Abstract
P2X4 receptors (P2X4Rs), a subtype of the purinergic P2X family, play important roles in regulating neuronal and glial functions in the nervous system. We have previously shown that the expression of P2X4Rs is upregulated in activated microglia after peripheral nerve injury and that activation of the receptors by extracellular ATP is crucial for maintaining nerve injury-induced pain hypersensitivity. However, the regulation of P2X4R expression on the cell surface of microglia is poorly understood. Here, we identify the CC chemokine receptor CCR2 as a regulator of P2X4R trafficking to the cell surface of microglia. In a quantitative cell surface biotinylation assay, we found that applying CCL2 or CCL12, endogenous ligands for CCR2, to primary cultured microglial cells, increased the levels of P2X4R protein on the cell surface without changing total cellular expression. This effect of CCL2 was prevented by an antagonist of CCR2. Time-lapse imaging of green fluorescent protein (GFP)-tagged P2X4R in living microglial cells showed that CCL2 stimulation increased the movement of P2X4R-GFP particles. The subcellular localization of P2X4R immunofluorescence was restricted to lysosomes around the perinuclear region. Notably, CCL2 changed the distribution of lysosomes with P2X4R immunofluorescence within microglial cells and induced release of the lysosomal enzyme β-hexosaminidase, indicating lysosomal exocytosis. Moreover, CCL2-stimulated microglia enhanced Akt phosphorylation by ATP applied extracellularly, a P2X4R-mediated response. These results indicate that CCL2 promotes expression of P2X4R protein on the cell surface of microglia through exocytosis of P2X4R-containing lysosomes, which may be a possible mechanism for pain hypersensitivity after nerve injury.
Keywords: P2X4 receptor, CCR2, Lysosome, Cell-surface expression, Microglia
Introduction
Accumulating evidence indicates that microglia, a type of immune cells, play crucial roles in diverse pathologies in the nervous system, including neuronal injury, trauma, ischemia, infection, and several neurological diseases [1–3]. Once activated under such pathological conditions, microglia dramatically change the expression of a variety of genes including cell-surface receptors [1, 4, 5]. We have previously shown that the ionotropic P2X receptor subtype P2X4R is upregulated in activated microglia in the spinal cord after peripheral nerve injury [6]. The upregulation of microglial P2X4R expression has also been reported in animal models of stroke [7], brain tumor [8], traumatic brain injury [9, 10], and spinal cord injury [11], and in human acute inflammatory demyelinating polyradiculoneuropathy [12]. Stimulation of microglial P2X4R by extracellular ATP causes a release of brain-derived neurotrophic factor [13, 14], which is necessary for maintaining pain hypersensitivity after nerve injury, and also induces phosphorylation of Akt, which is a crucial step for microglial chemotaxis toward a high concentration of ATP [15–17]. In addition, activation of P2X4Rs evokes prostaglandin E2 production in macrophages [18], which is essential for inflammatory pain. Thus, P2X4R has emerged as a key regulator of microglia/macrophage functions and thereby of pathologies in the nervous system.
Recent studies have identified extracellular bioactive factors that upregulate P2X4R expression in microglia. These include lipopolysaccharide [19], interferon-γ (IFN-γ) [20], and fibronectin [21–24]. We have demonstrated a molecular mechanism underlying fibronectin-induced P2X4R upregulation at transcriptional and translational levels [22, 24]. However, a large amount of P2X4R protein within microglia/macrophages has been shown to localize predominantly to intracellular lysosomal compartments [25]. Notably, P2X4R protein remains stable within the proteolytic environment of lysosomes, and when microglia cells are stimulated artificially by ionomycin, trafficking of P2X4R protein to the cell surface occurs. Microglial activity is influenced by the extracellular milieu [1, 26], but the identity of bioactive factors regulating P2X4R trafficking to the cell surface of microglia remains elusive. In the present study, we focused on extracellular signaling molecules that are reported to target microglia in vivo and to critically contribute to nerve injury-induced mechanical hypersensitivity because microglial P2X4Rs are necessary for neuropathic pain [6]. Among factors fulfilling these two criteria, we demonstrated that a member of the C–C subfamily of chemokines CCL2 [chemokine (C–C) ligand 2, also known as MCP-1] has an ability to rapidly promote P2X4R trafficking to the cell surface of microglia with lysosomal exocytosis without changing the total cellular level of P2X4R protein.
Materials and methods
Microglial cells
Rat primary cultured microglia were prepared according to a method described previously [6, 27]. In brief, a mixed glial culture was prepared from neonatal Wistar rats (Kyudo, Saga, Japan) and maintained for 9–24 days in Dulbecco’s modified eagle medium with 10% fetal bovine serum. Microglia were obtained as floating cells over the mixed glial culture. The floating cells were collected by a gentle shake and transferred to appropriate dishes or coverslips, and the microglia attached to them were then used for biotinylation, Western blotting, time-lapse imaging and immunocytochemistry.
Microglial cells were stimulated by applying either recombinant rat CCL2 (10, 30, or 100 ng/mL; R&D Systems, Lille, France), mouse CCL12 (10 ng/mL; R&D Systems), rat CX3CL1 (100 ng/mL; R&D Systems), or rat IFN-γ (100 U/mL; Calbiochem, San Diego, CA, USA) 30 min before biotinylation. A CCL2 neutralizing antibody (2.5 and 25 μg/mL; R&D Systems) and the dynamin inhibitor dynasore (80 μM; Sigma, St. Louis, USA) were added to cultures 30 min before CCL2 stimulation. RS-504393 (1 and 10 μM; Tocris, Bristol, UK) and forskolin (10 μM; Sigma) were added 20 and 10 min, respectively, before CCL2 stimulation. For treatment with fibronectin; microglia were plated on culture dishes coated with fibronectin (10 μg/mL; Invitrogen, Carlsbad, CA, USA), cultured for 24 h in a cell surface biotinylation assay and in measurement of CCL2 release at various time points.
