Abstract
Gliomas are the most common and devastating type of primary brain tumor. Many non-neoplastic cells, including immune cells, comprise the tumor microenvironment where they create a milieu that appears to dictate cancer development. ATP and the phosphohydrolytic products ADP and adenosine by activating P2 and P1 receptors may participate in these interactions among malignant and immune cells. Purinergic receptor-mediated cell communication is closely regulated by ectonucleotidases, such as by members of the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family, which hydrolyze extracellular nucleotides. We have shown that gliomas, unlike astrocytes, exhibit low NTPDase activity. Furthermore, ATP induces glioma cell proliferation and the co-administration of apyrase decreases progression of injected cells in vivo. We have previously shown that NTPDase2 reconstitution dramatically increases tumor growth in vivo. Here we evaluated whether NTPDase2 reconstitution to gliomas modulates systemic inflammatory responses. We observed that NTPDase2 overexpression modulated pro-inflammatory cytokine production and platelet reactivity. Additionally, pathological alterations in the lungs were observed in rats bearing these tumors. Our results suggest that disruption of purinergic signaling via ADP accumulation creates an inflammatory state that may promote tumor spread and dictate clinical progression.
Keywords: Gliomas, ATP, ADP, E-NTPDases, Lung, Inflammation
Introduction
Glioblastoma multiforme (GBM) is the most common and devastating type of primary brain tumor, characterized by diffuse infiltration of the brain parenchyma, recurrent local growth, vascular compromise, and dismal prognosis [1]. Recent studies have shown that immune cells and secretory products create a growth factor-rich environment linked to tumor maintenance and growth [2]. The progression of GBM is also associated with inflammatory changes in the tumor microenvironment [3].
Cytokines, chemokines, and their receptors might regulate cross-talk among cancer cells, immune cells, and vascular endothelium [4]. Purinergic signaling, involving ATP released from neural and immune cells and the respective breakdown or hydrolytic products such as ADP and adenosine activate their own responses and modulate cross-talk with chemokines [5]. ATP modulates cytokine gene expression within the nervous and immune system [6] and also controls the secretion of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α [7, 8]. Platelet activation by ADP has also been implicated in inflammation. Platelets are essential in the initiation of an inflammatory response [9] and adhesive interactions between platelets, leukocytes, and cancer cells play an important role in tumor progression and metastasis [10, 11]. Adenosine, a product of extracellular nucleotide hydrolysis may accumulate in the tumor interstitium [12] where it modulates cell proliferation, angiogenesis, and suppresses anticancer immune responses [13, 14]. Nucleotide/nucleoside receptor-mediated cell communication is controlled by ectonucleotidases, such as members of the ectonucleoside triphosphate diphosphohydrolases (E-NTPDases), ectonucleotide pyrophosphatase phosphodiesterases (E-NPPs), and ecto-5′-nucleotidase (ecto-5′-NT or CD73), which efficiently hydrolyze ATP, ADP, and AMP to adenosine [15–17].
Extracellular degradation of ATP in C6 glioma cells proceeds by a cascade of cell-surface-bound enzymes that, in addition to E-NTPDases, also includes the E-NPPs. The functional role of these enzyme families in the different biological systems is related to substrate availability and physio/pathological situation [15–17]. Grobben and colleagues [18] demonstrated that NPP1 is one of the main ATP-hydrolyzing enzymes associated with the plasma membrane of C6 at physiological ATP concentrations. However, in pathological situations such as cancer, the nucleotide release promoted by cell death, and infiltrating inflammatory cells results in ATP accumulation in the tumor interstitium at hundreds micromolar range, while it is undetectable in healthy tissues [19]. In this context, NTPDases become important regulators of P2 receptor activation.
We have previously demonstrated that different glioma cell lines exhibit low levels of NTPDase activity/expression, which might favor a disruption in P2 receptor activation and tumor progression [20–23]. As NTPDase2 is the major ectonucleotidase present in astrocytes in culture, we analyzed whether the overexpression of a preferential ecto-ATPase would affect the in vivo glioma growth. Surprisingly, NTPDase2 overexpression dramatically increased tumor malignity [24]. In the present study, we evaluated whether NTPDase2 reconstitution to gliomas could modulate the systemic inflammatory response. Our results show that NTPDase2 overexpression modulates the pro-inflammatory cytokine production and platelet function in vivo. Additionally, pathological alterations in the lungs were observed in rats bearing these tumors. These data support the notion that disruption of purinergic signaling creates an inflammatory microenvironment that modulates tumor cell progression and local invasiveness.
