Abstract
Multidimensional proteins such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) exhibit distinct activities unrelated to their originally identified functions. Apart from glycolysis, GAPDH participates in iron metabolism, membrane trafficking, histone biosynthesis, the maintenance of DNA integrity and receptor mediated cell signaling. Further, multifunctional proteins exhibit distinct changes in their subcellular localization reflecting their new activities. As such, GAPDH is not only a cytosolic protein but is localized in the membrane, the nucleus, polysomes, the ER and the Golgi. In addition, although the initial subcellular localizations of multifunctional proteins may be of significance, dynamic changes in intracellular distribution may occur as a consequence of those new activities. As such, regulatory mechanisms may exist through which cells control multifunctional protein expression as a function of their subcellular localization. The temporal sequence through which subcellular translocation and the acquisition of new GAPDH functions is considered as well as post-translational modification as a basis for its intracellular transport.
Keywords: multifunctional proteins, subcellular translocation, glyceraldehyde-3-phosphate dehydrogenase, post-translational modification, post-transcriptional regulation, membrane transport
“Follow the yellow brick road”
The Munchkins to Judy Garland as Dorothy in the “Wizard of Oz”
Multifunctional proteins may represent a new mechanism through which cells expand their limited amount of genomic information. Although such proteins were identified and categorized based on a single activity, each is now known to display multiple, independent functions beyond those from which they were identified originally (Jeffrey, 1999; Kim and Dang, 2005). Structure function analyses suggest the utilization of both their classically identified active sites as well as their known binding sites as the bases for these new activities (Sirover, 1999).
In accord with their varied functions, the subcellular location of such proteins may be diverse with each protein displaying its own intracellular “fingerprint”. These multiple in vivo localizations may be an obligatory requirement for their expression of each separate and distinct activity. Further, such subcellular localizations may not be invariable but may be dynamic in nature, constantly changing, subject to the demands required for the performance of multiple activities. This suggests the possibility that regulatory mechanisms may exist which function to modulate the cell distribution and thus the functional diversity of each protein.
Three such possible mechanisms are illustrated in Table I. Each is based on the temporal interrelationship between subcellular translocation and the acquisition of new activities, i.e., which occurs first and which second. In constitutive regulation, the new activity is exhibited subsequent to an alteration in cell localization. In inducible regulation, subcellular translocation is secondary to the new activity. Semi-constitutive regulation is more complex involving two subcellular translocations with the new activity subsequent to the first change but preceding the second.
Table I. Regulation of Multifunctional Protein Subcellular Translocation.
| Mechanism | Definition | New Function (GAPDH) |
|---|---|---|
| Constitutive | Change in subcellular localization → acquisition of new function | Maintenance of genomic integrity |
| Inducible | Acquisition of new function → change in subcellular localization | Regulation of gene expression |
| Semi-constitutive | Change in subcellular localization → acquisition of new function → change in subcellular localization | Intracellular membrane trafficking |
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, EC 1.2.1.12) was long thought to be a classical glycolytic protein, bereft of significance or the need for inquiry. It was relegated to the “housekeeping” category reserved for genes and proteins of seemingly little interest, useful as internal controls for the analysis of “important” genes and proteins or as samples for training purposes. However, as Nature would have it, in a curious twist of fate, studies indicate now that GAPDH is, in reality, a multifunctional protein exhibiting numerous activities unrelated to its role in energy production (Sirover, 1999, 2005, 2011); and as a protein intimately involvement in the etiology of a variety of human pathologies (Mazzola and Sirover, 2002; Du et. al., 2003; Hara and Snyder, 2006; Colell et. al., 2009; Butterfield et. al., 2010). For that reason, GAPDH may be considered as an archetypical multidimensional protein. As such, its analysis may be of importance not only in itself but also as a means to understand the relationship between protein structure and functional diversity.
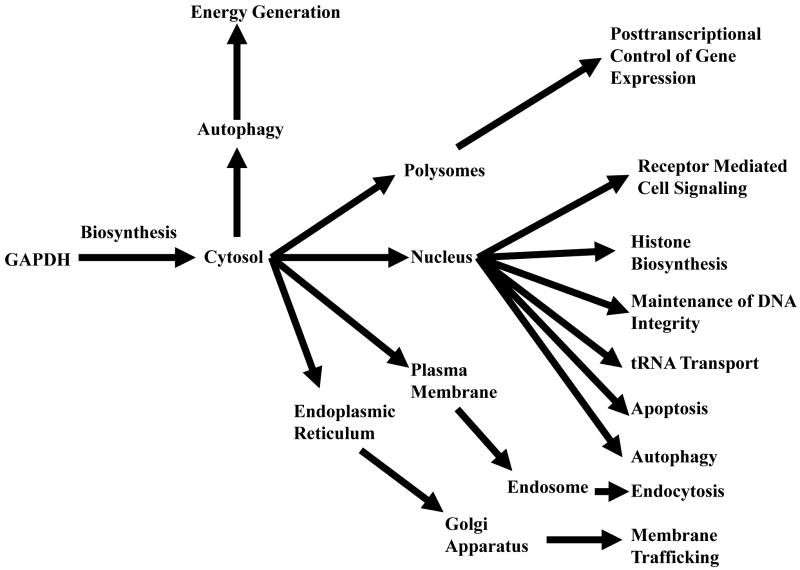
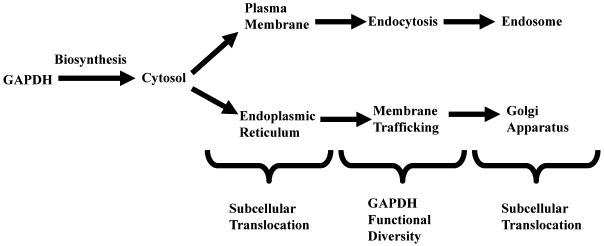
In accord with its multiple activities, the intracellular localization of GAPDH is not restricted to the cytosol for energy production but is detected in the plasma and nuclear membrane, the ER, Golgi, and in the nucleus (Tristan et. al., 2011). Further, distinct subcellular translocations may be fundamental to its functional diversity (Figure I). This complex intracellular “fingerprint” suggests the possibility that GAPDH may provide also a useful model to consider the mechanisms through which cells regulate the subcellular localization of multifunctional proteins as a means to increase exponentially proteomic utilization. Such studies would demonstrate not only the dynamic nature of multifunctional protein intracellular movement but also the basic means through which such subcellular transport occurs, i.e., how do such proteins traverse their own intracellular yellow brick road to Oz, thereby achieving their unique functional diversity?
