Abstract
We investigated the possible modulation of the intestinal contractility by uracil nucleotides (UTP and UDP), using as model the murine small intestine. Contractile activity of a mouse ileum longitudinal muscle was examined in vitro as changes in isometric tension. Transcripts encoding for uracil-sensitive receptors was investigated by RT-PCR. UDP induced muscular contractions, sensitive to PPADS, suramin, or MRS 2578, P2Y6 receptor antagonist, and mimicked by PSB 0474, P2Y6-receptor agonist. UTP induced biphasic effects characterized by an early inhibition of the spontaneous contractile activity followed by muscular contraction. UTP excitatory effects were antagonized by PPADS, suramin, but not by MRS 2578, whilst the inhibitory effects were antagonized by PPADS but not by suramin or MRS 2578. UTPγS, P2Y2/4 receptor agonist but not 2-thio-UTP, P2Y2 receptor agonist, mimicked UTP effects. The inhibitory effects induced by UTP was abolished by ATP desensitization and increased by extracellular acidification. UDP or UTP responses were insensitive to TTX, atropine, or L-NAME antagonized by U-73122, inhibitor of phospholipase C (PLC) and preserved in the presence of nifedipine or low Ca2+ solution. Transcripts encoding the uracil nucleotide-preferring receptors were expressed in mouse ileum. Functional postjunctional uracil-sensitive receptors are present in the longitudinal muscle of the mouse ileum. Activation of P2Y6 receptors induces muscular contraction, whilst activation of P2Y4 receptors leads to inhibition of the contractile activity. Indeed, the presence of atypical UTP-sensitive receptors leading to muscular contraction is suggested. All uracil-sensitive receptors are linked to the PLC pathway.
Keywords: UDP, UTP, P2Y2 receptors, P2Y4 receptors, P2Y6 receptors, Intestinal motility
Introduction
Nucleotides, such as, ATP, ADP, UTP, and UDP are important extracellular signaling molecules, activating cell membrane receptors, designed as P2 receptors, subdivided into two large families, ionotropic P2X and G-coupled P2Y receptors. So far, seven mammalian P2X-receptor subtypes (P2X1-7) and eight mammalian P2Y-receptors subtypes (P2Y1,2,4,6,11,12,13,14) have been cloned and recognized. P2X receptors are ligand-gated ion channels activated by ATP. Indeed, P2Y receptors can be subdivided into (1) adenine nucleotide-preferring receptors mainly responding to ADP and ATP; (2) uracil nucleotide-preferring receptors; (3) receptors of mixed selectivity; and (4) receptors responding to the sugar nucleotides UDP–glucose and UDP–galactose [1]. In particular, ADP is the most potent physiological agonist at humans and rodents P2Y1, P2Y12, and P2Y13 receptors, whilst ATP is the agonist at the human P2Y11 receptor (although this receptor may be activated by UTP). UTP is an agonist for P2Y2 and P2Y4 receptors, whilst UDP is agonist for P2Y6 receptors. UTP is up to two orders of magnitude more potent than UDP at P2Y2 and P2Y4 receptors, while the reverse is true at P2Y6 receptors. Indeed, P2Y2 receptors are activated by both UTP and ATP, and in rodents (rats and mice), differently from the human orthologues, P2Y4 receptors are equally activated by UTP and ATP. Lastly, P2Y14 receptors initially considered to be activated solely by UDP–sugar derivatives can be also activated by UDP [2–4]. Although considerable progress has been done, most of the P2Y receptor subtypes are still lacking potent and selective synthetic agonists and antagonists. Thus, a combined use of subtype preferential agonists and antagonists may allow the pharmacological characterization of a P2Y subtype. Moreover, some of the orphan P2Y-like receptors are still waiting for deorphanization.
So far, adenine-based purines have been mainly studied as regulators of gastrointestinal physiology [5]; however, other nucleotides, such as, uridine triphosphate (UTP), are released in the extracellular space, and they can be endogenous ligands for the uracil nucleotide-preferring receptors, which have reported to be widely distributed in the intestine [6]. It is suggested that purinergic signaling pathways, via activation of uracil nucleotide-preferring receptors, contribute to the regulation of ion transport in the intestinal, biliary, and pancreatic duct epithelium [7]. P2Y2 and P2Y4 receptors are prevalent luminal receptors [8], whilst basolateral P2Y6 receptors have been shown in colonic epithelia cells to stimulate NaCl secretion [9]. Previous studies suggested that pyrimidines may also modulate gastrointestinal motility. UTP has been reported to induce muscular contractile responses in the rat proximal stomach [10], duodenum [11], rat colon muscularis mucosae [12], whilst a relaxant response has been shown in a rat distal colon due to activation of neuronal P2Y2 receptors and nitric oxide release [13]. UTP relaxant responses have been shown also in a mouse distal colon [14]. In addition, P2Y4 receptors are suggested to be the main functional receptor underlying the purinergic neuron-to-glia signaling in the enteric nervous system [15, 16].
Due to the scarce information on the physiological function of the pyrimidines as modulators of gastrointestinal motor activity and in consideration that extracellular nucleotides and their receptors have been implicated in the pathogenesis of inflammatory bowel disease [17], we aimed to investigate the possible modulation of the intestinal contractility by uracil nucleotides (UTP and UDP), and the P2Y receptor subtype(s) involved, using as model the murine small intestine, in which the functionality of the adenine nucleotide-preferring receptors has been established [18–20].
