Abstract
Several purine receptors have been localised on skeletal muscle membranes. Previous data support the hypothesis that extracellular guanosine 5′-triphosphate (GTP) is an important regulatory factor in the development and function of muscle tissue. We have previously described specific extracellular binding sites for GTP on the plasma membrane of mouse skeletal muscle (C2C12) cells. Extracellular GTP induces an increase in intracellular Ca2+ concentrations that results in membrane hyperpolarisation through Ca2+-activated K+ channels, as has been demonstrated by patch-clamp experiments. This GTP-evoked increase in intracellular Ca2+ is due to release of Ca2+ from intracellular inositol-1,4,5-trisphosphate-sensitive stores. This enhances the expression of the myosin heavy chain in these C2C12 myoblasts and commits them to fuse into multinucleated myotubes, probably via a phosphoinositide-3-kinase-dependent signal-transduction mechanism. To define the signalling of extracellular GTP as an enhancer or modulator of myogenesis, we investigated whether the gene-expression profile of differentiated C2C12 cells (4 and 24 h in culture) is affected by extracellular GTP. To investigate the nuclear activity and target genes modulated by GTP, transcriptional profile analysis and real-time PCR were used. We demonstrate that in the early stages of differentiation, GTP up-regulates genes involved in different pathways associated with myogenic processes, including cytoskeleton structure, the respiratory chain, myogenesis, chromatin reorganisation, cell adhesion, and the Jak/Stat pathway, and down-regulates the mitogen-activated protein kinase pathway. GTP also increases the expression of three genes involved in myogenesis, Pp3ca, Gsk3b, and Pax7. Our data suggests that in the myogenic C2C12 cell line, extracellular GTP acts as a differentiative factor in the induction and sustaining of myogenesis.
Keywords: Transcriptome, Myogenesis, GTP
Introduction
Myogenesis is a highly ordered process that can be subdivided into a sequence of transiently separable events: proliferation of myoblasts, irreversible cell-cycle withdrawal, cell fusion to form myotubes, and maturation of these myotubes into various classes of myofibres. Successful myogenic differentiation requires numerous interactions between diverse cellular processes, including growth factors, hormones, receptors, transcription factors, kinases, and histone deacetylases [1–7]. These processes are controlled by muscle-specific transcriptional regulators that determine cell fate and differentiation, and by external signals that couple myogenesis to development and growth of the organism [4, 8, 9]. At the molecular level, myogenic determination and activation of muscle-specific gene expression involve the association of skeletal-muscle-specific helix-loop-helix myogenic regulatory factors (MRFs) with the MEF2 family of MADS-box myocyte-enhancer binding factors [10, 11].
MEF2 factors cannot activate muscle-specific gene expression themselves, but rather, they potentiate the myogenic activities of MRFs. MRFs include MyoD, Myf5, myogenin, and MRF4, and the MRF proteins can act as transactivators of muscle-specific genes by binding to a conserved consensus sequence that is termed the Ebox, which is located in the regulatory region of several muscle-specific genes. Recently, it was suggested that MRFs recruit chromatin-remodelling proteins to activate gene expression.
The transcriptional activities of MRFs can be negatively regulated by a family of inhibitors of DNA binding, the Id1-4 proteins. It is also of note that expression of MRFs in several non-myogenic cell lines is sufficient to induce myogenic differentiation [12–14]. Nevertheless, the specific role of different MRFs in skeletal muscle differentiation has not yet been completely defined, essentially due to the existence of auto-regulatory and cross-regulatory loops between the MRFs [15]. In particular, MRF4 appears to be involved in the last stages of differentiation, probably during myofibrillogenesis [13, 16–19], and/or it is expressed both in the early stages of myogenesis, and later during muscle development and in adult muscle tissue [20]. However, the potential for myoblasts to proliferate or to switch into a differentiation pathway is regulated by the balance of positive and negative cell-cycle regulators [21].
It appears that MRFs have a role only when the negative regulators of muscle differentiation are inactivated. Withdrawal from the cell cycle requires expression of cyclin-dependent kinase inhibitors [22, 23], which leads to fusion and maturation of multinucleate myotubes. This process is associated with the expression of late differentiation markers, such as myosin heavy and light chains, muscle creatine kinase, and the cholinergic receptor, which are among the downstream targets of MRFs and MEF2 family factors. All of these findings discussed above have been confirmed in recent studies performed using gene-expression analysis, which revealed groups of gene products that are directly involved in cell-cycle withdrawal, muscle differentiation, and apoptosis, and that are differentially expressed during myoblast differentiation [24, 25].
GTP might be an important regulatory factor for development, function, and myogenesis of muscle tissue. The present study was performed using C2C12 cells, a well-characterised model of murine skeletal muscle cells. Undifferentiated mononucleate C2C12 cells (myoblasts) proliferate in the presence of 20% foetal bovine serum, with a doubling time of about 12 h. When near to confluency, myoblasts can be committed to fuse and differentiate within 24–48 h, forming multinucleated myotubes in the presence of starvation medium. Our previous studies have shown that with C2C12 cells, extracellular GTP binds to specific, P2Y-receptor-like sites [26]. This induces an increase in intracellular calcium ion concentration ([Ca2+]i), which, in turn, causes membrane hyperpolarisation through the activation of K+ channels [27]. In C2C12 cells, effective concentrations of extracellular GTP provoke an increase in [Ca2+]i due to Ca2+ release from intracellular inositol-1,4,5-trisphosphate-sensitive stores. This enhances the expression of MyHC in C2C12 myoblasts, which are then committed to fuse into multinucleated myotubes [27], probably via a phosphoinositide-3-kinase-dependent signal-transduction mechanism [28].
Extracellular GTP has been shown to modulate the development of isometric twitch tension in frog muscle fibres. Moreover, extracellular purines affect the behaviour of Paramecium tetraurelia and Tetrahymena thermophila, inducing backwards swimming. As these studies only hint at the mechanisms of GTP-induced signal-transduction pathways, we used high-density oligonucleotide arrays and real-time PCR to investigate transcriptional changes that occur during the early stages of differentiation in C2C12 cells.
To obtain a “snapshot” of the gene expression patterns associated with GTP exposure, we analysed the transcriptional profiles of cells after 4 and 24 h of incubation with 500 μM GTP, using two different experimental set-ups:
C2C12 myoblasts committed to differentiation by incubation in normal differentiation medium (DM) containing 2% horse serum.
C2C12 myoblasts committed to differentiation by incubation in synthetic differentiation medium (SM) containing 1% bovine serum albumin (BSA).
We demonstrate here that in the early stages of differentiation, GTP up-regulates genes involved in different pathways associated with myogenic processes: cytoskeleton structure, the respiratory chain, myogenesis, chromatin reorganisation, cell adhesion, and the Jak/Stat pathway. In contrast, GTP down-regulates the mitogen-activated protein kinase (MAPK) pathway.
Materials and methods
Cell culture
C2C12 cells (CRL 1772; American Type Culture Collection, Rockville, MD, USA) were cultured as exponentially growing myoblasts in growth medium (GM): Dulbecco's modified Eagle's medium (DMEM; Euroclone, Pero, Italy) containing 20% foetal bovine serum (Euroclone), 4 mM l-glutamine (Euroclone) and 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin (Euroclone). The cells were seeded into 100-mm diameter Petri dishes at a density of 1,500–2,000 cells cm−2 in GM and subcultured by standard trypsinisation every 3 days. After 2 days in GM, differentiation was induced using one of two differentiation media: (1) DM, as DMEM containing 2% horse serum (Euroclone), and l-glutamine and antibiotics as for GM; and (2) SM, as DMEM containing 1% (w/v) BSA (Sigma-Aldrich, Milan, Italy), and l-glutamine and antibiotics as for GM.