Figure I. Subcellular GAPDH Dynamics.
Constitutive Subcellular Translocation
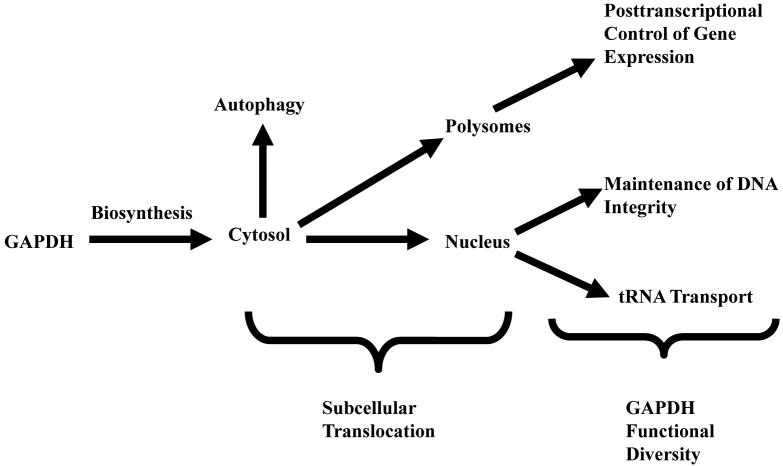
Constitutive subcellular translocation may be defined as a two step process in which a “free” multifunctional protein is transported first to a specific intracellular locale where it is “recruited” in some manner to acquire a new function (Figure II). For GAPDH, this change to a non-cytosolic localization results in the acquisition of several separate activities, each distinct from its role in glycolysis (Table II).
Figure II. Constitutive GAPDH Translocation.
Table II. “Constitutive” GAPDH Functions.
| Function | Activity | Reaction | Subcellular Localization |
|---|---|---|---|
| Regulation of Posttranscriptional Gene Expression | mRNA binding | Cytosolic, Polysomal | |
| ET-1 | destabilization | ||
| CSF-1 | stabilization | ||
| AT1R | inhibition of translation | ||
| Maintenance of DNA Integrity | Telomere Structure | Prevention of telomere shortening | Nuclear |
| DNA Repair | AP endonuclease reactivation | Nuclear | |
| Autophagy | Energy Production | ATP generation | Cytosolic |
| RNA Processing | Precursor tRNA binding | Transport | Nuclear |
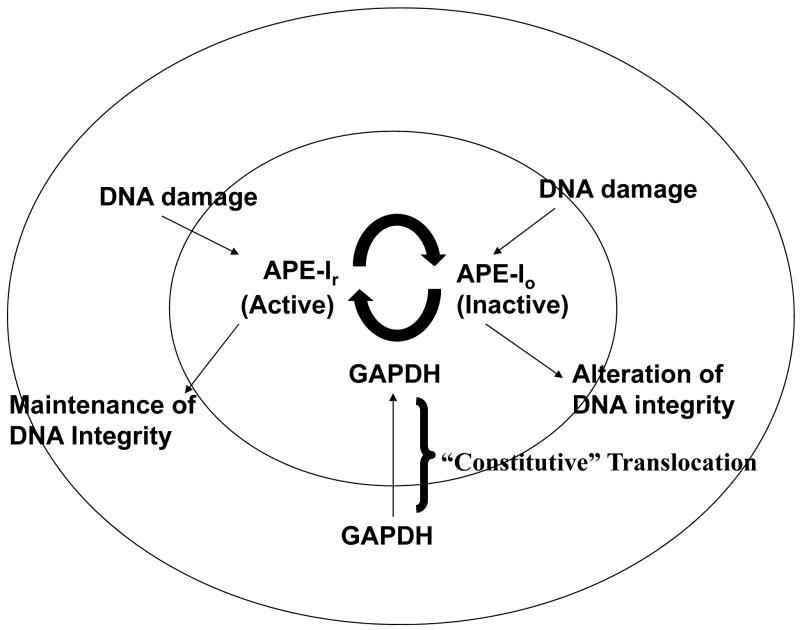
Constitutive regulation of a multifunctional protein is illustrated in Figure III which describes a new, nuclear function of GAPDH, i.e. its facilitation of mechanisms to ensure the integrity of DNA through its restoration of the catalytic activity of a DNA repair enzyme, the apurinic/apyrimidinic acid (AP) endonuclease-1 (APE-1, Azam et. al., 2008). The latter is a critical DNA repair enzyme initiating the removal of miscoding DNA lesions. The physiological significance of GAPDH in DNA repair is indicated by siRNA studies which demonstrated that spontaneous and agent-induced mutational frequencies are demonstrably higher in GAPDH siRNA treated cells. These latter studies are reflective of numerous studies on the functional diversity of GAPDH, i.e., in each study RNA interference was used to demonstrate its role in the specific activity of interest (Sirover, 2011).