Methods
Experiments, authorized by the Ministero della Sanità (Rome, Italy), were performed on adult male mice (C57BL/10SnJ; 25.5 ± 0.5-g body weight; 15 weeks old), obtained from Charles River Laboratories, Calco-Lecco, Italy) and maintained in a light (12-h/12-h light) and temperature (23°C)-controlled environment with free access to food and water. The animals were sacrificed by cervical dislocation; the abdomen was immediately opened, the ileum was removed and placed in Krebs solution, and the contents of the excised segments were gently flushed out. Segments (20 mm in length) were suspended in a four-channel organ bath containing 10 ml of oxygenated (95% O2 and 5% CO2) Krebs solution maintained at 37°C.
Recording of mechanical activity
The distal end of each segment was tied to an organ holder, and the proximal end was secured with a silk thread to an isometric force transducer (FORT 10, Ugo Basile, Biological Research Apparatus, Comerio VA, Italy). Mechanical activity was amplified and digitized via an analog/digital interface (Quad Bridge and PowerLab/400, AD Instruments, Ugo Basile, Biological Research Apparatus, Comerio VA, Italy) and then acquired onto a personal computer. Longitudinal preparations were subjected to an initial tension of 200 mg (unless otherwise stated) and were allowed to equilibrate for at least 30 min. Rhythmic spontaneous contractions of varying amplitude developed in all preparations. After the equilibration time, preparations were challenged with 10 μM of carbachol until reproducible responses were obtained. The amplitude of the contractile response to carbachol was 968.2 ± 64.1 mg (n = 20). Moreover, preparations were challenged with 1 μM isoproterenol for 2 min, to verify if they were able to relax. The amplitude of the relaxant response to isoprotenerol was 216.2 ± 21.3 mg (n = 20). Since activation of P2 receptors can be controlled by ecto-nucleotidases capable of hydrolyzing nucleoside tri- and diphosphates [21], UDP and UTP were tested in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM).
Concentration–response curves for uracil nucleotides or related drugs were constructed by noncumulative addition of the drug. The contact time between tissue and agonist was 3 min, which was followed by two washings with 1 min between each wash. Twenty-minute intervals between doses were assessed. The antagonists were allowed to maintain contact with the tissue for at least 30 min before eventually repeating the curve of agonists. Time control experiments showed that a second curve to the agonist was reproducible. Tetrodotoxin (TTX), atropine, or Nω-nitro-L-arginine methyl ester (L-NAME) were tested against a submaximal dose of UDP or UTP. Each preparation was tested with a single agonist and antagonist, except when otherwise stated.
ATP-sensitive P2 receptor desensitization was induced by prolonged perfusion with ATP (30 μM for 10 min). A transient relaxation occurred on addition of ATP to the bath after which the basal tone returned back to the baseline value and after 10 min ATP response was abolished. UTP effects were also studied at pH 6.5 Krebs solution, prepared by adding, to the physiological pH (7.4) solution, 1.0 N HCl to achieve the desired level. Lastly, UDP and UTP were tested in the presence of U-73122, a widely used specific inhibitor of phospholipase C, nifedipine, L-type voltage-dependent calcium channel blocker, and low calcium solution.
Solution and drugs
Krebs solution consisted of (in millimolar): NaCl, 119; KCl, 4.5; MgSO4, 2.5; NaHCO3, 25; KH2PO4, 1.2; CaCl2, 2.5; glucose, 11.1. Solution with low calcium was made by equimolar substitution with sodium. The following drugs were used: adenosine 5′-triphosphate (ATP), atropine sulphate, carbachol, 4-[[4-Formyl-5-hydroxy-6-methyl-3-[(phosphonooxy) methyl]-2-pyridinyl] azo]-1,3-benzenedisulfonic acid tetrasodium salt (PPADS), indomethacin,{1-[6 ((17β-3-methox-yestra-1,3,5 (10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione} (U-73122), L-NAME, nifedipine, 6-N,N-diethyl-d-b,g-dibromomethyleneATP trisodium salt (ARL 67156), suramin, tetrodotoxin (TTX), uridine 5′-diphosphate sodium salt (UDP), uridine 5′-triphosphate tris salt (UTP), (Sigma-Aldrich, Inc., St. Louis, USA). N,N˝-1,4-butanediylbis[N′-(3-isothiocyanatophenyl)thiourea (MRS 2578), 3-(2-oxo-2-phenylethyl)-uridine-5′-diphosphate disodium salt (PBS 0474), 2-thiouridine 5′-triphosphate tetrasodium salt (2-thio-UTP), uridine-5′-(γ-thio)-triphosphate trisodium salt (UTPγS) (Tocris Cookson Ltd., Avonmouth). Indomethacin solution was prepared fresh in 2% sodium carbonate solution and the pH was adjusted to 7.4. MRS 2578 was dissolved in dimethyl sulphoxide, U-73122 and nifedipine in ethanol, and further diluted in Krebs. All the other drugs were dissolved in distilled water. Control experiments using the different solvents alone showed that none has effect on the contractility of the ileum segments. The working solutions were prepared fresh on the day of the experiment by diluting the stock solutions in Krebs. Concentrations of the drugs used were determined from literature.
