Abstract
Objective
This study aimed to compare the levels of TB-antigen specific Interferon gamma (IFN-γ) and IFN-γ inducible protein (IP)-10 in culture of whole blood samples from healthy controls (HC) and healthy household contacts (HHC).
Methodology
A total of 386 study subjects, which included 186 HC and 200 HHC, were recruited. QuantiFERON TB-Gold in-tube (QFT-IT) assay was employed to measure IFN-γ levels. IP-10 levels were measured in the supernatants collected from QFT-IT tubes. Tuberculin skin test was also performed.
Results
The levels of TB antigen specific IFN-γ and IP-10 were significantly higher in HHC compared to HC. There was no significant difference observed between positivity of QFT-IT and IP-10 in HC and HHC. The positivity of TST was significantly lower in subjects <17 year, when compared to IP-10 (p<0.005). The reduced cut-off point 0.22 IU/ml significantly increased the positivity of QFT-IT among children with high risk for latent TB infection (LTBI).
Conclusions
Measurement of TB antigen specific IFN-γ and IP-10 can be potential markers for the detection of LTBI.
Keywords: Tuberculosis, diagnosis, QuantiFERON-TB Gold, Interferon gamma inducible protein-10
INTRODUCTION
Tuberculin skin test (TST) remains the most commonly used test to detect the latent TB infection (LTBI).1 However, TST is not specific, as the outcome could be influenced by exposure to non-tuberculous mycobacteria (NTM) and prior BCG vaccination. In addition to several practical disadvantages, TST also fails to detect LTBI particularly in high-risk groups, such as children and human immunodeficiency (HIV) infected individuals that are targeted for preventive therapy.1,2
The introduction of interferon gamma release assays (IGRA) for the detection of LTBI seems to be a significant upgrade of the century-old TST.3 Unlike TST, IGRAs are less influenced by environmental mycobacteria, since the antigens used in the assay are absent in most of the NTM.4 The utility of IGRA in LTBI detection is extensively studied and reviewed elsewhere.5–7
Recently, Interferon gamma inducible protein-10 is also suggested as alternative marker for diagnosis of TB. 7–10 IP-10 is predominantly expressed by monocytes and macrophages11,12 by the stimulation of IFN-γ, which is secreted by antigen specific T cells. Higher level of IP-10 was observed in antigen or mitogen stimulated TB blood samples in earlier studies.13 Our earlier study also evidenced the higher secretion of IP-10 in response to TB specific antigens in TB patients that decreases after successful therapy. 9,10,14,15,16 Although some studies available, the data on IGRA and IP-10 from TB endemic countries for LTBI diagnosis particularly in children are still limited.17–22 India is a country with 1.8 million new TB cases per year and accounting for one third of the global burden of TB. The estimated incidence of TB disease in this setting was 168/100 000/year in 2007 and classified as high TB endemic area by WHO.23
In this study, we compared the positivity of QFT-IT, TST and IP-10 between healthy household contacts (HHC) and healthy controls (HC) to evaluate the potential of the three tests in detecting LTBI
MATERIAL AND METHODS
The study subjects were recruited at Tuberculosis Research Centre, India between January 2008 and February 2009. This study was approved by the Scientific Advisory Committee and Institutional Ethical Committee of Tuberculosis Research Centre. Informed written consent was obtained from all the eligible subjects.
Study subjects were recruited under two groups: they were (i) healthy controls (HC) and (ii) healthy household contacts (HHC). HHC were identified from families, where there was at least one case of sputum positive pulmonary TB living in the same household, for at least 3 months prior to the start of treatment of the index case24. HC were selected from families, in which no pulmonary TB patient was living. Individuals with previous history of TB and those who underwent TST in the past 16 months were excluded from the study. After registering the eligible subjects, the chest radiological examination was carried out. Blood was drawn from all the study subjects for HIV testing, QFT-IT and IP-10 assays. Then, the TST was carried out.
Tuberculin skin test
The 2 TU (tuberculin unit) of purified protein derivative (PPD) RT23 (Statens Serum Institute, Copenhagen, Denmark) was injected intradermally by Mantoux method and the induration was measured between 48–72 hrs after PPD injection by trained professionals. The cut-off point for TST positivity was considered as 10mm for this study.