Biotinylation of cell surface proteins
Biotinylation and isolation of cell surface proteins were performed using a Pierce Cell Surface Protein Isolation Kit (Pierce, Rockford, IL, USA). Microglial cells (1.5–2.0 × 106 cells/dish) were rinsed two times with ice-cold phosphate-buffered saline (PBS) and incubated with freshly prepared sulfo-NHS-SS-biotin (Pierce) for 30 min at 4°C. The cells were subsequently lysed in 500 μL of RIPA buffer [50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholic acid] containing protease inhibitors cocktail (Sigma). After centrifugation of the lysates, 50 μL of the supernatant was subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) to measure the total cellular levels of P2X4R protein. For isolation of cell surface proteins, 450 μL of supernatant was further incubated with streptavidin beads (Pierce) for 1 h at room temperature to pull down all biotinylated proteins. Beads were washed three times and eluted in Laemmli buffer with dithiothreitol (20 μM), and the eluted samples were subjected to SDS-PAGE for assessment of the cell surface levels of P2X4R protein.
Western blotting
For detection of P2X4R protein in fractions of the cell surface and whole cells, each sample was subjected to 10% SDS-PAGE, and the proteins were transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Buckinghamshire, UK). The membrane was blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween-20. The membrane was incubated with primary antibodies [anti-P2X4R (1:5000; Alomone, Jerusalem, Israel) and anti-β-actin (1:2000; Sigma)] for overnight at 4°C. The antibodies were detected using horse radish peroxidase conjugated anti-rabbit or anti-mouse IgG secondary antibody (GE Healthcare, 1:1,000). For Akt phosphorylation evoked by ATP, microglial cells in primary cultures that had been treated with CCL2 (100 ng/mL) for 30 min were stimulated by applying ATP (5 μM, Sigma). Whole-cell lysates of microglia were prepared 10 min after ATP stimulation and were subjected to 10% SDS-PAGE and transferred to PVDF membranes. Anti-phospho-Akt (Ser473) (1:1,000; Cell Signaling, Danvers, MA, USA) and anti-Akt (1:1,000; Cell Signaling) were used to detect phosphorylated and total Akt, respectively. In this experiment, some microglial cultures were pretreated with TNP-ATP (100 μM, Sigma), which can block P2X4R [6], for 5 min before ATP application. The blots were detected using an ECL western blotting detection system (GE Healthcare) and an LAS-3000 imaging system (Fujifilm, Tokyo, Japan). For densitometric quantification, each band was quantified using the software of the LAS-3000 imaging system. Equal amounts of protein loaded into each lane were verified based on the band intensity of β-actin. The relative P2X4R band density levels were obtained by normalizing the cell surface and total cellular P2X4R bands against the total β-actin band from the same sample.
Measurement of CCL2 release
Microglial cells (5 × 104 cells/well) were cultured on fibronectin-coated 24-well plates for each time point tested (1–24 h). The supernatants were collected and centrifuged (4°C, 1,500 rpm, 5 min). The resultant supernatants were diluted and used as samples for measuring CCL2 release. The concentrations of CCL2 in each sample were determined using OptiEIA™ ELISA Set rat MCP-1 (BD Bioscience, Erembodegem, Belgium). The assay was performed according to the manufacturer’s protocol. Absorbance was read at 450 nm and corrected at 570 nm.
Immunocytochemistry
Primary cultured microglia were fixed in 3.7% formaldehyde/PBS. After washing and incubating with blocking buffer [0.05% saponin/1% BSA/PBS] for 15 min at room temperature, the cells were incubated for 24 h at 4°C in primary antibodies [anti-P2X4R (1:5000; Alomone) and anti-LGP85 (85-kDa lysosomal membrane glycoprotein; 1:500)] [28]. Following incubation, microglial cells were washed and incubated for 1 h at room temperature in secondary antibody solution [Alexa 488 goat anti-rabbit IgG antibody and Alexa 546 goat anti-mouse IgG antibody (1:1,000, Molecular Probes, OR, USA)] and analyzed using an LSM510 Imaging System (Carl Zeiss, Oberkochen, Germany).
Time-lapse imaging of P2X4Rs in living microglial cells
Full-length complementary DNAs for rat P2X4R-GFP was cloned into the lentiviral CS2-EF-MCS vector (packaging plasmid; provided by Dr. Hiroyuki Miyoshi, RIKEN BioResource Center, Tsukuba, Ibaraki, Japan). The vector with pCAG-HIVgp and pCMV-VSV-G-RSV-Rev (packaging plasmid; provided by Dr. Hiroyuki Miyoshi) was cotransfected into HEK293T cells (RIKEN BioResource Center). After 100-fold concentration with polyethylene glycol, viral particles and 8 μg/mL polybrene were added onto primary cultured microglia plated on 24-well plates (1.2 × 105 cells/well). After a 12-h treatment with the lentivirus, cultured medium was changed to conditioned medium prepared from the mixed glial culture, and microglia were further cultured for 60 h. For time-lapse imaging of P2X4Rs, P2X4R-GFP lentivirus-transduced microglial cells were washed with Hank’s balanced salt solution (HBSS, 37°C). The P2X4R-GFP fluorescence was then recorded every 5 s for 270 s using an LSM5 Imaging System (Zeiss) with a 63× oil-immersion objective at room temperature. Obtained images were imported into ImageJ software, and the orbital and total distance of the movement of P2X4R-GFP fluorescence particles were obtained.
Lysosomal exocytosis assay
Microglial cells (5 × 105 cells/well) were plated onto 24-well plates and were cultured for 3 h. Lysosomal exocytosis was assayed by measuring release of β-hexosaminidase. Microglial cells were incubated with CCL2 (100 ng/mL) or ionomycin (5 μM; Sigma) for 30 min in HBSS at 37°C. Thirty microliters of supernatant was incubated with 60 μL of 1 mM 4-nitrophenyl N-acetyl-beta-d-glucosaminide in 0.05 M citrate buffer (0.025 M citric acid, 0.025 M trisodium citrate, pH 4.5) for 1 h at 37°C. Reactions were stopped by addition of 150 μL of 0.05 M sodium carbonate buffer (pH 10). Absorbance was then measured at 405 nm.
