Abstract
The dorsal root ganglion (DRG) is consisted of neurons that relay multiple types of spinal sensory stimuli to the central nervous system. Several neuroactive molecules may be involved in sensory modulation especially pain processing at the DRG, including the purinergic receptor P2X3 and calcitonin-gene-related peptide (CGRP). P2X3 receptor has been considered a promising pharmaceutical target for the development of new pain medicine. Currently, litter is known about the expression of P2X3 in the human DRG. The present study characterized the localization of P2X3 in prenatal human DRG obtained from fetuses at 4–8 gestational months, by comparing to CGRP expression as well as binding pattern of isolectin-B4 (IB4), a marker of small DRG neurons presumably relevant to nociception. P2X3 immunoreactivity (IR) appeared in most neuron-like perikarya, with their numerical density reduced during the gestational period studied. P2X3 IR was co-labeled very commonly with IB4 binding and infrequently with CGRP IR and was not colocalized with IR for the gliocyte marker glutamine synthetase. Together, the data show an early and broad expression of P2X3 in prenatal human DRG neurons, pointing to a biological role of purinergic signaling during the development of spinal sensory system.
Keywords: P2X3, CGRP, DRG, Immunohistochemistry, Human
Introduction
Purinergic receptors are a large family of proteins that are involved in modulation of broad cellular functions such as vascular/platelet activity, secretion, cytokine release, and apoptosis in different bodily systems [1–5]. In the nervous system, ATP released extracellularly from neurons and neuronal terminals bind to and activate cell-surface purinergic receptors, which are consisted of the G-protein-coupled P2Y receptors and the ligand-gated ion-conducting P2X receptors in mammals. The latter can be divided into at least seven subtypes, defined as P2X1 through P2X7 [3, 5–7]. The expression and physiology of purinergic receptors on the peripheral sensory and autonomic pathways have attracted great attention during the past decade, in part because pathological pain remains a clinical area of tremendous unmet medical need [6–8]. Among the P2X group, P2X3 has been found to express in the primary spinal sensory system, both the cord and dorsal root ganglion (DRG) of laboratory rodents [9–14]. Many experimental studies have linked P2X3 expression in the DRG to pain perception. For example, upregulation of P2X3 expression in the DRG has been observed in laboratory rodents under paradigms of neuropathic pain, associated with chronic inflammation, cancer nociception, and other conditions [10, 12, 15–21]. Thus, P2X3 antagonism is considered a potential breakthrough transformative approach to treat chronic pain associated with osteoarthritis, back pain, visceral pain, cancer, and neuropathy, with a number of leading compounds being tested in clinical trials [3, 7, 8, 22–24].
Several neuropeptides, including substance P and calcitonin-gene-related peptide (CGRP), are also important for neuronal modulation in the primary spinal sensory pathway, including pain processing [25–27]. In addition, the isolectin-B4 (IB4) from the plant griffonia simplicifolia binds selectively to relatively small DRG neurons that are associated with the group C afferent fibers involving largely in conduction of thermal, mechanical, and chemical stimuli [28, 29]. CGRP expression in the spinal cord and DRG has been demonstrated in a variety of mammalian species, including nonhuman primates and humans [25, 30–36]. Also, levels of CGRP mRNA and protein are elevated in the DRG and dorsal horn in a number of acute adjuvant-induced inflammatory pain models [34–36]. As with the purinergic receptors, CGRP receptor antagonists are being developed as new generations of pain relievers [37–39].
Currently, no data are available in regard to the expression and cellular localization of P2X3 in the human DRG (to our knowledge). Given its relevance to sensory neurobiology and pain pharmacology, the present study characterized the expression of P2X3 in prenatal human DRG. The pattern of P2X3 expression was compared to that of CGRP expression and IB4 binding in DRG neurons. A neuronal localization of this purinergic receptor in the DRG was also verified by a lack of colocalization of P2X3 immunoreactivity with the glial marker glutamine synthetase (GS) that is selectively localized to DRG satellite astrocytes.
Materials and methods
Tissue preparation
Twelve human fetuses were available, after legal abortion including that indicated by gynecological complications, with proper consent from parents, from the department of obstetrics of the affiliated hospital of Changzhi Medical School. Handling of human material met all necessary requirements and regulations set by the ethics committee of the medical school. All samples were obtained and used in a manner compliant with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Gestational ages, determined by the last menstrual date, body weight, and the crown-rump and foot lengths, were 4 (13–16 weeks, n = 2), 5 (17–20 weeks, n = 2), 6 (21–24 weeks, n = 3), 7 (25–28 weeks, n = 3), and 8 (29–32 weeks, n = 2) gestational months. The 7- and 8-month-old fetuses were aborted per parents request due to severe fetal heart malformation (n = 3) and maternal carcinoma pending chemo/radiotherapy (n = 2). The nervous system appeared normal in all fetuses before abortion by ultrasound.
