Abstract
Statins have both cholesterol lowering and anti-inflammatory activities, whether mechanisms underlying their activities are independent remains unclear. The ATP-gated P2X4 receptor is a pro-inflammatory mediator. Here, we investigate the action of fluvastatin and other cholesterol depleting agents on native and recombinant human P2X4 receptor. Fluvastatin and mβCD suppressed P2X4-dependent calcium influx in THP-1 monocytes, without affecting P2Y receptor responses. mβCD or filipin III suppressed the current density of recombinant human P2X4 receptors. Human P2X2 was insensitive to cholesterol depletion. Cholesterol depletion had no effect on intrinsic P2X4 receptor properties as judged by ATP concentration–response relationship, receptor rundown or current decay during agonist occupancy. These data suggest fluvastatin suppresses P2X4 activity in monocytes through cholesterol depletion and not by modulating intrinsic channel properties.
Keywords: ATP, P2X receptor, Statin, Inflammation, Cholesterol
Introduction
Statins are the most effective treatment for artherosclerosis and lowering cardiovascular risk [1]. Statins reduce cellular cholesterol through the inhibition of HMG-CoA reductase [1], though a second anti-inflammatory action is thought to contribute to their effectiveness as anti-atherosclerosis agents. Mechanisms of anti-inflammatory action by statins remains poorly described [2, 3]. Monocytes are major participants in the progression of atherosclerosis [4]. Monocyte recruitment and retention at sites of lesion contribute to plague development by direct secretion of pro-inflammatory molecules and monocyte maturation into macrophage and dendritic cells.
Signalling via extracellular ATP is important in the induction and resolution of inflammation. P2X receptors are a family of ATP-activated calcium channel, of which seven human receptor subunits (P2X1–7) have been cloned and characterised [5]. P2X4 is highly expressed in peripheral myeloid cells [6–8]. P2X4 expression is up-regulated in microglia following nerve damage [9, 10]. More recently, activation of P2X4 was demonstrated to mediate PGE2 release in macrophage [10]. In light of the pro-inflammatory action of P2X4, this study sought to investigate whether P2X4 is a target for fluvastatin and other cholesterol-depleting agents.
Materials and methods
Cell culture and transfection
Human THP-1 cells were cultured in RPMI medium containing 10% foetal calf serum. HEK293 cells were cultured in DMEM medium with 10% foetal calf serum at 37°C with 5% CO2 in a humidified incubator. For electrophysiology, cells were transfected with 1 μg plasmid-encoded human P2X4 with a C-terminal glu-glu tag or human P2X2 (both kind gifts from Prof. R. Alan North FRS, University of Manchester). Cells were co-transfected with 0.1 μg plasmid expressing GFP alone to allow identification of transfected cells.
Intracellular calcium measurements
Cells were pre-incubated with fura-2 AM for 1 h at 37°C followed by a 0.5-h wash at room temperature. Human THP-1cells were centrifuged in the plate at 2 × 105 cells/well. Measurements were made at room temperature on a 96-well plate reader (FlexStation, Molecular Devices). The change (∆) in intracellular calcium (Ca2+i) concentration is indicated as the ratio of fura-2 emission intensities for 340- and 380-nm excitation (F ratio). Wells within columns of the 96-well plate were loaded alternately for test and control conditions. The recording solution contained (millimolars): 130 NaCl, 5 KCl, 8 d-glucose, 10 HEPES and 1.2 MgCl2, titrated to pH 7.4 with NaOH. Ca2+-free extracellular solution (0 Ca2+) was prepared by excluding CaCl2. Cells were pretreated with the reagents for 0.5 h during the washing period of the fura-2 before Ca2+ measurements at room temperature.