Material and methods
Cell culture and transient transfection
The C6 rat glioma cell line was obtained from the ATCC (Rockville, Maryland, USA). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% (v/v) fetal bovine serum (FBS; Cultilab, Brazil) and kept at a temperature of 37°C, humidity of 95%/5% CO2 in air. Enhanced yellow fluorescent protein (EYFP)-NTPDase2 was constructed by inserting NTPDase2 into the PBS-SKII plasmid (Stratagene, La Jolla, CA, USA), and then inserting it into pEYFP-C1 (Clontech Laboratories Inc., Palo Alto, CA, USA). C6 cells at 80% confluence were transfected with lipofectamine 2000 (Life Technologies, Invitrogen Co., Carlsbad, CA, USA) according to the manufacturer’s instructions with 1 μg/well of pEYFP/NTPDase2 (C6NT2), or pEYFP empty vector (C6EYFP). Cell transfection and the evaluation of plasmid functionality were performed 72 h post-transfection, as previously described [24]. NTPDase2 expression was confirmed by Western blot using a mouse monoclonal anti-human NTPDase2 antibody [25] and by evaluating the ATPase/ADPase activities in C6 glioma-transfected cells.
Glioma implantation
The glioma implantation was made as described by Braganhol et al. [24]. Briefly, C6, C6EYFP, or C6NT2 glioma cells 72 h post-transfection (1 × 106 cells) were injected in the striatum of male Wistar rats (250–270 g, 8 weeks old) anesthetized by intraperitoneal administration of ketamine/xilazine. The negative control group (naive) was carried out via the same procedure and received an injection of 3 μL of DMEM/5% FBS in the right striatum. All procedures used in the present study followed the “Principles of Laboratory Animal Care” from National Institutes of Health (NIH) and were approved by the Ethical Committee of the Hospital de Clínicas de Porto Alegre.
Pathological analysis
Twenty days after glioma implantation, rats were decapitated and the liver, kidney, and lungs were removed, sectioned, and fixed in 4% PFA in PBS (pH 7.4). The fixed tissue sections were then stained with hematoxylin and eosin and pathological analysis of the slides was performed by a pathologist in a blinded manner.
Immunohistochemical staining
Cryostat sections (5 μm) from lung tissue were fixed in acetone, blocked in 1% albumin solution, and incubated overnight (4°C) with the following antibodies: rabbit anti-rat pAb P-selectin (1:500; BD Pharmingen, USA) and rabbit anti-rat mAb nestin (1:200; Chemicon, USA and Canada). Next, tissue sections were incubated with biotinylated secondary antibody and streptavidin–avidin–biotin (kit Lsab, Dako, CA, USA). The peroxidase reaction was performed using 3, 3′diaminobenzidine tetrahydrochloride, according to the manufacturer’s specifications. Sections were counterstained with Harris hematoxylin. The IHC slides were analyzed by a pathologist in a blinded manner.
Determination of cytokine levels in blood serum
Twenty days after glioma implantation, the blood samples of animals of the naive, C6, C6EYFP, and C6NT2 groups were collected by cardiac puncture. The blood samples were centrifuged at 1,300×g at 4°C for 10 min. The supernatant was rapidly frozen and stored at −80°C for the later measurement of TNF-α, IL-1β, IL-6, and IL-10 levels using specific enzyme-linked immunosorbent assay (ELISA) kits, according to the recommendations of the supplier (R&D Systems).
Platelet count
The blood used for platelets count was collected by cardiac puncture from anesthetized ketamin/xilazin control and glioma-implanted rats into vacutainer plastic tube containing 120 mM sodium citrate. Blood samples were analyzed in a Coulter JT counter. The data are expressed as the number of platelets × 103/μL.
Isolation of platelets
Platelet suspensions were prepared in accordance to the method of Lunkes and colaborators [26], with minor modifications. Total blood was collected by cardiac puncture into a flask containing 120 mM sodium citrate as anticoagulant. The total blood citrate system was centrifuged at 160×g during 15 min in order to obtain the platelet-rich plasma (PRP). The PRP was centrifuged at 1,400×g for 20 min and washed twice by centrifugation at 1,400×g for 10 min with 3.5 mM HEPES isosmolar buffer pH 7.5 containing 142 mM NaCl, 2.5 mM KCl, and 5.5 mM glucose. The washed platelets were suspended in HEPES buffer for subsequent assays.
Platelet aggregation
The blood samples for platelet aggregation assays were collected by cardiac puncture and then centrifuged at 160×g for 15 min at room temperature to achieve PRP suspensions. Platelet aggregation assays were performed on a SpectraMax microplate reader (Molecular Devices, USA). Briefly, platelet agonists 10 μM ADP, 2.0 mM CaCl2, and Tyrode/BSA buffer were mixed in 96-well flat-bottom plates. Aggregation was triggered by the addition of 100 μL of platelet suspension in a final reaction volume of 150 μL. The plate was incubated for 2 min at 37°C before the beginning of stirring. Readings were followed at 650 nm every 20 s for 20 min. Changes in turbidity were measured in absorbance units and the results were obtained as area under the aggregation curves.