Perfusion with 4% paraformaldehyde was carried out within less than 2 h after death via the ascending aorta following vascular rinse with 0.01 M cold phosphate-buffered saline (PBS; pH = 7.2). The fourth lumber DRGs were dissected out and postfixed in the perfusion fixative overnight a 4°C. The fifth lumber DGRs were collected for Western blot after vascular rinse. For anatomical studies, DRGs were embedded with paraffin according to standard protocol and then sectioned at 4 μm thick along the longitudinal axis using a Shandon microtome (England). For each case, sections passing the middle portion of the DRG (i.e., sections approaching the greatest cross-sectional area of the ganglion) were collected on six to ten microslides, with each mounted with two rows of five consecutive sections. A set of these sections was stained with cresyl violet for histological evaluation, while the remaining sets were used for immunohistochemistry with the avidin–biotin complex (ABC) method or by immunofluorescent light microscopy.
Immunohistochemistry
Paraffin sections were dewaxed with xylene and rehydrated through descending ethanol (100%, 95%, 75%, and 50%) solutions and PBS and then immunostained on-slide using the ABC method. Briefly, sections were first treated with 0.05% hydrogen peroxide for quenching endogenous peroxidase activity and then pre-incubated in PBS containing 5% normal horse serum to block nonspecific reactivity. Sections were further incubated in the above PBS buffer containing polyclonal rabbit anti-P2X3 (Abcam, 1:2,000) or monoclonal mouse anti-CGRP (Sigma-Aldrich, C7113, 1:3,000) with 0.3% Triton X-100 overnight at room temperature. After several rinses with PBS, sections were reacted in a solution containing 1% biotinylated horse anti-rabbit/mouse/goat (universal) IgG with Triton X-100 for 1 h at room temperature followed by 1 h incubation in 1% ABC solution (Vector Laboratories, Burlingame, CA, USA). The immunoreaction product was visualized using 0.005% hydrogen peroxide and 0.05% 3,3-diaminobenzidine. For quantitative analysis, sections from all cases were processed under identical conditions in a single final experiment for each antibody to eliminate experimental bias.
For double immunofluorescence, paraffin sections were dewaxed and rehydrated as aforementioned and pre-incubated in PBS containing 5% donkey serum for 1 h. Subsequently, sections were incubated in PBS buffer containing rabbit anti-P2X3 (1:2000) vs mouse anti-CGRP (1:3,000) and rabbit anti-P2X3 or rabbit anti-CGRP (Sigma-Aldrich, C8198, 1:4,000) vs mouse anti-GS (1:4,000, clone GS-6, Millipore) overnight at 4°C. Sections were then reacted for 2 h in Alexa Fluor® 488 and Alexa Fluor® 594 conjugated donkey anti-mouse and rabbit IgGs (1:200, Invitrogen, Carlsbad, CA, USA). For double or triple labeling for P2X3, CGRP, and IB4, biotinylated IB4 (Sigma-Aldrich, C8198, 1:4,000) was incubated together with P2X3 and/or CGRP primary antibodies. Immunofluorescent signals were visualized with Alexa Fluor® 488 and Alexa Fluor® 594 conjugated secondary antibodies (for P2X3 and CGRP), whereas IB4 binding was revealed using aminomethylcoumarin conjugated streptavidin (Jackson ImmunoResearch, 016-150-084, 1:200). Sections were then washed three times in PBS and coverslipped with anti-fading medium.
Western blot
We used Western blot to help evaluate the specificity of the P2X3 antibody in human tissue. DRGs from 4-, 7-, and 8-gestational month-old fetuses were sonicated on ice in a digestion buffer [150 mM NaCl, 25 mM Tris–HCl (pH 7.4), 2 mM EDTA, 1.0% Triton X-100, 1.0% sodium deoxycholate, 0.1% SDS] containing a cocktail of protease inhibitors. Homogenates were centrifuged at 15,000×g for 10 min at 4°C and supernatants collected and protein concentration determined by bicinchoninic acid assay (Pierce, Rockford, IL, USA). Fifty micrograms of total protein in 62.5 mM Tris (pH 6.8) containing 25% glycerol, 2% SDS, 0.01% bromophenol blue, and 5% β-mercaptoethanol (Bio-Rad, Hercules, CA, USA) was heated to 70°C for 10 min and then loaded in 4–20% linear gradient Tris–HCl ready gel (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA) and immunoblotted for P2X3, with and without addition of the immunogenic peptide at 1:10 (ABCAM, P2X3 peptide 383–397, ab46989). Immunoblotting signal was visualized with ECF chemifluorescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and captured using a Molecular Dynamics phosphorimager.
Imaging, cell density analysis, and statistical test
All sections were examined on a Nikon E800 fluorescent microscope equipped with the Motic advances 3.2 imaging system (Motic Instruments, Inc.). Images were taken from one randomly selected section among the five sections on each paraffin slide. Cell count and densitometry were performed in five sections per case with the aid of the Motic software by taking an unbiased stereological approach. Means were calculated for individual cases and were analyzed by one-way ANOVA using the SPSS13.0 statistic software. P value < 0.05 was set as statistically significant.