RNA isolation and RT-PCR
Total RNA was extracted from mouse ileum specimens devoid of mucosa layer, using PureLink™ RNA Mini Kit (Invitrogen, Paisley, UK) according to the manufacturer’s instructions. After quantification by spectrophotometry, 1 μg of total RNA was reverse-transcribed in a final volume of 50 μl using the High Capacity c-DNA Reverse Transcription Kit (Applied Biosystems, Foster city, CA, USA) and the following thermal cycle profile: 10 min at 25°C, 2 h at 37°C, and 5 min at 85°C as described by the kit. cDNA (100 ng per reaction) was denatured and subjected to PCR amplification. Each PCR cycle consisted of denaturing at 94°C for 45 s, annealing at 58°C (P2Y2, P2Y4 receptors and β-actin) or 56°C (P2Y6 receptor) for 45 s, and extension at 72°C for 1 min. This was repeated for 35 cycles, followed by extension at 72°C for 15 min. PCR analysis was performed in triplicate. The oligonucleotide primers for mouse P2Y2, P2Y4 and P2Y6 receptors, and β-actin were as follows: P2Y2: (forward) 5′-CTCACGCGCACCCTCTACTA-3′, (reverse) 5′-TCGGGTGCACTGCCTTTCTT-3′; P2Y4: (forward) 5′-CTTTGGCTTTCCCTTCTTGA-3′, (reverse) 5′-GTCCGCCCACCTGCTGAT-3′; P2Y6: (forward) 5′-GCCCTGTGCTGGAGACCTTC-3′, (reverse) 5′-CATGGCCCCAGTGACAAACA-3′; β-actin: (forward) 5′-CCGCCCTAGGCACCAGGGT-3′, (reverse) 5′-GGCTGGGGTGTTGAAGGTCTCAAA-3′.
The amplimers were separated on a 1% agarose gel containing 0.5 μg ml−1 of ethidium bromide for visualization, and the gel was scanned under UV light. The expected length of the PCR products for P2Y2, P2Y4 and P2Y6 receptors, and β -actin were 548, 427, 226, and 300 bp, respectively. The corrected band intensities of the specific P2Y receptors obtained using the ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA) were normalized with the corresponding β-actin mRNA expression. It was ensured that the band intensities of β-actin mRNA were similar before the comparison of the expression levels.
Statistical analysis
All data are given as means ± S.E.M. The variable “n” in the results section refers to the number of animal preparations on which observations were made. Contractile responses were expressed as a percentage of the amplitude of contraction induced by 10 μM carbachol (CCh). Inhibitory effects were estimated as a percentage of the decrease of the spontaneous contraction amplitude, 100% corresponding to the total suppression of spontaneous contractions. Responses to uracil nucleotides and related drugs were fitted to sigmoid curves (Prism 4.0, GraphPAD, San Diego, CA, USA), and EC50 values (with 95% confidence intervals (CIs)) were determined from these curves. Statistical analysis was performed by means of Student’s t test or by means of analysis of variance, followed by Bonferroni’s test, when appropriate. A probability value of less than 0.05 was regarded as significant.
Results
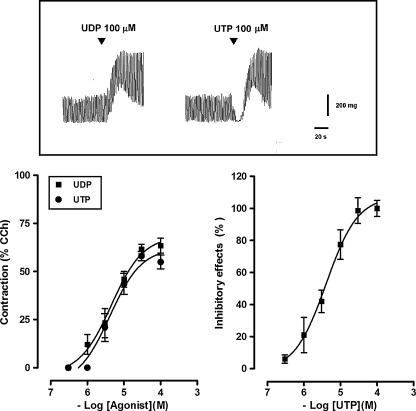
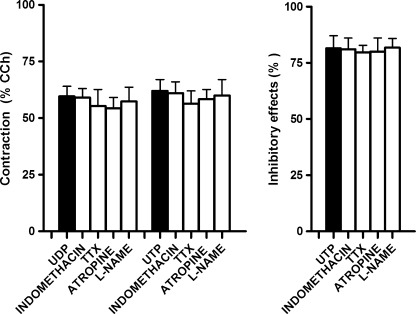
Isolated segments of the mouse ileum displayed spontaneous activity consisting of phasic contractions with an amplitude of 347.2 ± 31.0 mg (n = 20) and a frequency of 30.1 ± 3.2 cpm (n = 20). Noncumulative additions of UDP and UTP, at concentrations ranging from 0.3 to 100 μM, were tested in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM), which per se did not modify spontaneous mechanical activity. UDP induced a concentration-dependent muscular contraction, reaching a maximum of 641.5 ± 58.3 mg (n = 20) at a concentration of 100 μM, with an EC50 of 4.1 μM (95% CIs, 2.3–8.7 μM, n = 20) (Fig. 1). Indeed, UTP caused a concentration-dependent biphasic effect characterized by an early, transient inhibitory effect, consisting of a transient reduction of the amplitude of the spontaneous contractile activity up to the complete abolition (EC50 = 4.0 μM; 95% CIs, 2.7–7.2 μM; n = 20), followed by muscular contraction (EC50 = 4.5 μM; 95% CIs, 1.1–15.5 μM; n = 20) (Fig. 1). The maximal contractile response of administered UTP (100 μM) was 602.0 ± 24.1 mg (n = 20). No evident relaxation was observed also in preparations subjected to an initial tension of 500 mg. Indomethacin (10 μM), a cyclooxygenase inhibitor, TTX (1 μM), neural voltage-gated sodium channel blocker, atropine (1 μM), muscarinic receptor antagonist, or L-NAME (100 μM), a nitric oxide synthase inhibitor, failed to affect the responses to submaximal doses of UDP and UTP (10 μM each) (Fig. 2).
Fig. 1.