Assay of ectonucleotide pyrophosphatase/phosphodiesterase activity
The artificial substrate for E-NPPs, p-nitrophenyl 5′-thymidine monophosphate (p-Nph-5′-TMP), was used as a substrate to evaluate the enzymatic activity in C6EYFP and C6NT2 glioma cells [27]. The enzyme reaction was started by the addition of p-Nph-5′-TMP to a final concentration of 0.5 mM in a mixture medium containing 2 mM CaCl2, 120 mM NaCl, 5 mM KCl, 10 mM glucose, 20 mM HEPES (pH 7.4), or 50 mM Tris–HCl buffer (pH 8.9) in a final volume of 200 μL. After 60 min of incubation, the reaction was stopped by the addition of 200 μL 0.2 N NaOH and the samples were chilled on ice. Incubation times and substrate concentration were chosen to ensure the linearity of the reaction. All assays were carried out in triplicate. The amount of p-nitrophenol released from the substrate hydrolysis was measured at 400 nm using p-nitrophenol as standard. Specific activity was expressed as picomoles of p-nitrophenol released per minute per milligram of protein.
Assay of ectonucleoside triphosphate diphosphohydrolase activity
The analysis of platelet nucleotide hydrolysis was performed as described by Zanin and colleagues [28]. The enzyme reaction was started by the addition of ATP, ADP, or AMP as substrates (1.0 mM) in a reaction mixture containing washed platelets (20 μg of protein), 50 mM Tris–HCl (pH 7.4), 120 mM NaCl, 5 mM KCl, 6 mM glucose, 5 mM CaCl2, for ATP and ADP, or 5 mM MgCl2 for AMP, pH 7.4, in a final volume of 200 μL. The incubation was stopped by adding 200 μL trichloroacetic acid (5%, final concentration). The release of inorganic phosphate (Pi) was measured by the malachite green method [29] using KH2PO4 as Pi standard. The protein concentration was measured by the Coomassie Blue method [30] using bovine serum albumin as standard. Specific activity was expressed as nanomoles of Pi released per minute per milligram of protein.
Statistical analysis
Data were expressed as mean ± SD of at least three independent experiments and were subjected to one-way analysis of variance (ANOVA) followed by Tukey–Kramer post-hoc test (for multiple comparisons) or t student test, when necessary. Differences between mean values were considered significant when P < 0.05.
Results
Biochemical characterization of NTPDase2 overexpression in C6 glioma cell
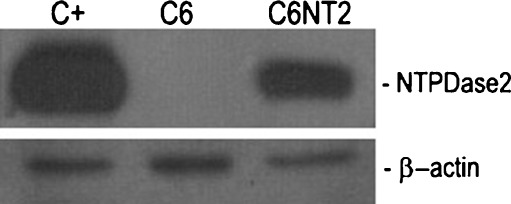
The C6 glioma transfection resulted in NTPDase2 protein expression as evaluated by Western blot (Fig. 1); green fluorescence at the C6 glioma cell surface 72 h post-transfection (data not shown) and in high ATPase activity, with an ATP/ADPase ratio of around 20 (170 ± 24 and 8.8 ± 0.7 nmol Pi/min/mg of protein for ATP and ADP, respectively), as expected for this enzyme. The influence of NTPDase2 overexpression on the whole pattern of nucleotide metabolism, especially on NPPs activity was also evaluated. The analysis was performed using the p-nitrophenyl 5′-thymidine monophosphate an artificial substrate for NPP1-3 [27]. The NTPDase2 overexpression did not alter the NPPase activity in C6 glioma cells (0.56 ± 0.25 vs 0.87 ± 0.51 pmol p-nitrophenol/min/mg of protein for C6EYFP and C6NT2, respectively at pH 7.4; 3.79 ± 1.08 vs 4.7 ± 1.51 pmol p-nitrophenol/min/mg of protein for C6EYFP and C6NT2, respectively, at pH 8.9).
Fig. 1.