Results
Overall histological evaluation of prenatal human DRG with Nissl stain
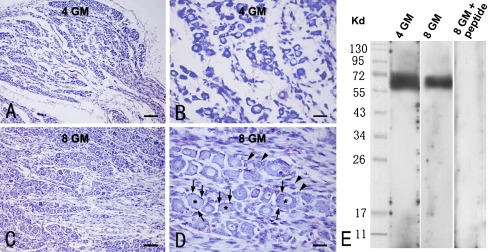
To evaluate the histological integrity of the DRG, a set of sections in each case was stained with cresyl violet. Cells were stained evenly across the DRG in each case (Fig. 1a, c). Relatively large-sized neuronal somata were arranged as islands or cord-like groups separated by unstained area. Dark blue Nissl substance was seen in the cytoplasm of these DRG neuronal cells, with the nucleus unlabeled except for the nucleolus (Fig. 1b, d). Small-sized glial cells and satellite astrocytes could be identified between the neuronal islands and surrounding individual neurons at high magnification (Fig. 1b, d). Overall, Nissl-stained DRG neurons appeared to be smaller and more densely packed in earlier relative to later gestational cases (Fig. 1b, d).
Fig. 1.
Histological evaluation of the prenatal human dorsal root ganglion (DRG) (a–d) and Western blot characterization of the rabbit anti-P2X3 antibody used in the present study. a–d Representative Nissl stain images of DRGs from 4 (a, b)- and 8 (c, d)-gestational month-old fetuses. The junction between the DRG and the peripheral spinal root is around the lower-right corner of the images (a, c). Nissl-stained neurons are round or oval with Nissl substance confined within the perikarya and the proximal neurites in some cases (b, d). Note that the cross-sectional area of the neurons appears larger in the 8-month (d) relative to the 4-month (b) samples. Nissl bodies are clearly seen in large DRG neurons in the 8-month case (d). Asterisks in d point to DRG neurons. Arrows point to satellite astrocytes (d). Arrowheads indicate other small DRG cells between neuronal islands (d). e The rabbit anti-P2X3 antibody blots a monomer band migrated at approximately 64 kd in DRG extracts from 4 (left lane) as well as 7 and 8 (mixed extract, middle lane) gestational month (GM) fetuses. This immunoblotting band is eliminated in the presence of the absorbing antigenic peptide in the primary antibody incubation buffer (right lane). Scale bar = 80 μm in a, c and 20 μm in b, d
Distribution and morphology of P2X3 and CGRP immunoreactive DRG cells
The specificity of P2X3 antibody was evaluated in previous studies in vivo and in vitro in rodent DRG [2, 20]. In the present study, the rabbit P2X3 antibody detected a monomer band in prenatal human DRG extract in Western blot, migrated at ~64 kd (Fig. 1e), which was consistent with previous characterization regarding the molecular weight of this protein in monkey and human tissues [40, 41]. The use of the mouse and rabbit anti-CGRP antibodies from Sigma-Aldrich has been widely documented in literature [30–34, 42, 43]. In pilot experiments, no specific immunolabeling was detectable in DRG sections processed in the absence of a given primary antibody.
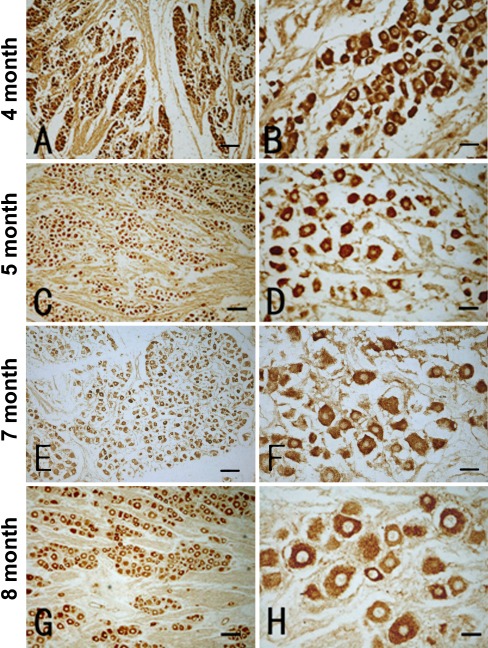
P2X3 immunoreactivity was detected in the DRG in all cases available in the present study. Labeled perikarya were packed as cell islands, with immunoreaction product present in the somata and proximal processes but not in the nucleus (Fig. 2 A, C, E, G). In general, labeled cells in the RDG of the earlier gestational cases were smaller in size but exhibited a stronger labeling intensity relative to later gestational cases (Fig. 2 B, D, F, H). Thus, labeled cells were generally less than 20 μm in larger somal diameter in 4- and 5-gestational month-old cases (Fig. 2 B, D), but often exceeded 20 μm in 7- and 8-month-old fetuses (Fig. 2 F, H). The labeling intensity was largely comparable among labeled DRG cells in 4–6-gestational month-old samples (Fig. 2 A–D). In 7- and 8-month-old cases, the labeling intensity of DRG cells varied substantially, with granule-like elements seen in some larger perikarya (Fig. 2 F, H). It should be noted that in 4- to 5-month-old fetuses, P2X3 immunoreactive processes occurred between the labeled cell islands and merged as fibrous bundles at the peripheral and central ends of the DRG (Fig. 2 A–D). By 7 to 8 gestational months, P2X3 immunoreactive processes were reduced or no longer detectable (Fig. 2 E–H).
Fig. 2.
