Abstract
The clinical management of neuroendocrine tumours is complex. Such tumours are highly vascular suggesting tumour-related angiogenesis. Adenosine, released during cellular stress, damage and hypoxia, is a major regulator of angiogenesis. Herein, we describe the expression and function of adenosine receptors (A1, A2A, A2B and A3) in neuroendocrine tumours. Expression of adenosine receptors was investigated in archival human neuroendocrine tumour sections and in two human tumour cell lines, BON-1 (pancreatic) and KRJ-I (intestinal). Their function, with respect to growth and chromogranin A secretion was carried out in vitro. Immunocytochemical data showed that A2A and A2B receptors were strongly expressed in 15/15 and 13/18 archival tumour sections. Staining for A1 (4/18) and A3 (6/18) receptors was either very weak or absent. In vitro data showed that adenosine stimulated a three- to fourfold increase in cAMP levels in BON-1 and KRJ-1 cells. The non-selective adenosine receptor agonist (adenosine-5′N-ethylcarboxamide, NECA) and the A2AR agonist (CGS21680) stimulated cell proliferation by up to 20–40% which was attenuated by A2B (PSB603 and MRS1754) and A2A (SCH442416) receptor selective antagonists but not by the A1 receptor antagonist (PSB36). Adenosine and NECA stimulated a twofold increase in chromogranin A secretion in BON-1 cells. Our data suggest that neuroendocrine tumours predominantly express A2A and A2B adenosine receptors; their activation leads to increased proliferation and secretion of chromogranin A. Targeting adenosine signal pathways, specifically inhibition of A2 receptors, may thus be a useful addition to the therapeutic management of neuroendocrine tumours.
Keywords: Adenosine, A2A adenosine receptor, A2B adenosine receptor, Human neuroendocrine tumours, Cell proliferation, Chromogranin A
Introduction
Neuroendocrine tumours (NETs) are a heterogeneous group of neoplasms of unknown aetiology which vary broadly both in their clinical presentation and biological behaviour. Such tumours are widely distributed and originate, as their name suggests, from neuroendocrine cells throughout the body. These tumours are often characterised by excessive secretion of hormones and biogenic amines. In a local setting, surgery is the treatment of choice for metastatic disease and clinical management is aimed at reducing hormone-associated symptoms. The mainstay of treatment for patients with metastatic disease consists of long-acting somatostatin analogues which improve quality of life by controlling symptoms related to hormonal hypersecretion [1]. Of the somatostatin analogues, octreotide has been shown to stabilise tumour progression in some well-differentiated NETs [2] and SOM230 (which displays broader receptor affinity) possesses anti-angiogenic activity [3]. In vitro experiments also show that somatostatin can induce morphological changes in neuroendocrine cells [4]. However, with these treatments there is virtually no tumour shrinkage in the majority of patients with metastatic disease.
The increased vascularity of endocrine tumours [5] suggests that agents targeting vascular endothelial growth factor (VEGF) and other angiogenic pathways might have value in the treatment of patients with NETs. VEGF levels in NET tissue show an inverse correlation with patient survival [6] suggesting that VEGF expression and the mediation of angiogenesis are associated with human NET growth. There are several clinical trials involving pharmaceutical agents (Bevacizumab and Sunitinib) that are aimed at targeting VEGF secretion or inhibition of the VEGF receptor [7]. Hypoxia is a major regulator of angiogenesis, mediated in part via up-regulation of hypoxia-inducible factor (HIF)-1 α [8]. The purine nucleoside adenosine, released from hypoxic tissues, appears to be a key mediator in this pathway, accounting for up to 70% of hypoxia-induced angiogenesis in some situations [9–11]. Adenosine mediates its actions through four adenosine receptors, A1, A2A, A2B and A3, which are differentially expressed in different tissues [12]. Therapeutic modulation of adenosine levels or signalling could therefore provide a basis for the treatment of many cancers [13–15]. In this study, we show that A2A and A2B receptors are the dominant adenosine receptors in human NET cell lines and in archival human NET tissue. Our in vitro data indicate that activation of these receptors can mediate cell proliferation and chromogranin A secretion. Thus the specific inhibition of A2 signal pathways may be useful for the therapeutic management of NETS.
Materials and methods
Materials
Cell culture materials and reagents were obtained from Lonza (Wokingham, UK), Biosera (Ringmer, UK), Fisher Scientific (Loughborough, UK), Invitrogen (Paisley, UK) and Sigma–Aldrich (Poole, UK). Specific polyclonal antisera to human A1 and A2A receptors were from Abcam (Cambridge, UK) and affinity-purified antisera to the A2B and A3 receptors were from Alpha Diagnostic International (San Antonio, USA). Adenosine receptor agonists and antagonists were obtained from Tocris (Avonmouth, UK). Vectastain Elite ABC kits (Vector Laboratories, Peterborough, UK) were used for immunocytochemistry. Molecular biology reagents, except TRIzol (from Invitrogen), were obtained from Promega, (Southampton, UK). Western blotting reagents were from GE Healthcare (Chalfont St. Giles, UK).