Biochemical characterization of NTPDase2 overexpression in C6 glioma cell. NTPDase2 protein expression in transfected glioma cells as evaluated by Western blot. COS-7 cells, transiently transfected with NTPDase2 cDNA construct were used as a positive control (C+). A specific band is detected at the expected molecular weight (66 kDa) only in the protein samples from NTPDase2 transfected glioma cells (C6NT2)
NTPDase2 expression in implanted gliomas promoted pulmonary histological alterations in vivo
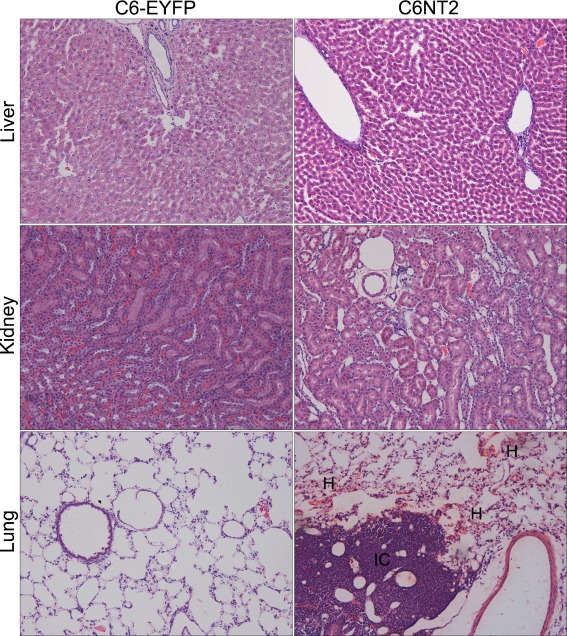
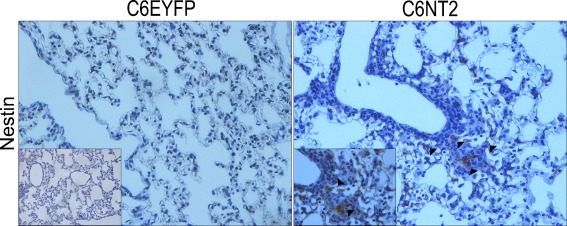
We have previously shown that the NTPDase2 restoration to C6 glioma promoted a dramatic tumor growth in vivo [24]. Here, we investigated whether NTPDase2 overexpression in gliomas could also impacts systemic responses. Twenty days after glioma implantation, the major sites for cancer metastasis and vascular injury, including lung, kidney, and liver, were excised and processed for histological analysis (Fig. 2). While no histological changes were observed in the liver and kidney, important alterations, such as focal hemorrhage, extensive lymphocytic infiltration and hypercellularity were observed in the vasculature of lungs from NTPDase2 glioma-implanted rats (Fig. 2 and Table 1). In addition, the expression of nestin, a marker of immature cells with high proliferative potential was evaluated by IHC (Fig. 3). Interestingly, lung tissues from NTPDase2 glioma-implanted rats exhibited the presence of nestin-positive cells.
Fig. 2.
NTPDase2 overexpression in gliomas promoted histological changes in the pulmonary tissue. To perform the histological analysis in peripheral organs, equal amounts of C6EYFP or C6NT2 cells (1 × 106 cells) were implanted in the right striatum of Wistar rat brains by stereotaxical surgery. The animals were killed 20 days later and the liver, kidney, and lung were dissected, processed for hematoxilin/eosin standard stain and the histological analysis was performed by a pathologist in a blinded manner. Representative sections of liver, kidney, and lung are shown. Histological characteristics exhibited by lung tissue from C6NT2 glioma-implanted rats indicate the presence of pathological alterations: extensive immune cell infiltrate (IC) and hemorrhage (H) (magnification ×20). The analyses were performed in five animals per group. The complete histological evaluation is presented in Table 1
Table 1.
Histological characteristics of lungs from rats implanted with gliomas
| Histology | C6 (n = 5) | C6-EYFP (n = 5) | C6NT2 (n = 5) |
|---|---|---|---|
| Focal hemorrhage | 1/5 | 2/5 | 5/5 |
| Lymphocytic infiltration | 1/5 | 0/5 | 4/5 |
| High cellularity | 0/5 | 0/5 | 5/5 |
Twenty days following glioma implantation, lungs from rats implanted with C6, C6-EYFP, or C6-EYFP/NTPDase2 (C6NT2) were processed for hematoxylin and eosin differential staining. The histological analysis was performed by a pathologist in a blinded manner. The histological variables (focal hemorrhage, lymphocytic infiltration, and high cellularity) were scored as present or absent. The results are expressed as the number of animals positive for the pathological characteristic in relation to the total number of animals per group
Fig. 3.