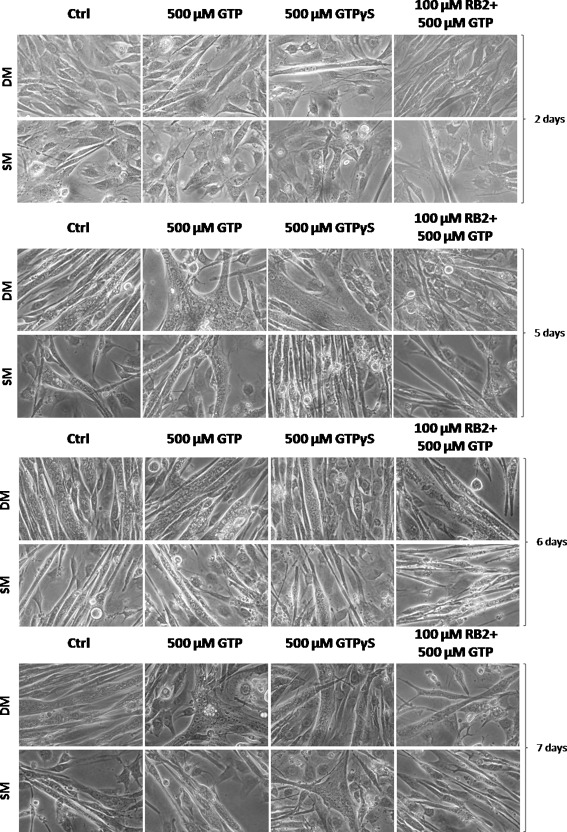
For the differentiation, the C2C12 cells were plated in 100-mm diameter Petri dishes at a density of 2,500 cells cm−2 and grown for 48 h in GM. Differentiation was induced by replacing the medium with DM or SM, and the cells were stimulated (as indicated) by addition of 500 μM GTP or 500 μM GTPγS to the medium, or following a preincubation with 100 μM reactive blue 2 (RB2), with 500 μM GTP then added. Images were captured after 2, 5, 6, and 7 days of differentiation, using a Canon Powershot S45 camera on an inverted microscope (Leica) at 400× magnification, and then stored on a host computer. The experimental procedures were repeated twice.
Transcriptional profile analysis
First experimental set-up
To perform the microarray experiments, MWG mouse 30k A microarray slides were used. C2C12 cells were plated into 100-mm diameter Petri dishes at a density of 2,500 cells cm−2 and grown for 48 h in GM. Differentiation was induced using SM, with the experiments performed in two stages: over 4 and 24 h of differentiation and in the absence and presence of 500 μM GTP (Sigma-Aldrich, Milan, Italy). Total RNA from these C2C12 cells was then purified using NucleoSpin RNA II kits (Macherey-Nagel, Duren, Germany), according to the manufacturer's instructions. The total RNA extractions were quantified spectrophotometrically (GeneQuant pro, Amersham). The analysis of RNA samples was performed using an Agilent Bioanalyser 2100 with a Nano chip, according to the RNA sample amounts. The RNA amplification and labelling reactions were carried out using two-step amino-allyl labelling.
The hybridisation was performed using the MWG mouse 30k A microarray slides according to the MWG array manual. Multiple scans with a dual laser scanner were performed using different photomultiplier gain settings (e.g., 250, 220, 190) for each channel. For a typical slide, six images were created: three in the Cy5 channel and three in the Cy3 channel, as TIFF images. The ImaGen software (BioDiscovery) was used to calculate the intensity of each spot and the corresponding background from the individual scanner TIFF images. For combining the data of the multiple scans per slide, the MAVI Pro 2.6.0 (MWG Biotech AG) background correction and normalisation software was used. The background-corrected and normalised intensities were used to calculate the Cy5:Cy3 ratios, as a direct measure of up-regulation or down-regulation of each gene analysed. For these ratios, the channel considered to be the experimental data was that coupled with Cy5, which was divided by that coupled with Cy3, which was considered to be the control data. Cy5:Cy3 ratios > 2 (i.e., twofold up-regulated) represent up-regulated gene expression (as experimental vs. control), while ratios <0.5 represent down-regulated gene expression. Ratios between 0.5 and 2 correspond to genes that were not considered to have been regulated under these conditions. Non-commercial tools were used to organise and visualise the gene-network data obtained from the microarray analysis, including GenMAPP and iHOP—http://www.ihop-net.org/. The microarray results have been deposited at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18531.
Second experimental set-up
The mouse oligonucleotide array consisted of 13,443 70mer oligonucleotides (Operon, version 1.1) that were designed on Mouse Unigene clusters, mainly in the 3′ terminal region. Each oligonucleotide was spotted by a robotic station (Biorobotics Microgrid II) onto MICROMAX glass slides (SuperChip I, PerkinElmer, Life Sciences Inc.) as two replicates. Details of the design of the slide and the sequence selection can be found at the manufacturer's website (http://microcribi.cribi.unipd.it/e-index.htm). For microarray experiments, the C2C12 cells were cultured in the presence of DM for 4 h, to induce cell differentiation, and in the absence and presence of 500 μM GTP. The RNA isolation, quantification, and analysis were as described for the first experimental set-up above.
Aliquots (1 μg) of each RNA sample were reverse transcribed using Amino Allyl MessageAmp II aRNA Amplification kits (Ambion, Monza, Italy). An aliquot of the modified aaRNA was coupled with the fluorophores Cy3 and Cy5 (CyDye Post-Labelling Reactive Dye Packs, GE Healthcare, Milan, Italy). Optimal amounts of aRNA-Cy3 and aRNA-Cy5 were co-precipitated by adding 0.8 volume of ammonium acetate 5 M and 2.5 volumes of absolute ethanol. The sample was dissolved using 120 μl hybridisation buffer (500 μl 20× SSC, 20 μl 10% [w/v] sodium dodecyl sulfate, SDS, 1 ml formamide, 480 μl distilled, Ambion water) and 3 μl RNase-free water (GIBCO, San Giuliano Milanese, Italy), denatured at 90°C for 2 min, and applied directly to the microarray slides and covered with a cover slip. The microarray slides were seeded and sealed in the hybridisation chamber and incubated for 2 days in a water bath at 48°C. After hybridisation, the slides were washed sequentially with solutions of 0.2% (w/v) SDS in 1× SSC, of 0.2% (w/v) SDS in 0.1× SSC, of 0.2× SSC, and of 0.1× SSC (4 min for each wash), in the dark at room temperature. The arrays were scanned using a GSI Lumonics LITE dual confocal laser scanner, with a ScanArray Microarray Analysis System (PerkinElmer), and the raw images were analysed using QuantArray Analysis Software (GSI Lumonics, Ottawa, Canada). The expression levels of all of the spot replicates were normalised by applying the Lowess (Locfit) normalisation procedure, using MIDAS (TIGR Microarray Data Analysis System) before performing any statistical analyses. As described above, the background-corrected and normalised intensities were used to calculate the Cy5:Cy3 ratios. Differentially expressed genes were identified using the significant analysis of microarrays permutation test procedure (STANFORD software, http://www-stat.stanford.edu/_tibs/SAM), which defines as significant those genes with computed scores greater than the threshold value. The false discovery rate associated with a given threshold was also calculated from the permutation data. A log base-2 of the ratio >1 (twofold up-regulated) represented up-regulated gene expression (experimental vs. control); while a log base-2 of the ratio <−1 (twofold down-regulated) represented down-regulated gene expression. A log base-2 of the ratio between 1 and −1 corresponded to genes that were not considered to have been regulated under these conditions. The non-commercial tools used to organise and visualise the gene-network data were as indicated above, and the microarray results are deposited at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18584.
Real-time PCR
For the real-time PCR, the C2C12 cells were prepared (in GM), differentiated (in SM) and treated (GTP/ GTPγS) as described above. The total RNA was isolated also as described above. For cDNA synthesis, 20 μl (1 μg) total RNA was directly processed with high-capacity cDNA Archive kits. For each sample, 20 μl total RNA was added to 5 μl 10× RT buffer, 2 μl 25× dNTP mix, 5 μl 10× RT random primers, 1.25 μl reverse transciptase, 16.75 μl nuclease-free water, to a final volume of 50 μl. The reactions were incubated in a GeneAmp PCR System 9700 at 25°C for 10 min, 42°C for 1 h, 95°C for 5 min and then at 4°C.
So-called singleplex real-time PCR was performed for the relative quantitation of the gene expression of Pax7 (paired box gene 7), Ppp3ca (calcineurin catalytic subunit), Gsk3b (glycogen synthase kinase 3 beta), Cox7a1 (cytochrome c oxidase, subunit VIIa 1), vs. beta-actin, using TaqMan technology on an ABI Prism 9700HT Sequence Detection System instrument, connected to Sequence Detector Software (SDS, version 2.0) for collection and analysis of data. The primer pairs and TaqMan probes for all of the target genes and for the beta-actin reference gene were provided as 20× mixes that were ready to use at a final concentration of 1×. According to the manufacturer recommendations, 25 μl reactions were performed in a MicroAmp Optical 96-well reaction plate using 12.5 μl 2× TaqMan Universal PCR Master mix, with 1.25 μl 20× Inventoried Gene Expression Product for the mouse Pax7 target gene, or 1.25 μl for the mouse Ppp3ca target gene, or 1.25 μl for the mouse Gsk3b target gene, or 1.25 μl for the mouse Cox7a1 target gene (FAM dye-labelled TaqMan MGB probe), or 1.25 μl 20× Inventoried TaqMan Assay reagent for the mouse beta-actin reference gene (FAM dye-labelled TaqMan MGB probe). For each sample, the cDNA was diluted in RNAse-free water to reach the final 25 μl reaction volume. PCR was performed at 50°C for 2 min, and at 95°C for 10 min, and then run for 45 cycles at 95°C for 15 s and at 60°C for 1 min. All of the reactions were performed in triplicate, and each experiment was repeated three times. The results were exported from the ABI Prism 9700HT Sequence Detection System into Microsoft Excel files for further analysis. The relative quantification of target gene expression was evaluated with data from the SDS software, using the arithmetical formula 2−DDCt, according to the comparative Ct method, which represents the amount of target, as normalised to the beta-actin endogenous control (reference). All materials, instruments and softwares were purchased by Applied Biosystems.
