Abstract
Adenosine is an endogenous nucleoside that modulates many physiological processes through four receptor subtypes (A1, A2a, A2b, A3). Previous work from our laboratory has uncovered a critical role for adenosine A1 receptor (A1 R) in osteoclastogenesis both in vivo and in vitro. Our current work focuses on understanding the details of how A1 R modulates the receptor activator of NF-κB ligand (RANKL)-induced signaling in osteoclastogenesis. Osteoclasts were generated from mouse bone marrow precursors in the presence of RANKL and macrophage-colony stimulating factor. A pharmacological antagonist of A1 R (DPCPX) inhibited RANKL-induced osteoclast differentiation, including osteoclast-specific genes (Acp5, MMP9, β3Integrin, αvIntegrin, and CTSK) and osteoclast-specific transcription factors such as c-fos and nuclear factor of activated T cells cytoplasmic 1 (NFATc1) expression in a dose-dependent manner. DPCPX also inhibited RANKL-induced activation of NF-κB and JNK/c-Jun but had little effect on other mitogen-activated protein kinases (p38 and Erk). Finally, immunoprecipitation analysis showed that blockade of A1R resulted in disruption of the association of tumor necrosis factor receptor-associated factor 6 (TRAF6) and transforming growth factor-β-activated kinase 1 (TAK1), a signaling event that is important for activation of NF-κB and JNK, suggesting the participation of adenosine/A1R in early signaling of RANKL. Collectively, these data demonstrated an important role of adenosine, through A1R in RANKL-induced osteoclastogenesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-012-9292-9) contains supplementary material, which is available to authorized users.
Keywords: Adenosine A1 receptor, Osteoclastogenesis, TAK1, TRAF6, NF-κB
Introduction
Osteoclasts, which are derived from monocytic/macrophagic hematopoietic cells in response to macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL), a member of the tumor necrosis factor (TNF) cytokine superfamily, play a critical role in bone remodeling. Increased osteoclast activity is seen in many osteopenic disorders, including postmenopausal osteoporosis [1, 2], Paget's disease [3, 4], bone metastases [5, 6], periodontitis [7, 8], and rheumatoid arthritis [9, 10]. Mature osteoclasts are giant, multinucleated cells that synthesize and directionally secrete bone matrix-degrading enzymes, including cathepsin K, matrix metalloproteinase-9 (MMP9), and tartrate-resistant acid phosphatase (TRAP). The bone-resorbing activity of osteoclasts requires their adherence to the bone surface and subsequent development of ruffled borders and sealing zones. A substantial body of evidence suggests that adhesion molecules, including the integrin αvβ3, play an important role in regulating migration, adhesion, polarization, and activation of osteoclasts [11–14].
Activation of the NF-κB complex is a key early event in RANKL-induced osteoclast formation. Mammalian cells have five NF-κB family members (RelA/p65, RelB, c-Rel, NF-κB1/p105, and NF-κB2/p100) which contain an N-terminal Rel homology domain (RHD) with sequences for dimerization, DNA binding, and nuclear localization. Mice that lack both the p50 and p52 subunits of NF-κB developed severe osteopetrosis due to a defect in osteoclast differentiation. Mice lacking c-Fos, a component of the transcription factor activator protein-1 (AP-1), also develop osteopetrosis due to a complete block in osteoclast differentiation [15]. AP-1 binding sites have been identified in the promoters of several osteoclast genes including Acp5, β3 integrin, carbonic anhydrase II, and NFATc1 [16–19]. Moreover, AP-1 cooperates with other transcription factors (e.g., NF-κB and NFATc1) to regulate RANKL-induced transcription osteoclast-specific genes [20]. Binding of RANKL to RANK activates other signals that are critical for osteoclast formation as well including activation of mitogen-activated protein kinases (MAPKs), namely the extracellular signal-regulated kinase (Erk), c-Jun N-terminal kinase (JNK), and p38 kinase. Genetic and biochemical studies indicate that the activation of JNK/c-Jun is indispensible for RANKL-induced osteoclast formation and mice from JNK null mice or c-Jun-deficient mice fail to form osteoclasts and suffer from osteopetrosis [21, 22].
Another signaling protein, transformation growth factor-β (TGF-β) activated kinase-1 (TAK1) has been implicated in RANKL-induced osteoclastogenesis [23–25]. Upon RANK receptor engagement, the cytoplasmic domain of RANK interacts with an adaptor protein, tumor necrosis factor-receptor-associated factor 6 (TRAF6), and endogenous TAK1 is recruited to the TRAF6 complex. The phosphorylation and activation of TAK1 subsequently leads to MAPKs and inhibitory κB kinase (IKK) activation, the prerequisite event necessary to induce NF-κB. In this process, TAK1-associated binding protein-2 (TAB2) acts as a bridge linking TRAF6 to TAK1 [26]. Although the mechanism by which TAK1 is activated is not fully understood, many studies have revealed the critical role of the lysine-63-linked polyubiquitination by TRAF6 in the activation of TAK1 [27, 28].
Adenosine is an endogenous nucleoside that modulates many physiological processes through four receptor subtypes (A1, A2a, A2b, A3). Recent studies in our laboratory have revealed a novel role for adenosine/A1 receptor (A1R) in osteoclastogenesis: A1R activation is required for both osteoclast formation and function in vitro and only function in vivo, as demonstrated using pharmacologic inhibitors and mice lacking adenosine A1 receptors [29, 30]. The disparity between in vitro and in vivo osteoclast formation is reminiscent of a similar disparity in osteoclast formation in vitro and in vivo in mice lacking either TRAF6 or Atp6v0d2 in which osteoclasts are present in vivo, although functionally defective [31] and do not form from precursors in vitro [32, 33]). One possible explanation for these discrepancies is the presence of other factors in the in vivo microenvironment which can partially compensate the A1R, TRAF6 or Atp6v0d2 deficiency, such as TGF-β [32]. In this work, we further probed the signaling pathways by which adenosine/A1R activation mediates its effect on osteoclastogenesis. We report here that adenosine A1R activation is required for appropriate formation of TRAF6/TAK1 complexes and the resulting activation of NF-κB, the critical signaling step in osteoclastogenesis.
Methods
Antibodies and reagents
Commercially available antibodies were purchased from the following resources: IκB, p-c-Jun, c-Jun, p-Erk, p65, TAK1, TRAF6, NFATc1 (Santa Cruz Biotechnology Inc), p-p-38, p38, Erk, p-JNK, JNK (Cell Signaling Technology), p84, and β-actin (abcam). Recombinant murine M-CSF and murine RANKL were from R&D System Inc. Sodium thiosulfate and silver nitrate were purchased from Sigma.