Original tracings showing the effects of the UDP and UTP (100 μM each) on spontaneous mechanical activity of the longitudinal muscle of a mouse ileum (top). Concentration–response curves for the contractile effects induced by UDP or UTP and for the inhibitory effects induced by UTP in the longitudinal muscle of a mouse ileum (bottom). Experiments were performed in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM). Contractile responses are expressed as a percentage of the amplitude of contraction induced by carbachol (CCh, 10 μM), whilst inhibitory responses as a percentage of the decrease of the spontaneous contraction amplitude, 100% corresponding to the total suppression of spontaneous contractions. Data are means ± S.E.M. (n = 20)
Fig. 2.
Histograms showing the lack of effect of the cyclooxygenase inhibitor, indomethacin (10 μM, n = 4), the Na+ voltage-gated neural channel blocker, TTX (1 μM, n = 5), the muscarinic receptor antagonist, atropine (1 μM, n = 5), or the NO synthase inhibitor L-NAME (100 μM, n = 5) on the response induced by UDP or UTP (10 μM each). Experiments were performed in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM). Contractile responses are expressed as a percentage of the amplitude of contraction induced by carbachol (CCh, 10 μM), whilst inhibitory responses as a percentage of the decrease of the spontaneous contraction amplitude, 100% corresponding to the total suppression of spontaneous contractions. Data are means ± S.E.M. (n = 5). The graphed values for the UDP or UTP bars are the means of the data obtained before each treatment (n = 19)
Pharmacological characterization of UDP responses
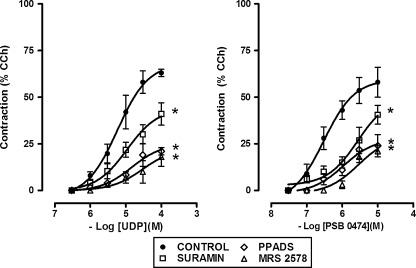
UDP excitatory effects were significantly reduced in the presence of the P2 receptor antagonists, PPADS (50 μM) or suramin (100 μM) (Fig. 3). The effect of PPADS and suramin appeared to be noncompetitive leading to a reduction in the maximal response. In addition, the UDP responses were markedly reduced by MRS 2578 (0.1 μM), noncompetitive P2Y6 receptor antagonist [22] (Fig. 3). The P2Y6-purinoreceptor agonist, PSB 0474 (0.03–10 μM) [23], induced, as well, a concentration-dependent contraction of mouse ileum longitudinal muscle (EC50 = 0.3 μM, 95% CIs, 0.2–0.4 μM, n = 5) (Figs. 3 and 4), antagonized by PPADS (50 μM), suramin (100 μΜ), or by the P2Y6 receptor antagonist, MRS 2578 (0.1 μΜ) (Fig. 3). None of the antagonists per se had any effects on the spontaneous activity of mouse ileum.
Fig. 3.
Concentration–response curves for the contractile effects induced by UDP or PSB 0474, P2Y6 selective receptor agonist, in the longitudinal muscle of a mouse ileum, in the absence or in the presence of the P2 receptor antagonists, PPADS (50 μM, n = 5), suramin (100 μM, n = 5) or of the P2Y6 receptor antagonist, MRS 2578 (0.1 μM, n = 5). UDP was tested in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM). Data are means ± S.E.M. and are expressed as a percentage of the amplitude of contraction induced by carbachol (CCh, 10 μM). The values for the control curves are the means of the control data obtained before each treatment (n = 15). *P ≤ 0.05 when the concentration–response curves were compared to those obtained in the respective control condition
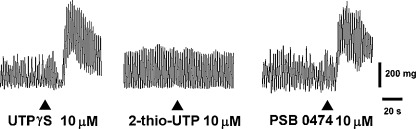
Fig. 4.
Original tracings showing the effects on the spontaneous mechanical activity of the longitudinal muscle of a mouse ileum induced by the P2Y2, P2Y2/4, and P2Y6 receptor agonists, 2-thio-UTP, UTPγS, and PSB 0474 (10 μM each), respectively
Pharmacological characterization of UTP responses
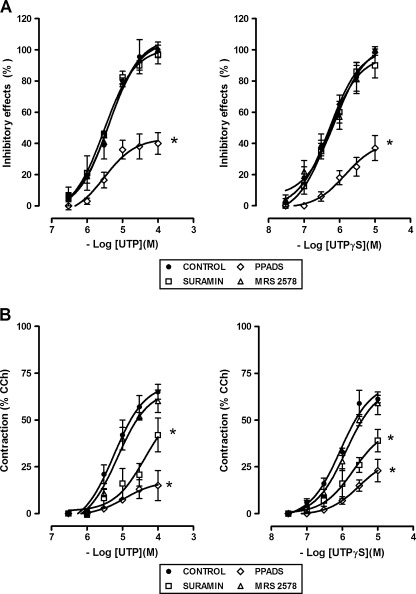
UTP inhibitory effects were markedly reduced by PPADS (50 μM), but not affected by suramin (100 μM) or by the P2Y6 receptor antagonist, MRS 2578 (0.1 μM) (Fig. 5a). Indeed, the excitatory effects were significantly antagonized by PPADS (50 μM), suramin (100 μM), but not by MRS 2578 (0.1 μM) (Fig. 5b). UTPγS (0.03–10 μM), P2Y2/4 receptor agonist, mimicked UTP responses, inducing a dose-dependent biphasic response characterized by the early inhibitory effect followed by the excitatory one (inhibitory effect: EC50 = 0.5 μM, 95% CIs, 0.3–0.9 μM; excitatory effect: EC50 = 0.9 μM, 95% CIs, 0.3–2.4 μM; n = 4) (Figs. 4 and 5). UTPγS-induced inhibitory effects were antagonized by PPADS (50 μM), but not by suramin (100 μM), whilst the contractile effects were significantly reduced by PPADS (50 μM) and suramin (100 μΜ) (Fig. 5). MRS 2578 (0.1 μM) was without any effect. 2-Thio-UTP (0.1–10 μM), P2Y2 selective agonist, did not cause any significant changes in the muscular activity (Fig. 4), even in preparation precontracted with 1 μM CCh (data not shown). Moreover, 2-thio-UTP (10 μM) did not attenuate or potentiate a subsequent response to UTP.