Figure III. GAPDH and the Maintenance of DNA Integrity.
As illustrated, GAPDH first undergoes nuclear translocation as a free protein then is “recruited” for its role in DNA repair. As these studies used hydrogen peroxide treated APE-1, GAPDH presumerably recognizes modified amino acid sequences within the APE-1 protein. Similarly, to ensure telomeric integrity, recruitment involves the NAD+ GAPDH binding site recognition of the T1, G5 and G6 of the TTAGGG telomeric DNA repeat (Demarse et. al., 2009). Comparable models may be constructed for GAPDH function in nuclear tRNA transport (Singh and Green, 1993) as well as its polysomal localization through which it regulates gene expression (Nagy and Rigby, 1995), i.e., the temporal sequence of subcellular GAPDH regulation is transport first, new function second. The latter is especially intriguing as GAPDH: mRNA binding produces three separate effects, i.e., mRNA stabilization, destabilization or inhibition of translation (Bonafe et. al., 2005; Zhou et. al., 2008; Rodriguex-Pascual et. al., 2008; Backlund et. al., 2009). As described, the sequences within each mRNA or the changes in GAPDH structure which provide the foundation for such striking differences in activity are unknown at the present time (Sirover, 2011).
This begs the question of the manner in which GAPDH (or any multifunctional protein), as part of a normal and not a pathological, cell activity undergoes subcellular translocation which is then followed by the acquisition of a new function. Is there a specific transport mechanism to provide the foundation for the former as well as a defined targeting process for the latter? Further, given that each GAPDH molecule is presumerably identical, how does each mechanism select a GAPDH molecule A or molecule B for that process?
Two possibilities may be considered initially: the presence of targeting domains within the primary amino sequence of the multifunctional protein or specific post-translational modifications as a selection mechanism. In either instance, the presumption is that a carrier protein or a multiprotein complex would be required as a transport vehicle.
GAPDH in somatic cells is a highly conserved protein transcribed from a single gene without alternate transcripts (Bruns and Gerald, 1976; Bruns et. al., 1979; Mezquita et. al., 1998). For that reason, as all GAPDH molecules would contain the identical sequence, the probability of it containing unique targeting domains for its dispersal to multiple subcellular locales would be extremely unlikely, i.e., how would GAPDH molecule A be targeted for polysomal transport while GAPDH molecule B, containing the identical sequences, be selected for nuclear translocation?
In contrast, recent evidence indicates that post-translational modification of GAPDH is a relatively common event. GAPDH is phosphorylated at tyrosine (Tisdale and Artalejo, 2006) and serine residues (Tisdale, 2002), S-nitrosylated at its active site cysteine149 (Hara et. al., 2006; Nakajima et. al., 2007), acetylated at lys160 (Sen et. al., 2008), lys117, lys227, lys251 (Ventura et. al., 2010), 0-linked N-acetylglucosamine glycosylated at thr227 (Park et. al., 2009), poly ADP-ribosylated as a consequence of hyperglycemic stress (Du et. al, 2003); and pyruvylated by 3-bromopyruvate (Ganapathy-Kanniapann et. al, 2009). The functional changes arising from these structural modifications have been recently reviewed (Sirover, 2011).
Accordingly, such differential post-translational modifications present a likely mechanism for the constitutive subcellular regulation not only of GAPDH but also of other multifunctional proteins as well. For GAPDH, this may be experimentally verified by the analysis of GAPDH: RNA, DNA or GAPDH protein: protein complexes with mRNA, telomeric DNA or its association with individual cellular proteins or with polyprotein complexes. The presumption is that each will contain a GAPDH species characterized by a unique post-translational modification. Similar studies may be performed with other such multidimensional proteins.