QFT-IT
The IFN-γ release assay was performed using QFT-IT In-tube test (Cellestis Ltd., Victoria, Australia). One ml of blood was taken in each of the three tubes precoated with TB–antigen, phytohemaglutinin (PHA) for the positive control or no antigen for the negative control. The blood samples were drawn and taken to the lab within 2 hours of phlebotomy. The tubes were incubated for 16–24 hours at 37°C and plasma were collected after centrifugation
The plasma samples were brought to Chennai and stored at 4°C until assayed. Within 2 weeks of time, QFT-IT enzyme linked immunosorbant assay (ELISA) was carried out. The test results were interpreted using software supplied by the manufacturer (Cellestis Ltd., Victoria, Australia) and the cut-off point for the diagnosis was followed as per manufacturer’s instruction. If the IFN-γ secretion in response to TB antigen, after subtracting nil control IFN-γ, was ≥0.35 IU/ml, it was considered as positive for QFT-IT and if the value was <0.35 IU/ml, it was considered as negative. If the negativity was associated with poor PHA response (i.e. IFN-γ secretion in response to PHA was <0.5 IU/ml), it was considered as indeterminate or invalid result for QFT-IT. The subjects with IFN-γ secretion >8.0 IU/ml in the nil control samples were also considered as indeterminate for QFT-IT.
IP-10 assay
The IP-10 levels were measured in duplicates in the supernatants collected from QFT-IT tubes using BD Opt EIA kits (BD Biosciences, USA) as per manufacturer’s instructions.14,15. Briefly, 100 μl of capture antibody (mouse anti human IP-10 monoclonal antibody) at the recommended concentration was coated in the 96-well flat bottom polystyrene plates (NUNC maxisorp, Roskilde, Denmark). After overnight incubation, the excess antibodies were washed off using PBST. The sample was added to the plate, incubated for 2 hours and then the plates were washed off. The secondary antibody (biotinylated anti human IP-10 monoclonal antibody) conjugated with HRP was incubated for 1 hour and the excess antibodies were washed off. Then tetra methyl benzidine (TMB) was used as substrate and incubated for 30 minutes and the reaction was arrested by the addition of 2N H2SO4.
The cut-off point for IP-10 for TB specific antigens and mitogen were set as 300 pg/ml and 200 pg/ml based on our earlier studies.15 The subjects with ≥300 pg/ml for TB antigens (TB antigen – nil), irrespective of mitogen response were considered as positive; <300 pg/ml for TB antigens and ≥200 pg/ml for mitogen were considered as negative; others (<300 pg/ml for TB antigen and <200 pg/ml for mitogen) were considered as indeterminate.14,15
Statistical analysis
Data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, California, USA) and GraphPad software available in their website (www.graphpad.com/quickcals.cfm). Mann-Whitney U test was carried out to calculate the differences of IFN-γ and IP-10 levels between the groups. The proportion of positivity between the tests was compared using Fisher exact test. The agreement between the tests was quantified using kappa statistics.
RESULTS
A total of 386 study subjects were recruited during the study period. Among them, 186 were HC and 200 were HHC. The demographic profile of study subjects is given in Table 1.
Table 1.