Electrophysiology
Whole-cell or perforated patch recordings were made 48 following plasmid transfection. HEK293 cells were seeded onto glass covers and allowed to adhere for at least 1 h prior to recording. The extracellular solution contained (millimolars): 145 NaCl, 2 KCl, 2 CaCl2, 1 MgCl2, 13 d-glucose and 10 HEPES, pH 7.3. The intracellular pipette solution contained (millimolars): 145 NaCl, 10 EGTA and 10 HEPES, pH 7.3. The pipette solution was supplemented with 120 μg/mL amphotericin for perforated patch recordings; perforation typically occurred 5 mins after gigaseal formation. Pipettes had resistance of 3–5 MΩ. ATP was applied using an RSC 160 rapid solution changer (Biological Science Instruments, Intracell). ATP was applied for 2 s at 2-min intervals and cells were clamped at −60 mV. HEK293 cell treatment with cholesterol depleting agents was at 37°C.
Quantitative mRNA analysis
Total RNA was extracted from control and fluvastatin-treated cells using TriReagent (Sigma). First-strand cDNA was synthesised using Maxima Reverse Transcriptase (Fermentas) and Oligo(dT)18 priming. cDNA was amplified using sense and anti-sense oligonucleotide pairs selective for human P2X4 and GAPDH using an ABI Biosystems PCR Instrument. P2X4 mRNA abundance was expressed relative to GAPDH housekeeping gene.
Drugs and treatments
Fluvastatin sodium salt (Tocris) stock solutions were prepared to 10 mM in DMSO. For chronic treatment of monocytes, THP-1 cultures (<1 × 106 cells/mL) were supplemented with 10 μM fluvastatin or an equivalent volume of DMSO for 48 h prior to experimentation. For shorter treatments, 10 μM fluvastatin was applied 1 h prior to experimentation. Methyl-β-cyclodextrin (Sigma) was prepared in water and applied at 10 mM 1 h prior to experimentation.
Statistical analysis
Numerical data are expressed as mean ± S.E. Statistical significance was tested by Student’s t test and analysis of variance.
Results and discussion
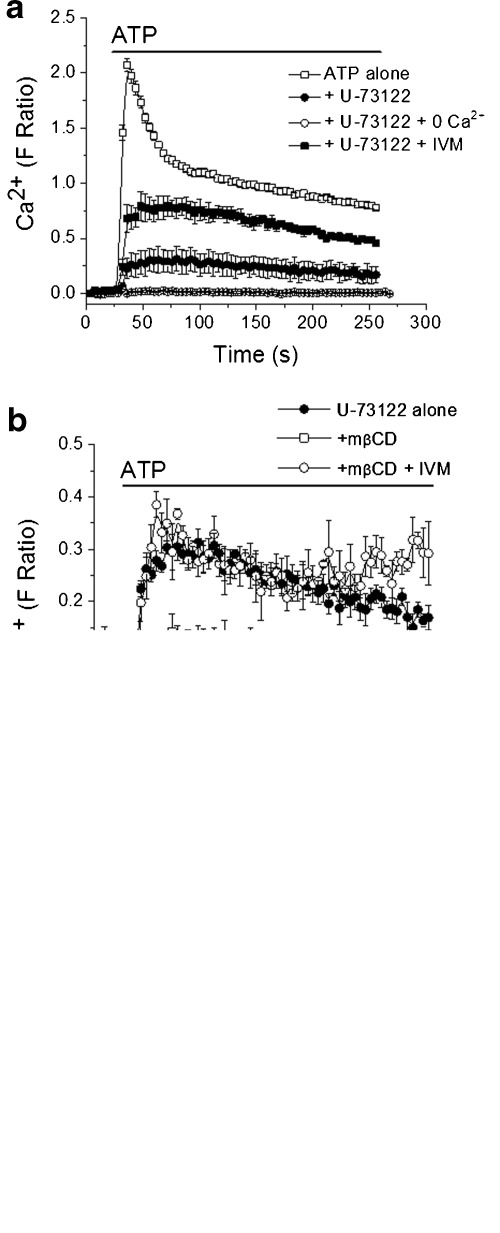
Application of ATP (100 μM) to fura-2 loaded THP-1 cells robustly increased [Ca2+]i (Fig. 1a). Intracellular calcium rapidly peaked then decayed to a sustained elevated phase (Fig. 1a). Pre-incubation with the phospholipase C inhibitor U-73122 (10 μM, 30 min) abolished the rapid peak in ATP-evoked in [Ca2+]i though a residual sustained calcium rise persisted (Fig. 1a). The U-73122-resistant calcium rise was not inhibited by the selective P2X7 antagonist AZ11645373 (1 μM, 30 min) (Fig. 1c). Moreover 100 μM ATP is sub-threshold for human P2X7 receptor activation. The peak residual calcium component was potentiated ∼2.5-fold by ivermectin (10 μM, 30 min) (Fig. 1a), a positive allosteric modulator of P2X4 [11, 12]. These data strongly suggest that P2X4 mediates the U-73122-resistant ATP-evoked calcium influx in THP-1 monocytes. P2X1, P2X4 and P2X7 mRNA transcripts have been detected in THP-1 monocytes though it is most unlikely that P2X1 would mediate a sustained calcium influx due to its rapid desensitisation. Functional P2X1 has been detected electrophysiologically in other inflammatory cell types [8, 13]. Pre-incubating