Representative images showing low (left panels) and high (right panel) power views of P2X3 immunoreactivity in the DRG from fetuses at four age points as indicated on the left. Immunoreactive cells are arranged as islands or clusters in all cases, with labeling occurring in the somata excluding the nucleus. Labeled perikarya are round, oval, or multipolar, sometimes with short proximal processes (B, D, F, H). Most labeled perikarya are less than 20 μm in diameter, but cells larger than 20 μm are increasingly seen in the 7- and 8-gestational month-old cases (compare D, F with B, D). The labeling intensity is heavy in most labeled cells in 4- and 5-month-old fetuses (B, D). The reactivity appears more differential in the DRGs of 7- and 8-month-old cases, wherein larger cells exhibiting lower intensity, with a granular appearance of the immunoreaction product in the cytoplasm (F, H). Note the immunoreactive fibers and bundles in A–F not in G, H. Scale bar = 80 μm in A, C and 20 μm in B, D
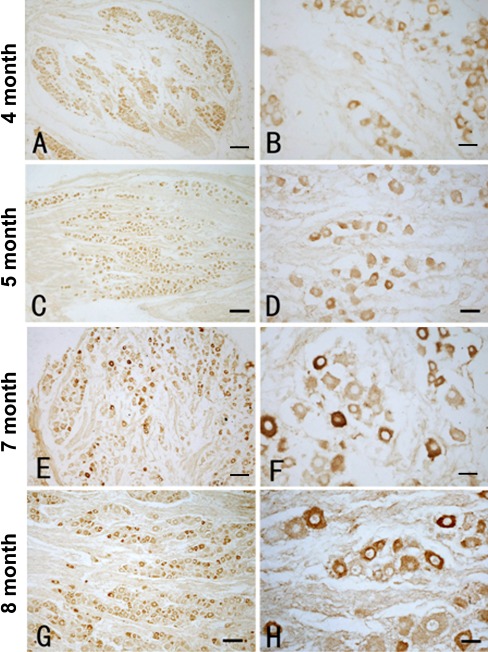
CGRP immunoreactivity was detected in the DRG in all cases available for the present study, with the mouse and rabbit antibodies exhibited identical cellular patterns. In 4- and 5-gestation month-old fetuses (Fig. 3 A–D), CGRP labeling was present in neuron-like perikarya in the DRG, with an overall weak reactivity among the labeled cells. The immunoreaction product occurred in the cell body and proximal processes as well (Fig. 3 B, D). In 7- to 8-gestational month-old samples, a differential CGRP labeling was noted (Fig. 3 E, G). Thus, a subset of DRG cells expressed fairly strong reactivity in the cytoplasm, while the remaining labeled cells exhibited moderate or light immunoreactivity (Fig. 3 E–H). It should be noted that the number of CGRP-labeled DRG somata appeared to be less numerous relative to P2X3 immunoreactive somata in all cases by a gross visual comparison (Figs. 2 and 3).
Fig. 3.
Representative images showing low (left panels) and high (right panel) power views of calcitonin-gene-related peptide (CGRP) immunoreactivity in the DRG at gestational ages as indicated on the left. In the DRG in 4- and 5-gestational month-old fetuses (A–D), a diffuse and weak CGRP labeling is present in the perikarya of many DRG neurons. In 7- and 8-gestational month-old samples, a subset of DRG neurons are strongly labeled, while some other neurons are weakly labeled (E–H). No immunoreactivity is seen in the nucleus. Scale bar = 80 μm in A, C and 20 μm in B, D
Densitometric analysis of P2X3 and CGRP immunoreactive DRG cells
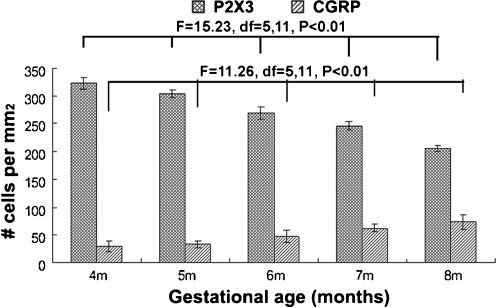
The density of P2X3-labeled somata in the DRG was the highest in the 4-month-old group, averaged at 322.8 ± 10.06 cells/mm2 (Fig. 4). The density reduced with the increase of gestational time. Thus, the density declined to 205.5 ± 5.45 cells/mm2 in the 8-month-old group. Statistical analysis indicated a gestational month-related significant difference (F = 15.23, df = 5, 11, P < 0.01) (i.e., age-dependent reduction). The average density of CGRP immunoreactive cells in the DRG was 29.84 ± 9.47, 33.2 ± 4.79, 47.12 ± 11.09, 62.16 ± 7.67, and 73.36 ± 13.67 cells/mm2 in the 4-, 5-, 6-, 7-, and 8-gestational month groups, respectively. There existed a significant difference among the five age groups (one-way ANOVA analysis; F = 11.26, df = 5, 11, P < 0.01).
Fig. 4.