Osteoclast culture
For generation of bone marrow-derived osteoclasts, primary bone marrow cells from 6 to 8-week-old mice were cultured as described previously [30]. Briefly, bone marrow was extracted from femora and tibia of mice. The cells were grown in complete α-MEM (Invitrogen) containing 10% fetal bovine serum for 24 h. Then the non-adherent BMMs were collected and replated in culture dishes at 1 × 105 cells/cm2 density with murine M-CSF (30 ng/ml) for 2 days. Cells at this stage were considered M-CSF-dependent bone marrow macrophages (BMMs) and used as osteoclast precursors. Induction of differentiation to osteoclasts was achieved by culturing the BMM cells with the osteoclastogenic medium containing M-CSF (30 ng/ml) and recombinant murine RANKL (30 ng/ml). The day cells were treated with differentiation medium (RANKL + M-CSF) was counted as day 0. Typically, cells were TRAP-positive multinuclear at day 5 after the initiation of culture with RANKL. To test the effect of A1R antagonist, DPCPX, these osteoclast precursors (BMMs) were cultured in the osteoclastogenic medium with or without different doses of DPCPX for 5 days. The culture medium was replaced with fresh medium containing these reagents every 3 days.
TRAP staining and bone resorption assay
Osteoclast differentiation was evaluated by staining for TRAP using a leukocyte acid phosphatase kit (Sigma-Aldrich). TRAP-positive multinucleated cells (≥3 nuclei) were counted as osteoclasts. For bone resorption assay, The BMMs were seeded on BD BioCoat Osteologic MultiTest slides (BD Biosciences) and cultured in the osteoclastogenic medium with or without different doses of DPCPX for 8 days. At the end of culture, cells were removed from chamber slides with bleach and stained by Von Kossa method. Briefly, slides were stained with 5% silver nitrate for 30 min and then fixed in 5% sodium carbonate in 25% formalin for 5 min to remove un-reacted silver nitrate. The surface areas of resorption pits were measured and analyzed using Labworks Image Acquisition and Analysis software 4.0 (UVP). Using the software, the percentile of pixels of white in a gray scale was measured as a representation of the percentage of resorption area to total area.
Real-time PCR
Total RNA was isolated from culture cells using RNeasy Kit (Qiagen). cDNA was synthesized from 1 μg of total RNA using the SuperScript First-Strand Synthesis System (Invitrogen) in a volume of 20 μl. Real-time PCR was performed using Master SYBR Green Kit (Strategene). Primers are listed in Table 1. PCR conditions were 95°C for 5 min followed by 38 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Each experiment was done in triplicate, and results were standardized against the message level of β-actin. The comparative CT method was used to calculate the expression levels of RNA transcripts.
Table 1.
Oligonucleotides used for quantitative real-time PCR
| Target mouse gene | Sequence | Gene bank reference |
|---|---|---|
| β-actin | (F) 5′-ACTATTGGCAACGAGCGGTT-3′ | NM_007393.3 |
| (R) 5′-CAGGATTCCATACCCAAGAAGGA-3′ | ||
| Acp5 | (F) 5′- CGTCTCTGCACAGATTGCAT-3′ | NM_007388 |
| (R) 5′-TGAAGCGCAAACGGTAGTA-3′ | ||
| Ctsk | (F) 5′- GGAGGCGGCTATATGACCA-3′ | NM_007802 |
| (R) 5′-ACAACTTTCATCCTGGGCCCA-3′ | ||
| MMP-9 | (F) 5′-CCTGTGTGTTCCCGTTCATCT-3′ | NM_013599.2 |
| (R) 5′-GCCATACAGTTTATCCTGGTCA-3′ | ||
| Integrin β3 | (F) 5′-TTTGCCCAGCCTTCCAGCCCA-3 | NM_016780.2 |
| (R) 5′-CGGTAATCCTCCTCAGAGCA-3′ | ||
| Integrin αv | (F) 5′-AACATCACCTGGGGCATTCA-3′ | NM_008402.2 |
| (R) 5′-TGAGGTGGTCGGACACGTTT-3 | ||
| NFATc1 | (F) 5′-CTCGAAAGACAGCACTGGA-3′ | AF309389.1 |
| (R) 5′-AGGTGCTGGAAGGTGTACT-3′ | ||
| c-fos | (F) 5′-GAACAACACACTCCATGCGG-3′ | NM_010234.2 |
| (R) 5′-GGAGGACCTTACCTGTTCGTGA-3′ |
Western blot
For RANKL signaling, bone marrow cells were grown in 30 ng/ml M-CSF for 2 days. Cells were then washed with PBS and treated with 30 ng/ml M-CSF and 50 ng/ml RANKL or without different doses of DPCPX for the indicated times. Total cell lysate (30 μg) were collected and subjected to western blot analysis with the indicated antibodies. Nuclear extracts (15 μg) were prepared using NE-PER kit (Pierce) according to the manufacturer's instruction and loaded in 10% SDS-PAGE gels and immunoblotted with different antibodies.
NF-κB p65 DNA-binding analysis
Nuclear extracts were obtained from primary osteoclast precursor cells as above and assayed for NF-κB transcriptional activity using an ELISA (Cayman Chemicals) according to the manufacturer's instruction. Nuclear extracts (15 μg) were incubated into a 96-well plate coated with oligonucleotide containing the consensus NF-κB response element. Absorbance at 450 nm was read in a plate reader, and the data were calculated as ratio to control (BMMs treated with M-CSF only).
Immunoprecipitation assay
Cells were washed once with ice-cold PBS and lysed in ice-cold IP lysis buffer (Pierce) according to the manufacturer's instruction with freshly added proteinase inhibitor cocktail (Sigma) and phosphatase inhibitor (Cayman Chemicals). Cleared lysates (3–5 mg total protein) were preincubated with TRAF6 antibody for overnight and complexes separated using protein G agarose (25 μl per sample, Pierce) before gel electrophoresis.