monocytes with the cholesterol depleting agent mβCD suppressed the magnitude of P2X4-dependent calcium influx threefold (Fig. 1b). The suppressed P2X4-dependent calcium entry could be potentiated ∼2.5-fold by ivermectin (Fig. 1b and c). α-Cyclodextran, an inactive analogue, had no effect (data not shown). mβCD did not suppress the ATP stimulated calcium rise observed in the absence of U-73122 (Fig. 1c) suggesting that P2Y receptor-dependent calcium signalling is unaffected by cholesterol depletion.
Fig. 1.
Cholesterol depletion suppresses P2X4-dependent calcium entry in human monocytes. a Pharmacological isolation of P2X4-dependent calcium entry in THP-1 cells. Elevation in [Ca2+]i in response to 100 μM ATP is significantly inhibited by U-73122 though a resistant component remains. (N = 6). The resistant P2X4 component is abolished in the absence of extracellular calcium (0 Ca2+) and potentiated by ivermectin (IVM). b Treatment with the cholesterol depleting agent mβCD (10 mM, 1 h) reduces P2X4-dependent calcium entry (N = 6). c Mean peak calcium response data for 100 μM ATP summarising effect of cholesterol depletion of P2Y and P2X4-dependent calcium entry. (N = 4–6; ***p < 0.01 vs ATP with U-71322; #p < 0.01 vs ATP with U-71322 and mβCD) Note that the U-71322-resistant component is not sensitive to AZ11645373 (1 μM, 30 min), a selective P2X7 antagonist
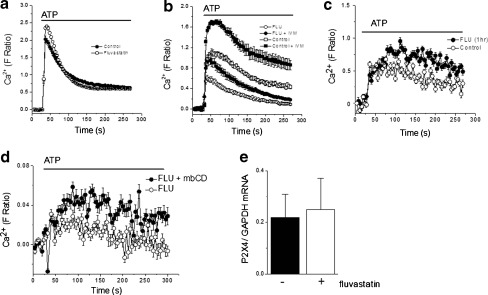
Next, we sought to determine whether clinically relevant cholesterol lowering drugs had a similar effect on P2X4-mediated calcium entry as acute chemical sequestration of cholesterol. THP-1 monocytes were cultured for 48 h with 10 μM fluvastatin to mimic clinical doses. As for mβCD treatment, fluvastatin treatment also had no effect on P2Y receptor-mediated calcium rises compared to vehicle controls (Fig. 2a). However, fluvastatin treatment suppressed peak P2X4-dependent calcium response by 2-fold compared to their vehicle control counterparts (Fig. 2b). Ivermectin was capable of potentiating suppressed P2X4-mediated calcium entry (Fig. 2b). The cholesterol depleting action of statins through HMG-CoA reductase inhibition takes many hours to manifest. To this end, we tested the effect of acute treatments of fluvastatin to rule out the possibility that fluvastatin inhibits P2X4-dependent Ca2+ entry via a different mechanism. Unlike 48-h treatment, 1-h treatment with 10 μM fluvastatin had no significant effect on P2X4-dependent Ca2+ entry (Fig. 2c) (N = 4; p < 0.05). Moreover, application of mβCD to cells treated with fluvastatin for 48 h showed no significant difference in P2X4-dependent Ca2+ entry compared to cells treated with fluvastatin alone (Fig. 2d). These data indicate that fluvastatin and mβCD suppress P2X4 receptor activity by the same mechanism. Furthermore, no significant difference was observed for the abundance of P2X4 mRNA in control monocytes or monocytes treated with fluvastatin for 48 h (Fig. 2e), suggesting fluvastatin does not reduce P2X4 activity through a transcriptional influence. These data suggest P2X4 activity is suppressed following depletion of cellular cholesterol.