Inducible Subcellular Translocation
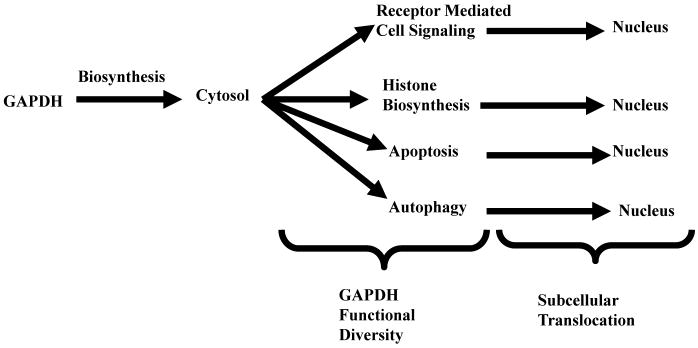
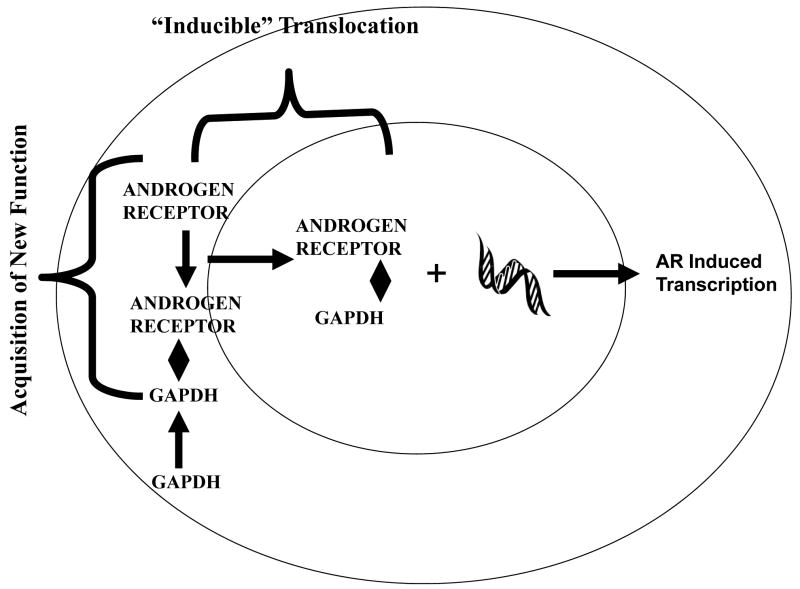
Inducible subcellular translocation may be also defined as a two step process. However, in contrast to constitutive translocation, GAPDH is first recruited for its new function in the cytosol then transported to a different subcellular locale where that new activity is manifested (Figure IV). For GAPDH, following its “recruitment”, it is transported within a protein: protein complex to a different intracellular locale, where that new function is expressed (Table III).
Figure IV. Inducible GAPDH Translocation.
Table III. “Inducible” GAPDH Functions.
| Function | Activity | Reaction | Initial Subcellular Localization | Final Subcellular Localization |
|---|---|---|---|---|
| Receptor Mediated Cell Signaling | Regulation of gene expression | Androgen Receptor Transactivation | Cytosolic | Nuclear |
| Chromosomal Replication | Histone Biosynthesis | Formation of Oct-1 OCA-S coactivator protein complex | Cytosolic | Nuclear |
| Apoptosis | Nuclear Protein Degradation; regulation of apoptotic gene expression | Binding to and stabilization of Siah1; induction of P300/CBP; Transnitrosylation | Cytosolic | Nuclear |
| Autophagy | Regulation of Gene Expression | Induction of Agt12 | Cytosolic | Nuclear |
This process is illustrated in Figure V which defines GAPDH facilitation of androgen receptor nuclear translocation resulting in androgen dependent regulation of gene expression (Harada et. al., 2007). A similar model may be constructed for the role of GAPDH in histone biosynthesis (Zheng et. al., 2003), in apoptosis (Hara et. al., 2005; Hara and Snyder, 2006) and in autophagy (Colell et. al., 2007).
Figure V. GAPDH and Receptor Mediated Cell Signaling.
In contrast to the unknown mechanisms which may underlie constitutive GAPDH subcellular translocation, those which provide the foundation for inducible transport are, for the most part, well defined. With respect to nuclear translocation, this is particularly critical as it is well established that, although GAPDH contains a nuclear export signal (Brown et. al., 2004), it does not contain a comparable nuclear localization signal (NLS). Thus, it would appear that other mechanisms need to be utilized for GAPDH translocation.
Recent evidence suggests that, with respect to each inducible function, a unique pathway appears to be utilized to provide for this dynamic intracellular GAPDH movement. For example, with respect to androgen receptor transactivation, cytosolic GAPDH binds initially to the androgen receptor followed by the nuclear translocation of this protein: protein complex. The latter is facilitated by the NLS contained in the androgen receptor (Jenster et. al., 1993).
With respect to histone gene expression, studies show an S phase dependent recruitment of GAPDH to the H2B promoter possibly involving a nuclear localization signal within another component of the OCA-S multiprotein complex (Zheng et. al., 2003) or a P13K/Akt, AMPK mediated nuclear/cytoplasmic signal transduction pathway (Schmitz, 2001; Kwon et. al., 2010). In either case, each possibility is in accord with the proliferative dependent cytosol to nuclear GAPDH translocation as defined by immunocytochemical and immunoblot analyses (Cool and Sirover, 1989; Schmitz et. al., 2003) as well as by the growth dependent nuclear localization of newly synthesized GAPDH (Lee and Sirover, 1989).