Results
To demonstrate the unique role of GTP in myogenesis, we investigated its effects using two different experimental conditions. We used normal DM and an unusual differentiation media of DMEM with BSA (SM). The rationale was that the absence of serum differentiating factors in SM would allow the GTP effects to be better discriminated. Indeed, the starved cells fuse with each other and form myotubes over a longer period of time with respect to DM. Figure 1 shows images of C2C12 cells during the differentiation process in DM and SM at 2, 5, 6, and 7 days. As can be seen, the fusion process and the differentiation timing are delayed in SM, by a few days. In SM, there are fewer myotubes after 6–7 days of differentiation, while in DM, the formation of myotubes starts after 4–5 days of differentiation.
Fig. 1.
Morphological effects of GTP, GTPγS, and RB2 in differentiating C2C12 cells. C2C12 cells were induced to differentiate using DM and SM in the absence and presence of 500 μM GTP, 500 μM GTPγS, and with preincubation with 100 μM RB2 before 500 μM GTP (all as indicated). The images show: (a) timing of myogenesis in DM is more rapid than in SM at all time points; (b) cells stimulated with 500 μM GTP show more numerous, longer, and thicker myotubes than control cells; (c) myotubes obtained in medium plus 500 μM GTP and 500 μM GTPγS show very similar morphologies; and (d) pre-incubation with RB2 leads to thinner and fewer myotubes and a higher myoblast percentage. Magnification, ×400
We also investigated the addition of 500 μM GTP and 500 μM GTPγS to C2C12 cells (Fig. 1). GTPγS is a non-hydrolysable analogue of GTP, and this was used to verify the GTP effect while excluding any effects that might be due to GTP degradation products. After 2 days of stimulation, the number of di-nucleated cells in the presence of GTP and GTPγS (cells that have started the fusion process) was twice that in the control samples in both DM and SM. Preincubation with 100 μM RB2, a purinergic receptor antagonist [29], in the medium in the presence of 500 μM GTP significantly reduced this GTP-induced increase in the number of di-nucleated cells.
After 5–7 days of stimulation in DM, the number of poly-nucleated cells (myotubes) obtained in the presence of GTP and GTPγS was again twice that in the control sample. The pre-incubation with RB2 interfered with the GTP effect, with the myotubes being fewer and thinner than those following GTP stimulation in the absence of RB2 (Fig. 1).
At 5 days of differentiation in SM, there were no myotubes in the control samples, while myotubes were seen in the presence of GTP and GTPγS. This effect was reduced by pre-incubation with RB2 (Fig. 1). Myotubes were seen after 6–7 days of differentiation in SM, with the addition of GTP and GTPγS inducing the fusion process, giving more hypertrophic myotubes. This effect was also blocked by the addition of RB2 (Fig. 1).
Transcriptional profile analysis
SM does not contain any differentiation-inducing factors, so although the starved cells do fuse with each other to form myotubes, this occurs with a delayed time with respect to DM. Under these conditions, the GTP-induced genes determined after a delay of 4 h in DM might be comparable with those determined after a delay of 24 h in SM. These different culture protocols (with the different media) were used to investigate whether GTP itself can induce and sustain C2C12 differentiation. We used high-density microarray to define potential genes related to GTP-induced myogenesis.
A list of the up-regulated and down-regulated genes in GTP-treated cells (relative to control cells, and that satisfied statistical criteria) was thus generated using a significant analysis of microarrays analysis (see “Materials and methods” for more details).
GTP-regulated genes in C2C12 cell differentiation in synthetic medium
Comparative statistical analyses revealed that in SM, 13 and 15 genes were differentially expressed at 4 and 24 h, respectively, in GTP-treated C2C12 cells. Nine genes were up-regulated, and four genes were down-regulated at 4 h, and 12 genes were up-regulated and three genes were down-regulated at 24 h (Table 1).
Table 1.
Differentially regulated gene transcripts following 4 and 24 h differentiation of C2C12 cells in SM with 500 μM GTP
| Gene symbola | Description | IGTP/ICtrl 4 h of differentiationb | IGTP/ICtrl 24 h of differentiationb |
|---|---|---|---|
| Cytoskeleton structure | |||
| Macs | Myristoylated alanine-rich protein kinase c substrate | 4.7 | |
| Sgcb | Sarcoglycan, beta (dystrophin-associated glycoprotein) | 2.1 | |
| Respiratory chain | |||
| ND1 | Nadh dehydrogenase subunit 1 | 2.3 | |
| Cox7b | Cytochrome c oxidase subunit VIIb | 2 | |
| Cox7a1 | Cytochrome c oxidase subunit VIIa 1 | 2 | |
| Protein balance | |||
| Ubc | Ubiquitin c | 0.5 | |
| Ubqln2 | Ubiquilin 2 | 0.13 | |
| Energetic metabolism | |||
| Cpt2 | Carnitine palmitoyltransferase 2 | 42.6 | |
| Acatn | Acetyl-coenzyme a transporter | 28 | |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | 2 | |
| Myogenesis | |||
| Ndn | Necdin | 2.7 | |
| Pax7 | Paired box transcription factor pax7 | 2.5 | |
| Myod1 | Myogenic differentiation 1 | 1.4 | |
| Rab17 | Rab17, member ras oncogene family | 2 | |
| Gsk3b | Glycogen synthase kinase 3 beta | 2.2 | |
| Ppp3ca | Calcineurin | 1.1 | 1.2 |
| Cell adhesion | |||
| Catna1 | Catenin alpha 1 | 0.46 | |
| Vcam1 | Vascular cell adhesion molecule 1 | 1.7 | 0.9 |
| Ncam-180 | Neuronal cell adhesion molecule ncam-180 | 1.5 | 2.4 |
| – | Homologue to metastasis-associated gpi-anchored protein | 0.5 | |
| Mrc2 | Mannose receptor, c type 2 | 0.4 | 0.46 |
| Others | |||
| Saa3 | Serum amyloid a 3 | 2.5 | 5.5 |
| Hoxc4 | Homeo box c4 | 0.5 | |
aGene symbols as described in the NCBI database
bChanges in gene expression levels as log base-2 of the ratios (log2 IGTP-treated/Inon-treated): >2, up-regulated genes; <0.5, down-regulated genes
These differentially regulated genes were assigned to their functional categories and analysed using non-commercial tools, such as GenMAPP and iHOP. Their functional categories included: cytoskeleton structure, energetic metabolism and the respiratory chain, protein balance, cell adhesion, and myogenesis. Broadly speaking, all of the genes identified are linked to cellular processes that have roles in skeletal muscle growth and differentiation [30].
Cytoskeleton structure
The fusion process during differentiation requires reorganisation of the cytoskeleton and redistribution of membrane components. The myristoylated alanine rich protein kinase c substrate (Macs) gene, which expresses a major cytoskeletal protein substrate of protein kinase C, was up-regulated at 24 h. MACS proteolysis is necessary for fusion of myoblasts and its cleavage is mediated by calpain [31]. The sarcoglycan, beta (dystrophin-associated glycoprotein) gene was up-regulated at 24 h; its protein product forms a complex with three further subunits on the skeletal muscle cell surface membrane. A gene mutation can cause a loss or a marked decrease in the whole sarcoglycan complex, resulting in autosomal recessive muscular dystrophy: sarcoglycanopathy [32].
