pH 7.4, using an Amicon centrifugal concentrator, analyzed by Western blot using either anti-CKAP4 (Alexis Biochemicals) or anti-histidine (Invitrogen) monoclonal antibody, and quantitated using the Bradford assay.
Electrophoretic mobility shift assay
CKAP4 binding to the CCN2 proximal promoter was assessed by electrophoretic mobility shift assay. 5′-Biotinylated CCN2 proximal promoter probes spanning bases +1 to −744 (743–base pair probe), bases +1 to −600 (600–base pair probe), bases +1 to −500 (500–base pair probe), or bases −500 to −600 (100–base pair probe) were created by PCR amplification of the human CCN2 promoter (S722188; Switchgear Genomics) using biotinylated primers obtained from Integrated DNA Technologies (Coralville, IA). For the CCN2 promoter +1 to −744, the forward primer used was 5′-biotin/GCTGTCGTCTCGGGGCTGTC-3′ and reverse primer was 5′-biotin/ACGCGTCTTTGTTCTCTTTC-3′ for PCR amplification. The primers used for PCR amplification of the other three probes are as follows: for the 600–base pair probe, the forward primer used was 5′-biotin/TTTGGGGCGGGCGGCCCGAG-3′ and the reverse primer was 5′-biotin/TGGAGAGTTTCAAGAGCCTA-3′ for PCR amplification; for the 500–base pair probe, the forward primer used was 5′-biotin/GCTGTCGTCTCGGGGCTGTC-3′ and the reverse primer was 5′-biotin/CTCTTCAGCTACCTACTTCC-3′ for PCR amplification; and for the 100–base pair probe, the forward primer used was 5′-biotin/GGCAAACAGCAGGAATTCCT-3′ and the reverse primer was 5′-biotin/TGGAGAGTTTCAAGAGCCTA-3′. Smaller, overlapping (10 base pairs), 40-mer DNA probes spanning the 100–base pair region (probes A–C) and one 20–base pair probe (oligo D) were synthesized courtesy of IDT. Sequence for oligo A is 5′-biotin/GATTTGCGTTTTAGAGGCTATAGGCTCTTGAAACTCTCCA-3′, sequence for oligo B is 5′-biotin/AAAAGGTTTCTCCCCCCCAACCCTTAGCAATGATTTGCGTT-3′, sequence for oligo C is 5′-biotin/GGCAAACAGCAGGAATTCCTAAAAAATTCGAAAAGGTTTCT-3′, and sequence for oligo D is 5′-biotin/CAATGATTTGCGTTTTAG-3′. Nonlabeled forms of all four probes were also synthesized courtesy of IDT. Then, 0.5 μg of rCKAP4 was incubated with 5′-biotinylated CCN2 probe in binding buffer (20 mM Tris pH 7.9, 2 mM dithiothreitol [DTT], 10% glycerol, and 1 mM EDTA) for 30 min at room temperature. As controls, samples were prepared with probe only and no rCKAP4, or, in some instances, samples were preincubated with a 50 M excess of unlabeled competitor probe (COMP +) to test for specificity. Samples were separated by electrophoresis on a NuPAGE 7% Tris-Acetate gel (Invitrogen) in 1× Tris-Glycine Native Running Buffer (25 mM Tris base, 192 mM glycine pH 8.3) and transferred electrophoretically to a Biodyne B Pre-Cut Modified Nylon Membrane (0.45 μm, ThermoFisher Scientific) in NuPAGE Transfer Buffer (Invitrogen). The membrane was then UV cross-linked for 20 s and developed using the Chemiluminescent Nucleic Acid Detection Module (ThermoFisher Scientific).