As indicated, GAPDH functions not only in “normal” cellular pathways but also in several intracellular “pathological” processes. The latter represent important cellular responses to injury either from endogenous or exogenous toxins. In these instances as well, inducible GAPDH movement is required. In apoptosis, detailed analyses revealed that GAPDH cytosolic to nuclear translocation involves an oxidative stress-induced S-nitrosyl post-translational modification at its active site cys149 yielding SNO-GAPDH (Hara et. al., 2005). The latter binds to Siah1, an ubiquitin ligase, which contains its own nuclear localization sequence thereby facilitating the intracellular transfer of this protein: protein complex (Hara et. al., 2005; Hara and Snyder, 2006). Lastly, the autophagic function of GAPDH would also require nuclear transport in order to activate the autophagic gene agt12 which subsequently induces downstream autophagic gene expression (Colell et. al., 2007). The process by which this occurs is unknown at the present time. However, it probably requires a fourth transport mechanism as it would be unlikely that Siah1 would be involved, i.e., how would a cell determine that one GAPDH: Siah1 complex would function in apoptosis and another in autophagy? As such, this requires also further detailed analysis for clarification.
Semi-Constitutive GAPDH Subcellular Translocation
Semi-constitutive GAPDH subcellular translocation may be thought of as a mixture of the aforementioned mechanisms through which the intracellular movement of GAPDH is directed. As such, it represents a more complex pathway requiring three different steps (Figure VI). Its temporal sequence is movement to a new intracellular locale first, GAPDH acquisition of a new function second, followed third by its translocation to another intracellular position.
Figure VI. Semi-Constitutive GAPDH Translocation.
This is indicated in Table IV with respect to GAPDH function in iron metabolism as a transferrin binding protein (Raje et. al., 2007) . In that capacity, GAPDH is transported first to the cell membrane as a “free” protein, where, second, it binds transferrin. Subsequently, the GAPDH-transferrin complex translocates to the endosome, presumerably by endocytosis (Robbins et. al., 1995).
Table IV. “Semi-Constitutive” GAPDH Functions.
| Function | Activity | Reaction | Initial Subcellular Localization | Final Subcellular Localization |
|---|---|---|---|---|
| Iron Metabolism | Transferrin Binding Protein | Endocytosis | Plasma Membrane | Endosome |
| Membrane Trafficking | Formation of Vesicular Tubular Complexes | Modification of Tubulin Structure | Endoplasmic Reticulum | Golgi Apparatus |
Implicit in the role of GAPDH as an iron receptor/transport protein is the initial control of its subcellular localization, i.e., GAPDH transport to the plasma membrane. As recent findings indicate that GAPDH recruitment to the plasma membrane may be a function of extracellular iron concentration, there needs to be not only a specific mechanism through which a GAPDH molecule becomes membrane associated but also how that process may be iron-dependent (Raje et. al., 2007).
Given the nature of GAPDH gene and protein structure, this presumerably can not be accomplished through the use of a membrane localization signal within the GAPDH protein. This would indicate again the necessity of a post-translational modification as the targeting signal. In support of that assertion, previous studies indicated the presence of two GAPDH membrane isoforms distinguished not only by structural differences but also by unique catalytic activities (Glazer and Gross, 1995). With respect to the former, the difference in the two forms (pI 7.8 and 8.5, respectively) are indicative of functional group addition. With respect to the latter, selective exhibition of either membrane fusion activity or of glycolytic activity indicate catalytic changes arising from the above referenced structural modification. As GAPDH retains its catalytic activity upon transferrin binding; this indicates that one isoform may be involved in this new GAPDH function.
A similar model may be constructed for the role of GAPDH in intracellular membrane trafficking. However, in this instance, there is clear evidence for the role of GAPDH post-translational modification as a controlling, targeting mechanism. Two phosphorylation events have been identified, one at GAPDHtyr41 (Tisdale and Artalejo, 2006) and another at a serine residue (Tisdale, 2002). The first phosphorylation controls vesicular tubular complex formation in the ER while the second is responsible for both tubulin rearrangement and movement of the complex to the Golgi (Tisdale, 2009).
Apart from the complexity of semi-conservative GAPDH regulation, two other parameters may distinguish this type of subcellular GAPDH translocation from those discussed earlier. Both its iron transport and its trafficking functions require GAPDH association with large, well-defined high molecular weight protein complexes which function in highly ordered cell transport systems. With respect to the former, previous studies indicate the interrelationship between membrane bound GAPDH and endocytosis (Robbins et. al., 1995). With respect to the latter, recent investigations identified and characterized the set of proteins involved in rab2 mediated membrane transport (Tisdale, 2001, 2002; Tisdale and Artalejo, 2006; Tisdale et. al., 2009).
Discussion
Multifunctional proteins may represent a new mechanism through which cells expand the limited amount of their genetic information. In particular, given the restricted number of cellular macromolecules specified by coding sequences as compared with functional requirements in vivo, it may have become necessary for a cell to devise procedures through which it would be able to expand its capacity to utilize the information contained within its DNA sequences. As one may consider alternate splicing and post-translational modification as such methods, it is also possible to consider multifunctional proteins in a similar manner. A further rationale for the development of multifunctional proteins resides in their potential utility as key regulators for more than one cellular biochemical pathway. By definition, specific alterations of a single multifunctional protein could have a pleiotropic effect in vivo. This would permit the inhibition or activation of multiple pathways through that single change in post-translational protein structure. Further, such alterations could, by themselves, result in the appearance of a new, previously unknown, catalytic activity. For example, recent studies indicate that SNO-GAPDH may act as a transnitrosylase donating its NO moiety to a different protein (Kornberg et. al., 2010).