Fig. 2.
Fluvastatin treatment inhibits P2X4-dependent calcium entry in human monocytes. a Comparison of P2Y receptor-mediated Ca2+ responses evoked by 100 μM ATP in control and fluvastatin-treated THP-1 cells (10 μM, 48 h) (N = 8). b Effect of fluvastatin (FLU) on the U-73122-resistant P2X4 component. Control cells are untreated. Ivermectin (IVM; 10 μM, 30 min) potentiates P2X4-dependent calcium entry in control and FLU-treated cells (N = 8). c No acute effect of FLU (10 μM, 1 hr) on P2X4-dependent calcium entry (N = 4). d No effect of mβCD (10 mM, 1 h) on P2X4-dependent Ca2 + -dependent entry in FLU-treated cells (10 μM, 48 h) (N = 3). e qRT-PCR analysis of P2X4 mRNA in naïve and fluvastatin-treated (10 μM, 48 h) monocytes. mRNA abundance is expressed relative to GAPDH housekeeper gene (N = 4)
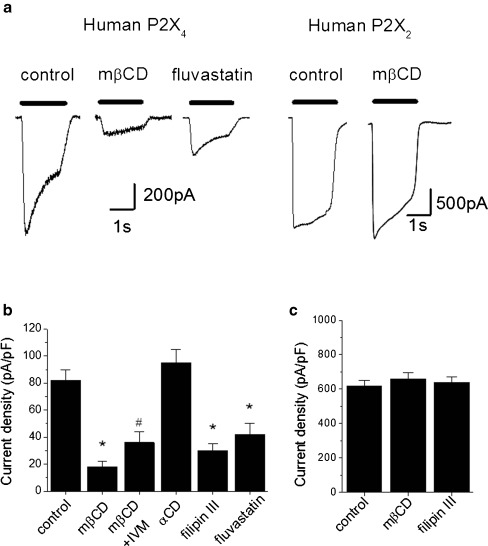
To investigate the mechanism further, we explored the effects of cholesterol depletion on human P2X4 heterologously expressed in HEK293T cells [12]. ATP (100 μM) evoked robust inward currents that moderately desensitised during agonist application (Fig. 3a). The mean whole-cell current density was 82 ± 8 pA/pF (N = 10 cells). To examine the influence of membrane cholesterol on human P2X4 activity, we employed mβCD and filipin III, two chemically unrelated cholesterol sequestering agents. Treatment of HEK293T cells with 10 mM mβCD for 1 h significantly reduced P2X4 current density compared to untreated cells (18 ± 4 pA/pF; N = 10, p < 0.01 vs control) (Fig. 3a). The effect was not mimicked by 10 mM mαCD (95 ± 10pA/pF; N = 8, p > 0.05 vs control) (Fig. 3b). Treatment with 10 μM filipin III for 30 min also significantly reduced P2X4 current density (30 ± 5 pA/pF; N = 10, p < 0.01 vs control) (Fig. 3b). Previous studies have revealed some selectivity of cholesterol depletion on P2X receptor function. As for THP-1 monocytes, ivermectin was able to potentiate P2X4 suppressed by mβCD (35 ± 8 pA/pF; N = 5, p < 0.01 vs control) (Fig. 3b). Forty-eight-hour treatment with fluvastatin (10 μM) significantly reduced P2X4 peak current density (Fig. 3a and b).
Fig. 3.