Densitometry of P2X3 and calcitonin-gene-related peptide (CGRP) immunoreactive neurons in prenatal human DRG from 4 to 8 gestational months. The average density of P2X3 immunoreactive cells is 322.8 ± 10.06, 303.76 ± 7.38, 269.48 ± 11.12, 246.3 ± 8.25, and 205.52 ± 5.45 cells/mm2 at 4, 5, 6, 7, and 8 gestational months, respectively. The average density of CGRP immunoreactive cells is 29.84 ± 9.47, 33.2 ± 4.79, 47.12 ± 11.09, 62.16 ± 7.67, and 73.36 ± 13.67 at the above age points, respectively. One-way ANOVA analysis for P2X3 (P < 0.01, F = 15.23, df = 5, 11) and CGRP (P < 0.01, F = 11.26, df = 5, 11) cells shows statistically significant difference among groups at the above ages
Phenotype characterization on P2X3 immunoreactive DRG cells
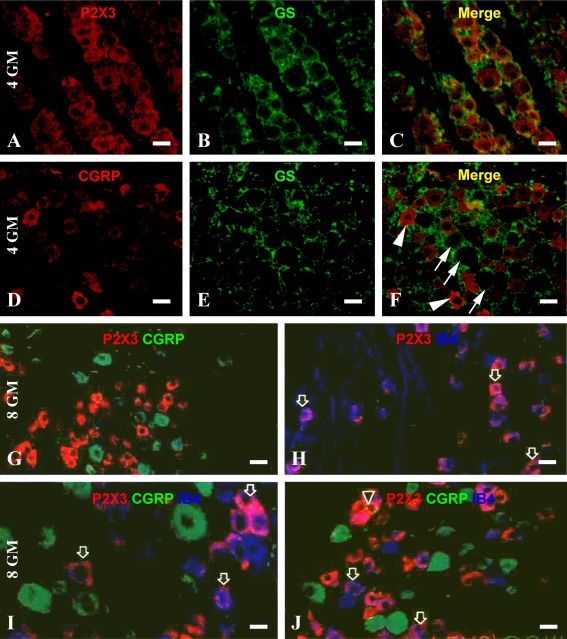
Satellite astrocytes are arranged around the DRG neurons, which express GS [44]. Therefore, we checked the double labeling for P2X3 and CGRP with GS. P2X3/GC double immunofluorescence showed no colocalization of P2X3 or CGRP with GS in the prenatal human DRG. For example, GC immunoreactive satellite astrocytes were distributed around DRG perikarya that were mostly labeled by P2X3 in 4-month-old cases (Fig. 5a–c). The spatial arrangement between GC immunoreactive satellite astrocytes and CGRP-labeled perikarya was similar to that seen in P2X3/GC double labeling. However, CGRP immunoreactivity was seen in only a small subpopulation of DRG somata (Fig. 5d–f). Therefore, P2X3 as well as CGRP immunolabeled perikarya likely represented neurons rather than glial cells in prenatal human DRG. In additional double immunofluorescent preparations, P2X3 and CGRP (mouse antibody) appeared to be largely expressed in separate subpopulations of DRG neurons (Fig. 5g, i, j). Thus, P2X3 immunoreactive cells were relatively small whereas CGRP immunoreactive cells relatively large (Fig. 5g, i). It should be noted that P2X3 and CGRP colocalization was detected in a few DRG cells that were invariably small in size (Fig. 5j). A preliminary cell count was carried out in the DRG from 7- to 8-month-old cases, which yielded a 4.6 ± 1.1% (44 of 948) colocalization of CGRP among P2X3-positive neurons.
Fig. 5.
Representative double immunofluorescent images illustrating the cellular expression of P2X3 and CGRP in prenatal human DRG. a–f A 4-gestational month fetus and g–j an 8-month fetus. Neither P2X3 (a–c) nor CGRP (d–f) immunoreactive cells are colocalized with glutamine synthetase (GS). Arrows in f point to CGRP negative neurons, whereas arrowheads indicate CGRP-positive DRG neurons, both of which are surrounded by GS-labeled satellite astrocytes. Most P2X3-positive cells are not colocalized with CGRP (g, i), although coexpression of these two markers is seen occasionally (j, indicated by a triangle). Note that CGRP-labeled cells appear somewhat larger in somal size relative to P2X3 cells (g, i, j). In double and triple labeling preparations (h–j), P2X3 immunoreactive cells are frequently colocalized with IB4 binding (examples are indicated by arrows). Scale bar = 20 μm for a–h, j, and 10 μm in i
IB4 binding can be used to visualize small dorsal DRG neurons that are involved in nociception [28, 29]. Double fluorescent labeling detected a frequent colocalization of P2X3 with IB4 binding in the DRG in all cases (Fig. 5h–j). A preliminary quantification (using sections from 7- to 8-month-old cases) indicated that 89.5 ± 4.1% (847 of 946) P2X3-positive cells colocalized with IB4 binding and, vice versa, 92.6 ± 3.8% (847 of 915) IB4 binding cells colocalized with P2X3 immunolabeling. A small fraction of DRG neurons were concurrently labeled for P2X3, CGRP, and IB4 in the triple labeling preparation (Fig. 5j).
Discussion
P2X3 is a key member of the ligand-gated ion-conducting P2X receptors, and its expression can be found in the central and peripheral nervous systems in laboratory rodents during development and at adult stage [45–52]. Specifically, P2X3 expression has been shown in adult rat DRG neurons and cultured DRG neurons [8–15, 53]. Several studies demonstrate that P2X3 expression in rodent adult rodent DRG is present in relatively small-sized neurons that are also labeled by IB4 binding, implicating a role for this purinergic receptor in pain processing [54, 55].