For the use of a protein for multiple purposes, two practical problems need resolution. First, such proteins, identified by a single activity with a specific subcellular localization, might need to be transported to a different intracellular locale for the performance of that new activity. Second, mechanisms must exist through which said protein is selected for transport. As such, each adds an additional layer of complexity for multidimensional protein function and requires the cell to devise a means to resolve each concern.
With respect to the former, using GAPDH as a model, three mechanisms were postulated through which multifunctional proteins not only may undergo subcellular translocation but also may be able to assume their new functions. Each is based on the temporal sequence between intracellular movement and the new activity in question. Each model is consistent not only with the known, diverse subcellular locales in which GAPDH is detected (Sirover, 1999; 2005; 2011; Tristan et. al., 2011) but also with its inherent movement to a second intracellular location when necessary.
With respect to the latter, analyses of GAPDH structure and function reveal separate protein: protein interactions which mediate GAPDH subcellular translocation as a function of receptor mediated cell signaling (Haraday et. al., 2007), iron metabolism (Raje et. al., 2007), membrane trafficking (Tisdale, 2001) and oxidative stress (Hara et. al, 2005). In contrast, mechanisms through which GAPDH becomes membrane associated (Robbins et. al., 1995) as well as its nuclear localization in the absence of a “multifunctional stimulus” remain unclear (Singh and Green, 1993, Azam et. al, 2008, Demarse et. al., 2009). However, with respect to those nuclear functions, once in the nucleus it would appear that specific signals exist through which GAPDH is then recruited for its new activities.
Physiological changes in the cell may also affect the regulation of multiprotein subcellular localization. For GAPDH, perhaps the greatest magnitude change in intracellular distribution may be that observed with cell proliferation. Immunofluorescent analyses and quantitation of the localization of newly synthesized GAPDH indicate a growth dependent movement of the protein from the cytosol to the nucleus (Lee and Sirover, 1989; Cool and Sirover, 1989; Schmitz et. al., 2003). Even a change from low to high serum induces this intracellular movement (Schmitz, 2001). The latter change in nutritional status is reminiscent of that observed for membrane bound GAPDH as a function on iron concentration (Raje et. al., 2007).
Although this review focuses on the normal and the “cellular pathological” regulation of GAPDH subcellular translocation, significant changes may occur in specific disease states, most notably cancer, diabetes and age-related neurodegenerative disorders. The Warburg effect, establishing higher levels of glycolysis in cancer cells (Warburg et. al., 1927) may provide the foundation for tumor-specific increases in GAPDH expression (rev. in Sirover, 2011). Considering the multidimensional nature of GAPDH, such increases may have a pleiotropic effect as indicated by its post-transcriptional control of colony-stimulating factor-1 in ovarian cancer cells (Bonafe et. al., 2005; Zhou et. al., 2008). Chronic hyperglycemic dependent changes in GAPDH expression in association with the induction of oxidative stress may also result in noticeable changes in GAPDH function perhaps contributing not only to endothelial cell destruction but also to diabetic retinopathy (Du et. al, 2003; Yego et. al., 2009; 2010). Similarly, GAPDH binding to neurodegenerative proteins results in perturbations in its intracellular localization (Mazzola and Sirover, 2002). As the intracellular location of multifunctional proteins may be in equilibrium subject to the demands of the cell, a pathological state may induce considerable dysregulation of multifunctional protein location and thus function in vivo. This may be verified experimentally.
Acknowledgments
The author is grateful for the studies of his colleagues (Tristan et. al., 2011) which stimulated his thinking and for his conversations with Clifford Schweinfest which led to a cellular understanding of the meaning of Dorothy and the yellow brick road. The author has no conflicts of interest to declare.
Grant Information: Work in the author's laboratory was funded by a grant from the National Institutes of Health (CA 119285).