Statistical analyses
Statistical analyses of the results were evaluated using the Student’s t test and the Tukey’s multiple comparison test after one-way ANOVA.
Results
CCR2 agonists increase levels of P2X4R protein on the surface of cultured microglial cells
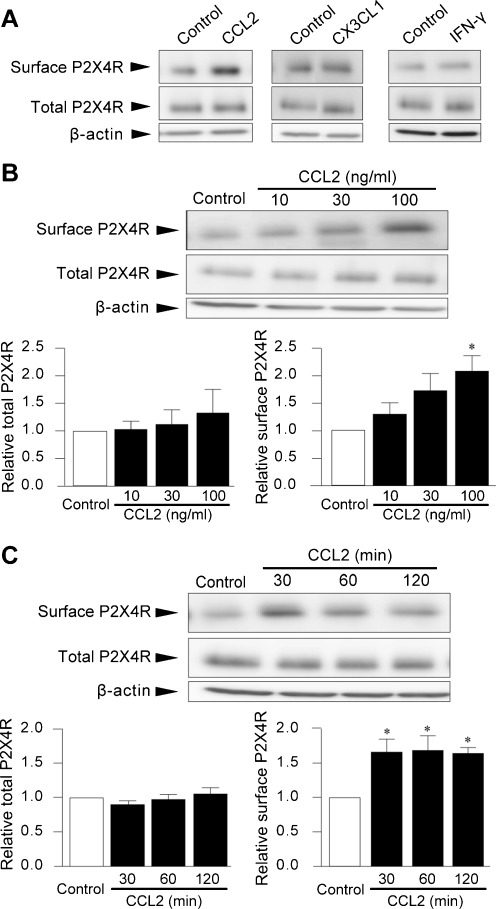
By searching for endogenous signaling molecules that target microglia in vivo and that critically contribute to nerve injury-induced mechanical hypersensitivity, from a number of published papers, we focused on the following molecules as candidates: CCL2, CX3CL1 [chemokine (C-X3-C motif) ligand 1, also known as fractalkine] and IFN-γ. First, the effects of these factors on the levels of P2X4R protein on the cell surface of microglial cells in primary culture were examined. Cell surface biotinylation followed by Western blot analysis of P2X4R protein using its antibody demonstrated that only CCL2 produced a pronounced increase in the P2X4R protein levels on the cell surface at 30 min post-stimulation (Fig. 1a). The specificity of the P2X4R antibody was confirmed using P2X4R-deficient microglial cells (data not shown). We therefore further explored the role of CCL2 in P2X4R protein localization.
Fig. 1.
CCL2 increases cell surface expression of P2X4R protein in microglial cells. P2X4R protein on the surface of primary cultured microglial cells was detected by cell surface biotinylation followed by Western blot analysis of P2X4R protein using its specific antibody. Total cellular P2X4R protein was detected in whole-cell lysates of microglial cells. Microglial cells in primary cultures were treated with either CCL2 (100 ng/mL), CX3CL1 (100 ng/mL), or IFN-γ (100 U/mL) for 30 min (a) with CCL2 (10, 30, and 100 ng/mL) for 30 min (b) and with CCL2 (100 ng/mL) for 30, 60, and 120 min (c). b, c histograms of the relative band density ratio of surface and total P2X4R protein normalized to the levels of β-actin. The experiments were repeated four to five times, and the data are means ± SEM of fold changes over the value of unstimulated control microglia. *P < 0.05 compared with unstimulated control microglia
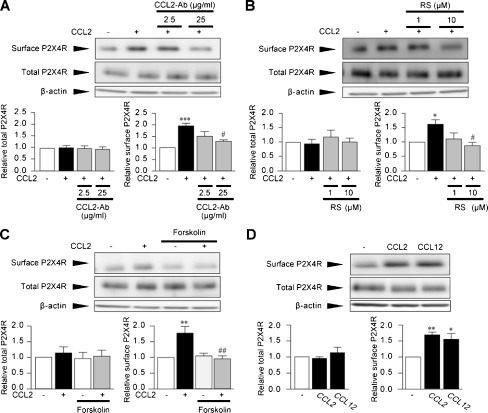
The increase in cell surface levels of P2X4R proteins by CCL2 occurred in a concentration-dependent manner, and a significant effect was observed in the presence of 100 ng/mL CCL2 (2.1-fold, P < 0.05, Fig. 1b). However, P2X4R protein levels in whole-cell lysates of CCL2-stimulated microglia were not significantly changed at any of the concentrations tested (Fig. 1b). CCL2-induced surface P2X4R expression peaked at 30 min (P < 0.05, Fig. 1c), persisted for up to 120 min (P <0.05, Fig. 1c) and returned to the basal level at 24 h (data not shown). Furthermore, pretreatment of microglial cells with a neutralizing antibody for CCL2 inhibited the CCL2-induced upregulation of P2X4R protein expression on the cell surface (P < 0.05, Fig. 2a), confirming the promoting effect of CCL2 on the cell surface expression of P2X4Rs in microglia.
Fig. 2.