The cellular localization of P2X3 in prenatal human DRG is generally consistent with the aforementioned data from rodents. Thus, P2X3 immunoreactive cells in 4–8-gestational month-old fetuses appear to be mostly small-sized neurons, generally <20 μm in diameter. This is established based on their neuronal morphology, distribution pattern, binding to IB4, and lack of colocalization with GS. Although some P2X3-positive cells in 7- to 8-gestational month cases appear fairly large (>20 μm), double labeling indicates that these P2X3 neurons are still smaller than CGRP neurons in general. Thus, we propose that in humans P2X3 is predominantly expressed in small-sized DRG neurons. Given that these neurons also overwhelmingly exhibited IB4 binding, P2X3-expressing DRG neurons in humans might be associated with C-type afferents conducting pain and other senses [28, 29].
In a previous developmental study in mice [54], P2X3 immunolabeling is found in almost all DRG sensory neurons at embryonic day 14, but only in about 50% of neurons at birth. P2X3-labeled neurons in prenatal mouse DRG are relatively small in size. However, from postnatal day 7, both small and large neurons are labeled. P2X3-positive DRG neurons in mice do not exhibit IB4 binding until after birth. By postnatal day 14, about 50% DRG neurons bind IB4 and co-express P2X3. Unlike P2X3, CGRP is expressed in about 10% DRG neurons in the mouse embryos, which colocalize with P2X3. CGRP expressing neurons in mouse DRG increases during postnatal development, reaching up to 35–40% in the adult [54].
In the present study, the density of P2X3-positive DRG neurons is the highest in the youngest gestational group (4 months) and tends to reduce with the increase of gestation. This finding is similar to the decline of P2X3 neurons in mice with development [54]. Another finding comparable to mice, P2X3 immunoreactive neurons in human DRG are generally small in size and show strong labeling at earlier gestational stages. These labeled neurons increase in size but decrease in labeling intensity with prenatal development. It is possible that the increase in cell size and decrease in cell density with gestation may be related to the growth of neuronal somata and development of neuronal processes and glial components in the DRG. The latter events would cause a decline of neuronal density due to a “cell dilution” effect. However, it remains to exclude whether neuronal death or apoptosis may occur in the human DRG leading to the observed decline in numerical density of P2X3 immunoreactive neurons.
Somewhat different from rodents, P2X3-labeled DRG neurons in 4–8-gestational month-old fetuses are commonly colocalized with IB4 binding. This finding may suggest an earlier occurrence of IB4 binding activity in developing human DRG neurons relative to mice. Also different from mice is that P2X3 and CGRP colocalization appears much infrequent (~5%) in prenatal human relative to prenatal mouse DRG [54]. It is possible that in mice there exists a transient expression of CGRP in small DRG neurons. Alternatively, P2X3 and CGRP coexpression might appear later during development (e.g., postnatal), as speculated from the densitometric data showing a noticeable trend of increase of CGRP expressing neurons in human DRG with the increase of gestational months.
In summary, the present study demonstrates that the purinergic receptor P2X3 is expressed early and fairly widely in prenatal human DRG. P2X3 immunolabeling occurs in a large amount of relatively small-sized DRG neurons that also bind to IB4, but is absent in satellite astrocytes. There exists a noticeable decline in density of the labeled cells with gestational age. These human data implicate that developing purinergic antagonists for pain therapy in rodent models are of future clinical relevance.
Acknowledgments
This work was supported in part by the Natural Science Foundation of China (A.P., Z.L, grant nos. 30771135 and 30770825); Hunan Provincial Natural Science Foundation of China (no. 09JJ3070), and Central South University (A.P., X.X.Y). We thank Drs. Xuegang Luo and Jufang Huang for constructive comments.
Contributor Information
Xiao-Xin Yan, Email: yanxiaoxin@csu.edu.cn.
Zhiyuan Li, Email: li_zhiyuan@gibh.ac.cn.