Effect of cholesterol-depleting agents on human P2X4 and P2X2 receptors in HEK293T cells. a Representative traces showing effect of mβCD treatment of P2X2 and P2X4 currents in HEK293 cells, and the effect of fluvastatin (10 μM, 48 h) on P2X4 currents. b Pooled averages demonstrated reduction in P2X4 current density following cholesterol depletion by methyl-β-cyclodextrin or filipin III, but not by αCD. Ivermectin (IVM; 10 μM, 30 min). Fluvastatin (10 μM, 48 h). N = 8; *p < 0.01 vs control, #p < 0.01 vs mβCD treatment. c P2X2 currents are unaffected cholesterol depletion, N = 8. All currents evoked by 100 μM ATP
Cholesterol depletion suppresses P2X1 function when expressed in HEK293 cells [14, 15] but not P2X2 or P2X3 [15]. Native P2X3 currents are also unaffected by cholesterol depletion [16]. Consistent with previous studies [15], ATP-evoked currents were unaffected by cholesterol depletion in HEK293T cells expressing human P2X2 receptor (Fig. 3a). ATP (100 μM) evoked an average current density of 620 ± 30 pA/pF (N = 9 cells) in control cells which was unaffected by mβCD (661 ± 30pA/pF, N = 8cells) or filipin III (640 ± 30pA/pF) (Fig. 3c). Our observations of the effect of cholesterol depletion on native and transiently expressed P2X4 receptor are in contrast to those of Allsopp et al. (2010) who employed stable P2X4 HEK293 cells. A main difference between this study and that of Allsopp et al. (2010) is that electrophysiological recordings have been made using the perforated patch configuration. Statins may therefore impinge on intracellular factors that regulate P2X4R function and/or trafficking which are dialysed out during whole-cell recordings. Indeed, patch perforation minimises the extensive rundown of P2X4R activity observed in whole-cell recordings [12]. The data presented here suggests that the effect of cholesterol depletion is consistent between native and recombinant human P2X4 receptors.
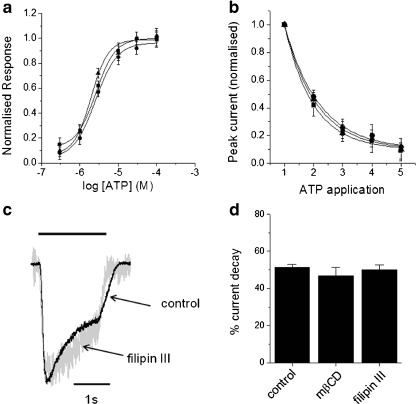
P2X receptor current density is governed by multiple factors including responsiveness to ATP, receptor desensitisation and receptor number. ATP concentration–response curves were not significantly different between control and cholesterol depleted cells (Fig. 4a). The EC50 value for control cells was 2.5 ± 1.2 μM (N = 10 cells), comparable to previous studies [17], and 2.1 ± 2.0 μM and 2.2 ± 1.4 μM for mβCD and filipin III-treated cells, respectively (both N = 10 cells; p > 0.05 vs control). Responses were recorded using the perforated patch configuration to minimise receptor rundown [12]. The effects of cholesterol depletion on two modes of receptor desensitisation were also tested. The human P2X4 receptor runs down with repeated agonist application [12]. In control cells, repeated application of 100 μM ATP at 2 min intervals was marked with an exponential decay in peak whole-cell current (τ = 1.19 ± 0.05; N = 8). The rate of rundown was unaffected by cholesterol depletion; τ = 1.19 ± 0.08 and 1.10 ± 0.08 (N = 8 cells each, p > 0.05 vs control) for mβCD and filipin III-treated cells, respectively (Fig. 4b). Human P2X4 currents decayed by 51.5 ± 1.5% (N = 8 cells) during application of 100 μM ATP (Fig. 4c). Again, cholesterol depletion had no effect on the magnitude of current decay; 46.9 ± 4.3% and 50.2 ± 2.5% (N = 8 cells each; p > 0.05 vs control) for mβCD and filipin III-treated cells, respectively (Fig. 4c and d). These data support the notion that P2X4 current density and receptor desensitisation during agonist occupancy are independent [12].
Fig. 4.