Streptavidin-bead pull-down assay
APF-inducible changes in DNA binding were assessed by streptavidin-bead pull-down assay. Briefly, nuclear extracts were prepared from serum-starved T24 cells treated with or without APF using the NE-PER Nuclear and Cytoplasmic Extraction Kit according to the manufacturer's protocol (ThermoFisher Scientific). The protein concentration was determined by the Micro BCA Protein Assay Kit (Pierce) using bovine serum albumin as a standard. Five microliters of 5′-biotinylated, 743–base pair, CCN2-proximal promoter probe (200 pM) were incubated with 5 μg of nuclear extract and 100 μl of Dynal streptavidin-coated magnetic beads (Invitrogen) prewashed in binding buffer (20 mM Tris, pH 7.9, 2 mM DTT, 10% glycerol, and 1 mM EDTA). The biotinylated probe and bead concentrations were in excess to ensure a complete pulldown of DNA–protein complexes. The mixture was placed on a rotating shaker with gentle mixing at room temperature for 1 h. The sample was centrifuged at 5000 × g in a microcentrifuge for 30 s. The supernatant was removed, and the pellet was washed four times each with 1 ml of iced PBS. After the last wash, the pulled-down mixture was resuspended in 50 μl of LDS sample buffer, boiled for 5 min, and separated by electrophoresis on a NuPAGE 10% Bis-Tris gel. The proteins were transferred to a nitrocellulose membrane and immunoblotted using a CKAP4-specific antibody (anti-CLIMP-63, diluted 1:1000; Alexis Biochemicals).
Acknowledgments
We thank Jun Ling (The Commonwealth Medical College) for supplying the p53 antibody and for providing his technical expertise. This work was supported by funding from the National Institutes of Health (1R03AR057193-01), a Keystone Innovation Zone grant, and The Commonwealth Medical College.
Abbreviations used:
- APF
antiproliferative factor
- CKAP4
cytoskeleton-associated protein 4/p63
- CTGF/CCN2
connective tissue growth factor
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- IC
interstitial cystitis
- NSCLC
non–small cell lung cancer
- PBS
phosphate-buffered saline
- siRNA
small interfering RNA
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-08-0714) on March 21, 2012.
REFERENCES
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SR. P63 (CKAP4) as an SP-A receptor: implications for surfactant turnover. Cell Physiol Biochem. 2010;25:41–54. doi: 10.1159/000272062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Brigstock DR, et al. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56:127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Shih JY, Jeng YM, Su JL, Lin BZ, Chen ST, Chau YP, Yang PC, Kuo ML. Connective tissue growth factor and its role in lung adenocarcinoma invasion and metastasis. J Natl Cancer Inst. 2004;96:364–375. doi: 10.1093/jnci/djh059. [DOI] [PubMed] [Google Scholar]
- Chaqour B, Whitbeck C, Han JS, Macarak E, Horan P, Chichester P, Levin R. Cyr61 and CTGF are molecular markers of bladder wall remodeling after outlet obstruction. Am J Physiol Endocrinol Metab. 2002;283:E765–E774. doi: 10.1152/ajpendo.00131.2002. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien W, Yin D, Gui D, Mori A, Frank JM, Said J, Kusuanco D, Marchevsky A, McKenna R, Koeffler HP. Suppression of cell proliferation and signaling transduction by connective tissue growth factor in non-small cell lung cancer cells. Mol Cancer Res. 2006;4:591–598. doi: 10.1158/1541-7786.MCR-06-0029. [DOI] [PubMed] [Google Scholar]
- Chuang JY, Yang WY, Lai CH, Lin CD, Tsai MH, Tang CH. CTGF inhibits cell motility and COX-2 expression in oral cancer cells. Int Immunopharmacol. 2011;11:948–954. doi: 10.1016/j.intimp.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem. 2006;281:37836–37843. doi: 10.1074/jbc.M604581200. [DOI] [PubMed] [Google Scholar]
- Deng WG, Zhu Y, Montero A, Wu KK. Quantitative analysis of binding of transcription factor complex to biotinylated DNA probe by a streptavidin-agarose pulldown assay. Anal Biochem. 2003;323:12–18. doi: 10.1016/j.ab.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Deng YZ, Chen PP, Wang Y, Yin D, Koeffler HP, Li B, Tong XJ, Xie D. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem. 2007;282:36571–36581. doi: 10.1074/jbc.M704141200. [DOI] [PubMed] [Google Scholar]