Statistical analysis
Data are shown as the means ± S.D from at least three independent experiments. Statistical analysis was done by using the Prism 4.02 (GraphPad Software). All data was evaluated using an analysis of variance (ANOVA) followed by Bonferroni post hoc. P < 0.05 was considered to be significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
A1R blockade suppresses osteoclastogenesis and bone-resorbing activity in primary BMMs cultured with RANKL and M-CSF in a dose-dependent manner
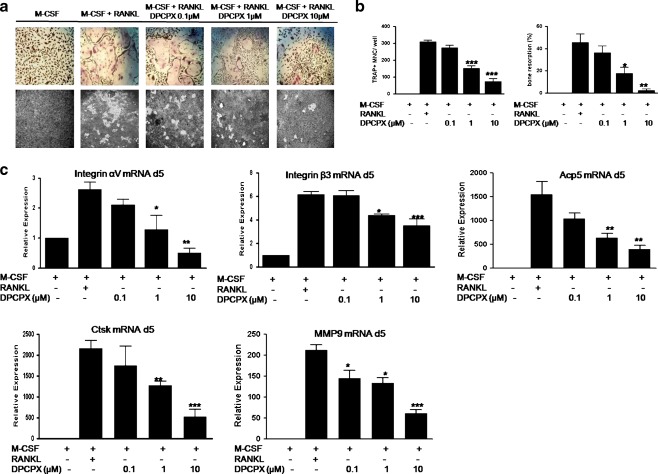
We have previously demonstrated that adenosine regulates osteoclast formation through A1R [30]. We therefore examined the effect of A1R blockade on osteoclast formation and function in vitro using pharmacological tools. After 5-day culture in osteoclast differentiation medium (M-CSF and RANKL (30 ng/ml each)), murine primary osteoclast precursors were differentiated into large, multinucleated TRAP+ cells (Fig. 1a). When the A1R-selective antagonist, DPCPX, was added at the beginning of culture (day 0), a dose-dependent inhibition of osteoclastogenesis was observed, as we previously reported (Fig. 1a). DPCPX at 1 and 10 μM inhibited RANKL-induced osteoclast formation by about 51% and 76%, respectively (P < 0.001 compared with control treated with RANKL + M-CSF, for both). Even though DPCPX may also block A2A and A2B receptors, it is unlikely that interaction with these receptors plays any role in inhibiting osteoclastogenesis since stimulation of both A2A and A2B receptors inhibits osteoclast differentiation ([34] and Supplemental Figure 1). Consistent with the effect on osteoclast formation, resorption pit formation by mature osteoclasts at day 8 of culture was inhibited by 1 μM DPCPX by 61% (Fig. 1a and b, P < 0.05) and nearly eliminated by 10 μM DPCPX (by 95%) (Fig. 1a, b, P < 0.01). DPCPX treatment is not cytotoxic at the concentrations used (MTT assay, data not shown), indicating that the anti-osteoclastogenic effect of DPCPX was not due to toxic effects on osteoclast precursor cells.
Fig. 1.
Suppression of osteoclast formation and function by A1R-selective antagonist. Murine BMMs (1 × 105 cell/cm2) were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of DPCPX for 5 days in 48-well plates for TRAP staining (aupper panel), or 8 days on calcium phosphate-coated slides (BD biosciences) and for pit formation assay (alower panel), respectively. b Numbers of TRAP-positive multinuclear cells containing more than three nuclei (TRAP+ MNC) were counted. For pit formation assay, the slides were stained with von Kossa reagent. Bone resorption percentage was quantified using Labworks Image Acquisition and Analysis software 4.0. The percentile of pixels of white in a gray scale was measured as a representation of the percentage of resorption area to total area (bone resorption, percent). c BMMs were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of DPCPX in 6-well plates for 5 days prior to RNA extraction and real-time PCR for Integrin αv, β3, Acp5, Ctsk, and MMP9. β-actin served as PCR control. Relative expression was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to RANKL + M-CSF cells
A similar pattern of dose-dependent inhibition by adenosine A1R blockade was observed with regard to RANK-induced expression of known osteoclast marker genes, including Acp5, Ctsk, MMP9, and Integrin αvβ3 (Fig. 1c). DPCPX at a concentration of 1 μM or more significantly reduced the transcription of these genes at day 5 of culture. Similarly, rolofylline (KW3902, a more selective and potent A1R antagonist) inhibited RANKL-induced gene expression of Ctsk by KW3902 (1 μM, supplemental Figure 2, P ≤ 0.001 compared with control treated with RANKL ± M-CSF), further confirming the receptor specificity of the inhibitory effect of DPCPX we observed in RANKL-induced osteoclastogenesis. Because the selective A1 receptor agonist N6-cyclopentyladenosine neither directly affected Ctsk expression nor reversed the DPCPX-mediated inhibition of Ctsk expression (Supplemental Figure 3), our results are consistent with the hypothesis that the A1 receptor is constitutively active and DPCPX acts as an inverse agonist to inhibit osteoclastogenesis.
A1R blockade diminishes induction of the transcription factors c-Fos and NFATc1 in primary BMMs
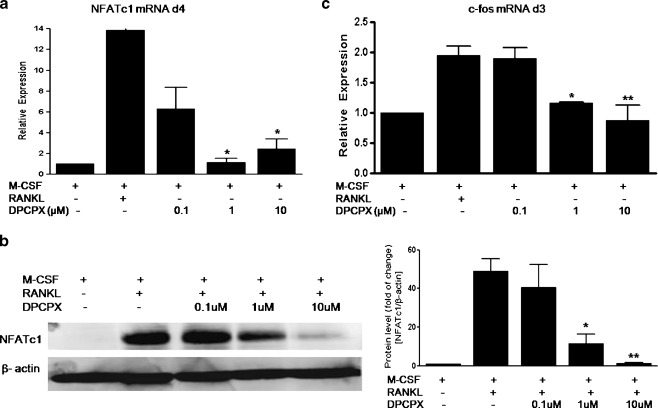
The essential role of transcription factors c-Fos and NFATc1 in RANKL signaling is now well established. Hence, we determined whether the inhibitory effect of A1R blockade also leads to regulation of these factors. NFATc1 is a master switch for terminal differentiation of osteoclasts; we therefore examined the expression of NFATc1 at day 4 of culture. As shown in Fig. 2a, b, whereas NFATc1 mRNA levels were augmented about 13.88-fold and protein levels were increased about 49.1-fold by RANKL treatment (relative to M-CSF only), these increases were completely abolished by DPCPX at concentrations of 1 and 10 μM.
Fig. 2.