CCR2 mediates CCL2-induced surface P2X4R expression. a–c Microglial cells in primary cultures were pretreated with either CCL2 neutralizing antibodies (CCL2-Ab, 2.5 and 25 μg/mL) for 30 min (a), RS-504393 (RS, 1 and 10 μM) for 20 min (b) or forskolin (10 μM) for 10 min (c) before CCL2 (100 ng/mL) stimulation. d Microglial cells were stimulated by CCL12 (10 ng/mL) for 30 min. Surface proteins of microglial cells were biotinylated 30 min after CCL2 (a–c) and CCL12 (d) stimulation. Surface and total P2X4R proteins were detected by Western blot analysis using its specific antibody. Histograms of the relative band density ratio of surface and total P2X4R protein normalized to the levels of β-actin. The experiments were repeated four to five times, and the data are means ± SEM of fold changes over the value of unstimulated control microglia. *P < 0.05, **P < 0.01, ***P < 0.001 compared with unstimulated control microglia; #P < 0.05, ##P < 0.01 compared with CCL2-stimulated microglia
CCL2 is known to be an endogenous agonist of CCR2. To determine the role of CCR2, we pretreated microglial cells with RS-504393, an antagonist of CCR2 [29, 30], and found that RS-504393 completely abolished the CCL2-induced increase in P2X4R protein on the cell surface without changing total cellular P2X4R protein expression (P < 0.05, Fig. 2b). Furthermore, the increase in the surface P2X4R protein expression by CCL2 was also markedly inhibited by pretreating cells with forskolin (P < 0.01, Fig. 2c), which indirectly counteracts inhibitory effects of CCL2 on the Gi/adenylyl cyclase (AC)/cyclcic AMP (cAMP) signaling cascade downstream of CCR2 [31–33]. Moreover, microglial cells stimulated with CCL12, another agonist of CCR2 [34], produced a comparable increase in the cell surface level of P2X4R proteins, as observed in CCL2-stimulated microglia (P < 0.05, Fig. 2d). Together, these results indicated that CCL2 rapidly promotes P2X4R expression on the cell surface of microglia via CCR2.
CCL2 is involved in the fibronectin-induced increase in surface P2X4R
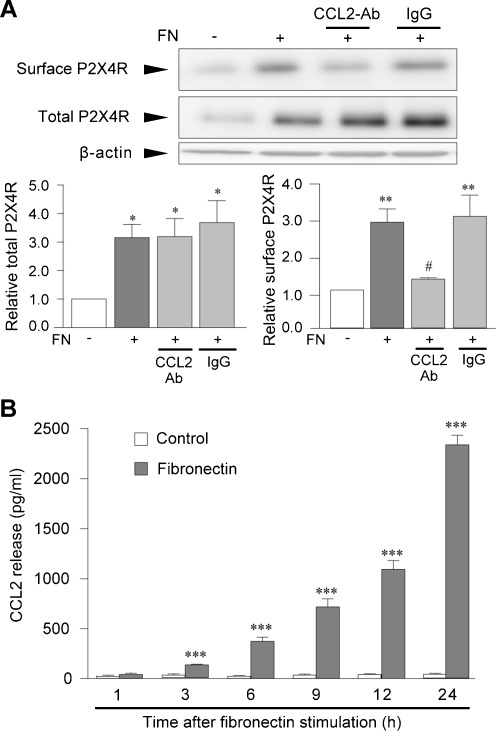
As shown in our previous studies [21, 23, 35], microglial cells treated with fibronectin for 24 h expressed increased levels of total cellular P2X4R protein (P < 0.05, Fig. 3a). We further found that fibronectin-stimulated microglia showed increased expression of P2X4R protein on the cell surface (P < 0.01, Fig. 3a). Interestingly, the increase in the level of surface P2X4R induced by fibronectin was significantly reduced by pretreatment of cells with the CCL2 neutralizing antibody (P < 0.05, Fig. 3a) with no effect on the increase in total cellular levels of P2X4R protein. We next examined the levels of CCL2 in supernatants of microglial cultures. The levels of CCL2 were low in the supernatants of non-stimulated microglia cultures but were strikingly increased in those of fibronectin-stimulated microglia cultures in a time-dependent manner (P < 0.001, Fig. 3b). These results indicate that fibronectin not only increases expression of P2X4R messenger RNA and protein [21, 22] but also enhances the level of P2X4R protein on the cell surface, which is dependent on CCL2 released from microglia following fibronectin stimulation.
Fig. 3.
CCL2 is involved in fibronectin-induced surface P2X4R upregulation. a Biotinylation assay of surface P2X4R proteins. CCL2 neutralizing antibody (CCL2-Ab, 25 μg/mL) and normal goat IgG (IgG, 25 μg/mL) were pretreated for 30 min before fibronectin (FN, 10 μg/mL) stimulation. Surface proteins of microglial cells were biotinylated 24 h after fibronectin stimulation. Surface and total P2X4R proteins were detected by Western blot analysis using its specific antibody. Histograms of the relative band density ratio of surface and total P2X4R protein normalized to the levels of β-actin. The experiments were repeated five times and the data are means ± SEM of fold changes over the value of unstimulated control microglia. *P < 0.05, **P <0.01 compared with unstimulated control microglia; #P < 0.05 compared with fibronectin-stimulated microglia. b CCL2 levels in supernatants of microglial cultures were measured by ELISA; primary cultured microglial cells were treated with or without fibronectin (10 μg/mL) for various time points of testing. The experiments were repeated six times, and the data are means ± SEM of the concentration of CCL2 (pg/mL). ***P < 0.001 compared with control at 1 h
CCL2 enhances P2X4R trafficking from lysosomes to the cell surface
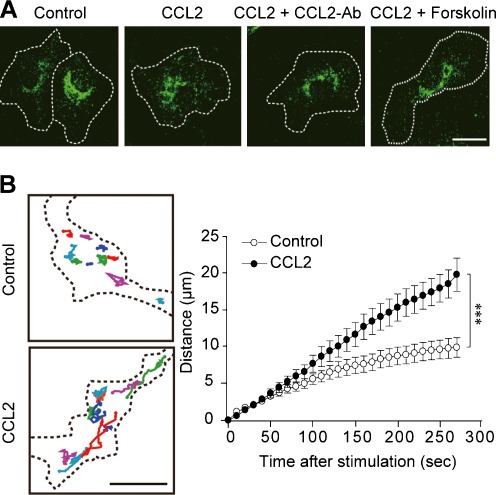
We next examined the subcellular distribution of P2X4R protein in microglia by immunocytochemical analyses. In unstimulated control microglial cells, immunofluorescence for P2X4R protein accumulated around the perinuclear region. By contrast, 30 min after application of CCL2, the subcellular distribution of P2X4R immunofluorescence was changed: The normally dotted P2X4R immunofluorescence was distributed more diffusely within the cell. The CCL2-induced change in P2X4R distribution was prevented by pretreating microglial cells either with the CCL2 neutralizing antibody or with forskolin (Fig. 4a). Furthermore, to visualize the behavior of P2X4R protein in living microglial cells, we transduced primary cultured microglia with a lentiviral vector encoding P2X4R in conjunction with AcGFP1 (GFP) at the C-terminus of P2X4R (P2X4R-GFP) and monitored the movement of GFP fluorescence by confocal microscopy. Time-lapse imaging showed that, in unstimulated microglia, particles of P2X4R-GFP fluorescence moved slightly within a restricted area. By contrast, after CCL2 application, P2X4R-GFP fluorescence particles were highly dynamic within microglial cells. The total distance of movement of P2X4R-GFP fluorescence particles was significantly increased after CCL2 stimulation (P < 0.001, Fig. 4b). Thus, these results indicate that CCL2 stimulation facilitates the movement of P2X4R protein in microglial cells.