NTPDase2 overexpression promoted the presence of nestin-positive cells in lung tissue. The presence of glioma cells on lung tissue was assessed by immunohistochemical analysis of nestin-positive cells, a glioblastoma multiform marker. Arrows indicate nestin-positive cells around blood vessels and inflammatory infiltrate in the lung tissue from C6NT2 glioma-implanted rats (magnification ×40). The C6EYFP and C6NT2 inserts represent the isotype control Ab for nestin and higher magnification (×100) of nestin-positive cells, respectively. Experiments were performed in five animals per group
NTPDase2 overexpression in implanted gliomas induced an increase in pro-inflammatory cytokine levels
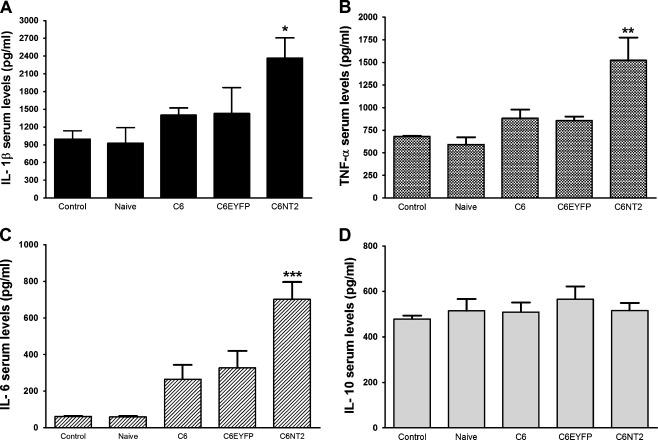
Taking in consideration that nestin-positive cells in lung tissues were localized within the inflammatory infiltrate and around blood vessels (Fig. 3), we tested whether cytokines could be involved in the mediation of the alterations observed in the lung. Figure 4 shows that blood serum samples from rats implanted with C6NT2-glioma had two times more of the pro-inflammatory cytokine IL-1β (1,040 ± 204 vs 2,345 ± 676 pg/mL), TNF-α (680 ± 7 vs 1,500 ± 500 pg/mL) and IL-6 (327 ± 161 vs 698 ± 163 pg/mL) when compared to C6EYFP glioma-implanted rats. In contrast, the serum level of the anti-inflammatory cytokine IL-10 remained unchanged (Fig. 4). This is consistent with our previous study that reports the increased malignant potential of NTPDase2-derived gliomas [24], and suggests that the regulation of inflammatory process by purinergic signals could be involved in the peripheral effects caused by NTPDase2 overexpression in gliomas.
Fig. 4.
NTPDase2 overexpression in gliomas promoted an increase in the serum cytokine levels of glioma-implanted rats. Following 20 days of C6EYFP or C6NT2 glioma implantation, the rats were anesthetized with ketamin/xilazin and the blood was obtained by cardiac puncture. The A IL-1β, B TNF-α, C IL-6, and D IL-10 serum levels (picograms per milliliter) were evaluated by ELISA following the manufacturer’s instructions. Animals not submitted to the surgery (Control) or animals injected with DMEM/5% FBS in absence of tumor cells (Naive) were taken as controls. The values represent the means ± SD of five animals per group. Data were analyzed by ANOVA followed by post-hoc comparisons (Tukey’s test). *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different from all groups
Inflammatory cytokines modulated platelet activation in C6NT2 implanted gliomas
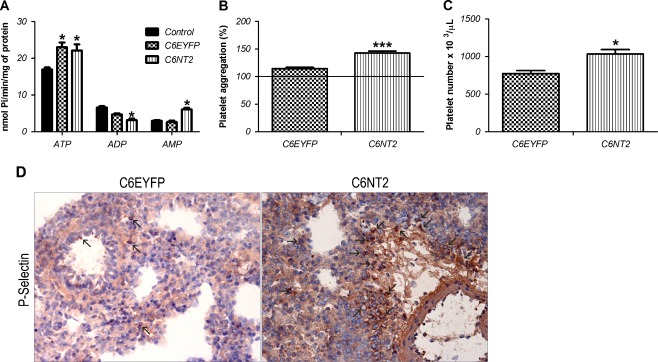
Cytokines produced during inflammatory responses coupled with low levels of ADP can serve as strong stimuli for activating platelet function [31]. Therefore, ectonucleotidase activities, important modulators of P2-mediated platelet activation, were evaluated. Figure 5 shows that ATP hydrolysis was increased in platelets derived from C6EYFP and C6NT2 glioma-implanted rats, when compared to control rats. However, the ADP hydrolysis was significantly decreased only in platelets from C6NT2 glioma-implanted rats. In addition, a significant increase on AMP hydrolysis was observed in platelets from these rats. Platelet number and platelet aggregation have shown a significant increase in C6NT2 glioma-implanted rats (35% and 25%, respectively) when compared to control group (Fig. 5B, C). Finally, lung cryostat sections from rat-implanted gliomas were immunostained with a P-selectin antibody which revealed increased platelet sequestration in the lung tissue from C6NT2 glioma-implanted rats (Fig. 5D).
Fig. 5.