Suppression of the RANKL-induced expression of transcription factors NFATc1 and c-fos by A1R-selective antagonist. BMMs were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of DPCPX for 4 days (a, b) or 3 days (c). Total RNA was isolated and NFATc1 (a) and c-fos (c) mRNA levels were quantified by real-time PCR. Relative expression in mRNA levels was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of four independent experiments. b The NFATc1 protein expression was determined by immunoblotting. Protein band intensities were quantified by densitometry and corrected with β-actin. Protein level (fold of change) was expressed as fold change compared with M-CSF only. Values are shown as means ± S.D. of three independent experiments. *P < 0.05, **P < 0.01 compared to RANKL + M-CSF cells
Previous studies of signaling pathways in osteoclast formation indicate that NFATc1 is downstream of c-Fos in osteoclastogenesis regulated by RANKL [17, 20]. We next determined whether adenosine A1R blockade inhibits c-fos expression at day 3 of culture and found that DPCPX abrogated RANKL-induced c-fos expression was observed (Fig. 2c). The increase in the c-Fos message induced by RANKL (about twofold) was significantly decreased by DPCPX in a dose-dependent fashion. These results indicate that A1R blockade directly diminishes the transcription of c-Fos and NFATc1 by RANKL.
A1R blockade inhibits RANKL-induced JNK/c-Jun activation, but not that of p38 and Erk in primary BMMs
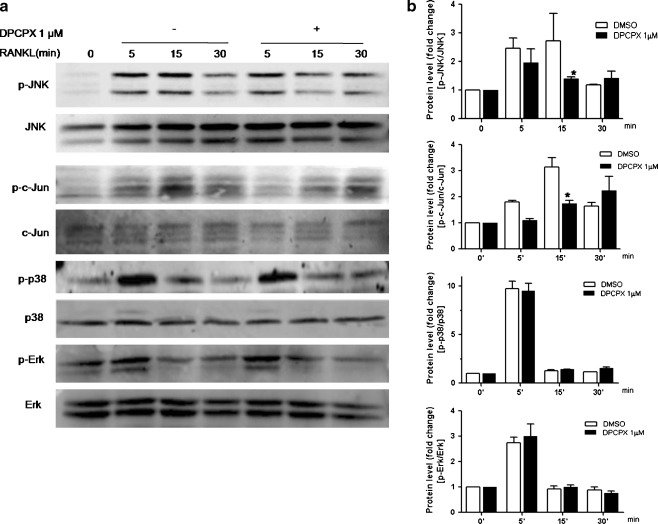
An increasing body of information indicates that RANKL stimulates NF-κB and MAPK activation and subsequently elicits the activation of essential transcription factors, such as AP-1 and NFATc1, required for osteoclast differentiation. Since we found that DPCPX profoundly blocks the induction of c-fos and NFATc1 expression by RANKL, we next examined the two major RANKL-induced signaling pathways, MAPKs and NF-κB. Our findings confirm prior reports that RANKL induces rapid phosphorylation of the JNK, c-Jun, p38, and Erk in primary bone marrow culture (Fig. 3a). With the addition of DPCPX to the cultures, we observed a substantial suppression of the RANKL-induced phosphorylation of JNK and c-Jun at 15 min (P < 0.05 for both, Fig. 3b), whereas the activation of p38 and Erk were not affected. The activation of c-Jun by JNK is an important mechanism involved in osteoclastogenesis [21]. Since the expression of c-fos was down-regulated by A1R blockade (Fig. 2c) and it is known that AP-1 comprises Fos/Jun dimers, our findings indicate that blockade of adenosine A1R impaired the RANKL-induced activation of AP-1, the critical factor in osteoclast differentiation.
Fig. 3.
Inhibition of RANKL-induced activation of JNK/c-Jun, but not that of Erk and p38, by A1R-selective antagonist. BMMs were stimulated with RANKL/M-CSF, with or without 1 μM DPCPX for various times. a Cells were lysed and equal amounts of proteins were separated by SDS-PAGE and immunoblotted with the indicated antibodies. b Protein band intensities were quantified by densitometry, and the levels of phosphorylated MAPKs were normalized to the total levels of corresponding MAPKs. The ratio of phosphorylated MAPKs/total MAPKs in the RANKL-stimulated cells was relative to that of cells treated with M-CSF only (fold value 1). The densitometry values are shown as means ± S.D. of three independent experiments. The figure shows representative data from one of three replicate experiments. *P < 0.05 using an analysis of variance (ANOVA) followed by Bonferroni post hoc
A1R blockade inhibits RANKL-induced NF-κB activation in primary BMMs
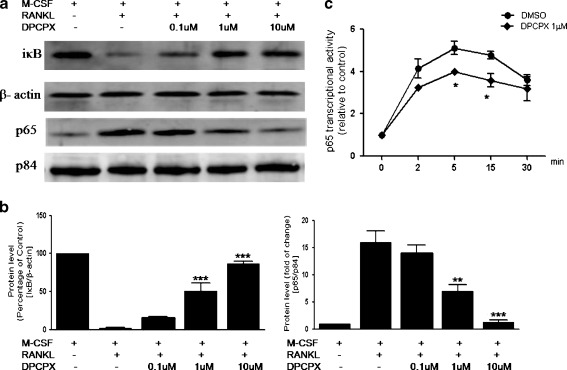
NF-κB signaling in osteoclasts has been extensively studied. Recent study suggests that NF-κB activation is the upstream signaling molecule of c-Fos in osteoclastogenesis [35]. Deletion of the NF-κB subunits p50 and p52 causes severe osteopetrosis through the absence of osteoclasts [36, 37]. We therefore examined the effects of DPCPX on the activation of NF-κB by RANKL. To this end, we stimulated BMMs with RANKL in the presence or absence of different doses of DPCPX and assessed the degradation of IκB in whole cell lysates at 5 min and the presence of p65 in nuclei at 10 min by western blot analysis. As previously reported, there is cytosolic degradation of IκB and nuclear translocation of p65 in response to RANKL (Fig. 4a). DPCPX markedly reduced IκB degradation in the cytosol and nuclear translocation of p65 in a dose-dependent manner (Fig. 4b). Consistent with these findings, we observed that RANKL induced significant p65 DNA binding activity starting from 2 min and continuing as long as 30 min (Fig. 4c). With the addition of 1 μM of DPCPX to the cultures, we observed a significant inhibition of RANKL-induced p65 transcriptional (DNA binding) activity at 5 min and 15 min (P < 0.05 for both). These results indicate that the A1R-mediated inhibition of RANKL-induced osteoclastogenesis is mediated through two major pathways, inhibition of NF-κB and JNK pathways, which regulate transcription of c-Fos and NFATc1 in osteoclast precursors.
Fig. 4.