Adenosine receptor immunocytochemistry in human NETS
Eighteen representative blocks of NETs (eight ileocaecal, four pancreatic, four appendiceal, one ovarian and one liver metastasis) were retrieved from the histopathology tissue archives at the University Hospital of Cardiff, Wales. For this, ethical approval was obtained from the local research ethics committee (approval number 07/WSE02/1). H and E sections (haematoxylin and eosin) from each of these blocks were scanned using Mirax Scan (Carl Zeiss, Gottingen, Germany) and the digital images viewed by a trained pathologist to identify tumour bearing tissue. Two or three 1.5-mm tissue cores were punched out from each of these areas using a microarray device to prepare two tissue microarrays containing tissue cores from nine patients each. Serial sections were cut from each of the resulting tissue microarray blocks and immunostained for adenosine receptors using an automated Shandon Varistain Gemini programme (Fisher Scientific, Loughborough, UK) coupled to the DAKO Autostainer Plus (DAKO UK Ltd, Ely, Cambridgeshire, UK) procedure based on Vectastain ABC Elite kits (Vector Laboratories, Peterborough, UK). Staining was conducted using polyclonal antibodies to A1, A2A, A2B and A3 adenosine receptors at optimal dilutions of 1:200, 1:400, 5 μg/ml and 1:400, respectively, and applied for 1 h at room temperature. Sections were then incubated for 30 min in the appropriate second antibody coupled to biotin and then visualised with diaminobenzidine (5 min). Nuclei were counter-stained with haematoxylin. For negative controls, non-immune mouse serum (at 1:200 dilution) was used. Immunostaining was reviewed independently by two pathologists (JN and BJ) and scored to be either negative (0), weak (1+), moderate (2+) or strong (3+).
Cell culture
BON-1 cells (a generous gift from Dr. Ursula Plockinger, Interdisziplinares Stoffwechsel-Centrum, Charite-Universitatsmedizin Berlin, Campus Virchow-Klinikum, Berlin, Germany) were cultured in DMEM/Ham’s F12 (50% v/v) and 10% (v/v) FBS (foetal bovine serum). KRJ-I cells [16–18] were cultured in Ham’s F12 and 10% (v/v) FBS.
RT-PCR
Total cellular RNA was prepared using TRIzol reagent and treated with RQ1 ribonuclease-free deoxyribonuclease. RNA (0.2 μg) was reverse transcribed using oligodeoxythymidilic acid [oligo(dT)15] for 1 h at 37°C and the cDNA generated was subjected to PCR amplification using primers specific for human adenosine receptors. Primer sequences (shown in Table 1) were designed using the Primer 3 software programme and gene sequences obtained from GenBank. For the PCR reaction 30–40 cycles were carried out as follows: 94°C, 65°C and 72°C for 30 s, 1 min and 1 min, respectively, and a final extension step of 72°C for 10 min. Amplified products were electrophoresed in 2% (w/v) agarose and visualised with ethidium bromide.
Table 1.
PCR primer sequences and amplicon sizes
| Gene | Forward | Reverse | Base pairs, bps |
|---|---|---|---|
| A1R | GTCCTCATCCTCACCCAGAG | CAGATTGTTCCAGCCAAACA | 189 |
| A2aR | TCTTCAGTCTCCTGGCCATC | TCCAACCTAGCATGGGAGTC | 156 |
| A2bR | CTCCATCTTCAGCCTTCTGG | GGAATGGAGTCAATCCGATG | 152 |
| A3R | GGGCATCACAATCCACTTCT | AGGGCCAGCCATATTCTTCT | 171 |
| CD73 | ACCAGGGCACTATCTGGTTC | AGTGGCTCGATCAGTCCTTC | 133 |
| Adenosine deaminase | GACCCGCTCATCTTCAAGTC | GGTCGAGAAGCTCCCTCTTT | 151 |
| Adenosine kinase | TCACCCAAGGGAGAGATGAC | ATAGTGGCCAGCACGGATAC | 185 |
| Chromogranin A | AGAAACACAGCGGTTTTGAAGA | TCTGCCTCCTTGGAATCCTCT | 136 |
Quantitative (q) RT-PCR, which is much more sensitive than conventional PCR, was also used to assess adenosine receptor expression in BON-1 and KRJ-1 cells. qRT-PCR was performed on the MX3000P thermal cycler system (Stratagene, Cambridge, UK). The reaction mix was: 12.5 μl Platinum SYBR Green qPCR Supermix-UDG (Invitrogen), 1 μl cDNA, 1 μl (10 nmol) forward and reverse primers and 10.5 μL nuclease-free water. The thermal profile was 1 cycle (95°C 10 min), 40 cycles (95°C 30 s, 60°C 1 min, 72°C 30 s) and 1 cycle (95°C 1 min, 60°C 30 s, 95°C 30 s). All four genes were assessed in the same sample, performed in triplicate and in three separate experiments.
Western blotting analysis
Western blotting analysis was used to demonstrate expression of adenosine receptor (A1, A2A, A2B and A3) protein in BON-1 and KRJ-I cells. BON-1 monolayers and pelleted KRJ-I cells were lysed in 200 μl RIPA buffer containing 1 mM sodium orthovanadate, 0.1 mg/ml PMSF and chymostatin, leupeptin, antipain and pepstatin A (all at 10 μg/ml). Lysates after centrifugation were stored at −80°C. Aliquots (15 μl) from each treatment were mixed with an equal volume of electrophoresis buffer, boiled and electrophoresed in 10% (w/v) polyacrylamide. Proteins were transferred onto PVDF membranes and incubated overnight at 4°C with antisera to adenosine receptors at a concentration of 1:1,000, except for the A2A receptor (A2AR) antiserum which was used at 1:2,000. Secondary anti-rabbit IgG conjugated to horseradish peroxidase, at 1:5,000, was applied for 1 h at room temperature and proteins were visualised with ECL Plus reagent.