References
- Azam S, Jouvet N, Jilani A, Vongsamphanh R, Yang X, Yang S, Ramotar D. Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1. J Biol Chem. 2008;283:30632–30641. doi: 10.1074/jbc.M801401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund M, Paukku K, Daviet L, De Boer RA, Valo E, Hautaniemi S, Kalkkinen N, Ehsan A, Kontula KK, Lehtonen JA. Posttranscriptional regulation of angiotensin II type 1 receptor expression by glyceraldehyde-3-phosphate dehydrogenase. Nucl Acids Res. 2009;37:2346–235. doi: 10.1093/nar/gkp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafe N, Gilmore-Hebert M, Folk NL, Azodi M, Zhou Y, Chambers SK. Glyceraldehyde-3-phosphate dehydrogenase binds to the AU-rich 3′untranslated region of colony-stimulating factor-1 (CSF-1) messenger RNA in human ovarian cancer cells: possible role of CSF-1 posttranscriptional regulation and tumor phenotype. Cancer Res. 2005;65:3762–3771. doi: 10.1158/0008-5472.CAN-04-3954. [DOI] [PubMed] [Google Scholar]
- Brown VM, Krynetski EJ, Krynetskaia NF, Grieger D, Mukatira ST, Murti KG, Slaughter CA, Park HW, Evans WE. A novel CRM1-mediated nuclear export signal governs nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase following genotoxic stress. J Biol Chem. 2004;279:5984–5992. doi: 10.1074/jbc.M307071200. [DOI] [PubMed] [Google Scholar]
- Bruns GAP, Gerald PS. Human glyceraldehyde-3-phosphate dehydrogenase in man-rodent somatic cell hybrids. Science. 1976;192:54–56. doi: 10.1126/science.176725. [DOI] [PubMed] [Google Scholar]
- Bruns G, Gerald PS, Lalley P, Francke U, Minna J. Gene mapping of the mouse by somatic cell hybridization. Cytogenet Cell Genet. 1979;25:139. [Google Scholar]
- Butterfield DA, Hardas SS, Bader Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to Neurodegeneration. J Alzheimers Dis. 2010;20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio-Carrion A, Waterhouse NJ, Li CW, Mari B, Barbry P, Newmeyer DD, Beere HM, Green DR. GAPDH and autophagy preserve survival after cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death and Diff. 2009;16:1573–1581. doi: 10.1038/cdd.2009.137. [DOI] [PubMed] [Google Scholar]
- Cool BL, Sirover MA. Immunocytochemical analysis of the base excision repair enzyme uracil DNA glycosylase in quiescent and proliferating normal human cells. Cancer Res. 1989;49:3029–3036. [PubMed] [Google Scholar]
- Demarse NA, Ponnusamy S, Spicer EK, Apohan E, Baatz JE, Ogretman B, Davies C. Direct binding of glyceraldehyde-3-phosphate dehydrogenase to telomeric DNA protects telomeres against chemotherapy-induced rapid degradation. J Mol Biol. 2009;394:789–803. doi: 10.1016/j.jmb.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly (ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy-Kannlapan S, Geschwind JF, Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, Cole RN, Syed LH, Rao PP, Ota O, Vali M. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cell death. Antican Res. 2009;29:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- Glaser PE, Gross RW. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3 phosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry. 1995;34:12194–12203. doi: 10.1021/bi00038a013. [DOI] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature Cell Biology. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Hara MR, Snyder SH. Nitric oxide-GAPDH-Siah: A novel cell death cascade. Cell Mol Neurobiol. 2006;26:525–536. doi: 10.1007/s10571-006-9011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara MR, Cascio MB, Sawa A. GAPDH as a sensor of NO stress. Biochimica et Biophysica Acta. 2006;1762:502–509. doi: 10.1016/j.bbadis.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Harada N, Yasunaga R, Higashimura Y, Yamaji R, Fujimoto K, Moss J, Inui H, Nakano Y. Glyceraldehyde-3-phosphate dehydrogenase enhances transcriptional activity of androgen receptor in prostate cancer cells. J Biol Chem. 2007;282:22651–22661. doi: 10.1074/jbc.M610724200. [DOI] [PubMed] [Google Scholar]
- Jeffery CJ. Moonlighting Proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- Jenster G, Trapman J, Brinkmann AO. Nuclear import of the human androgen receptor. Biochem J. 1993;293:761–768. doi: 10.1042/bj2930761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JVK, Snowman AM, Law L, Hester LD, Snyder SH. GAPDH mediates nitrosylation of nuclear proteins. Nature Cell Biol. 2010;12:1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Rhim JH, Jang IS, Kim GE, Park SC, Yeo EJ. Activation of AMP-activated kinase stimulates the nuclear localization of glyceraldehydes-3-phosphate dehydrogenase in human diploid fibroblasts. Exp Mol Med. 2010;42:254–269. doi: 10.3858/emm.2010.42.4.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Sirover MA. Physical association of base excision repair enzymes with parental or replicating DNA in BHK–21 cells. Cancer Res. 1989;49:3037–3044. [PubMed] [Google Scholar]
- Mazzola JL, Sirover MA. Alteration of Intracellular Structure and Function of Glyceraldehyde-3-Phosphate Dehydrogenase: A Common Phenotype of Neurodegenerative Disorders? NeuroToxicology. 2002;23:603–609. doi: 10.1016/s0161-813x(02)00062-1. [DOI] [PubMed] [Google Scholar]