Inhibition of RANKL-induced activation NF-κB by A1R-selective antagonist. BMMs were stimulated with RANKL/M-CSF, with or without various concentrations of DPCPX. IκB degradation in whole lysates was determined at 5 min and nuclear localization of p65 was determined at 10 min with western blot analysis (a). Protein band intensities were quantified by densitometry (b). For IκB degradation, the levels of IκB were normalized to β-actin, and the data are expressed as percentages of control (M-CSF only). For p65 nuclear translocation, the levels of p65 were normalized to p84 and the data are expressed as the fold changes to control (M-CSF only). The densitometry values are shown as means ± S.D. of three independent experiments. The figure shows representative data from one of three replicate experiments. p65 DNA binding activity was measured with an ELISA-based assay (c). BMMs were stimulated with RANKL/M-CSF, with or without 1 μM DPCPX for various times. Equal amounts of nuclear proteins were collected and incubated into a 96-well plate coated with oligonucleotide containing the consensus NF-κB response element. Absorbance at 450 nm was read in a plate reader and the data were calculated as ratio to control (M-CSF only). Values are shown as means ± S.D. of four independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to RANKL + M-CSF cells
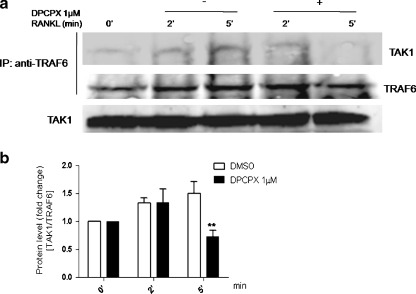
A1R blockade disrupts RANKL-induced formation of TRAF6-TAK1 complex in primary BMMs
TAK1, a MAPK kinase kinase can form complexes with RANK and TRAF6, and was up-regulated in RANKL-induced osteoclastogenesis ([23–25]. RANKL-induced osteoclast differentiation requires TAK1 for NF-κB activation [25]. Herefore, we determined whether DPCPX affects the recruitment, and interaction of TAK1 with TRAF6 by RANKL. In line with previous reports, we found a striking induction of TAK1 association with TRAF6 following stimulation with RANKL. The addition of 1 μM DPCPX in culture medium completely abolished the RANKL-induced TAK1/TRAF6 association without affecting the total levels of TAK1 at 5 min (P < 0.01, Fig. 5a, b). Taken together, our results suggest that adenosine A1R activation is required for association of TAK1 with RANK and that blockade of A1R prevents this activation step thereby diminishing TAK1-mediated NF-κB activation.
Fig. 5.
Disruption of RANKL-induced formation of TRAF6/TAK1 complex by A1R-selective antagonist. a BMMs were stimulated with RANKL/M-CSF, with or without 1 μM DPCPX for various times. Cell extracts were immunoprecipitated (IP) with anti-TRAF6 antibody. The immunoprecipitates were then analyzed by immunoblotting with anti-TAK1 antibody and anti-TRAF6 antibody (upper two panels). Whole-cell extracts were immunoblotted with anti-TAK1 antibody (bottom panel). Protein band intensities were quantified by densitometry (b). The level of TAK1 were normalized to TRAF6 and the data are expressed as fold change to control (M-CSF only). Values are shown as means ± S.D. of four independent experiments. The figure shows representative data from one of four replicate experiments. **P < 0.01 using an analysis of variance (ANOVA) followed by Bonferroni post hoc
Discussion
In the present study, we have confirmed, using the selective A1R antagonist DPCPX, that endogenous adenosine, acting via A1 receptor is an important regulator of RANKL-induced osteoclastogenesis. Moreover, we found that A1R blockade impairs the activation of NF-κB by RANKL, blocks phosphorylation of JNK and inhibits the transcription of c-fos and NFATc1. These findings indicate that adenosine A1R activation is required for signaling at RANK, and A1R blockade leads to disruption in the signaling pathway for osteoclast formation. These findings also provide a solid explanation for our prior observation that A1R-selective antagonists are most effective in preventing osteoclast formation when included at early stages of osteoclastogenesis (before day 3 of culture) [30]. Activation of NF-κB by RANKL is a crucial event in the early stages of osteoclastogenesis. A recent study using p50/p52 double knockout splenocytes and ectopic expression of c-Fos shows that NF-κB is upstream from c-Fos and required for c-Fos activation in RANKL-stimulated osteoclast precursors [35]. Similarly, in the present study, we found that induction of c-fos by RANKL was prevented by A1R blockade-mediated abrogation of NF-κB activation. Although the consensus NF-κB binding site has not been recognized in the c-Fos promoter, and there is no direct evidence of activation of c-Fos by NF-κB, our findings and others support the hypothesis that NF-κB is upstream of c-Fos in osteoclastogenesis.
Adenosine has been reported to activate the JNK pathway through A1R in many other systems [38, 39]. Our study is the first report to demonstrate that adenosine/A1R is also required for RANKL-induced JNK/c-Jun activation in BMMs. Although the precise role of JNK/c-Jun in osteoclastogenesis has yet to be determined, there is increasing evidence that JNK/c-Jun activation is essential for efficient osteoclastogenesis [22]. It has been reported that osteopetrosis develops in transgenic mice with dominant-negative c-Jun in their osteoclast lineage cells [21]. A more recent study showed that the JNK/c-Jun pathway is required for an anti-apoptotic effect of RANKL in multinucleated osteoclasts [40]. However, unlike mature osteoclasts, c-Jun activation by RANKL is not involved in the anti-apoptotic effect of JNK in bone marrow macrophages [22]. In line with these observations, blockade of A1R had little effect on cell viability in mouse BMMs (data not shown). These findings collectively argue for an essential role of JNK/c-Jun in osteoclastogenesis but not prevention of osteoclast apoptosis.
Our observation that inhibition of activation of JNK and NF-κB by blockade of A1R suggests that these molecules may share a common pathway to induce osteoclast precursor differentiation. Recent studies have provided more insights into the molecular mechanism underlying the activation of these pathways. In particular, new findings reveal that TRAF6 associates with TAK1 through an adaptor protein TAB2. The formation of the complex of TRAF6, TAB2, and TAK1 leads to the activation of TAK1 which subsequently phosphorylates NF-κB-inducing kinase (NIK) and eventually leads to the activation of the NF-κB pathway [23, 41, 42]. In addition, activated TAK1 also activates the JNK pathway downstream of RANK [24, 43]. In support of this hypothesis, we found that the association of RANKL/RANK in osteoclast precursors derived from bone marrow cells evokes a rapid and dramatic accumulation of TRAF6/TAK1 complex, which is clearly diminished by A1R blockade. This observation is consistent with the previous report that adenosine A1R blockade results in loss of cellular TRAF6 in RAW 264.7 cells [30]. These results suggest that adenosine A1R blockade-mediated blockade of TRAF6-TAK1 complexes triggers TRAF6 degradation. We therefore postulate that the pivotal effect of adenosine A1R in promotion of osteoclast formation is the proper formation of TRAF6-TAB2-TAK1 complex (Fig. 6). Further studies will be required to clarify the role of each component of this complex in mediating adenosine/A1R regulation of osteoclastogenesis as well as the mechanism by which A1R regulates formation of this complex.