Fig. 4.
CCL2 enhances movement of P2X4Rs. a Immunocytochemical analysis of P2X4Rs detected by its specific antibody. Primary cultured microglia were stimulated by CCL2 (100 ng/mL) for 30 min and were fixed by formaldehyde. Cells were pretreated with CCL2 neutralizing antibody (CCL2-Ab, 25 μg/mL) and forskolin (10 μM) for 30 and 10 min, respectively, before CCL2 stimulation. b Monitoring of P2X4R-GFP fluorescence particles in living microglial cells transduced with a lentiviral vector encoding P2X4R-GFP. Left Typical trajectories of P2X4R-GFP fluorescence particles in microglia treated with HBSS (control) or CCL2 (100 ng/mL). Right The total distance (μm, means ± SEM, n = 19) of the trajectories of P2X4R-GFP fluorescence particles from initial position. ***P < 0.001 compared with the control group by two-way ANOVA. Scale bar: 10 μm
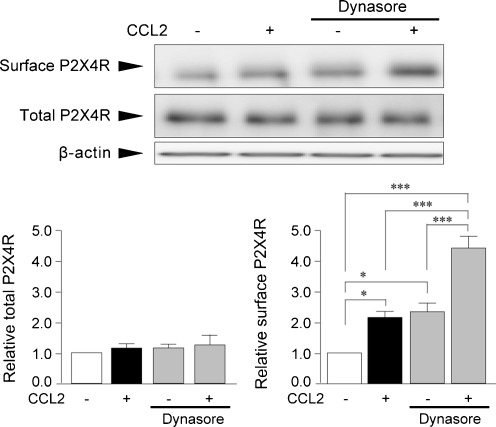
It has been reported that P2X4R undergoes rapid constitutive internalization in a manner that depends on dynamin [19, 36, 37]. Consistent with these studies, unstimulated control microglia treated solely with dynasore, an inhibitor of dynamin that is known to inhibit P2X4R internalization [19], showed an increase in the cell surface level of P2X4R protein (P < 0.05, Fig. 5), indicating an active dynamin-dependent P2X4R internalization in cultured microglia under our experimental condition. If CCL2 enhances P2X4R expression on the cell surface by inhibiting P2X4R internalization, then the effect of CCL2 might not be observed in dynasore-treated microglia. We found that CCL2-induced surface P2X4R expression was not inhibited by pretreatment of cells with dynasore (P < 0.001, Fig. 5).
Fig. 5.
Dynamin inhibitor does not affect the effect of CCL2 on cell surface P2X4R expression. Microglial cells in primary cultures were pretreated with the dynamin inhibitor dynasore (80 μM) for 30 min before CCL2 (100 ng/mL) stimulation. Surface proteins of microglial cells were biotinylated 30 min after CCL2 stimulation. Surface and total P2X4R proteins were detected by Western blot analysis using its specific antibody. Histograms of the relative band density ratio of surface and total P2X4R protein normalized to the levels of β-actin. The experiments were repeated four times and the data are means ± SEM of fold changes over the value of unstimulated control microglia. *P < 0.05, ***P < 0.001 compared with unstimulated control microglia
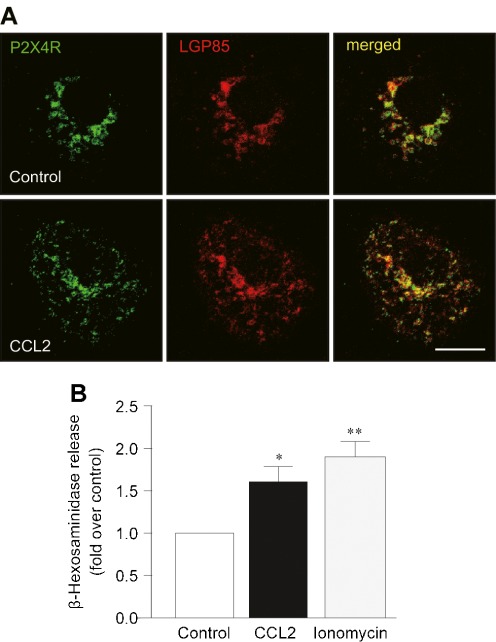
A recent study showed that P2X4Rs are localized predominantly to lysosomes [25]. In unstimulated control microglia, immunofluorescence for LGP85, a marker of lysosomes [28, 38], was observed around the perinuclear region and co-localized with P2X4R immunofluorescence. Thirty minutes after CCL2 stimulation, the distribution of LGP85-positive lysosomes was more diffuse throughout the cell, a change that was similar to the change in the P2X4R distribution in CCL2-stimulated microglia. Indeed, overlapping immunofluorescence for P2X4R and LGP85 was observed not only in the perinuclear region but also the area distal to the nucleus of microglial cells after CCL2 stimulation (Fig. 6a). Exocytosis of lysosomes has been shown to result in a delivery of P2X4R protein to the plasma membrane [25]. To further examine whether CCL2 causes lysosomal exocytosis, the levels of the lysosomal enzyme β-hexosaminidase in supernatants of cultured microglia were measured. We found a significant increase in the levels of β-hexosaminidase 30 min after application of CCL2 as well as ionomycin (as a positive control known to cause lysosomal exocytosis) [25] (P < 0.05, Fig. 6b). These results suggest that CCL2 causes lysosomal exocytosis, which may result in enhancing P2X4R expression on the cell surface of microglia.