- Erickson DR. Interstitial cystitis: update on etiologies and therapeutic options. J Womens Health Gend Based Med. 1999;8:745–758. doi: 10.1089/152460999319075. [DOI] [PubMed] [Google Scholar]
- Goldschmeding R, Aten J, Ito Y, Blom I, Rabelink T, Weening JJ. Connective tissue growth factor: just another factor in renal fibrosis. Nephrol Dial Transplant. 2000;15:296–299. doi: 10.1093/ndt/15.3.296. [DOI] [PubMed] [Google Scholar]
- Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Fujimoto M, Grotendorst GR, Takehara K. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J Invest Dermatol. 1996;106:729–733. doi: 10.1111/1523-1747.ep12345771. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Watkins G, Fodstad O, Douglas-Jones A, Mokbel K, Mansel RE. Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer. Endocr Relat Cancer. 2004;11:781–791. doi: 10.1677/erc.1.00825. [DOI] [PubMed] [Google Scholar]
- Kazi AS, Tao JQ, Feinstein SI, Zhang L, Fisher AB, Bates SR. Role of the PI3-kinase signaling pathway in trafficking of the surfactant protein A receptor P63 (CKAP4) on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2010;299:L794–L807. doi: 10.1152/ajplung.00372.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S. Cell signaling in interstitial cystitis/painful bladder syndrome. Cell Signal. 2008;20:2174–2179. doi: 10.1016/j.cellsig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Keay S, Kleinberg M, Zhang CO, Hise MK, Warren JW. Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J Urol. 2000;164:2112–2118. [PubMed] [Google Scholar]
- Keay S, Seillier-Moiseiwitsch F, Zhang CO, Chai TC, Zhang J. Changes in human bladder epithelial cell gene expression associated with interstitial cystitis or antiproliferative factor treatment. Physiol Genomics. 2003a;14:107–115. doi: 10.1152/physiolgenomics.00055.2003. [DOI] [PubMed] [Google Scholar]
- Keay S, Zhang CO, Chai T, Warren J, Koch K, Grkovic D, Colville H, Alexander R. Antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor in men with interstitial cystitis versus chronic pelvic pain syndrome. Urology. 2004a;63:22–26. doi: 10.1016/j.urology.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Keay S, Zhang CO, Shoenfelt JL, Chai TC. Decreased in vitro proliferation of bladder epithelial cells from patients with interstitial cystitis. Urology. 2003b;61:1278–1284. doi: 10.1016/s0090-4295(03)00005-0. [DOI] [PubMed] [Google Scholar]
- Keay S, Zhang CO, Trifillis AL, Hise MK, Hebel JR, Jacobs SC, Warren JW. Decreased 3H-thymidine incorporation by human bladder epithelial cells following exposure to urine from interstitial cystitis patients. J Urol. 1996;156:2073–2078. [PubMed] [Google Scholar]
- Keay SK, Szekely Z, Conrads TP, Veenstra TD, Barchi JJ, Jr, Zhang CO, Koch KR, Michejda CJ. An antiproliferative factor from interstitial cystitis patients is a frizzled 8 protein-related sialoglycopeptide. Proc Natl Acad Sci USA. 2004b;101:11803–11808. doi: 10.1073/pnas.0404509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay SK, Zhang CO, Shoenfelt J, Erickson DR, Whitmore K, Warren JW, Marvel R, Chai T. Sensitivity and specificity of antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor as urine markers for interstitial cystitis. Urology. 2001;57:9–14. doi: 10.1016/s0090-4295(01)01127-x. [DOI] [PubMed] [Google Scholar]
- Kim J, Keay SK, Dimitrakov JD, Freeman MR. p53 mediates interstitial cystitis antiproliferative factor (APF)-induced growth inhibition of human urothelial cells. FEBS Lett. 2007;581:3795–3799. doi: 10.1016/j.febslet.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–6177. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Klumperman J, Lustig A, Kammerer RA, Oorschot V, Hauri HP. Subdomain-specific localization of CLIMP-63 (p63) in the endoplasmic reticulum is mediated by its luminal alpha-helical segment. J Cell Biol. 2001;153:1287–1300. doi: 10.1083/jcb.153.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lin BR, et al. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005;128:9–23. doi: 10.1053/j.gastro.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Moussad EE, Brigstock DR. Connective tissue growth factor: what's in a name. Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- Oemar BS, Luscher TF. Connective tissue growth factor. Friend or foe. Arterioscler Thromb Vasc Biol. 1997;17:1483–1489. doi: 10.1161/01.atv.17.8.1483. [DOI] [PubMed] [Google Scholar]