cAMP
BON-1 and KRJ-I cells were each plated out at a density of 0.1 × 106 cells/well into untreated (BON-1) 48-well multidishes or dishes that had been pre-coated with poly-D-lysine (70–150 Kd, 0.1 mg/ml) (KRJ-1). After 1–2 days in culture, cells (replicates × 4) were gently washed in serum-free culture medium and incubated for 15 min with adenosine, adenosine-5′N-ethylcarboxamide (NECA) and CGS21680 at a concentration range of 10 nM to 0.1 mM in the presence of 0.1 mM Ro 20-1724 (cyclic nucleotide phosphodiesterase inhibitor). Cells were solubilised in 0.2 ml 0.1 M HCl and stored at −20°C; the acid was removed by rotary vacuum evaporation and the residues dissolved in 0.5 ml cAMP assay buffer (50 mM sodium acetate, 0.25% w/v bovine serum albumin, pH 5.2). cAMP measurements were carried out as previously described [19].
Cell growth
Growth of BON-1 and KRJ-I cells was determined using the MTS proliferation assay kit from Promega (Southampton, UK). Briefly, BON-1 (2,000/well) and KRJ-I (25,000/well) cells were plated into 96-well multiwell plates in a volume of 0.05 ml, respectively, in DMEM/Ham’s F12 1% (v/v) FBS and Ham’s F12 1.25% (v/v) FBS. After an overnight incubation, replicate wells (×8) were treated with adenosine, NECA and CGS21680 at a concentration range 10 nM to 10 μM for 48 and 72 h. At the end of each time point, 0.02 ml MTS reagent was added and the formazan product measured by absorbance at 490 nm after 1 or 4 h (KRJ-I cells). A blank absorbance reading (culture medium alone) was subtracted from each value. The quantity of formazan produced is directly proportional to the number of living cells in culture. In some experiments, cells were incubated with adenosine receptor antagonists, PSB36 (A1), SCH442416 (A2A), PSB603 and MRS1754 (A2B), in the presence of agonist for 3 days.
Direct cell counting was also carried out by image cytometry using the Cellometer Auto T4 counter (Nexcelom Bioscience LLC, Lawrence, MA, USA). Images of cells in a disposable counting chamber (very similar to a haemocytometer) are taken and analysed using the attached software from which cell number per milliliter is calculated.
Chromogranin A
BON-1 cells (0.2 × 106 cells/well) were plated out into 24-well multidishes and allowed to become confluent. Replicate wells (×4) were treated with the AR agonists, adenosine, NECA and CGS21680 over the concentration range 10 nM to 0.1 mM for 1 h at 37°C in serum-free DMEM/Ham’sF12. Supernatants were collected and stored at −20°C prior to assay for chromogranin A using the radioimmunoassay kit from BioSource (Nivelles, Belgium), cat. no. KIPERB321. Similar experiments were carried out with KRJ-1 cells plated into poly-D-lysine-coated plates; these were left for 3 days and incubated with adenosine receptor agonists for 1–8 h.
Statistical analysis
Experiments were performed three to five times (n) with up to eight replicates for each treatment. Results were analysed with INSTAT 3 software (GraphPad Software, La Jolla, USA) and expressed as mean ± SEM and compared by ANOVA and the Tukey comparison test. P < 0.05 is deemed to be significant.
Results
Expression of adenosine receptors in archival human NETS
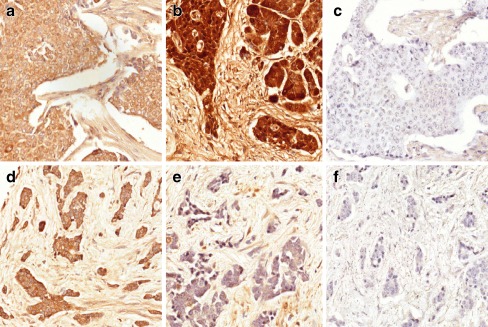
Adenosine receptor expression in human NETS patients was investigated with immunocytiochemistry on a limited number of archival samples. A summary of the staining is shown in Table 2. Staining for A1 and A3 receptors were either absent or only weakly positive and only in a minority (4/18 and 6/18, respectively) of the tumours. In contrast, the A2AR was expressed in all of the tumours tested (15/15, there was insufficient material in three sections) whilst the A2B receptor (A2BR) was positive in most (13/18) of the tumours; the staining intensity although variable was overall much stronger than that for the A1 and A3 receptors. In addition, tumours that were negative for A2BR were restricted to the small bowel where 4/5 tumours showed little or no staining, on the other hand, all of these tumours stained heavily for the A2AR. Figure 1a and b respectively show strong immunostaining for A2A and A2B receptors in a pancreatic tumour and strong A2AR but little or no A2BR staining in a small bowel tumour (Fig. 1d–e). There was little cellular staining in the absence of antisera as shown in Figure 1c and f. Closer examination of the slides also showed apparent staining for both A2A and A2B receptors in the connective tissue, this is likely to be non-specific staining as it was also apparent in the negative controls. The three staining images shown for each tumour type are from the same tumour. Archival placenta and stomach tissues were used as positive controls for adenosine receptors (not shown).
Table 2.