Fig. 6.
CCL2 causes lysosomal exocytosis in microglial cells. a Immunofluorescence for P2X4R (green) and LGP85 (red), a marker of lysosome, in primary cultured microglial cells treated with or without CCL2 (100 ng/mL) for 30 min. b The release of the lysosomal enzyme β-hexosaminidase from microglial cells was assayed by measuring its activity in the supernatants of microglial cultures collected at 30 min after CCL2 (100 ng/mL) or ionomycin (5 μM) stimulation. The experiments were repeated six times and the data are means ± SEM. *P < 0.05, **P < 0.01 compared with unstimulated control microglia. Scale bar: 10 μm
CCL2-stimulated microglia enhance the P2X4R-mediated response
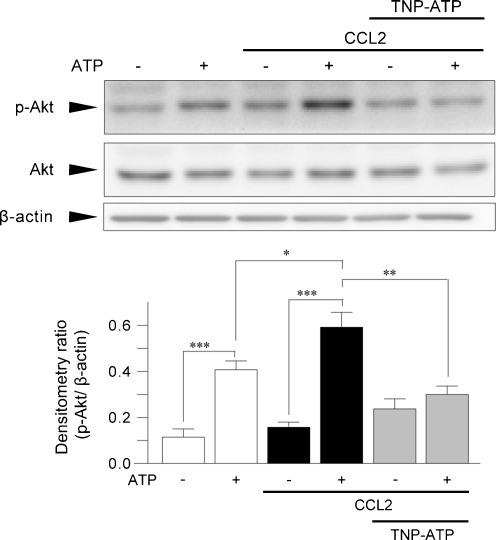
If CCL2 promotes P2X4R trafficking to the cell surface, then the P2X4R-mediated cellular response must be enhanced. To test this prediction, we measured phosphorylation of Akt evoked by ATP, a P2X4R-dependent response in microglia [15]. Consistent with previous results [15], ATP rapidly increased the level of phosphorylated Akt. In microglial cells that had been treated with CCL2, ATP stimulation further increased Akt phosphorylation as compared with untreated microglia with ATP stimulation (P < 0.05, Fig. 7). The enhancement of ATP-induced Akt phosphorylation in CCL2-treated microglia was suppressed by TNP-ATP (P < 0.01, Fig. 7), which can block P2X4R [6]. These results suggest that CCL2-stimulated microglia show an enhanced in P2X4R-mediated cellular response.
Fig. 7.
CCL2 enhances P2X4R-mediated Akt phosphorylation in microglial cells. Primary cultured microglia were treated with or without CCL2 (100 ng/mL) for 30 min. Western blot analysis of the levels of phosphorylated and total Akt protein in whole-cell lysate of CCL2-treated microglia 10 min after ATP (5 μM) stimulation. Cells were pretreated with TNP-ATP (100 μM) 5 min before ATP stimulation. A histogram of the relative band density ratio of Akt phosphorylation normalized to the levels of β-actin. The experiments were repeated five times, and the data are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
A rapidly growing body of evidence indicates that expression of the purinergic ionotropic receptor P2X4Rs is dramatically increased in activated microglia in animal models of neuropathic pain [6, 39] and other CNS diseases [7–12] and implicated in the disease processes [6, 13, 39, 40]. P2X4R trafficking to the plasma membrane must be a critical event for sensing extracellular ATP. Despite evidence that microglial activity is influenced by the extracellular milieu [1, 26] and might modulate P2X4R trafficking [25, 41, 42], the bioactive factors that control this trafficking remain unknown. Here, we identify, for the first time, CCL2 as a potential candidate. In the present study, we found that CCL2 enhanced the expression of P2X4R protein on the cell surface of cultured microglia without affecting total cellular levels of P2X4R protein. By showing that the effect was prevented by the CCR2 antagonist RS-504393 and mimicked by another CCR2 agonist, CCL12, we provide evidence that CCR2 mediates this effect of CCL2. Enhanced surface P2X4R expression induced by fibronectin in a manner that depends on CCL2 implies that P2X4R expression on the cell surface is also promoted by endogenous CCL2. We further found that 30 min after CCL2 stimulation, when the levels of P2X4R on the cell surface peaked, the subcellular distribution of P2X4Rs within microglial cells was changed, and the movement of P2X4R protein was also increased. Moreover, P2X4R-mediated Akt phosphorylation was upregulated in microglia 30 min after CCL2 stimulation. These results all are consistent with our view that CCL2 is an enhancer of P2X4R trafficking to the surface of microglial cells and suggest that CCR2 activation may render microglial cells hyper-responsive to extracellular ATP by enhancing P2X4R expression on their cell surfaces.