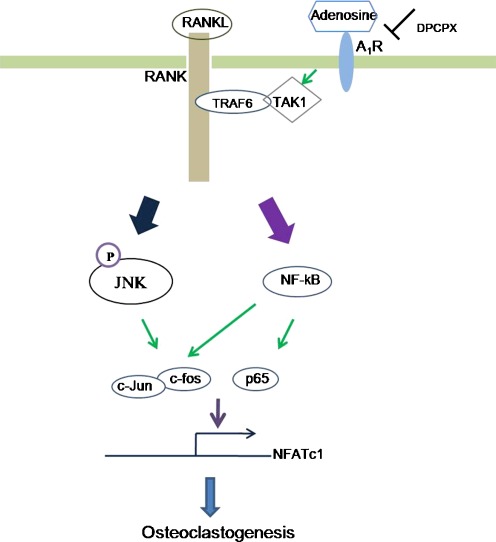
Fig. 6.
A proposed scheme of adenosine/A1R-mediated regulation of RANKL-induced osteoclast formation. During osteoclastogenesis, the critical transcription factors c-fos and NFATc1 are induced by RANKL. NFATc1 is known to be master switch of osteoclastogenesis. Blockade of A1R with DPCPX inhibits osteoclast formation through interfering the RANKL-induced formation of TRAF6/TAK1 signaling complex followed by de-activation of NF-κB and JNK and subsequently leading to the reduction of osteoclast formation by RANKL
Recent studies suggest that the usual classification of GPCR-active agents as agonists, antagonists, or inverse agonists (agents that diminish activity of a constitutively active receptor) needs refinement. Activation of GPCRs regulates cellular function by activating G proteins and, as more recently described, activating β-arrestin with distinct downstream signaling and functional consequences ([44]). It has recently been recognized that some receptor active agents preferentially stimulate G protein signaling whereas others stimulate the alternative pathway and this phenomenon has recently been shown to be important for adenosine receptor physiology [44]. Because we found that stimulation of the A1 receptor does not affect osteoclastogenesis whereas antagonism of A1 receptors inhibits osteoclastogenesis, our results are most consistent with the hypothesis that the A1 receptor is constitutively active and that blockade of the A1 receptor inhibits osteoclastogenesis by acting as an inverse agonist. Thus, it is possible that DPCPX is acting as an inverse agonist in the setting of A1R-mediated regulation of osteoclast function. Alternatively, many cell types release or generate adenosine at the cell surface and these low levels of adenosine are sufficient to activate A1R, a phenomenon blocked by DPCPX and other receptor antagonists.
Previous studies have demonstrated a role for adenosine and adenosine A1R in regulating formation of multinucleated giant cells from peripheral blood monocytes [45–47], a finding consistent with the observations here. Similarly, in preliminary studies, we have observed that both DPCPX and rolofylline block osteoclast formation from human bone marrow-derived precursors (He, Mazumder and Cronstein, unpublished observations). Thus, it is likely that the observations on murine osteoclast formation reported here are relevant to human osteoclasts as well.
Adenosine may either be released from cells via bidirectional adenosine transporters on the cell membrane or adenosine may be generated by hydrolysis of extracellular adenosine nucleotides by such extracellular phosphohydrolases as nucleoside triphosphate phosphohydrolase (CD39), ecto-5′ nucleotidase (CD73), tissue non-specific alkaline phosphatase (TNAP), among others. CD39 and CD73 are expressed on the surface of murine osteoclast precursors (He, W. and Cronstein, BN, unpublished), although their roles in producing extracellular adenosine have not been established. Because adenosine A1R can be fully activated by adenosine levels which are in the subnanomolar range, it is not surprising that even extremely low concentrations of adenosine present in the extracellular milieu are capable of fully activating A1R, an event required for osteoclast formation. Nonetheless, because treatment with the A1 receptor agonist CPA neither directly affected osteoclastogenesis nor reversed the effect of A1 receptor blockade on osteoclastogenesis, it is likely that the receptor is constitutively active and antagonists act as inverse agonists, a phenomenon previously described for adenosine A1 receptors [48].
We have recently reported that stimulation of adenosine A2A receptors inhibits RANKL-induced osteoclastogenesis in murine bone marrow-derived precursors [34]. Moreover, we have observed dose-dependent inhibition of RANKL-induced osteoclast formation and expression of osteoclast markers by the A2bR specific agonist BAY 60–6,583 in murine BMMs (supplemental Figure 1). These observations contrast, at least at a superficial level, with those of Teramachi et al. [49] who reported that adenosine abolished MTX-induced osteoclast inhibition via stimulation of A2b receptors. However, the effects described by Teramachi and colleagues are most likely mediated by A2b receptors on osteoblasts since, as noted in their work, the primary reason for inhibition of osteoclast formation in these bone marrow cultures was inhibition of RANKL expression without diminution of osteoprotegerin expression. Since RANKL, the principal stimulus for osteoclast differentiation, is primarily expressed on osteoblasts, which have been previously shown to express A2b receptors which affect their function [50–52], the most likely explanation for the observed effects of A2b receptor ligation on osteoclastogenesis is inhibition of osteoblast-mediated stimulation of osteoclastogenesis. It is also interesting to note that it is likely that the inhibitory effects of methotrexate on osteoclast-mediated bone destruction are mediated by the higher levels of adenosine released by methotrexate-treated cells and tissues which interact with A2A and A3 receptors [53]. Indeed, in the adjuvant arthritis model studied by Teramachi and colleagues, the adenosine receptor antagonists theophylline and caffeine reverse the anti-inflammatory effects of methotrexate [54].
We have recently reported that adenosine A2A receptor stimulation inhibits osteoclast formation and A2A receptor knockout mice have increased numbers of functionally active osteoclasts in their bones associated with diminished bone density [34]. These observations suggest that even the minimal activation of A2A receptors that occurs at ambient adenosine concentrations is sufficient to diminish the A1 receptor-mediated promotion of osteoclast differentiation. Thus, A2A receptors likely act as the natural brake on adenosine A1 receptors in regulating bone metabolism.