Summary of adenosine receptor immunostaining in archival human NETs. (staining intensity was graded 0 (absent), 1+ (weak), 2+ (moderate) and 3+ (strong)
| Receptor | Appendix | Midgut | Pancreas | Miscellaneous | Total | Staining grade |
|---|---|---|---|---|---|---|
| A1 | 1/4 | 1/8 | 1/4 | 1/2 | 4/18 | 0–1+ |
| A2A | 4/4 | 8/8 | 3/3 | 3 IMa | 15/15 | 1–3+ |
| A2B | 4/4 | 4/8 | 4/4 | 1/2 | 13/18 | 2–3+ |
| A3 | 2/4 | 1/8 | 2/4 | 1/2 | 6/18 | 0–1+ |
aInsufficient material for analysis
Fig. 1.
A2A and A2B receptor immunostaining in a human pancreatic and a small bowel tumour. Immunostaining for the A2AR (a and d), the A2BR (b and e) and with non-immune sera (c and f) in a pancreatic (a–c) and a small bowl tumour (d–f). Note the absence of cellular staining for the A2BR in e. Non-specific staining of connective tissue was also noted, even in control cells
Expression of adenosine receptors in BON-1 and KRJ-1 cells
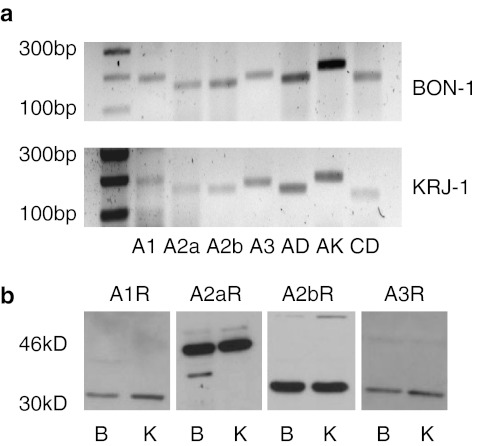
Adenosine receptor gene expression in BON-1 and KRJ-1 cells was determined with PCR, Fig. 2a shows that all four adenosine receptors were present in both cell lines together with adenosine deaminase, adenosine kinase and CD73 (ecto-5/-nucleotidase), enzymes that are involved in the direct synthesis and metabolism of adenosine. Expression of A1R and A3 receptor (A3R) in both KRJ-I and BON-1 cells appeared to be particularly weak and detection was only possible when the number of PCR cycles was increased to 40. DNA sequencing however did confirm their presence (data not shown).
Fig. 2.
Adenosine receptor and adenosine metabolic enzyme expression in BON-1 and KRJ-I cells. a RT-PCR for adenosine receptor and adenosine metabolic enzyme mRNA in KRJ-I and BON-1 cells, AD-adenosine deaminase, AK-adenosine kinase, CD-CD73 (ecto-5/-nucleotidase). The expected amplicon sizes are shown in Table 1 and the numbers on the left indicate the number of base pairs. b Western blotting analyses of adenosine receptors in BON-1 (B) and KRJ-1 (K) cells; the positions of the marker proteins are indicated
qRT-PCR was also used to confirm adenosine receptor gene expression; in the same cDNA sample cycle threshold (Ct) values for BON-1 cells and KRJ-I cells were, respectively, A1R 34 ± 2, 36 ± 3; A2AR 21 ± 2, 21 ± 1; A2BR 21 ± 2, 26 ± 2 and A3R 35 ± 3, 31 ± 3. The lower the Ct value, the higher the expression of mRNA and an increase of 1 equates to a halving of expression. Although it is not valid to compare Ct values from different genes, these data also indicate that A2AR and A2BR are the dominant receptors in BON-1 and KRJ-I cells. Western blotting analysis showed that the proteins for all four receptors were also expressed in both cell lines (Fig. 2b), although it is not possible to compare band intensities from different antisera, this data, as with gene expression, could also suggest that the A2AR and A2BR are the dominant receptors.
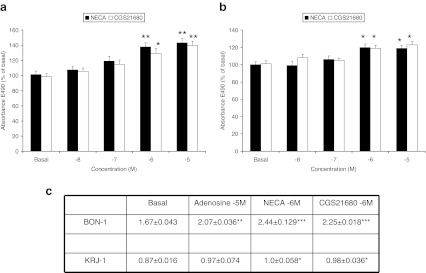
In order to confirm which receptors had the highest expression, we carried out functional cAMP measurements as A2 receptors primarily stimulate cAMP and A1 and A3 receptors inhibit cAMP. Figure 3 shows that adenosine, in a dose-related manner, stimulated endogenous cAMP levels in both KRJ-I cells (Fig. 3a) and BON-1 cells (Fig. 3b) by up to three- to fourfold and ED50 of 5–10 × 10−7 M. Interestingly, the stable non-selective adenosine receptor agonist, NECA, was most potent (up to sixfold) in BON-1 cells (Fig. 3b) whereas the selective A2AR agonist, CGS21680, was most potent in KRJ-1 cells (up to seven fold; Fig. 3a).
Fig. 3.