Energy metabolism and the respiratory chain
Stimulation of C2C12 cells with 500 μM GTP during their differentiation in SM resulted in the up-regulation of a number of genes, including glyceraldehyde-3-phosphate dehydrogenase (Gapdh), which codes for an enzyme that catalyses the sixth step of glycolysis. GTP stimulation also enhanced the expression of genes involved in mitochondrial transfer of electrons during synthesise of ATP. Nadh dehydrogenase subunit 1 (ND1) and cytochrome c oxidase subunit VIIb (Cox7b) were both up-regulated at 4 h, while cytochrome c oxidase subunit VIIa 1 (Cox7a1) was up-regulated at 24 h. Carnitine palmitoyltransferase 2 (cpt2) was also up-regulated at 24 h, and, together with carnitine palmitoyltransferase I, its gene product oxidises long-chain fatty acids in mitochondria. A Cpt2 deficiency is a metabolic myopathy that affects transport of fatty acids into mitochondria, which leads to an impaired energy supply under stress conditions. This can result in muscle weakness and rhabdomyolysis [33, 34]. The acetyl-CoA transporter (Acatn) gene was up-regulated at 24 h; this codes for a multiple transmembrane protein in endoplasmic reticulum, Ac-CoA, which is transported to the lumen of Golgi apparatus. Here, Ac-CoA serves as a substrate for acetyltransferases that modify sialyl residues of gangliosides and glycoproteins [35].
Protein balance
Proteasomes are among the major mechanisms by which cells regulate concentrations of particular proteins and degrade misfolded proteins. In our study, two genes that are linked to protein metabolism were differentially expressed during GTP-induced myogenesis in SM. In particular, after 4 h of differentiation, there was down-regulation of ubiquitin c; in combination with proteasomes, the ubiquitin c protein mediates degradation of myosin D and inhibition of DNA binding 1 (Id1) proteins in C2C12 cells during differentiation. Another gene involved in protein balance was down-regulated after 24 h: ubiquilin 2. However, the role of the ubiquilin 2 protein in skeletal muscle tissue is not clear, while in the nervous system, it is related to early-onset Alzheimer's disease [36].
Cell adhesion
Cell–cell and cell–substrate adhesion is essential for maintenance of cell polarity and for cell differentiation and tissue architecture [37]. Catenin alpha 1 was down-regulated at 24 h, and this codes for an actin-binding and bundling protein that mediates attachment of F-actin to the membrane adhesion complex [38]. It is an intracellular protein that associates with cadherins and the cytoskeleton, and it is required for the formation and maintenance of functional intercellular adhesion complex. Neuronal cell adhesion molecule (ncam)-180 was up-regulated at 4 and 24 h of differentiation, and a predominant trend for up-regulation was observed for vascular cell adhesion molecule 1 at both times; both of these code for cell adhesion molecules that are expressed on the surface of skeletal muscle satellite cells, and which contribute to myotube formation through the fusion of myoblasts [39, 40]. Cell adhesion is a process that is highly correlated to myoblast fusion and myogenesis. The only gene down-regulated at both 4 and 24 h of differentiation in SM was mannose receptor, c type 2, which is involved in many cellular functions, such as Ca2+ and collagen binding, and receptor activity; the collagen binding reaction might lead to adhesive functions as well as contribute to cellular degradation of collagen matrices [41].
Myogenesis
Stimulation of these C2C12 myoblasts with 500 μM GTP in SM for 4 h up-regulated necdin, an important factor that is required for myoblast differentiation in vitro and in vivo, for the first phase of muscle fibre growth and for efficient repair upon muscle injury. Pax genes are also involved in tissue differentiation and have important roles in the regulation of stem cell behaviour. In the case of skeletal muscle, Pax7, which was up-regulated at 4 h of differentiation in SM, performs this function both during development and regeneration in the adult. The Pax7 protein regulates the entry of satellite cells into the myogenic programme via activation of the myogenic determination gene MyoD, which was also up-regulated at 24 h of differentiation. Real-time PCR also confirmed these data, revealing a key role for Pax7 in the early stages of GTP-dependent myogenesis (at 4 h of differentiation). Among the genes involved in myogenesis, calcineurin (Ppp3ca) was up-regulated both at 4 and 24 h. Homeo box c4 regulates self-renewal and differentiation of stem cells, including both mesenchymal and embryonic stem cells [42]. The Rab17 gene was up-regulated, and it has been linked to the control of myogenesis, although it had never been identified and studied in skeletal muscle. Glycogen synthase kinase 3 beta (Gsk3b) was up-regulated at 24 h of differentiation in SM, which is the gene that codes for the protein GSK3ß. This gene facilitates the Notch signalling that takes place during myoblast proliferation. This gene was also confirmed to be up-regulated following real-time PCR. Furthermore, our study revealed that serum amyloid a 3, a gene involved in inflammatory processes, was up-regulated both at 4 and 24 h of differentiation in SM. Inflammation in response to muscle injury or disease is intimately associated with muscle regeneration. The number of up-regulated and down-regulated genes was similar, both at 4 and at 24 h.
GTP-regulated genes in normal differentiation
We also analysed the transcriptional profile of cells after 4 h incubation with 500 μM GTP with the second experimental set-up of gene expression analysis of myoblasts committed to differentiation by incubation in DM, which contained 2% horse serum. In GTP-treated C2C12 cells at 4 h of DM, eight genes were up-regulated and five genes were down-regulated (Table 2).
Table 2.
Differentially regulated gene transcripts following 4 h differentiation of C2C12 cells in DM with 500 μM GTP
| Gene symbola | Description | Log2 IGTP/ICtrl after 4 h of differentiationb |
|---|---|---|
| Jak/Stat pathway | ||
| IL1Ra | Interleukin 1 receptor antagonist homologue 1 | 2.5 |
| IL6 | Interleukin 6 | 1.4 |
| Pim-1 | Pim-1 associated protein | 2.0 |
| Hrk | BH3 interacting (with BCL2 family) domain, apoptosis agonist | 1.6 |
| Hspa9 | Heat shock protein, 74 kDa, A | −1.2 |
| Psme2 | Protease (prosome, macropain) 28 subunit, beta | 1.4 |
| Ereg | Epiregulin | 1 |
| MAPK pathway | ||
| Mif | Macrophage migration inhibitory factor | −1.28 |
| Rhoc | Aplysia ras-related homologue 9 | −1.7 |
| Chromatin reorganization | ||
| Mbd2 | Methyl-CpG binding domain protein 2 | 1.1 |
| Bub3 | Budding uninhibited by benzimidazoles 3 homologue (S. cerevisiae) | 1.2 |
| Sumo1 | Ubiquitin-like 1 | −1.35 |
| Others | ||
| Itga7 | Integrin alpha 7 | 1.3 |
| Sp1 | Trans-acting transcription factor 1 | −1 |
aGene symbols as described in the NCBI database
bChanges in gene expression levels as log base-2 of the ratios (log2 IGTP-treated/Inon-treated): >1, up-regulated genes; <−1, down-regulated genes
The differentially regulated genes were again assigned to their functional categories and were analysed using the same non-commercial tools. The functional categories revealed here included the Jak/Stat pathway, the MAPK pathway, and chromatin reorganisation.
The Jak/Stat pathway
Among pathways that regulate the cell cycle and cell differentiation, Jak/Stat and NF-κB activation have key roles. The up-regulated interleukin 6 gene codes for the endogenous ligand that activates Jak/Stat signalling, IL6. At the end of this signalling, Pim-1 is expressed, which was also up-regulated. Pim-1 is associated with two main effects: it enhances cell survival through cooperation with and regulation of Bcl-2, and it enhances Ca2+ dynamics associated with increased expression of SERCA2a [43–45]. It has been shown that the Pim-1 protein is targeted for degradation by ubiquitin, and that Hsp70 is associated with Pim-1 under these circumstances. An indirect confirmation of the Jak/Stat pathway stimulation is derived from the suppression of the NF-κB pathway, via up-regulation of the IL1Ra gene.
Up-regulation of protease (prosome, macropain) 28 subunit, beta could be linked to turnover of Pim-1 [46]. Furthermore, it results in down-regulation of heat shock protein, 74 kDa (Hspa9), a gene that encodes a member of the heat-shock cognate protein 70 gene family. This protein has a role in cell proliferation, stress responses, and maintenance of the mitochondria. Epiregulin belongs to the epidermal growth factor (EGF) family and binds to the EGF receptor. In the present study, epiregulin was also up-regulated at 4 h of differentiation in DM.
The MAPK pathway
In the present study, there was down-regulation of both Mif and Rhoc with GTP stimulation in DM, suggesting that GTP depresses the MAPK pathway in differentiating C2C12 cells.
Chromatin reorganisation
The level of methyl CpG-binding proteins was up-regulated, as was the expression of budding uninhibited by benzimidazoles 3 homolog (Saccharomyces cerevisiae), which codes for a member of a group of mitotic checkpoint proteins that are essential for the segregation of chromosomes during cell division [47, 48]. Ubiquitin-like 1 (Sumo1), which is involved in the control of cell transformation, was down-regulated.