Fig. 5.
Concentration–response curves for the excitatory and inhibitory effects induced by UTP or UTPγS, P2Y2/4 receptor agonist, in the longitudinal muscle of a mouse ileum, in the absence or in the presence of the P2 receptor antagonists, PPADS (50 μM, n = 5), suramin (100 μM, n = 5) or of the P2Y6 receptor antagonist, MRS 2578 (0.1 μM, n = 5). UTP was tested in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM). Contractile responses are expressed as a percentage of the amplitude of contraction induced by carbachol (CCh, 10 μM), whilst inhibitory responses as a percentage of the decrease of the spontaneous contraction amplitude, 100% corresponding to the total suppression of spontaneous contractions. Data are means ± S.E.M. The values for the control curves are the means of the control data obtained before each treatment (n = 10–15). *P ≤ 0.05 when the concentration–response curves were compared to those obtained in the respective control condition
As previously reported, in a mouse ileum, ATP evoked a rapid and transient concentration-dependent relaxation, followed by a contraction at the higher concentrations [18]. Since in rodents P2Y4 receptors are equally activated by UTP and ATP [3], we tested the effects of ATP desensitization on the response induced by UTP. Desensitization of purinoreceptors with ATP abolished the inhibition of mechanical activity induced by UTP (10 μM), without affecting the contractile response (Fig. 6). In addition, due to the reported effects of extracellular acidification in altering the potency of agonists at P2Y4 receptors in contrast to P2Y2 receptors [24], the effect of changing the pH was investigated on the response to UTP. Changing the pH of the Krebs solution from 7.4 to 6.6 resulted in an increase in the inhibitory effect of UTP (10 μM), leaving the excitatory effect unaltered (Fig. 6). Lowering the pH to 6, the spontaneous activity of the ileum segments was destroyed, and the effect could not be investigated.
Fig. 6.
Original tracings showing the effects on the UTP (10 μM)-induced responses by ATP receptor desensitization and by changing the pH of the Krebs solution from 7.4 to 6.6. Experiments were performed in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM)
Signal transduction mechanisms
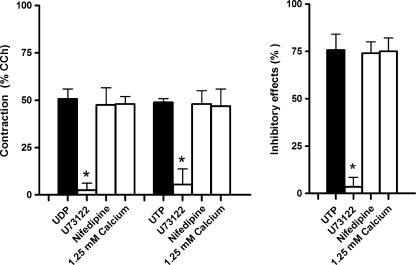
As shown in Fig. 7, the responses induced by UDP and UTP (10 μM each) were significantly antagonized in the presence of U-73122 (1 μM), a widely used inhibitor of phospholipase C (PLC). On the contrary, the responses to UDP and UTP (10 μM each) were preserved in the presence of nifedipine (0.5 μM) or low Ca2+ solution (1.25 mM).
Fig. 7.
Histograms showing the effects induced on the responses to UDP and UTP (10 μM each) by U-73122 (1 μM, n = 4), a widely used specific inhibitor of phospholipase C, nifedipine (0.5 μM, n = 4), L-type calcium channel blockers, or low Ca2+ solution (1.25 mM, n = 4). Experiments were performed in the continued presence of the ecto-nucleotidase inhibitor ARL67156 (30 μM). Contractile responses are expressed as a percentage of the amplitude of contraction induced by carbachol (CCh, 10 μM), whilst inhibitory responses as a percentage of the decrease of the spontaneous contraction amplitude, 100% corresponding to the total suppression of spontaneous contractions. Data are means ± S.E.M. The graphed values for the UDP or UTP bars are the means of the data obtained before each treatment (n = 12). *P ≤ 0.05 when compared to the respective own control
Transcripts encoding uracil nucleotide-preferring receptors in mouse ileum
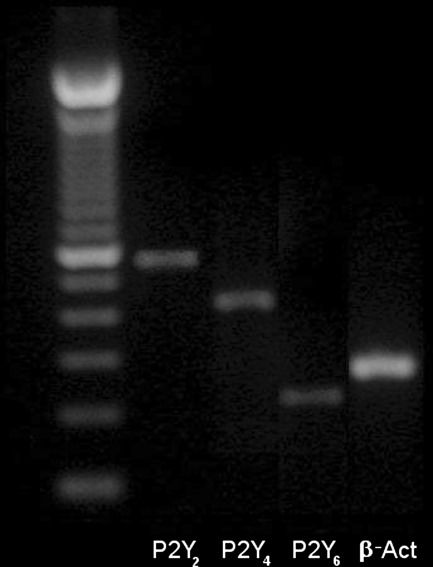
RT-PCR was used to investigate the expression of mRNA encoding for P2Y2, P2Y4, and P2Y6, receptors on preparation. Transcripts encoding each of receptors were found in our preparation (Fig. 8).
Fig. 8.