Effects of adenosine receptor agonists on intracellular cAMP accumulation in a KRJ-I and b BON-1 cells. Replicate cultures were incubated with adenosine receptor agonists and the phosphodiesterase inhibitor, Ro 20–1724 for 15 min; cells were extracted into HCl, vacuum dried and reconstituted in assay buffer for cAMP radioimmunoassay. Values shown are percentage changes (mean ± SEM) from five experiments with four replicates for each treatment. *P < 0.05, **P < 0.01, ***P < 0.001 when compared with untreated (basal) cells
Effects of adenosine on growth of BON-1 and KRJ-1 cells
Our data show that BON-1 and KRJ-1 cells express predominantly A2A and A2B receptors, we thus investigated if activation of these receptors can influence cell growth. Using the MTS proliferation assay, a single dose of NECA and CGS21680 and incubation for 3 days, stimulated growth of BON-1 and KRJ-I cells in a dose-related manner by 20–40% (Fig. 4a, b). The growth effects of NECA and CGS21680 were markedly greater in BON-1 than in KRJ-I cells. When the actual number of cells were physically counted similar results were obtained (20–45% stimulation in BON-1 cells and 10–15% in KRJ-I cells after three days of exposure to adenosine receptor agonists; Fig. 4c).
Fig. 4.
Growth effects of adenosine receptor agonists on BON-1 and KRJ-I cells as determined using the MTS proliferation assay and cell number counting. BON-1 (a) and KRJ-1 (b) cultures (eight replicates in duplicate) were incubated with varying concentrations of NECA or CGS21680 (single treatment) for 3 days; MTS solution was then added and the absorbance reading taken at 490 nm. Values shown are percentage changes (mean ± SEM) from three experiments. c BON-1 and KRJ-I cells were incubated with the indicated concentrations (single treatment) of adenosine receptor agonists for 3 days; cells were harvested and numbers counted with the Cellometer cell counter. Values shown are mean ± SEM × 106 of four experiments. *P < 0.05, **P < 0 < 0.01, ***P < 0 < 001 when compared with untreated (basal) cells
Selective adenosine receptor antagonists were used to confirm which of the receptors were involved in the mediation of growth; these experiments were carried out on BON-1 cells as these cells showed the largest changes in growth. When PSB603 (A2BR antagonist) was incubated along with NECA, the growth stimulating effects of the latter were attenuated in a dose-related fashion (Fig. 5a). SCH 442416 (A2AR antagonist) and PSB36 (A1R antagonist) at the same molar concentrations did not inhibit NECA-stimulated growth (Fig. 5a). We also used a second A2BR antagonist, MRS1754; this also attenuated NECA-stimulated growth in a dose-related manner (Fig. 5b). Although SCH 442416 failed to inhibit NECA-mediated growth of BON-1 cells, it did in fact reduce growth stimulation by the A2AR agonist, CGS21680 (Fig. 5c). These data together confirm that A2A and A2B receptors are both involved in adenosine-mediated cell growth. As these receptors act through cAMP we also showed that dibutyryl cAMP (115 ± 4% at 1 μM) and forskolin (140 ± 5% at 0.1 μM) stimulated cell growth in BON-1 cells, similar but less prominent data were seen in KRJ-1 cells.
Fig. 5.
Effect of adenosine receptor antagonists on NECA and CGS21680 mediated growth of BON-1 cells. BON-1 cells were incubated with NECA (a and b) or CGS21680 (c) for 3 days in the presence of varying concentrations of adenosine receptor antagonists: MRS1754 and PSB603 (A2BR antagonists), SCH442416 (A2AR antagonist) and PSB36 (A1R antagonist). Values shown are percentage changes (mean ± SEM) of three to four experiments. ***P < 0.001 when compared with untreated (basal) cells. #P < 0.05, ##P < 0.01 when compared with agonist only treated (0) cells
Effect of adenosine receptor agonists on chromogranin a secretion from BON-1 cells
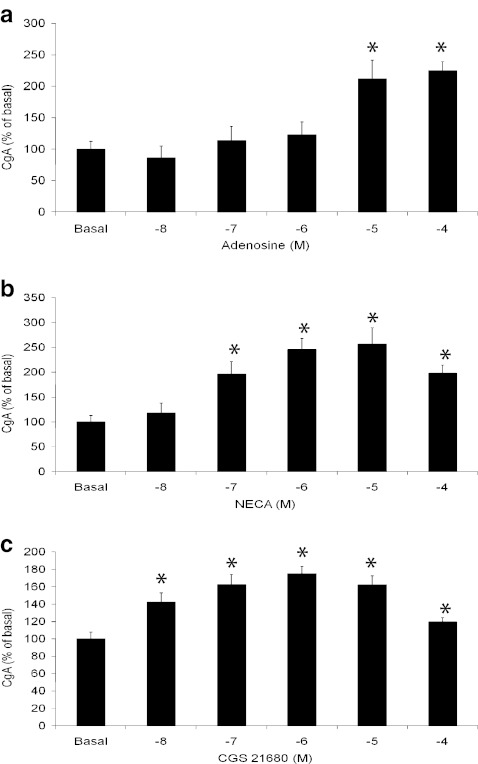
Adenosine, NECA and CGS21680 stimulated a twofold increase in chromogranin A secretion from BON-1 cells over a 1-h incubation period (Fig. 6a–c). As expected, NECA and CGS21680 were considerably more potent than adenosine itself and probably reflect the labile nature of adenosine and its short half-life in culture media. These data are consistent with the findings observed with cAMP and cell growth (Figs. 3 and 4). Chromogranin A however was not measurable in the conditioned media from KRJ-I cells (data not shown) even after 8-h exposure to agonists; the mRNA and protein were detectable by RT-PCR (unpublished observation-J. Ham) and immunofluorescence, respectively [18].