NTPDase2 overexpression-induced alterations in platelet reactivity. A The ATPase, ADPase, and AMPase activities in platelets from control (animals not submitted to surgery), C6EYFP or C6NT2 glioma-implanted rats. B Platelet aggregation from C6EYFP or C6NT2 glioma-implanted rats. The platelet aggregation results are expressed as the percentage in relation to control (animals not submitted to surgery). C Platelet count from C6EYFP or C6NT2 glioma-implanted rats; C6EYFP was taken as control (platelet reference value for Wistar rats 700 × 103 μL). D The presence of platelet activation and recruitment to lung tissue was assessed by immunohistochemical analysis of P-selectin positive cells, a platelet activation marker. Arrows indicate P-selectin-positive platelets (magnification ×40). The values represent the mean ± SD of at least three independent experiments performed in triplicate. Data were analyzed by ANOVA followed by post-hoc comparisons (Tukey–Kramer test). *P < 0.05 and ***P < 0.001, significantly different from controls
Discussion
The data presented herein, allied to previous work showing that NTPDase2 overexpression is involved in increased glioma growth in vivo [24] suggest that this enzyme may play a general role on process related to tumor progress, including modulation of inflammatory response. An important finding of the present study is that C6NT2-gliomas-implanted rats exhibited histological alterations in the pulmonary tissue, including nestin immunopositivity. Nestin is a member of intermediate filaments expressed by bone-marrow-derived cells, developing arteries from rat embryos and by neural stem cells, being widely used as a glioma marker [32–36]. Considering that nestin expression was virtually absent in lung tissue from adult rats [35], the immunopositivity for this marker in the lungs of C6NT2 glioma-implanted rats suggest a lung inflammatory response and a progression of vascular remodeling uncommon in adult animals [36]. Although these processes are also related to an initial malignant process, additional cell markers are required to confirm this hypothesis.
Next, we hypothesized that NTPDase2 overexpression in C6 glioma cells induced changes in the glioma–environment interaction, leading to cytokine production and circulating platelet activation. The latter processes are thought to drive the tumor cell epithelial–mesenchymal transition, an important mechanism of tumor invasion [37, 38]. In agreement, C6NT2 glioma-implanted rats showed an important increase of IL-1β, TNF-α, and IL-6 pro-inflammatory cytokine serum levels when compared to control. The inflammatory cytokines detected in the serum may have multiple sources. They could be produced by endothelial cells as well as circulating and tumor-associated immune cells. However, the participation of tumor cells in the cytokine release, which is known to have important role in leukocyte/macrophage infiltration into the tumors and in organ-specific metastasis [39], cannot be excluded. For example, IL-1β affects the pattern of tumor–host interactions [40], and genetic ablation in mice results in absence of metastatic tumors in vivo [41]. Additionally, TNF-α up-regulates selectin overexpression on endothelial cells, which promotes tumor cell adhesion and migration [42, 43]. Finally, IL-6 accelerates tumorigenesis and its administration during tumor initiation resulted in increased malignant potential of the tumor [44].
In addition, we also demonstrate that C6NT2-gliomas implanted rats exhibited an increase in platelet count, activation, and sequestration to lungs. While the increase in the ATP hydrolysis was similar in both groups of glioma-implanted rats, the platelets from C6NT2 glioma-implanted rats exhibited a decrease in ADP and an increase in AMP hydrolysis. Data from the literature indicate that the NTPDase1 is the main enzyme responsible for ATP and ADP hydrolysis in platelets [45]. However, the presence of other NTPDases cannot be excluded, since the modulation of ATPase/ADPase activities in platelets from C6NT2 glioma-implanted rats was not parallel. Although the presence of ectonucleotidases is critical for maintaining the vascular homeostasis, little is known about its expression regulation at the molecular level. Modulated expression of NTPDase1 and ecto-5′-NT/CD73 has been closely associated with inflammatory cytokines, oxidative stress, and hypoxia [46–49]. Therefore, the pro-inflammatory environment present in C6NT2 glioma-implanted rats may modulate the ectonucleotidase expression in platelets. In addition, considering that inflammatory cytokines and ADP have a synergical effect on platelet activation [31], we suggest that the alterations in the nucleotide metabolism by platelets would modulate its aggregation and recruitment properties. Although it remains to be elucidated whether the platelet recruitment and inflammatory response is a cause or consequence of larger C6NT2-gliomas, results from our group showing that both apyrase/NTPDase1 treatment (unpublished data) and NTPDase2 overexpression did not affect the in vitro C6 glioma proliferation [24] suggest that something other than the proliferative rate of C6 co-injected with apyrase and C6NT2-derived gliomas accounts for the difference in growth potential in vivo [23, 24]. These results indicate that the effects of anti- or pro-tumor mediated by P2R activation are related to the modulation of extracellular nucleotide level in the in vivo tumor environment.
In summary, the data presented in this work reinforce previous data from our laboratory and points to a new role of purinergic signaling in the glioma biology. Furthermore, our findings reveal an important function of extracellular nucleotides, probably ADP, in modulating cancer-related inflammation events at distant sites.
Acknowledgments
We thank N. Copetti, J.A. Lopes, and L.R. Blazina for their excellent technical assistance; M.S.L. Pereira for helping in the image edition; Dr. G. Guidotti for the pGW1-hNTPDase2 vector and the Clinical Analysis laboratory of Pharmacy—UFRGS for the biochemical assays performed. This work was supported by the Brazilian funding agencies: CNPq-BR (to A.M.O. Battastini, E. Braganhol, A. Bernardi, L.S. Bergamin, and L.F. Campesato) and FIPE-HCPA (to M.I.A. Edelweiss). This work was also facilitated by NIH grants (HL094400-01, HL087203-02 to S.C. Robson) and by grants from the Canadian Institutes of Health Research (CIHR to J. Sévigny).