Although the concentrations of CCL2 to enhance P2X4R protein on the cell surface of microglia (Fig. 1) are similar to the EC50 of CCL2 for activation of CCR2 [18], the concentrations of CCL2 used in this study are higher than those of CCL2 measured in supernatant of spinal cord slices taken from nerve-injured animals [43]. Similarly, CCL2 concentration in supernatant of cultured microglia stimulated by fibronectin (Fig. 3b) was lower than that of CCL2 directly applied to enhance surface P2X4R protein (Fig. 1). Given that CCL2 released from microglia might act to the microglia itself in an autocrine manner or to neighboring microglial cells in a paracrine manner, it is possible that the local concentration of CCL2 around the microglial cells may be enough to enhance P2X4R expression on the cell surface. However, to clarify, this issue is a general technical problem because the concentration of CCL2 nearby microglial cells and the CCL2 concentration in the parenchyma of the spinal cord are difficult to quantify.
By showing an increase in P2X4R expression on the cell surface induced by the dynamin inhibitor dynasore, we confirmed a dynamin-dependent constitutive internalization of P2X4Rs in microglia [19]. However, CCL2 enhanced P2X4R protein expression on the cell surface in dynasore-pretreated microglial cells, the magnitude of which was comparable to that in control microglial cells. If CCL2 had promoted P2X4R expression on the surface by inhibiting P2X4R internalization, the effect of CCL2 would have been suppressed in microglia treated with dynasore. Therefore, the enhancing effect of CCL2 may not be associated with this process.
Recent studies have revealed that lysosomes are an organelle that pools intracellular P2X4R protein [25, 44]. In the primary cultured microglia we used, lysosomal localization of intracellular P2X4R protein was observed. In the present study, we found that CCL2 stimulation resulted in a diffused distribution of lysosomes within microglia at 30 min post-stimulation in parallel with the change in P2X4R protein distribution. Furthermore, CCL2 caused a release of the lysosomal protein β-hexosaminidase that also occurred 30 min later, as did ionomycin, which causes lysosomal exocytosis [25]. These results together suggest that CCL2 induces lysosomal exocytosis and subsequently results in a delivery of lysosomal P2X4R protein to the surface of microglia.
CCR2 is coupled with Gi-protein, which inhibits AC and reduces cAMP levels. The AC activator forskolin was effective in preventing CCL2-induced surface P2X4R expression and the change in P2X4R distribution, raising the possibility that the effect of CCL2 on trafficking of P2X4R-containing lysosomes might involve the Gαi/AC/cAMP pathway downstream of CCR2. Intracellular Ca2+ signaling is known to be crucial for fusion of lysosomes with the plasma membrane and/or phagosomes [45, 46]. Ionomycin induced release of the lysosomal enzyme and P2X4R expression on the cell surface of microglia [25]. It is thus possible that a mobilization of intracellular Ca2+, presumably through Gβγ subunit-mediated phospholipase C activation [45, 47–49], may be involved cooperatively with the Gαi/AC/cAMP pathway in CCL2-induced P2X4R trafficking to the cell surface. Identification of the intracellular signaling pathway from CCR2 to P2X4R trafficking will be an important issue that needs to be investigated in future studies. It is interesting to note that cell surface P2X4R expression was not promoted by CX3CL1 whose receptor, CX3CR1, is also coupled with the Gαi/AC/cAMP pathway. While several signaling pathways downstream of CCR2 have been demonstrated [50], a recent study has identified FROUNT as a protein that interacts with CCR2 and its functions [51]. Although it remains unknown whether FROUNT is expressed in microglia and whether FROUNT is involved in CCL2-induced P2X4R trafficking, CCR2 may utilize such unique molecules that closely interact with the P2X4R trafficking pathway.
The in vivo relevance of the interaction between CCR2 and P2X4R in regulating cell surface P2X4R expression in microglia remains to be elucidated. In models of diverse neuropathology, such as brain tumor, ischemia, and neuropathic pain, expression of both P2X4R and CCR2 is observed in microglia [6–8, 23, 52–56]. In particular, CCR2 critically contributes to the maintenance of neuropathic pain [55, 57]. Similar to a reversal effect of an acute pharmacological blockade of P2X4Rs on nerve injury-induced pain hypersensitivity [6], a recent paper using AZ889, a newly developed CCR2 antagonist, reverses both mechanical hypersensitivity in nerve-injured rats and innocuous brush-evoked activity of dorsal horn neurons in vivo [49]. These findings imply the existence of ongoing activation of P2X4R and CCR2 in microglia under neuropathic pain conditions. It is thus conceivable that ongoing activation of CCR2, probably activated by CCL2, may tonically promote P2X4R protein trafficking to the cell surface of microglia, which may be crucial for activating these cells by extracellular ATP and for maintaining neuropathic pain.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M.T., K.I.) and from the Core-to-Core program “Integrated Action Initiatives” of the Japan Society for the Promotion of Science (JSPS) (M.T., K.I.).