Fig. 6.
Effects of adenosine receptor agonists on chromogranin A secretion from BON-1 cells. BON-1 cultures were treated with varying concentrations of adenosine receptor agonists, adenosine, NECA and CGS21680 for 1 h as indicated. Secreted chromogranin A was measured by radioimmunoassay. Values shown are percentage changes (mean ± SEM) from three experiments. *P < 0.05 when compared with untreated (basal) cells
Discussion
The aetiology of NETS remains unclear and therapeutic management is focussed either on surgery or, if resection is not possible, then somatostatin analogues are the drugs of choice. More recent treatments have been based either on mTOR (mammalian target of rapamycin) or VEGF and related receptors. Since adenosine is a mediator of VEGF release acting through HIF-1α, we explored the expression and function of adenosine receptors in NETs. A number of other studies in other cancers suggest there could be a possible role for adenosine in the development and survival of NETs. For example adenosine has been shown to modulate the migration of colon cancer cells via an HIF-1α/VEGF/IL (interleukin)-8-dependent mechanism [14, 20] and A3R agonism inhibits proliferation or stimulates apoptosis in breast cancer and colon carcinoma cells [21–23]. Agonism at the A1R has also been reported to induce apoptosis of human colonic cancer cells [24] whereas other studies suggest that the A1R in breast carcinoma cells mediate cell cycle progression and cell proliferation [25].
The presence of adenosine receptors on NET cells has by and large been unexplored; our data with archival material show that A2A and A2B receptors are dominant with much weaker expression of the A1 and A3 receptors. Although the numbers of samples investigated were limited, our results and those of others [26] using the human BON-1 and KRJ-1 cell lines concur with these findings. The qRT-PCR suggests that there may be similar expression of A2AR in BON-1 and KRJ-1 cells but significantly higher (×30) expression of A2BR in BON-1 cells. This was reflected in a greater cAMP stimulation in response to NECA in BON-1 cells but a greater effect of CGS21680 in KRJ-1 cells (Fig. 3). Similarly, NECA showed a greater growth-promoting effect than CGS21680 in BON-1 cells whereas both compounds were equally active in KRJ-1 cells (Fig. 4). Interestingly, 4/5 small bowel tumours were negative for the A2BR, the significance of this is however unclear. We also do not know if our findings represent over expression of A2A and A2B receptors or under expression of A1 and A3 receptors. Whether adenosine receptors on tumour cells are activated depends on the microenvironment surrounding the tumour; large tumours in particular possess a hypoxic core with high concentrations of extracellular ATP and adenosine [27, 28]. In animals with experimentally induced tumours, ATP levels have been reported to be greater than 100 μM in tumour tissue and essentially undetectable in healthy tissues [28]. The half-life of ATP is however very short and its degradation product, adenosine has also been shown to accumulate in tumours [27, 29] where it can stimulate growth and angiogenesis and mediate immunosuppression. It is thus likely that the concentrations of adenosine seen in the microenviroment of tumours would be sufficient to activate adenosine receptors including the low affinity, A2BR.
The A2BR has been shown, in gene expression profiling data, to be expressed at a higher level in some cancer tissues and may thus play a role in cancer progression [30–32]. Our data showing that A2 receptors mediate growth of BON-1 and KRJ-I neuroendocrine cells would support these findings. The proinvasive action of adenosine in human colon cancer cells is also mediated through the A2BR and co-activation of the other adenosine receptor subtypes [33]. On the other hand, activation of A2BR on immune cells and the associated production of VEGF can also promote tumour growth [34]. There is also evidence, using adenosine receptor-deficient animals, that cancerous cells which express the A2AR and possibly the A2BR may be more resilient to the anti-tumour effects of T lymphocytes [35, 36]. Adenosine may itself be beneficial for tumour cells as it can suppress the activity of inflammatory cells and also stimulate the local production of endothelial cells and subsequent angiogenesis. The actions of the A2AR can be further complicated via possible synergism with dopamine D2 receptors [37]; whether this actually occurs in NET cells is not known but D2 receptors are co-expressed with somatostatin 2 and 5 receptors [38].
Adenosine, itself, could also be directly involved in tumour growth, as targeted inhibition of the enzyme, CD73 (the enzyme that dephosphorylates AMP to adenosine), has been shown to inhibit breast cancer growth and metastasis [39]. These data suggest that the accumulation of adenosine stimulates breast cancer cell growth which is further supported by the report that the A2AR agonist, CGS21680, stimulates proliferation of MCF-7 cells [40]. In our growth experiments, the observed changes were small, typically 30–40% in the presence of NECA or CGS21680 for BON-1 cells. These values are in line with those reported for the growth effects of 30 μM adenosine on HT-29 colectoral carcinoma and MCF-7 breast carcinoma cells over an incubation time of 8–10 days [41, 42]. As for HT-29 cells [41], prolonged agonist exposure (10–12 days) could perhaps lead to markedly greater growth effects in our experiments. The affinities of adenosine for the different adenosine receptors are quite different; A1 and A2A being essentially high affinity (nanomolar) and the A2B and A3 receptors low affinity (micromolar) [12]. Thus, the concentrations of the adenosine receptor agonists and antagonists used in our experiments were fairly high but necessary to activate the A2BR expressed on both BON-1 and KRJ-1 cells.