Influence of cholesterol depletion of intrinsic human P2X4 receptor properties. a Concentration–response relationships for ATP in control cells (squares), methyl-β-cyclodextrin (triangles) or filipin III (circles) treated cells (N = 10 cells each). Whole-cell currents in perforated patch configuration normalised to maximum response. b Rundown of peak current with repeated ATP application in control (squares), methyl-β-cyclodextrin (triangles) or filipin III (circles) treated cells (N = 8 cells each). Peak currents are normalised to peak after first application. c Representative current traces showing current decay in control and fillipin III-treated cells. Currents are normalised to peak response to 100 μM ATP. d Pooled average demonstrating current decay during agonist occupancy is independent of membrane cholesterol (N = 8 cells each)
In summary, this study suggests that fluvastatin inhibits P2X4 through its cholesterol depleting activity, as fluvastatin, mβCD and filipin III produce the same outcome. The mechanism underlying suppression of P2X4 does not involve modulation of intrinsic channel properties and is therefore likely to be a consequence of interfering in mechanisms controlling receptor trafficking or regulation by signal transduction or protein–protein interaction. Disruption of lipid raft-dependent signalling is a possible mechanism of action of fluvastatin. The existence of P2X4 in lipid rafts has been demonstrated in epithelia [18] and HEK293 cells [15]. Indeed, fluvastatin inhibits raft-dependent signalling in monocytes [19].
Acknowledgements
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC). SJF is generously supported by a BBSRC David Phillips Fellowship Award.
Glossary
- MβCD
Methyl-β-cyclodextrin
References
- 1.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Scalia R, et al. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apolipoprotein E-deficient mice. Circulation. 2001;103:2598–2603. doi: 10.1161/01.cir.103.21.2598. [DOI] [PubMed] [Google Scholar]
- 3.Pruefer D, et al. Simvastatin inhibits inflammatory properties of Staphylococcus aureus α-toxin. Circulation. 2002;106:2104–2110. doi: 10.1161/01.CIR.0000034048.38910.91. [DOI] [PubMed] [Google Scholar]
- 4.Østerud B, BjÈ-Rklid E. Role of monocytes in atherogenesis. Physiol Rev. 2003;83:1069–1112. doi: 10.1152/physrev.00005.2003. [DOI] [PubMed] [Google Scholar]
- 5.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi OS, Paramasivam A, Yu JCH, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120:3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 8.Sim JA, Park CK, Oh SB, Evans RJ, North RA. P2X1 and P2X4 receptor currents in mouse macrophages. Br J Pharmacol. 2007;152:1283–1290. doi: 10.1038/sj.bjp.0707504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 10.Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281:15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- 13.Wareham K, Vial C, Wykes RCE, Bradding P, Seward EP. Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells. Br J Pharmacol. 2009;157:1215–1224. doi: 10.1111/j.1476-5381.2009.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem. 2005;280:30705–30711. doi: 10.1074/jbc.M504256200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allsopp RC, Lalo U, Evans RJ. Lipid raft association and cholesterol sensitivity of P2X1-4 receptors for ATP. J Biol Chem. 2010;285:32770–32777. doi: 10.1074/jbc.M110.148940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Huang W, Wu D, Priestley JV. TRPV1, but not P2X3, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci. 2006;24:1–6. doi: 10.1111/j.1460-9568.2006.04889.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones CA, Chessell IP, Simon J, Barnard EA, Miller KJ, Michel AD, Humphrey PPA. Functional characterization of the P2X4 receptor orthologues. Br J Pharmacol. 2000;129:388–394. doi: 10.1038/sj.bjp.0703059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barth K, Weinhold K, Guenther A, Linge A, Gereke M, Kasper M. Characterization of the molecular interaction between caveolin-1 and the P2X receptors 4 and 7 in E10 mouse lung alveolar epithelial cells. Int J Biochem Cell Biol. 2008;40:2230–2239. doi: 10.1016/j.biocel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Hillyard DZ, Jardine AG, McDonald KJ, Cameron AJM. Fluvastatin inhibits raft dependent Fc[gamma] receptor signalling in human monocytes. Atherosclerosis. 2004;172:219–228. doi: 10.1016/j.atherosclerosis.2003.11.004. [DOI] [PubMed] [Google Scholar]