References
- 1.Davis FG, McCarthy BJ. Current epidemiological trends and surveillance issues in brain tumors. Expert Rev Anticancer Ther. 2001;1:395–401. doi: 10.1586/14737140.1.3.395. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A. Inflaming metastasis. Nature. 2009;457:36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 3.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Bours MJ, Swennen EL, Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- 7.Virgilio F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprague DL, Sowa JM, Elzey BD, Ratliff TL. The role of platelet CD154 in the modulation in adaptive immunity. Immunol Res. 2007;39:185–193. doi: 10.1007/s12026-007-0074-3. [DOI] [PubMed] [Google Scholar]
- 10.Sierko E, Wojtukiewicz MZ. Inhibition of platelet function: does it offer a chance of better cancer progression control? Semin Thromb Hemost. 2007;33:712–721. doi: 10.1055/s-2007-991540. [DOI] [PubMed] [Google Scholar]
- 11.Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352:1775–1777. doi: 10.1016/S0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 12.Melani A, Micheli E, Pinna G, Alfieri A, Corte LD, Pedata F. Adenosine extracellular levels in human brain gliomas: an intraoperative microdialysis study. Neurosci Lett. 2003;31(1–2):93–96. doi: 10.1016/S0304-3940(03)00596-2. [DOI] [PubMed] [Google Scholar]
- 13.Spychala J. Tumor promoting functions of adenosine. Pharmacol Ther. 2000;87:161–173. doi: 10.1016/S0163-7258(00)00053-X. [DOI] [PubMed] [Google Scholar]
- 14.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;29(35):13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationship and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles AF. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 2011;7:21–45. doi: 10.1007/s11302-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. BBA. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Grobben B, Anciaux K, Roymans D, Stefan C, Bollen M, Esmans EL, Slegers H. An ecto-nucleotide pyrophosphatase is one of the main enzymes involved in the extracellular metabolism of ATP in rat C6 glioma. J Neurochem. 1999;72:826–834. doi: 10.1046/j.1471-4159.1999.0720826.x. [DOI] [PubMed] [Google Scholar]
- 19.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wink MR, Lenz G, Braganhol E, Tamajusuku AS, Schwartsmann G, Sarkis JJ, Battastini AM. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett. 2003;198:211–218. doi: 10.1016/S0304-3835(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 21.Morrone FB, Jacques-Silva MC, Horn AP, Bernardi A, Schwartsmann G, Rodnight R, Lenz G. Extracellular nucleotides and nucleosides induce proliferation and increase nucleoside transport in human glioma cell line. J Neurooncol. 2003;64:211–218. doi: 10.1023/A:1025699932270. [DOI] [PubMed] [Google Scholar]
- 22.Morrone FB, Horn AP, Stella J, Spiller F, Sarkis JJ, Salbego CG, Lenz G, Battastini AM. Increased resistance of glioma cell lines to extracellular ATP cytotoxicity. J Neurooncol. 2005;71:135–140. doi: 10.1007/s11060-004-1383-1. [DOI] [PubMed] [Google Scholar]
- 23.Morrone FB, Oliveira DL, Gamermann P, Stella J, Wofchuk S, Wink MR, Meurer L, Edelweiss MI, Lenz G, Battastini AM. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:3–226. doi: 10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braganhol E, Morrone FB, Bernardi A, Huppes D, Meurer L, Edelweiss MI, Lenz G, Wink MR, Robson SC, Battastini AM. NTPDase2 expression modulates in vivo rat glioma growth. Cancer Sci. 2009;100(8):1434–1442. doi: 10.1111/j.1349-7006.2009.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelletier J, Lavoie EG, Fausther M, Munkonda MN, Lecka J, Tremblay A, Sévigny J (2010) Production d’anticoprs contre les NTPDases. 9e journée scientifique du CRRI. Hôtel-Musée Premières Nations, Wendake, Qc. Cahier des résumés p. 31. 8 nov
- 26.Lunkes GI, Lunkes DS, Morsch VM, Mazzanti CM, Morsch AL, Miron VR, Schetinger MR. NTPDase and 5′-nucleotidase activities in rats with alloxan-induced diabetes. Diabetes Res Clin Pract. 2004;65(1):1–6. doi: 10.1016/j.diabres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Sakura H, Nagashima S, Nakahima A, Maeda M. Characterization of fetal serum 5′-nucleotide phosphodiesterase: a novel function as a platelet aggregation inhibitor in fetal circulation. Thromb Res. 1998;91:83–89. doi: 10.1016/S0049-3848(98)00073-5. [DOI] [PubMed] [Google Scholar]