References
- 1.Ralevic V. P2 receptors in the central and peripheral nervous systems modulating sympathetic vasomotor tone. Auto Nerv Syst. 2000;81:205–211. doi: 10.1016/S0165-1838(00)00139-9. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Migita K, Samways DS, Voigt MM, Egan TM. Gain and loss of channel function by alanine substitutions in the transmembrane segments of the rat ATP-gated P2X2 receptor. J Neurosci. 2004;24:7378–7386. doi: 10.1523/JNEUROSCI.1423-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Liang D, Chen L. Potential therapeutic targets for ATP-gated P2X receptor ion channels. Assay Drug Dev Techno. 2008;16:277–284. doi: 10.1089/adt.2007.121. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 5.Fountain SF, Burnstock G. An evolutionary history of P2X receptors. Purinergic Signalling. 2009;5:269–272. doi: 10.1007/s11302-008-9127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan TM, Samways DS, Li Z. Biophysics of P2X receptors. Pflugers Arch. 2006;452:501–512. doi: 10.1007/s00424-006-0078-1. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G, Fredholm BB, Verkhratsky A. Adenosine and ATP receptors in the brain. Curr Top Med Chem. 2011;11:973–1011. doi: 10.2174/156802611795347627. [DOI] [PubMed] [Google Scholar]
- 8.Ballini E, Virginio C, Medhurst S, Summerfield S, Aldegheri L, Buson A, Carignani C, Chen Y, Giacometti A, Lago I, Powell A, Jarolimek W. Characterization of three diaminopyrimidines as potent and selective antagonists of P2X3 and P2X2/3 receptors with in vivo efficacy in a pain model. Br J Pharmacol. 2011;163:1315–1325. doi: 10.1111/j.1476-5381.2011.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JNA. P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 11.Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 12.Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/S0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- 13.Petruska JC, Cooper BY, Gu JG, Rau KK, Johnson RD. Distribution of P2X1, P2X2, and P2X3 receptor subunits in rat primary afferents: relation to population markers and specific cell types. J Chem Neuroanat. 2000;20:141–162. doi: 10.1016/S0891-0618(00)00080-6. [DOI] [PubMed] [Google Scholar]
- 14.Aoki Y, Ohtori S, Takahashi K, Ino H, Ozawa T, Douya H, Chiba T, Moriya H. P2X3-immunoreactive primary sensory neurons innervating lumbar intervertebral disc in rats. Brain Res. 2003;989:214–220. doi: 10.1016/S0006-8993(03)03365-1. [DOI] [PubMed] [Google Scholar]
- 15.Kage K, Niforatos W, Zhu CZ, Lynch KJ, Honore P, Jarvis MF. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res. 2002;147:511–519. doi: 10.1007/s00221-002-1263-x. [DOI] [PubMed] [Google Scholar]
- 16.Hwang IK, Lee HY, Yoo KY, Seong NS, Chung HG, Kim JH, Lee HJ, Lee WH, Kang TC, Won MH. Chronological alterations of P2X3 receptor expression in the trigeminal ganglion after ischaemic insult in the Mongolian gerbil. Anat Histol Embryol. 2004;33:220–224. doi: 10.1111/j.1439-0264.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 17.Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y. Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain. 2005;116:42–51. doi: 10.1016/j.pain.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci USA. 2008;105:16773–16778. doi: 10.1073/pnas.0801793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang Z, Xiong Y, Yan N, Li X, Mao Y, Ni X, He C, LaMotte RH, Burnstock G, Sun J. Functional up-regulation of P2X 3 receptors in the chronically compressed dorsal root ganglion. Pain. 2008;140:23–34. doi: 10.1016/j.pain.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan AH, Lu DH, Luo XG, Chen L, Li ZY. Formalin-induced increase in P2X(3) receptor expression in dorsal root ganglia: implications for nociception. Clin Exp Pharmacol Physiol. 2009;36:e6–e11. doi: 10.1111/j.1440-1681.2009.05179.x. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Liu H, Deng L, Zhu G, Xu C, Li G, Liu S, Xie J, Liu J, Kong F, Wu R, Li G, Liang S. Effect of emodin on neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Res Bull. 2011;84:406–413. doi: 10.1016/j.brainresbull.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Gever JR, Soto R, Henningsen RA, Martin RS, Hackos DH, Panicker S, Rubas W, Oglesby IB, Dillon MP, Milla ME, Burnstock G, Ford AP. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–1398. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behavior in rats. Brain. 2010;133:2549–2564. doi: 10.1093/brain/awq194. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev. 2010;63:222–232. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Bonfanti L, Bellardi S, Ghidella S, Gobetto A, Polak JM, Merighi A. Distribution of five peptides, three general neuroendocrine markers, and two synaptic-vesicle-associated proteins in the spinal cord and dorsal root ganglia of the adult and newborn dog: an immunocytochemical study. Am J Anat. 1991;191:154–166. doi: 10.1002/aja.1001910203. [DOI] [PubMed] [Google Scholar]
- 26.Bergman E, Johnson H, Zhang X, Hökfelt T, Ulfhake B. Neuropeptides and neurotrophin receptor mRNAs in primary sensory neurons of aged rats. J Comp Neurol. 1996;375:303–319. doi: 10.1002/(SICI)1096-9861(19961111)375:2<303::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Li XQ, Verge VM, Johnston JM, Zochodne DW. CGRP peptide and regenerating sensory axons. J Neuropathol Exp Neurol. 2004;63:1092–1103. doi: 10.1093/jnen/63.10.1092. [DOI] [PubMed] [Google Scholar]
- 28.Hubscher CH, Petruska JC, Rau KK, Johnson RD. Co-expression of P2X receptor subunits on rat nodose neurons that bind the isolectin GS-I-B4. Neuroreport. 2001;12:2995–2997. doi: 10.1097/00001756-200109170-00048. [DOI] [PubMed] [Google Scholar]