Expression of transcripts encoding the P2Y receptor subtypes, P2Y2, P2Y4 and P2Y6 in mouse ileum. β-Actin (β-Act) primer was used as a control for cDNA integrity. A 100 bp DNA ladder was used as marker
Discussion
Results from the present study indicate the presence, in the longitudinal muscle of a mouse ileum, of functional UTP- and UDP-sensitive receptors. Activation of these receptors induces both excitatory and inhibitory effects and thus modulates the spontaneous contractile activity. UDP, via activation of postjunctional P2Y6 receptors, induces muscular contraction. UTP, via activation of postjunctional P2Y4 receptors, induces inhibition of the spontaneous contractile activity, whilst via activation of postjunctional receptors, which cannot be accounted for by the properties of current molecular identified/characterized P2Y receptors, induces muscular contraction. All uracil nucleotide-preferring receptors are linked to the PLC pathway.
The role of the P2Y receptor activated by adenine-based purines in the control of intestinal motility has been extensively studied in the last years [7], but so far, only few studies investigated a possible involvement of uracil nucleotides [10–12, 14, 25]. Presently known uracil-sensitive receptors are belonging to the P2Y2, P2Y4, and P2Y6 subtypes, which show differential sensitivity to UTP or UDP. P2Y6 is considered to be specific for UDP, whereas UTP fails to discriminate between P2Y2 and P2Y4.
Data from our experiments indicate that transcripts for P2Y2, P2Y4, and P2Y6 receptors subtypes are expressed in the mouse ileum and that both nucleotides are able to modulate the contractility of the intestinal muscle, via activation of distinct uracil nucleotide-sensitive receptors. UDP exerts exclusively excitatory effects, whilst UTP induces biphasic effects, inhibition of the mechanical activity followed by muscular contraction. Contractions generated by UTP and UDP in ileum muscle are identical, indicating that the tissue responds similarly to UTP and UDP, as reported in rat mesenteric and basilar arteries [26, 27] or in rat urinary bladder [28].
The sensitivity of the responses to the nonselective purinergic antagonists, PPADS and suramin was, at first, determined. As previously reported before, the nonselective P2 purinoreceptor antagonist PPADS is able to antagonize equipotently P2Y4 and the P2Y6 purinoreceptor in mouse, whereas suramin antagonizes the responses to activation of P2Y2, P2Y6 purinoreceptor, while it is ineffective at the P2Y4 receptor subtype [3]. Data from our experiments revealed that the contractile effects induced by UDP were antagonized by PPADS and suramin, suggesting an involvement of P2Y6 receptors. A conclusion was corroborated by the antagonism of the response in the presence of MRS 2578, an insurmountable noncompetitive P2Y6 antagonist and by the observation that UDP effect was mimicked by the selective P2Y6-receptor agonist, PSB 0474. As expected, activation of P2Y6 receptors in our preparation induces activation of PLC and increases in intracellular calcium concentration via release of Ca2+ from internal stores. We are aware that recently it has been reported that UDP is an important cognate agonist of the P2Y14 receptors [4] and that activation of P2Y14 receptors induces contractile effects on rat isolated stomach [29]. Whether or not UDP effects may be due also to coactivation of P2Y14 receptors in our preparation have not been investigated and deserve further experiments.
UTP induced in a mouse ileum an inhibition of the contractile activity, as reported in other regions of a mouse intestine [14, 30, 31]. No relaxation was observed although ileum was able to relax in response of isoprotenerol. This inhibitory effect was potently antagonized by PPADS, but not affected by suramin, giving evidence of pharmacological interaction with the P2Y4 receptors, since suramin antagonizes all P2Y receptor subtypes except the P2Y4 receptor subtype [3]. Indeed, UTPγS, P2Y2/4 agonist [32], but not the P2Y2 selective agonist, mimicked the effect induced by UTP. The involvement of P2Y4 receptors is corroborated by the observation that the inhibitory response to UTP was abolished by desensitization of the receptors by ATP, since, as previously reported, murine P2Y4 receptors can be equally activated by ATP and UTP. Moreover, the inhibitory effect was increased by decreasing the pH of the medium, which has been shown to potentiate P2Y4 receptor responses induced by stimulation with UTP [24]. Therefore, these observations indicate that activation of P2Y4 receptors is responsible for the genesis of the early inhibition of the contractile activity induced by UTP. Activation of P2Y4 receptors induces inhibition of the ileum contractile activity via the PLC pathway, likely causing a highly directional and local Ca2+ transients who would increase the open probability of calcium dependent K+ channels [33, 34].
Indeed, from our results, the pharmacological profile of UTP contractile responses cannot allow us to allocate them to the properties of current molecular identified and characterized P2Y receptors. In fact, the possibility that UTP may interact per se with P2Y6 receptors can be excluded since the contractile effects evoked by UTP were not affected by MRS 2578. The contractile response to UTP resulted to be antagonized by PPADS and suramin, letting us hypothesize an involvement of both P2Y2 and P2Y4 receptor activation. As already reported, UTPγS, P2Y2/4 agonist, mimicked the effect induced by UTP, including as well contractile responses. The observation that P2Y2 selective agonist did not cause any significant changes in the muscular activity, even in precontracted preparation, did not allow us to assign a role of P2Y2 in UTP-induced effects. Moreover, we did not observe any synergic/antagonist effects between P2Y2 agonist and UTP. Although the sensitivity to PPADS of the contractile responses could indicate a partial involvement of P2Y4 receptors, the following observations do not corroborate such a possibility: (1) as previously demonstrated, ATP, in the mouse ileum, induces by activation of P2Y receptors only muscular relaxation [18]; (2) desensitization of P2Y4 receptors by prolonged exposure to ATP did not affect UTP-induced contraction; (3) the contractile response was not affected by acidification of extracellular medium. Altogether, these results may allow us to exclude even the involvement of P2Y4 receptors in the contractile effects induced by UTP. Therefore, UTP contractile responses appear to be mediated by atypical UTP-sensitive receptors, resembling those described in the dorsal spinal astrocytes of rats [35] and in the superior cervical ganglion neurons of mice [36]. However, the pharmacological profile of UTP contractile responses is the same as UTPγS, which, as already underlined, is nominally a selective P2Y2/4 receptors agonist. Therefore, it is possible also to speculate that, in our preparations, P2Y4 receptors may directly associate as either homo- or hetero-oligomers, with other P2Y receptor subtypes or non-P2Y receptors, to form receptors with novel pharmacological properties. The structural propensity of the P2Y4 protein to form homo-oligomers has been demonstrated in neuronal cells from the central and peripheral nervous systems [37]. Moreover, UTP-induced contraction appears to be mediated by G-protein-coupled receptors activating PLC, leading to increase in intracellular calcium concentration by release from intracellular stores. The observation that UTP-induced contraction was preserved in low calcium medium and in the presence of nifedipine discarding the possibility that UTP may activate P2X receptor cation channels promoting calcium influx via nifedipine-sensitive calcium channels, as reported in rat vascular muscle [38, 39].