- Mezquita J, Pau M, Mezquita C. Several novel transcripts of glyceraldehyde-3-phosphate dehydrogenase expressed in adult chicken testis. J Cell Biochem. 1998;71:127–139. doi: 10.1002/(sici)1097-4644(19981001)71:1<127::aid-jcb13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Nagy E, Rigby WFC. Glyceraldehyde-3-phosphate dehydrogenase selectively binds AU-rich RNA in the NAD+ binding region (Rossman Fold) J Biol Chem. 1995;270:2755–2763. doi: 10.1074/jbc.270.6.2755. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Amano W, Fujita A, Fukuhara A, Azuma YT, Hata F, Inui T, Takeuchi T. The active site cysteine of the proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase is essential in oxidative stress-induced aggregation and cell death. J Biol Chem. 2007;282:26562–26574. doi: 10.1074/jbc.M704199200. [DOI] [PubMed] [Google Scholar]
- Park J, Han D, Kim K, Kang Y, Kim Y. O-GlcNAcylation disrupts glyceraldehyde-3-phosphate dehydrogenase homo-tetramer formation and mediates its nuclear translocation. Biochim et Biophys Acta. 2009;1794:254–262. doi: 10.1016/j.bbapap.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Raje CI, Kumar S, Harle A, Nanda JS, Raje M. The macrophage cell surface glyceraldehyde-3-phosphate dehydrogenase is a novel transferrin receptor. J Biol Chem. 2007;282:3252–3261. doi: 10.1074/jbc.M608328200. [DOI] [PubMed] [Google Scholar]
- Robbins AR, Ward RD, Oliver C. A mutation in glyceraldehyde-3-phosphate dehydrogenase alters endocytosis in CHO Cells. J Cell Biol. 1995;130:1093–1104. doi: 10.1083/jcb.130.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguex-Pascual F, Redondo-Horcajo M, Magan-Marchal N, Lagares D, Martinez-Ruiz A, Kleinert H, Lamas S. Glyceraldehyde-3-phosphate dehydrogenase regulates endothelin-1 expression by a novel, redox-sensitive mechanism involving mRNA stability. Mol Cell Biol. 2008;28:7139–7155. doi: 10.1128/MCB.01145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz HD. Reversible nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase upon serum depletion. Eur J Cell Biol. 2001;80:419–427. doi: 10.1078/0171-9335-00174. [DOI] [PubMed] [Google Scholar]
- Schmitz HD, Dutine C, Bereiter-Hahn J. Exportin 1-independent nuclear export of GAPDH. Cell Biol Int'l. 2003;27:511–517. doi: 10.1016/s1065-6995(03)00096-9. [DOI] [PubMed] [Google Scholar]
- Sen N, Hara MR, Kornberg MD, Cascio MB, Bae BI, Shahani N, Thomas B, Dawson TM, Dawson VL, Snyder SH, Sawa A. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nature Cell Biology. 2008;10:866–873. doi: 10.1038/ncb1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Green MR. Sequence-specificity binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993;259:365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Sirover MA. New nuclear functions of the glycolytic protein glyceraldehyde-3-phosphate dehydrogenase. J Cell Biochem. 2005;95:45–52. doi: 10.1002/jcb.20399. [DOI] [PubMed] [Google Scholar]
- Sirover MA. New insight into an old protein: The functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1999;1432:159–184. doi: 10.1016/s0167-4838(99)00119-3. [DOI] [PubMed] [Google Scholar]
- Sirover MA. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: Biochemical mechanisms and regulatory control. Biochim Biophys Acta. 2011;1810:741–751. doi: 10.1016/j.bbagen.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ. Glyceraldehyde-3-phosphate dehydrogenase is required for vesicular transport in the early secretory pathway. J Biol Chem. 2001;276:2480–2486. doi: 10.1074/jbc.M007567200. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ. Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by protein kinase Cί/λ and plays a role in microtubule dynamics in the early secretory pathway. J Biol Chem. 2002;277:3334–3341. doi: 10.1074/jbc.M109744200. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Artalejo CR. Src-dependent aprotein kinase C ί/λ (aPKCί/λ) tyrosine phosphorylation is required for aPKCί/λ association with Rab2 and glyceraldehyde-3-phosphate dehydrogenase on pre-golgi intermediates. J Biol Chem. 2006;281:8436–8442. doi: 10.1074/jbc.M513031200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Azizi F, Artalejo CR. Rab2 utilizes glyceraldehyde-3-phosphate dehydrogenase and protein kinase Cί to associate with microtubules and to recruit dynein. J Biol Chem. 2009;284:5876–5884. doi: 10.1074/jbc.M807756200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristan C, Shahani N, Sedlak TW, Sawa A. The diverse functions of GAPDH: views from different subcellular compartments. Cell Signal. 2011;23:317–323. doi: 10.1016/j.cellsig.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, Mateo F, Serratosa J, Salaet I, Carujo S, Bachs O, Pujol MJ. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase is regulated by acetylation. Int'l J Biochem Cell Biol. 2010;42:1672–1680. doi: 10.1016/j.biocel.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Phys. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yego ECK, Vincent JA, Sarthy VP, Busik I, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Muller cells by IL-1beta and IL-6. Invest Opthalmol Vis Sci. 2009;50:1920–1928. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- Yego ECK, Mohr S. Siah-1 protein is necessary for high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation and cell death in Muller cells. J Biol Chem. 2010;285:3181–3190. doi: 10.1074/jbc.M109.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng I, Roeder RG, Luo Y. S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell. 2003;114:255–266. doi: 10.1016/s0092-8674(03)00552-x. [DOI] [PubMed] [Google Scholar]
- Zhou y, Yi X, Stofffer JB, Bonafe N, Gilmore-Hebert M, McAlpine J, Chambers SK. The multifunctional protein glyceraldehyde-3-phosphate dehydrogenase is both regulated and controls colony-stimulating factor-1 messenger RNA stability in ovarian cancer. Mol Cancer Res. 2008;6:1375–1380. doi: 10.1158/1541-7786.MCR-07-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]