In summary, the results reported herein provide further evidence for the role of adenosine/A1R as a local signaling molecule in osteoclast formation and highlight the significant influences of extracellular nucleosides in maintenance of bone homeostasis.
Electronic supplementary material
Suppression of osteoclast formation by A2bR-selective agonist. Murine BMMs (1 × 105 cell/cm2) were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of BAY 60–6,583 for 5 days in 48-well plates for TRAP staining (a). b Numbers of TRAP-positive multinuclear cells containing more than three nuclei (TRAP + MNC) were counted. c BMMs were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of BAY 60–6,583 in 6-well plates for 5 days prior to RNA extraction and real-time PCR for Ctsk. β-actin served as PCR control. Relative expression was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to RANKL + M-CSF cells (JPEG 96 kb)
Suppression of the RANKL-induced expression of Ctsk by A1R-selective antagonist, Rolofylline. BMMs were cultured with M-CSF and RANKL (30 ng/ml each), in the presence or absence of 1 μM KW3902 for 5 days. Total RNA was isolated and Ctsk mRNA levels were quantified by real-time PCR. Relative expression in mRNA levels was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of three independent experiments. ***P < 0.001 compared to RANKL + M-CSF cells (JPEG 11 kb)
A1R-selective agonist N6-cyclopentyladenosine (CPA) neither directly affected Ctsk expression nor reversed the DPCPX-mediated inhibition of Ctsk expression. BMMs were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of DPCPX in the presence or absence of 1 μM CPA for 5 days. Total RNA was isolated and Ctsk mRNA levels were quantified by real-time PCR. Relative expression in mRNA levels was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of four independent experiments. **P < 0.01, ***P < 0.001 compared to RANKL + M-CSF cells (JPEG 24 kb)
Acknowledgements
This work was supported by grants from the National Institutes of Health (AR56672, AR56672S1 and AR54897), the NYU-HHC Clinical and Translational Science Institute (UL1RR029893) and the Vilcek Foundation.
Disclosure
Bruce Cronstein, MD. Consultant (within the past 2 years), all <$10,000: Bristol-Myers Squibb, Novartis, CanFite Biopharmaceuticals, Cypress Laboratories, Regeneron (Westat, DSMB), Endocyte, Protalex, Allos, Inc., Savient,. Equity: CanFite Biopharmaceuticals received for membership in Scientific Advisory Board. Grants: King Pharmaceuticals, NIH, Vilcek Foundation, OSI Pharmaceuticals, URL Pharmaceuticals, Inc. Board Member: Vilcek Foundation. Intellectual Property: Patents on use of adenosine A2A receptor agonists to promote wound healing and use of A2A receptor antagonists to inhibit fibrosis. Patent on use of adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone. Patent on the use of adenosine A1 and A2B Receptor antagonists to treat fatty liver. Patent on the use of adenosine A2A receptor agonists to prevent prosthesis loosening.
References
- 1.Gruber HE, et al. Osteoblast and osteoclast cell number and cell activity in postmenopausal osteoporosis. Miner Electrolyte Metab. 1986;12(4):246–254. [PubMed] [Google Scholar]
- 2.Lozo P, et al. Bone histology in postmenopausal osteoporosis—variations in cellular activity. Acta Med Croatica. 2004;58(1):5–11. [PubMed] [Google Scholar]
- 3.Reddy SV. Etiology of Paget's disease and osteoclast abnormalities. J Cell Biochem. 2004;93(4):688–696. doi: 10.1002/jcb.20256. [DOI] [PubMed] [Google Scholar]
- 4.Neale SD, et al. Osteoclast differentiation from circulating mononuclear precursors in Paget’s disease is hypersensitive to 1,25-dihydroxyvitamin D(3) and RANKL. Bone. 2000;27(3):409–416. doi: 10.1016/S8756-3282(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 5.Roato I, et al. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005;19(2):228–230. doi: 10.1096/fj.04-1823fje. [DOI] [PubMed] [Google Scholar]
- 6.Roato I, et al. Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS One. 2008;3(11):e3627. doi: 10.1371/journal.pone.0003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjoa ST, et al. Formation of osteoclast-like cells from peripheral blood of periodontitis patients occurs without supplementation of macrophage colony-stimulating factor. J Clin Periodontol. 2008;35(7):568–575. doi: 10.1111/j.1600-051X.2008.01241.x. [DOI] [PubMed] [Google Scholar]
- 8.Sakellari D, Menti S, Konstantinidis A. Free soluble receptor activator of nuclear factor-kappab ligand in gingival crevicular fluid correlates with distinct pathogens in periodontitis patients. J Clin Periodontol. 2008;35(11):938–943. doi: 10.1111/j.1600-051X.2008.01314.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirayama T, et al. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology (Oxford) 2002;41(11):1232–1239. doi: 10.1093/rheumatology/41.11.1232. [DOI] [PubMed] [Google Scholar]
- 10.Gravallese EM. Bone destruction in arthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii84–ii86. doi: 10.1136/ard.61.suppl_2.ii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faccio R, et al. Dynamic changes in the osteoclast cytoskeleton in response to growth factors and cell attachment are controlled by beta3 integrin. J Cell Biol. 2003;162(3):499–509. doi: 10.1083/jcb.200212082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faccio R, et al. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest. 2003;111(5):749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHugh KP, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105(4):433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura I, Gailit J, Sasaki T. Osteoclast integrin alphaVbeta3 is present in the clear zone and contributes to cellular polarization. Cell Tissue Res. 1996;286(3):507–515. doi: 10.1007/s004410050720. [DOI] [PubMed] [Google Scholar]
- 15.Grigoriadis AE, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266(5184):443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 16.David JP, et al. Carbonic anhydrase II is an AP-1 target gene in osteoclasts. J Cell Physiol. 2001;188(1):89–97. doi: 10.1002/jcp.1099. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo K, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279(25):26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 18.Cao X, et al. Cloning of the promoter for the avian integrin beta 3 subunit gene and its regulation by 1,25-dihydroxyvitamin D3. J Biol Chem. 1993;268(36):27371–27380. [PubMed] [Google Scholar]
- 19.Reddy SV, et al. Characterization of the mouse tartrate-resistant acid phosphatase (TRAP) gene promoter. J Bone Miner Res. 1995;10(4):601–606. doi: 10.1002/jbmr.5650100413. [DOI] [PubMed] [Google Scholar]
- 20.Takayanagi H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda F, et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest. 2004;114(4):475–484. doi: 10.1172/JCI19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David JP, et al. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci. 2002;115(Pt 22):4317–4325. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- 23.Mizukami J, et al. Receptor activator of NF-kappaB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol. 2002;22(4):992–1000. doi: 10.1128/MCB.22.4.992-1000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SW, et al. TAK1-dependent activation of AP-1 and c-Jun N-terminal kinase by receptor activator of NF-kappaB. J Biochem Mol Biol. 2002;35(4):371–376. doi: 10.5483/BMBRep.2002.35.4.371. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, et al. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006;13(11):1879–1891. doi: 10.1038/sj.cdd.4401882. [DOI] [PubMed] [Google Scholar]