Results from the present study suggest also that uracil-sensitive receptors are located at postjunctional level, since the activation of neural sodium channels is not essential for the contractile or inhibitory induced effects. In addition, neither acetylcholine nor nitric oxide, nor prostaglandins are involved in the excitatory or inhibitory effects to pyrimidines due to the insensitivity of the response to atropine, L-NAME or indomethacin. Thus, uracil-sensitive receptors may be located directly on the smooth muscle, although there is the possibility that they can be expressed also on the membrane of other cell types composing the gut wall. For instance, recent studies have suggested that enteric glia would express P2Y4 receptors which can be the main functional receptor underlying the purinergic neuron-to-glia signaling in the enteric nervous system [15, 16]. Moreover, the “fibroblast-like cells,” recently proposed as new player in the regulation of GI motility, which may mediate a significant component of the purinergic postjunctional response in colonic muscles [40], might express also uracil-sensitive P2Y receptors.
Although defining the precise role of pyrimidines in regulating intestinal motility will require further investigation, the present findings imply that studies on uracil-sensitive P2Y receptors are a potentially promising target for the treatment of pathological conditions in the intestinal tract, including inflammation. Large amounts of extracellular nucleotides are rapidly released into the extracellular environment at the site of inflammation [41], and P2Y6 receptors have been reported to be upregulated in the context of inflammatory insult in a mouse colon [42].
In conclusion, functional postjunctional UTP- and UDP-sensitive P2Y receptors are present in the longitudinal muscle of a mouse ileum. P2Y6 receptors, activated by UDP, underlie muscular contraction, whilst P2Y4 receptors, activated by UTP, underlie inhibition of the contractile activity. We report, also, evidence for the presence of atypical UTP-sensitive receptors subserving the excitation of the contractility. All the uracil-sensitive receptors are linked to the PLC pathway.
Acknowledgments
This work was supported by a grant from Ministero dell’Università e della Ricerca Scientifica, Italy.
References
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 3.Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, Das A, Jacobson KA, Lazarowski ER, Harden TK. Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol. 2009;76:1341–1348. doi: 10.1124/mol.109.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornstein JC. Purinergic mechanisms in the control of gastrointestinal motility. Purinergic Signal. 2008;4:197–212. doi: 10.1007/s11302-007-9081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 7.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal. 2008;4:213–236. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol. 2005;564(Pt 1):269–279. doi: 10.1113/jphysiol.2004.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Köttgen M, Löffler T, Jacobi C, Nitschke R, Pavenstädt H, Schreiber R, Frische S, Nielsen S, Leipziger J. P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP-mediated transport. J Clin Invest. 2003;111:371–379. doi: 10.1172/JCI16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsuguro K, Ito S, Ohta T, Nakazato Y. Influence of purines and pyrimidines on circular muscle of the rat proximal stomach. Eur J Pharmacol. 1996;317:97–105. doi: 10.1016/S0014-2999(96)00694-2. [DOI] [PubMed] [Google Scholar]
- 11.Johnson CR, Hourani SM. Contractile effects of uridine 5′-triphosphate in the rat duodenum. Br J Pharmacol. 1994;113:1191–1196. doi: 10.1111/j.1476-5381.1994.tb17123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hourani SM, Bailey SJ, Johnson CR, Tennant JP. Effects of adenosine 5′-triphosphate, uridine 5′-triphosphate, adenosine 5′-tetraphosphate and diadenosine polyphosphates in guinea-pig taenia caeci and rat colon muscularis mucosae. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:464–473. doi: 10.1007/PL00005279. [DOI] [PubMed] [Google Scholar]
- 13.Crombruggen K, Lefebvre RA. Nitrergic–purinergic interactions in rat distal colon motility. Neurogastroenterol Motil. 2004;16:81–98. doi: 10.1046/j.1365-2982.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 14.Zizzo MG, Mule’ F, Serio R. Activation of P2Y receptors by ATP and by its analogue, ADPbetaS, triggers two calcium signal pathways in the longitudinal muscle of mouse distal colon. Eur J Pharmacol. 2008;595:84–89. doi: 10.1016/j.ejphar.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 15.Nassauw L, Costagliola A, Op V, Bosch J, Cecio A, Vanderwinden JM, Burnstock G, Timmermans JP. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton Neurosci. 2006;126–127:299–306. doi: 10.1016/j.autneu.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 17.Somers GR, Hammet FM, Trute L, Southey MC, Venter DJ. Expression of the P2Y6 purinergic receptor in human T cells infiltrating inflammatory bowel disease. Lab Invest. 1998;78:1375–1383. [PubMed] [Google Scholar]