- 28.Zanin RF, Campesato LF, Braganhol E, Schetinger MR, Wyse AT, Battastini AM. Homocysteine decreases extracellular nucleotide hydrolysis in rat platelets. Thromb Res. 2010;125(3):e87–e92. doi: 10.1016/j.thromres.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Chan K, Delfert D, Junger KD. A direct colorimetric assay for Ca2+−ATPase activity. Anal Biochem. 1986;157:375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:218–241. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Gear ARL, Suttitanamongkol S, Viisoreanu D, Polanowska-Grabowska RK, Raha S, Camerini D. Adenosine diphosphate strongly potentiates the ability of the chemokines MDC, TARC, and SDF-1 to stimulate platelet function. Blood. 2001;97(4):937–945. doi: 10.1182/blood.V97.4.937. [DOI] [PubMed] [Google Scholar]
- 32.Kitai R, Horita R, Sato K, Yoshida K, Arishima H, Higashino Y, Hashimoto N, Takeuchi H, Kubota T, Kikuta K. Nestin expression in astrocytic tumors delineates tumor infiltration. Brain Tumor Pathol. 2010;27(1):17–21. doi: 10.1007/s10014-009-0261-0. [DOI] [PubMed] [Google Scholar]
- 33.Zhou XD, Wang XY, Qu FJ, Zhong YH, Lu XD, Zhao P, Wang DH, Huang QB, Zhang L, Li XG. Detection of cancer stem cells from the C6 glioma cell line. J Int Med Res. 2009;37(2):503–510. doi: 10.1177/147323000903700226. [DOI] [PubMed] [Google Scholar]
- 34.Dell’Albani P. Stem cell markers in gliomas. Neurochem Res. 2008;33(12):2407–2415. doi: 10.1007/s11064-008-9723-8. [DOI] [PubMed] [Google Scholar]
- 35.Aihara M, Sugawara K, Torii S, Hosaka M, Kurihara H, Saito N, Takeuchi T. Angiogenic endothelium-specific nestin expression is enhanced by the first intron of the nestin gene. Lab Investig. 2004;84(12):1581–1592. doi: 10.1038/labinvest.3700186. [DOI] [PubMed] [Google Scholar]
- 36.Mokrý J, Nemecek S. Angiogenesis of extra- and intraembryonic blood vessels is associated with expression of nestin in endothelial cells. Folia Biol. 1998;44(5):155–161. [PubMed] [Google Scholar]
- 37.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;7(21):1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;15(20):3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson SC, Coussens LM. Soluble mediators of inflammation during tumor development. Adv Cancer Res. 2005;93:159–187. doi: 10.1016/S0065-230X(05)93005-4. [DOI] [PubMed] [Google Scholar]
- 40.Apte RN, Krelin Y, Song X, Dotan S, Recih E, Elkabets M, Carmi Y, Dvorkin T, White RM, Gayvoronsky L, Segal S, Voronov E. Effects of micro-environment- and malignant cell-derived interleukin-1 in carcinogenesis, tumour invasiveness and tumour–host interactions. Eur J Cancer. 2006;42(6):751–759. doi: 10.1016/j.ejca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100(5):2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoelcker B, Hafner M, Orosz P, Nieswandt B, Mannel DN. Role of adhesion molecules and platelets in TNF-induced adhesion of tumor cells to endothelial cells: implications for experimental metastasis. J Inflamm. 1995;46(3):155–167. [PubMed] [Google Scholar]
- 43.Mannel DN, Orosz P, Hafner M, Falk W. Mechanisms involved in metastasis enhanced by inflammatory mediators. Circ Shock. 1994;44(1):9–13. [PubMed] [Google Scholar]
- 44.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koziak K, Sevigny J, Robson SC, Siegel JB, Kaczmarek E. Analysis of CD39/ATPdophosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost. 1999;82:1538–1544. [PubMed] [Google Scholar]
- 46.Robson SC, Enjyoji K, Goepfert C. Modulation of extracellular nucleotide-mediated signaling by CD39/nucleoside triphosphate diphosphohydrolase-1. Drug Dev Res. 2001;53:193–207. doi: 10.1002/ddr.1188. [DOI] [Google Scholar]
- 47.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niemela J, Henttinen T, Yegutkin GG, Airas L, Kujari AM, Rajala P, Jalkanen S. IFN-alpha induced adenosine production on the endothelium: a mechanism mediated by CD73 (ecto-5′-nucleotidase) up-regulation. J Immunol. 2004;172:1646–1653. doi: 10.4049/jimmunol.172.3.1646. [DOI] [PubMed] [Google Scholar]
- 49.Liao H, Hyman MC, Baek AE, Fukase K, Pinsky DJ. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) Expression. JBC. 2010;285(19):14791–14805. doi: 10.1074/jbc.M110.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]