- 26.Besse A, et al. TAK1-dependent signaling requires functional interaction with TAB2/TAB3. J Biol Chem. 2007;282(6):3918–3928. doi: 10.1074/jbc.M608867200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorrentino A, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10(10):1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 28.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26(22):3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 29.Kara FM, et al. Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum. 2010;62(2):534–541. doi: 10.1002/art.27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kara FM, et al. Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J. 2010;24(7):2325–2333. doi: 10.1096/fj.09-147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lomaga MA, et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13(8):1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim N, et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202(5):589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito A, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4(6):353–362. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 34.Mediero A, et al. Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol. 2011;180:775–786. doi: 10.1016/j.ajpath.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita T, et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282(25):18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- 36.Iotsova V, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3(11):1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 37.Franzoso G, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11(24):3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brust TB, Cayabyab FS, MacVicar BA. C-Jun N-terminal kinase regulates adenosine A1 receptor-mediated synaptic depression in the rat hippocampus. Neuropharmacology. 2007;53(8):906–917. doi: 10.1016/j.neuropharm.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Liu AM, Wong YH. G16-mediated activation of nuclear factor kappaB by the adenosine A1 receptor involves c-Src, protein kinase C, and ERK signaling. J Biol Chem. 2004;279(51):53196–53204. doi: 10.1074/jbc.M410196200. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda F, et al. JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J Bone Miner Res. 2008;23(6):907–914. doi: 10.1359/jbmr.080211. [DOI] [PubMed] [Google Scholar]
- 41.Takaesu G, et al. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol Cell Biol. 2001;21(7):2475–2484. doi: 10.1128/MCB.21.7.2475-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morlon A, Munnich A, Smahi A. TAB2, TRAF6 and TAK1 are involved in NF-kappaB activation induced by the TNF-receptor, Edar and its adaptator Edaradd. Hum Mol Genet. 2005;14(23):3751–3757. doi: 10.1093/hmg/ddi405. [DOI] [PubMed] [Google Scholar]
- 43.Ninomiya-Tsuji J, et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398(6724):252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 44.Verzijl D, Ijzerman AP. Functional selectivity of adenosine receptor ligands. Purinergic Signal. 2011;7(2):171–192. doi: 10.1007/s11302-011-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merrill JT, et al. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 1997;40:1308–1315. doi: 10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 46.Merrill JT, et al. Inhibition of methotrexate-induced rheumatoid nodulosis by colchicine: evidence from an in vitro model and regression in 7 of 14 patients. J Clin Rheumatol. 1997;3(6):328–333. doi: 10.1097/00124743-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Merrill JT, et al. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 1997;40(7):1308–1315. doi: 10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 48.Shryock JC, Ozeck MJ, Belardinelli L. Inverse agonists and neutral antagonists of recombinant human A1 adenosine receptors stably expressed in Chinese hamster ovary cells. Mol Pharmacol. 1998;53(5):886–893. [PubMed] [Google Scholar]
- 49.Teramachi J, et al. Adenosine abolishes MTX-induced suppression of osteoclastogenesis and inflammatory bone destruction in adjuvant-induced arthritis. Lab Invest. 2011;91(5):719–731. doi: 10.1038/labinvest.2011.9. [DOI] [PubMed] [Google Scholar]
- 50.Gharibi B et al (2011) Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes doi:10.1038/ijo.2011.129 [DOI] [PubMed]
- 51.Russell JM, et al. Adenosine inhibition of lipopolysaccharide-induced interleukin-6 secretion by the osteoblastic cell line MG-63. Calcif Tissue Int. 2007;81(4):316–326. doi: 10.1007/s00223-007-9060-y. [DOI] [PubMed] [Google Scholar]
- 52.Evans BAJ, et al. Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res. 2006;21(2):228–236. doi: 10.1359/JBMR.051021. [DOI] [PubMed] [Google Scholar]
- 53.Chan ES, Cronstein BN. Methotrexate—how does it really work? Nat Rev Rheumatol. 2010;6(3):175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 54.Montesinos C, et al. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine. Evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000;43(3):656–663. doi: 10.1002/1529-0131(200003)43:3<656::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppression of osteoclast formation by A2bR-selective agonist. Murine BMMs (1 × 105 cell/cm2) were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of BAY 60–6,583 for 5 days in 48-well plates for TRAP staining (a). b Numbers of TRAP-positive multinuclear cells containing more than three nuclei (TRAP + MNC) were counted. c BMMs were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of BAY 60–6,583 in 6-well plates for 5 days prior to RNA extraction and real-time PCR for Ctsk. β-actin served as PCR control. Relative expression was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to RANKL + M-CSF cells (JPEG 96 kb)
Suppression of the RANKL-induced expression of Ctsk by A1R-selective antagonist, Rolofylline. BMMs were cultured with M-CSF and RANKL (30 ng/ml each), in the presence or absence of 1 μM KW3902 for 5 days. Total RNA was isolated and Ctsk mRNA levels were quantified by real-time PCR. Relative expression in mRNA levels was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of three independent experiments. ***P < 0.001 compared to RANKL + M-CSF cells (JPEG 11 kb)
A1R-selective agonist N6-cyclopentyladenosine (CPA) neither directly affected Ctsk expression nor reversed the DPCPX-mediated inhibition of Ctsk expression. BMMs were cultured with M-CSF and RANKL (30 ng/ml each), with or without various concentrations of DPCPX in the presence or absence of 1 μM CPA for 5 days. Total RNA was isolated and Ctsk mRNA levels were quantified by real-time PCR. Relative expression in mRNA levels was calculated relative to M-CSF only cells (fold value 1). Values are shown as means ± S.D. of four independent experiments. **P < 0.01, ***P < 0.001 compared to RANKL + M-CSF cells (JPEG 24 kb)