Footnotes
E. Toyomitsu and M. Tsuda are equally contributed to this work.
References
- 1.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 2.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 3.Prinz M, Mildner A. Microglia in the CNS: immigrants from another world. Glia. 2011;59:177–187. doi: 10.1002/glia.21104. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Koizumi S, Tsuda M. The role of nucleotides in the neuron–glia communication responsible for the brain functions. J Neurochem. 2007;102:1447–1458. doi: 10.1111/j.1471-4159.2007.04824.x. [DOI] [PubMed] [Google Scholar]
- 5.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 7.Cavaliere F, Florenzano F, Amadio S, et al. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. doi: 10.1016/S0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 8.Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor in rat C6 glioma by tumor-associated macrophages and activated microglia. J Neuroimmunol. 2004;152:67–72. doi: 10.1016/j.jneuroim.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol. 2005;163:120–127. doi: 10.1016/j.jneuroim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Zhang Z, Artelt M, et al. Dexamethasone attenuates early expression of three molecules associated with microglia/macrophages activation following rat traumatic brain injury. Acta Neuropathol. 2007;113:675–682. doi: 10.1007/s00401-007-0195-8. [DOI] [PubMed] [Google Scholar]
- 11.Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol. 2005;163:185–189. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Zhang ZY, Fauser U, et al. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152:495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Ohsawa K, Irino Y, Nakamura Y, et al. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 16.Ohsawa K, Irino Y, Sanagi T, et al. P2Y12 receptor-mediated integrin-beta1 activation regulates microglial process extension induced by ATP. Glia. 2010;58:790–801. doi: 10.1002/glia.20963. [DOI] [PubMed] [Google Scholar]
- 17.Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- 18.Charo IF, Myers SJ, Herman A, et al. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boumechache M, Masin M, Edwardson JM, et al. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284:13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuda M, Masuda T, Kitano J, et al. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasu-Tada K, Koizumi S, Tsuda M, et al. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia. 2006;53:769–775. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda M, Toyomitsu E, Kometani M, et al. Mechanisms underlying fibronectin-induced up-regulation of P2X4R expression in microglia: distinct roles of PI3K-Akt and MEK-ERK signalling pathways. J Cell Mol Med. 2009;13:3251–3259. doi: 10.1111/j.1582-4934.2009.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda M, Toyomitsu E, Komatsu T, et al. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M, Tozaki-Saitoh H, Masuda T, et al. Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi OS, Paramasivam A, Yu JC, et al. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120:3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz M, Butovsky O, Bruck W, et al. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima K, Shimojo M, Hamanoue M, et al. Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem. 1992;58:1401–1408. doi: 10.1111/j.1471-4159.1992.tb11356.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuronita T, Eskelinen EL, Fujita H, et al. A role for the lysosomal membrane protein LGP85 in the biogenesis and maintenance of endosomal and lysosomal morphology. J Cell Sci. 2002;115:4117–4131. doi: 10.1242/jcs.00075. [DOI] [PubMed] [Google Scholar]
- 29.Mirzadegan T, Diehl F, Ebi B, et al. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275:25562–25571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- 30.Nanki T, Nagasaka K, Hayashida K, et al. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol. 2001;167:5381–5385. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- 31.Jimenez-Sainz MC, Fast B, Mayor F, Jr, et al. Signaling pathways for monocyte chemoattractant protein 1-mediated extracellular signal-regulated kinase activation. Mol Pharmacol. 2003;64:773–782. doi: 10.1124/mol.64.3.773. [DOI] [PubMed] [Google Scholar]
- 32.Myers SJ, Wong LM, Charo IF. Signal transduction and ligand specificity of the human monocyte chemoattractant protein-1 receptor in transfected embryonic kidney cells. J Biol Chem. 1995;270:5786–5792. doi: 10.1074/jbc.270.43.25651. [DOI] [PubMed] [Google Scholar]
- 33.Wong LM, Myers SJ, Tsou CL, et al. Organization and differential expression of the human monocyte chemoattractant protein 1 receptor gene. Evidence for the role of the carboxyl-terminal tail in receptor trafficking. J Biol Chem. 1997;272:1038–1045. doi: 10.1074/jbc.272.2.1038. [DOI] [PubMed] [Google Scholar]
- 34.Sarafi MN, Garcia-Zepeda EA, MacLean JA, et al. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. J Exp Med. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- 36.Royle SJ, Qureshi OS, Bobanovic LK, et al. Non-canonical YXXGPhi endocytic motifs: recognition by AP2 and preferential utilization in P2X4 receptors. J Cell Sci. 2005;118:3073–3080. doi: 10.1242/jcs.02451. [DOI] [PubMed] [Google Scholar]
- 37.Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–4824. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–145. doi: 10.1016/S0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 39.Ulmann L, Hatcher JP, Hughes JP, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 41.Stokes L, Surprenant A. Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol. 2009;39:986–995. doi: 10.1002/eji.200838818. [DOI] [PubMed] [Google Scholar]
- 42.Toulme E, Garcia A, Samways D, et al. P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J Gen Physiol. 2010;135:333–353. doi: 10.1085/jgp.200910336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steenwinckel J, Reaux-Le Goazigo A, Pommier B, et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci. 2011;31:5865–5875. doi: 10.1523/JNEUROSCI.5986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murrell-Lagnado RD, Qureshi OS. Assembly and trafficking of P2X purinergic receptors (review) Mol Membr Biol. 2008;25:321–331. doi: 10.1080/09687680802050385. [DOI] [PubMed] [Google Scholar]
- 45.Colvin RA, Means TK, Diefenbach TJ, et al. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol. 2010;11:495–502. doi: 10.1038/ni.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czibener C, Sherer NM, Becker SM, et al. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol. 2006;174:997–1007. doi: 10.1083/jcb.200605004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 48.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serrano A, Pare M, McIntosh F, et al. Blocking spinal CCR2 with AZ889 reversed hyperalgesia in a model of neuropathic pain. Mol Pain. 2010;6:90. doi: 10.1186/1744-8069-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Old EA, Malcangio M (2011) Chemokine mediated neuron-glia communication and aberrant signalling in neuropathic pain states. Curr Opin Pharmacol (in press) [DOI] [PubMed]
- 51.Terashima Y, Onai N, Murai M, et al. Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nat Immunol. 2005;6:827–835. doi: 10.1038/ni1222. [DOI] [PubMed] [Google Scholar]
- 52.Schilling M, Strecker JK, Ringelstein EB, et al. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res. 2009;1289:79–84. doi: 10.1016/j.brainres.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 53.Galasso JM, Stegman LD, Blaivas M, et al. Experimental gliosarcoma induces chemokine receptor expression in rat brain. Exp Neurol. 2000;161:85–95. doi: 10.1006/exnr.1999.7249. [DOI] [PubMed] [Google Scholar]
- 54.Abbadie C, Bhangoo S, Koninck Y, et al. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbadie C, Lindia JA, Cumiskey AM, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wixey JA, Reinebrant HE, Carty ML, et al. Delayed P2X4R expression after hypoxia-ischemia is associated with microglia in the immature rat brain. J Neuroimmunol. 2009;212:35–43. doi: 10.1016/j.jneuroim.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Shi XQ, Echeverry S, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]