Other genes
Integrin alpha 7, a gene that encodes for a transmembrane protein of skeletal and cardiac muscle that links the cytoskeleton to the extracellular matrix, was up-regulated. Trans-acting transcription factor 1 (Sp1) was down-regulated; together with Sp3, the Sp1 protein activates the Dp71 promoter by binding to the Sp boxes.
Validation of microarray data by quantitative real-time PCR
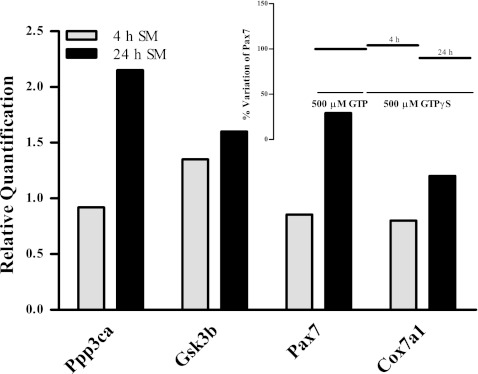
Based on the microarray findings, quantitative real-time PCR was performed to validate some of these putative GTP-sensitive genes, looking at the key players in the myogenic process and in metabolism. For this, the expression levels of mRNA for Ppp3ca, Gsk3b, Pax7, and Cox7a1 were evaluated by real-time PCR in C2C12 cells differentiated using SM for 4 and 24 h in the absence and presence of 500 μM GTP. In addition, Pax7 gene expression was evaluated under the same conditions (4 and 24 h) but with the cells incubated with 500 μM GTPγS.
Considering the microarray results that indicated an increase in aerobic metabolism, we investigated the GTP effects on fibre phenotype. A calcineurin (Ppp3ca) assay was performed because in considering skeletal muscle fibre type, calcineurin controls the slow muscle phenotype, driving expression of the type I myosin heavy chain (MyHC) gene, a myogenic marker [49].
As shown in Fig. 2, after 4 h of differentiation in SM in the presence of 500 μM GTP, the relative levels of Pp3ca, Gsk3b, Pax7, and Cox7a1 mRNAs were not significantly differentially regulated when compared to untreated control cells. In contrast, at 24 h of differentiation in SM in the presence of 500 μM GTP, the relative levels of mRNAs were significantly increased (Fig. 2), confirming both the microarray data and the GTP-dependent differentiation process towards the slow fibre phenotype. The inset of Fig. 2 shows that addition of GTPγS to the medium also enhances Pax7 expression to a similar level to that seen for GTP.
Fig. 2.
Real-time PCR after 4 and 24 h of differentiation with 500 μM GTP in SM in C2C12 cells. Ppp3ca, Gsk3b, Pax7, and Cox7a1 mRNA expression levels in C2C12 cells differentiated with SM for 4 and 24 h in the presence of 500 μM GTP and GTPγS (inset). Data are relative quantities of transcripts for these target genes, each versus beta-actin gene expression. At 24 h of differentiation in SM in the presence of 500 μM GTP, the relative levels of Ppp3ca, Gsk3b, Pax7, and Cox7a1 mRNAs were significantly increased. Each value represents the relative quantification of each gene at each time point. Insert: Pax7 mRNA expression levels comparing GTP (as 100% at 4 and 24 h) and GTPγS, with similar levels of expression seen. Data are representative of one of three independent experiments
Discussion
Our previous studies demonstrated that extracellular GTP can induce myogenic processes in C2C12 cells, initially enhancing proliferation and expression of the sarcomeric protein MyHC and subsequently through Ca2+-dependent intracellular signalling [27, 28]. In order to understand this process better, we analysed gene expression patterns at very early stages (4 and 24 h) of myogenesis, as associated with GTP exposure, and using SM and the standard DM.
During myogenesis, myoblasts (precursors of skeletal muscle cells), once they reach a critical density, cease to proliferate and start to differentiate. Extracellular factors, acting via cell-surface receptors, and adhesion molecules control cell proliferation arrest and differentiation of myoblasts through regulation of signalling pathways for the expression of muscle-specific transcription factors. This, in turn, coordinates the expression and activity of a cohort of factors that are responsible for phenotypic changes, including myoblast fusion into myotubes, the precursors of the mature myofibres [50]. Our data demonstrate the specific role of GTP as an enhancer of the myogenic process; indeed, the data in Fig. 1 shows that GTP and GTPγS stimulated the fusion process during the early stages of differentiation (2 days in DM and SM) and also after 5–7 days. These data confirm previously reported data showing that GTP-treated cells are 63 ± 9% thicker than control cells and 55 ± 8% longer than control myotubes [27]. Moreover, stimulation with GTPγS gave similar results to those of GTP, while pre-incubation with RB2, an antagonist of the P2Y purinoceptor, counteracts the GTP-induced fusion and differentiative processes [26, 51].
Initiating a differentiation pathway typically results in activation of genes that will have previously been maintained in an inactive state or repression of other genes that are normally activated. In C2C12 cells treated with GTP in SM for 4 h, our data show up-regulation of genes involved in the respiratory chain (nadh dehydrogenase subunit 1 and cytochrome c oxidase subunit VIIb), energy metabolism (glyceraldehyde-3-phosphate dehydrogenase), early stages of myogenesis (necdin, Pax7, and calcineurin), and cellular adhesion (Vcam1 and Ncam-180); this represents a very early phase of differentiation. The up-regulation of genes coding for proteins involved in mitochondrial respiration leading to a greater production of ATP shows a boost towards intense metabolism, to make up for new cellular functions that are related to muscle work, for which it needs more energy. These data are in agreement with up-regulation of cytochrome c oxidase subunit VIIa 1, carnitine palmitoyltransferase 2, and acetyl-coenzyme a transporter. The up-regulation of genes involved in the early stages of myogenesis demonstrates the role of GTP in the differentiative programme. Necdin is expressed in satellite cells upon activation and during their expansion phase, as it is co-expressed with Pax7 [52]. When myoblasts differentiate and start to fuse, necdin expression decreases. It has been shown that necdin acts at different levels: it cooperates to promote transcriptional activation of myogenin, thereby accelerating myoblast differentiation. In addition, necdin has a prosurvival, antiapoptotic action in vitro and in vivo, which can counteract the cytotoxic effects of several apoptotic agents [52]. The up-regulation at 4 h of differentiation suggests that the earlier stages of activation and proliferation start first in cells treated with GTP than in control cells. Pax7 expression is particularly important in muscle-derived stem cells because it induces satellite cell specification by restricting alternate developmental programmes [53]. In our study, Pax7 was up-regulated. This suggests that GTP can be considered a new important molecule that can induce specification towards muscle differentiation. Upon activation and proliferation of satellite cells, Pax7 has been identified in the up-regulation of MyoD through the recruitment of the histone methyltransferase complex [54]. The mechanisms by which MyoD induces myogenesis [10] involve both the activation of muscle-specific gene expression and the withdrawal from the cell cycle. After activation and proliferation, myoblasts undergo differentiation through an ordered process in which they first cease to proliferate via the sustained activation of p38 MAPK and Akt, and then they express the muscle-specific transcription factor, myogenin, and chromatin-associated proteins promoting assembly of the myogenic transcriptome. Thus, sustained activation of p38 MAPK and Akt, which both impact on MyoD, is a prerequisite for myoblasts to activate the myogenic programme. Other signalling pathways that regulate MyoD expression include the APC/GSK-3/β-catenin complex [55] and a small GTPase of the Rho family, RhoA [56], with these signals converging on the transcription factor, NF-kB [57].
GSK3ß is a protein that binds directly to the Notch 1 intracellular domain (Notch1IC) and facilitates Notch signalling, so it is possible that GSK3ß is a critical juncture between the Notch and Wnt pathways during postnatal myogenesis [58]. As the time course of muscle repair progresses from 1 to 4 days, Wnt signalling increases, and both GSK3ß and Notch activity decrease. It has been suggested that Notch activity is dominant during myoblast proliferation, after which there is a temporal switch to Wnt signalling and the subsequent myoblast differentiation and fusion into myotubes [59]. In our experiments with SM, in which the processes were slowed down, there was up-regulation at 24 h of the gene coding for the GSK3ß protein, confirming the activation of this pathway, which occurred first in cells stimulated with GTP. MyoD1, was also up-regulated at 24 h of differentiation, which is in agreement with the hypothesis of the promotion of differentiation by GTP. Furthermore, Hoxc4 was up-regulated, which supports the hypothesis of differentiation, because Hoxc4 is expressed in CD34+ cells as well as in satellite cells [60], and its over-expression induces an in vitro expansion of committed cells, as well as myogenic precursors. Moreover, at 24 h of differentiation, Rab17 was up-regulated. This once again supports the hypothesis of myogenesis, as in the early phases the process of differentiation requires a proliferative boost (contrary to what happens in cellular hypertrophy, where there is growth without division).