Plasma chromogranin A levels has been used as a diagnostic and prognostic indicator for NETS, particularly those of intestinal or pancreatic origin and one of the aims of therapeutic management is to lower hormone-associated symptoms [7, 43, 44]. Our data shows that activation of A2 receptors induces a rapid release of chromogranin A as well as stimulating cell growth. This stimulated secretion of chromogranin A is likely to be from preformed peptide stores rather than due to increased mRNA translation as we have data to show that message transcripts in BON-1 cells were unchanged after exposure to 1 μM NECA for up to 24 h (data not shown). A2 receptor-mediated chromogranin A secretion is probably via the activation of cAMP, this is consistent with the presence of a consensus cAMP element in the promoter sequence of the human chromogranin A gene [45].
In summary, we have shown that NETS express predominantly A2A and A2B adenosine receptors and adenosine acting via these receptors mediates cell growth and chromogranin A secretion. Selective inhibition of adenosine receptors or its signal pathways could serve as additional targets adjunct to the current therapeutic targets, somatostatin and VEGF.
Acknowledgements
The authors thank J. Stott for carrying out immunostaining of the human NET samples and financial support from Cardiff University (School of Medicine).
Conflicts of interest
None.
References
- 1.Rubin J, Ajani J, Schirmer W, Venook AP, Bukowski R, Pommier R, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 2.Arnold R, for the PROMID Study Group Placebo-controlled, double-blind, prospective, randomized study of the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Orthod. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 3.Adams RL, Adams IP, Lindow SW, Zhong W, Atkin SL. Somatostatin receptors 2 and 5 are preferentially expressed in proliferating endothelium. Brit J Cancer. 2005;92:1493–1498. doi: 10.1038/sj.bjc.6602503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saras J, Gronberg M, Stridsberg M, Oberg KE, Janson ET. Somatostatin induces rapid contraction of neuroendocrine cells. FEBS Lett. 2007;581:1957–1962. doi: 10.1016/j.febslet.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Oberg K. Biology, diagnosis and treatment of neuroendocrine tumors of the gastrointestinal tract. Curr Opin Oncol. 1994;6:441–451. doi: 10.1097/00001622-199407000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Jia Z, Li Q, Wang L, Rashid A, Zhu Z, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumours. Cancer. 2007;109:1478–1486. doi: 10.1002/cncr.22554. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, Gustafsson BI, et al. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Australia. 2010;193:46–52. doi: 10.5694/j.1326-5377.2010.tb03742.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Gene Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 9.Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol-Reg I. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- 10.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, et al. Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem Pharmacol. 2006;72:19–31. doi: 10.1016/j.bcp.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Ryzhov S, McCaleb JL, Goldstein AE, Biaggioni I, Feoktistov I. Role of adenosine receptors in the regulation of angiogenic factors and neovascularisation in hypoxia. J Pharmacol Exp Ther. 2007;320:565–572. doi: 10.1124/jpet.106.114850. [DOI] [PubMed] [Google Scholar]
- 12.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz K-N, Linden J. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 13.Merighi S, Mirandola P, Varani K, Gessi S, Leung E, Baraldi PG, et al. A glance at adenosine receptors: novel target for antitumor therapy. Pharmacol Therapeut. 2003;100:31–48. doi: 10.1016/S0163-7258(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Pfragner R, Wirnsberger G, Niederle B, Behmel A, Rinner I, Mandl A, et al. Establishment of a continuous cell line from a human carcinoid of the small intestine (KRJ-I): characterization and effects of 5-azacytidine on proliferation. Int J Oncol. 1996;8:513–520. doi: 10.3892/ijo.8.3.513. [DOI] [PubMed] [Google Scholar]
- 17.Modlin IM, Kidd M, Pfragner R, Eick GN, Champaneria C. The functional characterization of normal and neoplastic enterochromaffin cells. J Clin Endocr Metab. 2006;91:2340–2348. doi: 10.1210/jc.2006-0110. [DOI] [PubMed] [Google Scholar]
- 18.Kidd M, Eick GN, Modlin IM, Pfragner R, Champaneria MC, Murren J. Further delineation of the continuous human neoplastic enterochromaffin cell line, KRJ-I, and the inhibitory effects of lanreotide and rapamycin. J Mol Endocrinol. 2007;38:181–192. doi: 10.1677/jme.1.02037. [DOI] [PubMed] [Google Scholar]
- 19.Rees DA, Lewis MD, Lewis BM, Smith PJ, Scanlon MF, Ham J. Adenosine-regulated cell proliferation in pituitary folliculostellate and endocrine cells: differential roles for the A1 and A2B adenosine receptors. Endocrinology. 2002;143:2427–2436. doi: 10.1210/en.143.6.2427. [DOI] [PubMed] [Google Scholar]
- 20.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, et al. Caffeine inhibits adenosine-induced accumulation of HIF-1α, VEGF and IL-8 expression in hypoxic human colon cancer cells. Mol Pharmacol. 2007;72:395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Pierron A, Ravid K. An adenosine analogue, IB-MECA, down-regulates estrogen receptor alpha and suppresses human breast cancer cell proliferation. Cancer Res. 2003;63:6413–6423. [PubMed] [Google Scholar]