- 29.Fullmer JM, Riedl MS, Higgins L, Elde R. Identification of some lectin IB4 binding proteins in rat dorsal root ganglia. Neuroreport. 2004;15:1705–1709. doi: 10.1097/01.wnr.0000136037.54095.64. [DOI] [PubMed] [Google Scholar]
- 30.Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans RM. Calcitonin gene-related peptide immunoreactivity in the spinal cord of man and of eight other species. J Neurosci. 1984;4:3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merighi A, Kar S, Gibson SJ, Ghidella S, Gobetto A, Peirone SM, Polak JM. The immunocytochemical distribution of seven peptides in the spinal cord and dorsal root ganglia of horse and pig. Anat Embryol (Berl) 1990;181:271–280. doi: 10.1007/BF00174620. [DOI] [PubMed] [Google Scholar]
- 32.Biella G, Panara C, Pecile A, Sotgiu ML. Facilitatory role of calcitonin gene-related peptide (CGRP) on excitation induced by substance P (SP) and noxious stimuli in rat spinal dorsal horn neurons. An iontophoretic study in vivo. Brain Res. 1991;559:352–356. doi: 10.1016/0006-8993(91)90024-P. [DOI] [PubMed] [Google Scholar]
- 33.Nitsos I, Rees S. Development of immunoreactivity for calcitonin gene-related peptide, substance P and glutamate in primary sensory neurons, and for serotonin in the spinal cord of fetal sheep. Neuroscience. 1993;54:239–252. doi: 10.1016/0306-4522(93)90396-W. [DOI] [PubMed] [Google Scholar]
- 34.Traub RJ, Allen B, Humphrey E, Ruda MA. Analysis of calcitonin gene-related peptide-like immunoreactivity in the cat dorsal spinal cord and dorsal root ganglia provide evidence for a multisegmental projection of nociceptive C-fiber primary afferents. J Comp Neurol. 1990;302:562–574. doi: 10.1002/cne.903020312. [DOI] [PubMed] [Google Scholar]
- 35.Pohl M, Benoliel JJ, Bourgoin S, Lombard MC, Mauborgne A, Taquet H, Carayon A, Besson JM, Cesselin F, Hamon M. Regional distribution of calcitonin gene-related peptide-, substance P-, cholecystokinin-, Met5-enkephalin-, and dynorphin A (1–8)-like materials in the spinal cord and dorsal root ganglia of adult rats: effects of dorsal rhizotomy and neonatal capsaicin. J Neurochem. 1990;55:1122–1130. doi: 10.1111/j.1471-4159.1990.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X, Ju G, Elde R, Hökfelt T. Effect of peripheral nerve cut on neuropeptides in dorsal root ganglia and the spinal cord of monkey with special reference to galanin. J Neurocyto. 1993;122:342–381. doi: 10.1007/BF01195558. [DOI] [PubMed] [Google Scholar]
- 37.Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol. 2008;37(1):83–90. doi: 10.1007/s12035-008-8020-5. [DOI] [PubMed] [Google Scholar]
- 38.Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. 2009;9:9–14. doi: 10.1016/j.coph.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:573–582. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 40.Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/S0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- 41.Yiangou Y, Facer P, Birch R, Sangameswaran L, Eglen R, Anand P. P2X3 receptor in injured human sensory neurons. Neuroreport. 2000;11:993–996. doi: 10.1097/00001756-200004070-00019. [DOI] [PubMed] [Google Scholar]
- 42.Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides. 1985;6:721–745. doi: 10.1016/0196-9781(85)90178-0. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, Ribak CE, Yan XX, Giolli RA. Synaptic and neurochemical features of calcitonin gene-related peptide containing neurons in the rat accessory optic nuclei. Brain Res. 1999;838:119–130. doi: 10.1016/S0006-8993(99)01642-X. [DOI] [PubMed] [Google Scholar]
- 44.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Kidd EJ, Miller KJ, Sansum AJ, Humphrey PP. Evidence for P2X3 receptors in the developing rat brain. Neuroscience. 1998;87:533–539. doi: 10.1016/S0306-4522(98)00294-2. [DOI] [PubMed] [Google Scholar]
- 46.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/S0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 47.Dunn PM, Gever J, Ruan HZ, Burnstock G. Developmental changes in heteromeric P2X(2/3) receptor expression in rat sympathetic ganglion neurons. Dev Dyn. 2005;234:505–511. doi: 10.1002/dvdy.20466. [DOI] [PubMed] [Google Scholar]
- 48.Boldogköi Z, Schütz B, Sallach J, Zimmer A. P2X(3) receptor expression at early stage of mouse embryogenesis. Mech Dev. 2002;118:255–260. doi: 10.1016/S0925-4773(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 49.Cheung KK, Burnstock G. Localization of P2X3 receptors and coexpression with P2X2 receptors during rat embryonic neurogenesis. J Comp Neurol. 2002;443:368–382. doi: 10.1002/cne.10123. [DOI] [PubMed] [Google Scholar]
- 50.Cheung KK, Chan WY, Burnstock G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133:937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 51.Huang LC, Ryan AF, Cockayne DA, Housley GD. Developmentally regulated expression of the P2X3 receptor in the mouse cochlea. Histochem Cell Biol. 2006;125:681–692. doi: 10.1007/s00418-005-0119-4. [DOI] [PubMed] [Google Scholar]
- 52.Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC. Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. J Comp Neurol. 2008;506:627–639. doi: 10.1002/cne.21544. [DOI] [PubMed] [Google Scholar]
- 53.Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 54.Ruan HZ, Moules E, Burnstock G. Changes in P2X3 purinoceptors in sensory ganglia of the mouse during embryonic and postnatal development. Histochem Cell Biol. 2004;122:539–551. doi: 10.1007/s00418-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 55.Zhao SJ, Yin GP, Gu Y, Li Z, Hu D, Lin C, Dai J. Expression of adenosine triphosphate P2X3 receptor and its relation with substance P in rat primary sensory neurons. Chinese Journal of histochemistry and cytochemistry. 2006;15:91–94. [Google Scholar]