The up-regulation of calcineurin (at both 4 and 24 h), which is known to have an important regulatory role in phenotype specification, supports the hypothesis that GTP can induce a specific slow muscle phenotype [49]. Moreover, it has been demonstrated that calcineurin expression is sufficient to induce myogenic differentiation also in the absence of IGFs [61]. This result is also supported by the GTP stimulation of genes relating to mitochondrial metabolism.
During the process of myogenesis, myoblasts undergo a series of structural modifications and chromatin reorganisation, to allow gene transcription. In C2C12 cells with DM, in which these phases are not slowed down, at 4 h of differentiation, integrin alpha 7 was up-regulated and Sp1 was down-regulated. Integrin alpha 7 codes for a transmembrane protein of skeletal and cardiac muscle that links the cytoskeleton to the extracellular matrix. In C2C12 myoblasts, elevated levels of integrin result in increased adhesion to laminin, when serum is limiting [62]. Up-regulation of integrin alpha 7 mediates cytoskeleton–membrane–extracellular matrix interactions [63]. In addition, Integrin alpha 7 levels increase dramatically when myoblasts begin to differentiate into myotubes [64]. In myoblasts, Sp1 and Sp3 activate the Dp71 promoter by binding to Sp boxes. In myotubes, MyoD represses the expression of the Sp1 and Sp3 genes, and the resulting scarcity of these transcription factors causes down-regulation of the Dp71 promoter. In early myogenesis, Dp71 is expressed to participate in cytoskeletal remodelling [65]. Thus, the down-regulation of Sp1 suggests a crucial role for GTP in promoting differentiation. Furthermore, also in the presence of SM, other genes (Macs and Sgcb) related to the cytoskeleton structure pathway were up-regulated at 24 h of differentiation, demonstrating that there is a stimulus for cytoskeletal reorganisation.
Cell adhesion, migration, and invasion are fundamental properties of most cell types during development and normal tissue/cell function. For these cells, cell–cell and cell–substrate adhesion are essential for the maintenance of cell polarity and differentiation, and for tissue architecture. During development, some cells, including myoblasts, can migrate over large distances. Therefore, in the reorganisation that occurs during cell differentiation processes, adhesion molecules are involved [66]. We found Vcam1 and Ncam-180 up-regulated, both of which code for cell adhesion molecules and which contribute to myotube formation through fusion of myoblasts [39, 40]. Some studies have shown that for C2C12 myoblasts [67], Ncam expression is associated with the early stages of differentiation, and is concurrent with expression of T-type Ca2+ channels and inward rectifier K+ channels [68]. Our data again confirm the activation of these pathways during several phases of myogenesis.
During postnatal differentiation of myogenic precursors, several activated processes lead to chromatin reorganisation as a result of a proliferative boost that is followed by fusion of myoblasts into myotubes. More recently, there is increasing evidence of cross-talk between epigenetic modifications, such as histone and DNA methylation, which is recognised by the HP1 (Heterochromatin protein 1) and MeCP2 (methyl CpG-binding protein 2) regulators, respectively. It has been proposed that the dynamic interactions of HP1 and MeCP2 increase their concentrations at heterochromatin, which links two major gene-silencing pathways, to stabilise transcriptional repression during differentiation [47]. In agreement with this hypothesis, the levels of methyl CpG-binding proteins increased during the early phases of myogenesis (4 h in DM), which would lead to large-scale heterochromatin reorganisation. Furthermore, budding uninhibited by benzimidazoles 3 homolog (S. cerevisiae) was up-regulated, which is a member of a group of mitotic checkpoint proteins that are essential for segregation of chromosomes during cell division [48]. Genes that code for enzymes capable of altering chromatin structure are likely to be involved in the onset of differentiation events [69].
In the process of myogenesis, anabolic processes are promoted instead of catabolic ones, and proteolytic processes can be depressed. In the present study, genes such as ubiquitin-like 1 were down-regulated (at 4 h in DM), as was ubiquitin c (at 4 h in SM) and ubiquilin 2 (at 24 h in SM). It has been shown that ubiquitin-like 1 (Sumo1) modifies SnoN, which is involved in the control of cell transformation. The down-regulation of ubiquitin-like 1 found in our study can be explained in view of some recent results that have demonstrated that loss of sumoylation in the Lys-50 site of SnoN potently activates muscle-specific gene expression and enhances myotube formation. This would suggest a novel role for SUMO modification in the regulation of myogenic differentiation [70].
IL1Ra is the gene that codes for the IL-1 receptor antagonist protein. This protein recognises and binds to both types of IL-1 receptors, although it has no IL-l agonist activity. Once bound, it inhibits IL-l binding to its receptor, thus providing the body with a protective mechanism against the harmful effects of IL-1. Indeed, the literature supports a regulatory role for IL-1 as a mediator of muscle-protein synthesis, with alterations in body composition seen in catabolic states where IL-1 is over-expressed. Further heat shock protein 9 (which was down-regulated) appears to be involved in muscle atrophy [71], and it has also been detected in association with the IL-1 receptor, and is predicted to have a role in the internalisation of this receptor [72]. Inhibition of IL-1 signalling, which decreases the rate of protein synthesis of both myofibrillar and sarcoplasmic proteins through IL1Ra [73] (which was up-regulated) and probably Hspa9 (which was down-regulated at 4 h in DM), is in agreement with the hypothesis of anabolic processes during the myogenesis. We found that IL6 and Saa3 were up-regulated. In C2C12 cells, it has been seen that when these mediators increase, they act synergistically to increase SOCS3 expression in the muscle. This increase in SOCS3 can account for down-regulation of IRS-1 and impaired insulin/IGF-1 signalling, which promotes muscle proteolysis [74]. The up-regulation of Proteasome (prosome, macropain) 28 subunit, beta might be linked to the turnover of Pim-1 [46].
The proinflammatory peptide macrophage migration inhibitory factor (Mif) functions as an autocrine mediator of both growth factor-dependent and integrin-dependent sustained ERK/MAPK activation, and of cyclin D1 expression and cell-cycle progression. Mif promotes activation of the canonical ERK/MAPK cascade and cyclin D1 expression, by stimulating the activity of Rhoc GTPase and by its downstream signalling to stress fibre formation. Rho-dependent stress fibre accumulation promotes the sustained activation of ERK and subsequent cyclin D1 expression during the G1-S phase of cell-cycle progression. MIF regulates proliferative, migratory, and oncogenic processes [75]. In the present study, down-regulation of both Mif and Rhoc suggests that GTP depresses the MAPK pathway in C2C12 differentiating cells.
In purine metabolism, an increased production of GTP might have a role in skeletal muscle disease (e.g., denervated muscle). Indeed, we recently demonstrated the involvement of purine metabolism in the atrophied skeletal muscle of a 25-year-old male patient who was affected by complete lower motor-neuron lesion of his spinal cord. In studying the transcriptional profile of the skeletal muscle of this denervated patient, we observed stimulation of inosine monophosphate (IMP) dehydrogenase, which increases the conversion of IMP into xanthosine 5′-phosphate, an intermediate in purine metabolism that produces guanosine-based purines [76]. We concluded that increased production of guanosine-based purines might be associated with the denervated muscle trying to activate myogenesis. We know from the literature that the main sources of nucleotides in different tissues are the cells themselves [77]. In particular, the muscle cells might release GTP as a consequence of micro or more extensive lesions (e.g., during physical exercise), and several nucleotide species might be released. It is worth mentioning that the intracellular concentrations of GTP have been estimated to be high [78], and for this reason, cell damage or mechanical stress might release concentrations of guanosine nucleotides in the hundreds of micromolar range into the extracellular medium.
Conclusion
The data from our experiments add further information to the established effects of extracellular GTP on intracellular Ca2+ increases and cell hyperpolarisation, using as an experimental model the myogenic C2C12 cell line. The analysis of GTP-induced gene expression after 4 h of stimulation in the absence of any other differentiation factor provides us information on GTP-dependent early signal transduction pathways. We have demonstrated that GTP itself can activate genes that are directly involved in myogenesis and also in remodelling of the cytoskeleton structure and stimulating energy metabolism. Using real-time PCR, we have confirmed the expression patterns of three of the genes that were highlighted in our microarray experiments for C2C12 myogenesis, Gsk3b, Pax7, and Cox7a1. Furthermore, we have also demonstrated that GTP up-regulates calcineurin, a gene that can sustain the specification of the slow fibre phenotype. On the other hand, in the presence of serum factors, GTP mainly activates the Jak/Stat pathway, which affects basic cell functions, such as cell growth, differentiation, and death. Hence, under these conditions, GTP and serum cooperate with each other. In conclusion, we have demonstrated that GTP itself can initiate and sustain myogenesis in C2C12 myocytes.