- 18.Baldassano S, Zizzo MG, Serio R, Mule’ F. Interaction between cannabinoid CB1 receptors and endogenous ATP in the control of spontaneous mechanical activity in mouse ileum. Br J Pharmacol. 2009;158:243–251. doi: 10.1111/j.1476-5381.2009.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zizzo MG, Bonomo A, Belluardo N, Mule’ F, Serio R. A1 receptors mediate adenosine inhibitory effects in mouse ileum via activation of potassium channels. Life Sci. 2009;84:772–778. doi: 10.1016/j.lfs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Zizzo M, Mastropaolo M, Lentini L, Mule’ F, Serio R. Adenosine negatively regulates duodenal motility in mice: role of A(1) and A(2A) receptors. Br J Pharmacol. 2011;164:1580–1589. doi: 10.1111/j.1476-5381.2011.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 22.Mamedova LK, Joshi BV, Gao ZG, Kügelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Tayeb A, Qi A, Muller CE. Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem. 2006;49:7076–7087. doi: 10.1021/jm060848j. [DOI] [PubMed] [Google Scholar]
- 24.Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crombruggen K, Nassauw L, Timmermans JP, Lefebvre RA. Inhibitory purinergic P2 receptor characterisation in rat distal colon. Neuropharmacology. 2007;53:257–271. doi: 10.1016/j.neuropharm.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Malmsjö M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol. 2000;131:51–56. doi: 10.1038/sj.bjp.0703536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malmsjö M, Hou M, Pendergast W, Erlinge D, Edvinsson L. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between contractile cerebrovascular P2 receptors. Eur J Pharmacol. 2003;458:305–311. doi: 10.1016/S0014-2999(02)02787-5. [DOI] [PubMed] [Google Scholar]
- 28.Aronsson P, Andersson M, Ericsson T, Giglio D. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol. 2010;107:603–613. doi: 10.1111/j.1742-7843.2010.00554.x. [DOI] [PubMed] [Google Scholar]
- 29.Bassil AK, Bourdu S, Townson KA, Wheeldon A, Jarvie EM, Zebda N, Abuin A, Grau E, Livi GP, Punter L, Latcham J, Grimes AM, Hurp DP, Downham KM, Sanger GJ, Winchester WJ, Morrison AD, Moore GB. UDP-glucose modulates gastric function through P2Y14 receptor-dependent and -independent mechanisms. Am J Physiol. 2009;296:G923–G930. doi: 10.1152/ajpgi.90363.2008. [DOI] [PubMed] [Google Scholar]
- 30.Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/S0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- 31.Giaroni C, Knight GE, Zanetti E, Chiaravalli AM, Lecchini S, Frigo G, Burnstock G. Postnatal development of P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2006;50:690–704. doi: 10.1016/j.neuropharm.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Lazarowski ER, Watt WC, Stutts MJ, Brown HA, Boucher RC, Harden TK. Enzymatic synthesis of UTP gamma S, a potent hydrolysis resistant agonist of P2U-purinoceptors. Br J Pharmacol. 1996;117:203–209. doi: 10.1111/j.1476-5381.1996.tb15175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol. 2000;279:C126–C135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- 34.Kong ID, Koh SD, Bayguinov O, Sanders KM. Small conductance Ca2+-activated K+ channels are regulated by Ca2+-calmodulin-dependent protein kinase II in murine colonic myocytes. J Physiol. 2000;524(Pt 2):331–337. doi: 10.1111/j.1469-7793.2000.t01-1-00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvert JA, Atterbury-Thomas AE, Leon C, Forsythe ID, Gachet C, Evans RJ. Evidence for P2Y1, P2Y2, P2Y6 and atypical UTP-sensitive receptors coupled to rises in intracellular calcium in mouse cultured superior cervical ganglion neurons and glia. Br J Pharmacol. 2004;143:525–532. doi: 10.1038/sj.bjp.0705959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Ambrosi N, Iafrate M, Vacca F, Amadio S, Tozzi A, Mercuri NB, Volonté C. The P2Y(4) receptor forms homo-oligomeric complexes in several CNS and PNS neuronal cells. Purinergic Signal. 2006;2:575–582. doi: 10.1007/s11302-006-9014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaren GJ, Burke KS, Buchanan KJ, Sneddon P, Kennedy C. Evidence that ATP acts at two sites to evoke contraction in the rat isolated tail artery. Br J Pharmacol. 1998;124:5–12. doi: 10.1038/sj.bjp.0701772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugihara M, Morita H, Matsuda M, Umebayashi H, Kajioka S, Ito S, Nishida M, Inoue R, Futatsuki T, Yamazaki J, Mori Y, Inoue R, Ito Y, Abe K, Hirata M. Dual signaling pathways of arterial constriction by extracellular uridine 5′-triphosphate in the rat. J Pharmacol Sci. 2011;115:293–308. doi: 10.1254/jphs.10281FP. [DOI] [PubMed] [Google Scholar]
- 40.Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KS, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G401–G410. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 42.Grbic DM, Degagné E, Langlois C, Dupuis AA, Gendron FP. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J Immunol. 2008;180:2659–2668. doi: 10.4049/jimmunol.180.4.2659. [DOI] [PubMed] [Google Scholar]