- 22.Ohana G, Bar-Yehuda S, Arich A, Madi L, Deznick Z, Rath-Wolfson L, et al. Inhibition of primary colon carcinoma growth and liver metastasis by the A3 adenosine receptor agonist CF101. Brit J Cancer. 2003;89:1552–1558. doi: 10.1038/sj.bjc.6601315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panjehpour M, Karami-Tehrani F. Adenosine modulates cell growth in the human breast cancer cells via adenosine receptors. Oncol Res. 2007;16:575–585. doi: 10.3727/000000007783629981. [DOI] [PubMed] [Google Scholar]
- 24.Saito M, Yaguchi T, Yasuda Y, Nakano T, Nishizaki T. Adenosine suppresses CW2 human colonic cancer growth by inducing apoptosis via A(1) adenosine receptors. Cancer Lett. 2010;290:211–215. doi: 10.1016/j.canlet.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Mirza A, Basso A, Black S, Malkowski M, Kwee L, Pachter JA, et al. RNA interference targeting of A1 receptor-overexpressing breast carcinoma cells leads to diminished rates of cell proliferation and induction of apoptosis. Cancer Biol Ther. 2005;4:1355–1360. doi: 10.4161/cbt.4.12.2196. [DOI] [PubMed] [Google Scholar]
- 26.Christofi FL, Kim M, Wunderlich JE, Xue J, Suntres Z, Cardounel A, et al. Endogenous adenosine differentially modulates 5-hydroxytryptamine release from a human enterochromaffin cell model. Gastroenterology. 2004;127:188–202. doi: 10.1053/j.gastro.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 27.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Therapeut. 2000;87:161–173. doi: 10.1016/S0163-7258(00)00053-X. [DOI] [PubMed] [Google Scholar]
- 28.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Huang S, Peng SB. Overexpression of G-protein-coupled receptors in cancer: involvement in tumor progression. Int J Oncol. 2005;27:1329–1339. [PubMed] [Google Scholar]
- 31.Panjehpour M, Castro M, Klotz KN. Human breast cancer cell line MDA-MB-231 expresses endogenous A2B adenosine receptors mediating a Ca++ signal. Brit J Pharmacol. 2005;145:211–218. doi: 10.1038/sj.bjp.0706180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang HJ, Liu ZC, Wang DS, Chen Y, Yang YL, Dou KF. Adenosine A2B receptor is highly expressed in human hepatocellular carcinoma. Hepatol Res. 2006;36:56–60. doi: 10.1016/j.hepres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues S, Wever O, Bruyneel E, Rooney RJ, Gespach C. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene. 2007;26:5615–5625. doi: 10.1038/sj.onc.1210347. [DOI] [PubMed] [Google Scholar]
- 34.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, et al. Host A(2B) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, et al. A2A adenosine receptor protects tumors from antitumor T cells. P Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukashev D, Sitkovsky M, Ohta A. From “Hellstrom Paradox” to anti-adensinergic cancer immunotherapy. Purinerg Signal. 2007;3:129–134. doi: 10.1007/s11302-006-9044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudlacek O, Just H, Korkhov VM, Vartian N, Klinger M, Pankevych H, et al. The human D2 dopamine receptor synergizes with the A2A adenosine receptor to stimulate adenylyl cyclase in PC12 cells. Neuropsychopharmacol. 2003;28:1317–1327. doi: 10.1038/sj.npp.1300181. [DOI] [PubMed] [Google Scholar]
- 38.Srirajaskanthan R, Watkins J, Marelli L, Khan K, Caplin ME. Expression of somatostatin and dopamine 2 receptors in neuroendocrine tumours and the potential role for new biotherapies. Neuroendocrinology. 2009;89:308–314. doi: 10.1159/000179899. [DOI] [PubMed] [Google Scholar]
- 39.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. P Natl Acad Sci USA. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etique N, Grillier-Vuissoz I, Lecomte J, Flament S. Crosstalk between adenosine receptor (A2A isoform) and ERalpha mediates ethanol action in MCF-7 breast cancer cells. Oncol Rep. 2009;21:977–981. doi: 10.3892/or_00000311. [DOI] [PubMed] [Google Scholar]
- 41.Mujoomdar M, Hoskin D, Blay J. Adenosine stimulation of the proliferation of colorectal carcinoma cell lines. Roles of cell density and adenosine metabolism. Biochem Pharmacol. 2003;66:1737–1747. doi: 10.1016/S0006-2952(03)00548-3. [DOI] [PubMed] [Google Scholar]
- 42.Mujoomdar M, Bennett A, Hoskin D, Blay J. Adenosine stimulation of proliferation of breast carcinoma cell lines: evaluation of the [3H]thymidine assay system and modulatory effects of the cellular microenvironment in vitro. J Cell Physiol. 2004;201:429–438. doi: 10.1002/jcp.20089. [DOI] [PubMed] [Google Scholar]
- 43.Syversen U, Ramstad H, Gamme K, Qvigstad G, Falkmer S, Waldum HL. Clinical significance of elevated serum chromogranin A levels. Scand J Gastroentero. 2004;39:969–973. doi: 10.1080/00365520410003362. [DOI] [PubMed] [Google Scholar]
- 44.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Cl Ga. 2005;19:753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Canaff L, Bevan S, Wheeler DG, Mouland AJ, Rehfuss RP, White JH, et al. Analysis of molecular mechanisms controlling neuroendocrine cell specific transcription of the chromogranin A gene. Endocrinology. 1998;139:1184–1196. doi: 10.1210/en.139.3.1184. [DOI] [PubMed] [Google Scholar]