Acknowledgments
Grants
This study was supported by research grants from MIUR (COFIN 2002) and from “G. d'Annunzio” University of Chieti-Pescara, to Stefania Fulle and Tiziana Pietrangelo.
Glossary
- BSA
Bovine serum albumin
- [Ca2+]i
Intracellular calcium ion concentration
- DM
Standard differentiating medium
- GTP
Guanosine 5′-triphosphate
- MRFs
Myogenic regulatory factors
- MyHC
Myosin heavy chain
- SM
Synthetic differentiating medium
- RB2
Reactive blue 2
References
- 1.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 2.Chambers RL, McDermott JC. Molecular basis of skeletal muscle regeneration. Can J Appl Physiol. 1996;21:155–184. doi: 10.1139/h96-014. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JE. Murray L. Barr Award Lecture. Studies of the dynamics of skeletal muscle regeneration: the mouse came back! Biochem Cell Biol. 1998;76:13–26. doi: 10.1139/o98-007. [DOI] [PubMed] [Google Scholar]
- 4.McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev. 2001;11:497–504. doi: 10.1016/S0959-437X(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 5.Megeney LA, Rudnicki MA. Determination versus differentiation and the MyoD family of transcription factors. Biochem Cell Biol. 1995;73:723–732. doi: 10.1139/o95-080. [DOI] [PubMed] [Google Scholar]
- 6.Perry RL, Rudnicki MA. Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci. 2000;5:D750–D767. doi: 10.2741/Perry. [DOI] [PubMed] [Google Scholar]
- 7.Friday BB, Mitchell PO, Kegley KM, Pavlath GK. Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation. 2003;71:217–227. doi: 10.1046/j.1432-0436.2003.710303.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller JB, Schaefer L, Dominov JA. Seeking muscle stem cells. Curr Top Dev Biol. 1999;43:191–219. doi: 10.1016/S0070-2153(08)60382-8. [DOI] [PubMed] [Google Scholar]
- 9.Bailey P, Holowacz T, Lassar AB. The origin of skeletal muscle stem cells in the embryo and the adult. Curr Opin Cell Biol. 2001;13:679–689. doi: 10.1016/S0955-0674(00)00271-4. [DOI] [PubMed] [Google Scholar]
- 10.Lassar AB, Skapek SX, Novitch B. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr Opin Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 11.Molkentin JD, Olson EN. Combinatorial control of muscle development by basic helix-loop-helix and MADSbox transcription factors. Proc Natl Acad Sci USA. 1996;93:9366–9373. doi: 10.1073/pnas.93.18.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 13.Wright WE, Sassoon DA, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 14.Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10 T1/2 fibroblasts. EMBO J. 1989;8:701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thayer MJ, Tapscott SJ, Davis RL, Wright WE, Lassar AB, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- 16.Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 17.Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- 18.Rudnicki MA, Jaenisch R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays. 1995;17:203–209. doi: 10.1002/bies.950170306. [DOI] [PubMed] [Google Scholar]
- 19.Lindon C, Montarras D, Pinset C. Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J Cell Biol. 1998;140:111–118. doi: 10.1083/jcb.140.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinterberger TJ, Sassoon DA, Rhodes SJ, Konieczny SF. Expression of the muscle regulatory factor MRF4 during somite and skeletal myofiber development. Dev Biol. 1991;147:144–156. doi: 10.1016/S0012-1606(05)80014-4. [DOI] [PubMed] [Google Scholar]
- 21.Naya FS, Olson E. MEF2: a transcriptional target for signalling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol. 1999;11:683–688. doi: 10.1016/S0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 22.Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin DS, Xiong Y. Induction of p18INK4c and its predominant association with CDK4 and CDK6 during myogenic differentiation. Mol Biol Cell. 1996;7:1587–1599. doi: 10.1091/mbc.7.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomczak KK, Marinescu VD, Ramoni MF, Sanoudou D, Montanaro F, Han M, Kunkel LM, Kohane IS, Beggs AH. Expression profiling and identification of novel genes involved in myogenic differentiation. FASEB J. 2004;18(2):403–405. doi: 10.1096/fj.03-0568fje. [DOI] [PubMed] [Google Scholar]
- 25.Shen X, Collier JM, Hlaing M, Zhang L, Delshad EH, Bristow J, Bernstein HS. Genome-wide examination of myoblast cell cycle withdrawal during differentiation. Dev Dyn. 2003;226(1):128–138. doi: 10.1002/dvdy.10200. [DOI] [PubMed] [Google Scholar]
- 26.Pietrangelo T, Mariggiò MA, Lorenzon P, et al. Characterization of specific GTP binding sites in C2C12 mouse skeletal muscle cells. J Muscle Res Cell Motil. 2002;23:107–118. doi: 10.1023/A:1020288117082. [DOI] [PubMed] [Google Scholar]
- 27.Pietrangelo T, Fioretti B, Mancinelli R, Catacuzzeno L, Franciolini F, Fanò G, Fulle S. Extracellular guanosine-5′-triphosphate modulates myogenesis via intermediate Ca2+-activated K+ currents in C2C12 mouse cells. J Physiol. 2006;572(3):721–733. doi: 10.1113/jphysiol.2005.102194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrangelo T, Guarnieri S, Fulle S, Fanò G, Mariggiò MA. Signal transduction events induced by extracellular guanosine 5′ triphosphate in excitable cells. Purinergic Signal. 2006;2(4):633–636. doi: 10.1007/s11302-006-9021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue K, Nakazawa K, Ohara-Imaizumi M, Obama T, Fujimori K, Takanaka A. Antagonism by reactive blue 2 but not by brilliant blue G of extracellular ATP-evoked responses in PC12 phaeochromocytoma cells. Br J Pharmacol. 1991;102(4):851–854. doi: 10.1111/j.1476-5381.1991.tb12265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran JL, Li Y, Hill AA, Mounts WM, Miller CP. Gene expression changes during mouse skeletal myoblast differentiation revealed by transcriptional profiling. Physiol Genomics. 2002;10(2):103–111. doi: 10.1152/physiolgenomics.00011.2002. [DOI] [PubMed] [Google Scholar]
- 31.Dulong S, Goudenege S, Vuillier-Devillers K, Manenti S, Poussard S, Cottin P. Myristoylated alanine-rich C kinase substrate (MARCKS) is involved in myoblast fusion through its regulation by protein kinase Cα and calpain proteolytic cleavage. Biochem J. 2004;382:1015–1023. doi: 10.1042/BJ20040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakabayashi-Takai E, Noguchi S, Ozawa E. Identification of myogenesis-dependent transcriptional enhancers in promoter region of mouse gamma-sarcoglycan gene. Eur J Biochem. 2001;268(4):948–957. doi: 10.1046/j.1432-1327.2001.01954.x. [DOI] [PubMed] [Google Scholar]
- 33.Musumeci O, Aguennouz M, Comi GP, Rodolico C, Autunno M, Bordoni A, Baratta S, Taroni F, Vita G, Toscano A. Identification of the infant-type R631C mutation in patients with the benign muscular form of CPT2 deficiency. Neuromuscul Disord. 2007;17(11–12):960–963. doi: 10.1016/j.nmd.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Wieser T, Kraft B, Kress HG. No carnitine palmitoyltransferase deficiency in skeletal muscle in 18 malignant hyperthermia susceptible individuals. Neuromuscul Disord. 2008;18(6):471–474. doi: 10.1016/j.nmd.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Hirabayashi Y, Kanamori A, Nomura KH, Nomura K. The acetyl-CoA transporter family SLC33. Pflugers Arch. 2004;447(5):760–762. doi: 10.1007/s00424-003-1071-6. [DOI] [PubMed] [Google Scholar]
- 36.Massey LK, Mah AL, Ford DL, Miller J, Liang J, Doong H, Monteiro MJ. Overexpression of ubiquilin decreases ubiquitination and degradation of presenilin proteins. J Alzheimers Dis. 2004;6(1):79–92. doi: 10.3233/jad-2004-6109. [DOI] [PubMed] [Google Scholar]
- 37.Thompson O, Kleino I, Crimaldi L, Gimona M, Saksela K, Winder SJ. Dystroglycan, Tks5 and Src mediated assembly of podosomes in myoblasts. PLoS One. 2008;3(11):e3638. doi: 10.1371/journal.pone.0003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. αl(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. PNAS. 1995;92(19):8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capkovic KL, Stevenson S, Johnson MC, Thelen JJ, Cornelison DD. Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp Cell Res. 2008;314(7):1553–1565. doi: 10.1016/j.yexcr.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishido M, Uda M, Masuhara M, Kami K. Alterations of M-cadherin, neural cell adhesion molecule and beta-catenin expression in satellite cells during overload-induced skeletal muscle hypertrophy. Acta Physiol (Oxf) 2006;187(3):407–418. doi: 10.1111/j.1748-1716.2006.01577.x. [DOI] [PubMed] [Google Scholar]