- Perbal B. The CCN family of genes: a brief history. Mol Pathol. 2001;54:103–104. doi: 10.1136/mp.54.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Planey SL, Keay SK, Zhang CO, Zacharias DA. Palmitoylation of cytoskeleton associated protein 4 by DHHC2 regulates antiproliferative factor-mediated signaling. Mol Biol Cell. 2009;20:1454–1463. doi: 10.1091/mbc.E08-08-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planey SL, Zacharias DA. Palmitoyl acyltransferases, their substrates, and novel assays to connect them (review) Mol Membr Biol. 2009;26:14–31. doi: 10.1080/09687680802646703. [DOI] [PubMed] [Google Scholar]
- Rashid HH, Reeder JE, O'Connell MJ, Zhang CO, Messing EM, Keay SK. Interstitial cystitis antiproliferative factor (APF) as a cell-cycle modulator. BMC Urol. 2004;4:3. doi: 10.1186/1471-2490-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq TM, Bass R, Vines DJ, Werner F, Whawell SA, Ellis V. Functional regulation of tissue plasminogen activator on the surface of vascular smooth muscle cells by the type-II transmembrane protein p63 (CKAP4) J Biol Chem. 2003;278:42679–42685. doi: 10.1074/jbc.M305695200. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri HP. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J Cell Sci. 1993;104(Pt 3):671–683. doi: 10.1242/jcs.104.3.671. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Rohrer J, Hauri HP, Kornfeld S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J Cell Biol. 1994;126:25–39. doi: 10.1083/jcb.126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahjee HM, Koch KR, Guo L, Zhang CO, Keay SK. Antiproliferative factor decreases Akt phosphorylation and alters gene expression via CKAP4 in T24 bladder carcinoma cells. J Exp Clin Cancer Res. 2010;29:160. doi: 10.1186/1756-9966-29-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedrenne C, Hauri HP. Morphogenesis of the endoplasmic reticulum: beyond active membrane expansion. Traffic. 2006;7:639–646. doi: 10.1111/j.1600-0854.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- Vedrenne C, Klopfenstein DR, Hauri HP. Phosphorylation controls CLIMP-63-mediated anchoring of the endoplasmic reticulum to microtubules. Mol Biol Cell. 2005;16:1928–1937. doi: 10.1091/mbc.E04-07-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C, Acharya A, Taparowsky EJ. Deciphering B-ZIP transcription factor interactions in vitro and in vivo. Biochim Biophys Acta. 2006;1759:4–12. doi: 10.1016/j.bbaexp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Wang SC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917–8923. [PubMed] [Google Scholar]
- Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, Koeffler HP. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10:2072–2081. doi: 10.1158/1078-0432.ccr-0659-03. [DOI] [PubMed] [Google Scholar]
- Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- Yang W, et al. Quantitative proteomics identifies a beta-catenin network as an element of the signaling response to Frizzled-8 protein-related antiproliferative factor. Mol Cell Proteomics. 2011;10:M110 007492. doi: 10.1074/mcp.M110.007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CO, Wang JY, Koch KR, Keay S. Regulation of tight junction proteins and bladder epithelial paracellular permeability by an antiproliferative factor from patients with interstitial cystitis. J Urol. 2005;174:2382–2387. doi: 10.1097/01.ju.0000180417.11976.99. [DOI] [PubMed] [Google Scholar]
- Zhang J, Planey SL, Ceballos C, Stevens SM, Jr, Keay SK, Zacharias DA. Identification of CKAP4/p63 as a major substrate of the palmitoyl acyltransferase DHHC2, a putative tumor suppressor, using a novel proteomics method. Mol Cell Proteomics. 2008;7:1378–1388. doi: 10.1074/mcp.M800069-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]