- 41.Behrendt N, Jensen ON, Engelholm LH, Mørtz E, Mann M, Danø K. A urokinase receptor-associated protein with specific collagen binding properties. J Biol Chem. 2000;275(3):1993–2002. doi: 10.1074/jbc.275.3.1993. [DOI] [PubMed] [Google Scholar]
- 42.Phinney DG, Gray AJ, Hill K, Pandey A. Murine mesenchymal and embryonic stem cells express a similar Hox gene profile. Biochem Biophys Res Commun. 2005;338(4):1759–1765. doi: 10.1016/j.bbrc.2005.10.140. [DOI] [PubMed] [Google Scholar]
- 43.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13(12):1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, Magnuson NS. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation, differentiation and tumorigenesis. J Vet Sci. 2001;2(3):167–179. [PubMed] [Google Scholar]
- 45.Lilly M, Sandholm J, Cooper JJ, Koskinen PJ, Kraft A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene. 1999;18(27):4022–4031. doi: 10.1038/sj.onc.1202741. [DOI] [PubMed] [Google Scholar]
- 46.Shay KP, Wang Z, Xing PX, McKenzie IF, Magnuson NS. Pim-1 kinase stability is regulated by heat shock proteins and the ubiquitin-proteasome pathway. Mol Cancer Res. 2005;3(3):170–181. doi: 10.1158/1541-7786.MCR-04-0192. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal N, Hardt T, Brero A, Nowak D, Rothbauer U, Becker A, Leonhardt H, Cardoso MC. MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation. Nucleic Acids Res. 2007;35(16):5402–5408. doi: 10.1093/nar/gkm599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalitsis P, Fowler KJ, Griffiths B, Earle E, Chow CW, Jamsen K, Choo KH. Increased chromosome instability but not cancer predisposition in haploinsufficient Bub3 mice. Genes Chromosomes Cancer. 2005;44(1):29–36. doi: 10.1002/gcc.20215. [DOI] [PubMed] [Google Scholar]
- 49.Pandorf CE, Jiang WH, Qin AX, Bodell PW, Baldwin KM, Haddad F. Calcineurin plays a modulatory role in loading-induced regulation of type I myosin heavy chain gene expression in slow skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297(4):R1037–R1048. doi: 10.1152/ajpregu.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riuzzi F, Sorci G, Donato R. RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth. Am J Pathol. 2007;171(3):947–961. doi: 10.2353/ajpath.2007.070049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knight GE, Burnstock G. Responses of the aorta of the garter snake (Thamnophis sirtalis parietalis) to purines. Br J Pharmacol. 1995;114(1):41–48. doi: 10.1111/j.1476-5381.1995.tb14903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deponti D, François S, Baesso S, Sciorati C, Innocenzi A, Broccoli V, Muscatelli F, Meneveri R, Clementi E, Cossu G, Brunelli S. Necdin mediates skeletal muscle regeneration by promoting myoblast survival and differentiation. J Cell Biol. 2007;179(2):305–319. doi: 10.1083/jcb.200701027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/S0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 54.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172(1):91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cossu G, Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18(24):6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N, Fernandez A. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol Biol Cell. 1998;9(7):1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289(5488):2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 58.Tsivitse S. Notch and Wnt signaling, physiological stimuli and postnatal myogenesis. Int J Biol Sci. 2010;6(3):268–281. doi: 10.7150/ijbs.6.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brack A, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from Notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309(5743):2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 61.Hlaing M, Spitz P, Padmanabhan K, Cabezas B, Barker CS, Bernstein HS. E2F-1 regulates the expression of a subset of target genes during skeletal myoblast hypertrophy. J Biol Chem. 2004;279(42):43625–43633. doi: 10.1074/jbc.M408391200. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Burkin DJ, Kaufman SJ. Increasing alpha 7 beta 1-integrin promotes muscle cell proliferation, adhesion, and resistance to apoptosis without changing gene expression. Am J Physiol Cell Physiol. 2008;294(2):C627–C640. doi: 10.1152/ajpcell.00329.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allikian MJ, Hack AA, Mewborn S, Mayer U, McNally EM. Genetic compensation for sarcoglycan loss by integrin alpha7beta1 in muscle. J Cell Sci. 2004;117(Pt 17):3821–3830. doi: 10.1242/jcs.01234. [DOI] [PubMed] [Google Scholar]
- 64.Jethanandani P, Kramer RH. Alpha7 integrin expression is negatively regulated by deltaEF1 during skeletal myogenesis. J Biol Chem. 2005;280(43):36037–36046. doi: 10.1074/jbc.M508698200. [DOI] [PubMed] [Google Scholar]
- 65.León MB, Montañez C, Gómez P, Morales-Lázaro SL, Tapia-Ramírez V, Valadez-Graham V, Recillas-Targa F, Yaffe D, Nudel U, Cisneros B. Dystrophin Dp71 expression is down-regulated during myogenesis: role of Sp1 and Sp3 on the Dp71 promoter activity. J Biol Chem. 2005;280(7):5290–5299. doi: 10.1074/jbc.M411571200. [DOI] [PubMed] [Google Scholar]
- 66.Pietrangelo T, Puglielli C, Mancinelli R, Beccafico S, Fanò G, Fulle S. Molecular basis of the myogenic profile of aged human skeletal muscle satellite cells during differentiation. Exp Gerontol. 2009;44(8):523–531. doi: 10.1016/j.exger.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Moore SE, Thompson J, Kirkness V, Dickson JG, Walsh FS. Skeletal muscle neural cell adhesion molecule (N-CAM): changes in protein and mRNA species during myogenesis of muscle cell lines. J Cell Biol. 1987;105(3):1377–1386. doi: 10.1083/jcb.105.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kubo Y. Comparison of initial stages of muscle differentiation in rat and mouse myoblastic and mouse mesodermal stem cell lines. J Physiol. 1991;442:743–759. doi: 10.1113/jphysiol.1991.sp018817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kowenz-Leutz E, Leutz A. A C/EBPβ isoform recruits the SWI/SNF complex to activate myeloid genes. Mol Cell. 1999;4(5):735–743. doi: 10.1016/S1097-2765(00)80384-6. [DOI] [PubMed] [Google Scholar]
- 70.Wrighton KH, Liang M, Bryan B, Luo K, Liu M, Feng XH, Lin X. Transforming growth factor-beta-independent regulation of myogenesis by SnoN sumoylation. J Biol Chem. 2007;282(9):6517–6524. doi: 10.1074/jbc.M610206200. [DOI] [PubMed] [Google Scholar]
- 71.Dalla Libera L, Ravara B, Gobbo V, Tarricone E, Vitadello M, Biolo G, Vescovo G, Gorza L. A transient antioxidant stress response accompanies the onset of disuse atrophy in human skeletal muscle. J Appl Physiol. 2009;107(2):549–557. doi: 10.1152/japplphysiol.00280.2009. [DOI] [PubMed] [Google Scholar]
- 72.Sacht G, Brigelius-Flohe R, Kiess M, Sztajer H, Flohe L. ATPsensitive association of mortalin with the IL-1 receptor type I. Biofactors. 1999;9:49–60. doi: 10.1002/biof.5520090107. [DOI] [PubMed] [Google Scholar]
- 73.Vary TC, Owens EL, Beers JK, Verner K, Cooney RN. Sepsis inhibits synthesis of myofibrillar and sarcoplasmic proteins: modulation by interleukin-1 receptor antagonist. Shock. 1996;6(1):13–18. doi: 10.1097/00024382-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20(3):604–612. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swant JD, Rendon BE, Symons M, Mitchell RA. Rho GTPase-dependent signalling is required for macrophage migration inhibitory factor-mediated expression of cyclin D1. J Biol Chem. 2005;280(24):23066–23072. doi: 10.1074/jbc.M500636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mancinelli R, Kern H, Fulle S, Carraro U, Zampieri S, La Rovere R, Fanò G and Pietrangelo T (2011) Transcriptional profile of denervated vastus lateralis muscle derived from a patient 8 months after spinal cord injury: a case-report. International Journal of Immunopathology and Pharmacology (in press) [DOI] [PubMed]
- 77.Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4(8):793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- 78.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140(1):1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]