Demographic profile of the study subjects
| Category | Healthy controls | Healthy contacts |
|---|---|---|
| Total number of subjects | 186 | 200 |
| Sex, Number (%) | ||
| Male | 82 (44.1) | 73 (36.5) |
| Female | 104 (55.9) | 127 (63.5) |
| Age, Median in years (Range; IQR) | 23 (2–85; 12,30) | 29 (4–75; 16,38) |
| Age range (in years), Number (%) | ||
| ≤17 | 86 (46.2) | 59 (29.5) |
| 18–30 | 54 (29.0) | 62 (31) |
| 30–45 | 16 (8.6) | 41 (20.58) |
| >45 | 30 (16.2) | 38 (19) |
N - Number of subjects
IQR - Inter-quartile range
%- Percentage
IP-10 and IFN-γ levels
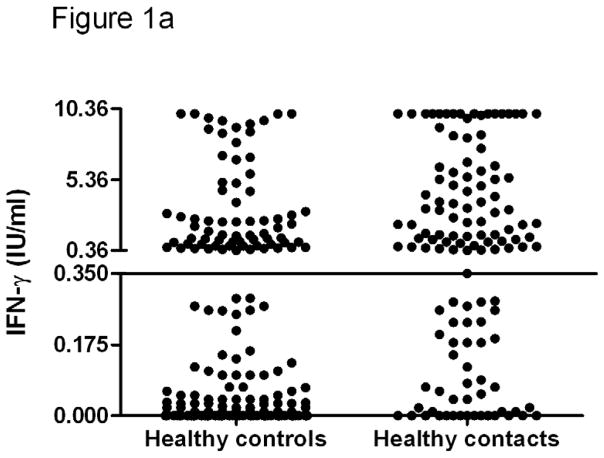
The levels of IFN-γ and IP-10 in HC and HHC are given in Figure 1. The median TB antigen specific IFN-γ in HC (0.05 IU/ml; IQR 0, 0.99) was significantly lower when compared to HHC (1.14 IU/ml; IQR 0.18, 5.4). Similarly, the median TB antigen specific IP-10 was also significantly lower in HC (160.3 pg/ml; IQR 0, 387) compared to HHC (2503 pg/ml; IQR 461, 5303).
Figure 1. The levels of IFN-γ (a) and IP-10 (b) secretion in response to TB antigens in healthy controls and household contacts.
The overall levels of IFN-γ and IP-10 in response to TB antigens, in adults and children as well, were significantly lower in healthy controls compared to healthy household contacts. The horizontal line shows the cut-off point. The difference between the groups was calculated by Mann-Whitney U test. QFT-IT – QuantiFERON-TB Gold in-tube, IP-10- Interferon gamma inducible protein-10, TB- Tuberculosis
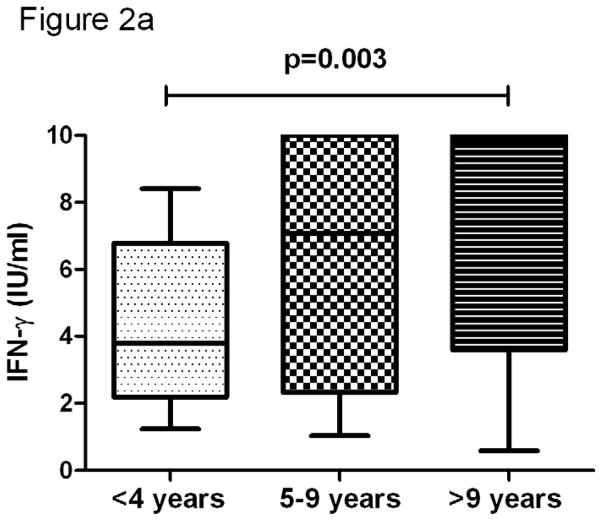
In all study subjects, the QFT-IT mitogen specific IFN-γ level was significantly lower in subjects <4 years when compared to subjects >9 years (p=0.003) (Figure 2). However, we did not observe significant difference, when we compared subjects with age of <4 years and 5–9 years and subjects with age of 5–9 years and >9 years. On the other hand, IP-10 did not correlate with age.
Figure 2. The association of age and mitogen specific IFN-γ (a) and IP-10 (b).
The decline of mitogen of specific IFN-γ (a), but not IP-10 (b) was associated with age. Box and Whisker plots show range, inter-quartile range and median. **significant difference p<0.01 by Kruskal-Wallis tests.
Positivity of tests
The positivity of QFT-IT, IP-10 and TST are given in Table 2. Of 186 HC and 200 HHC tested, QFT-IT was positive in 46 (24.7%; 95% confidence interval (CI): 18.5%–30.9%) HC and 128 (64.0%; 95%CI: 57.3%–70.7%) HHC. IP-10 was positive in 53 (26.5% 95%CI: 20.2%–32.8%) HC and 136 (68%; 95%CI: 61.4%–74.8%) HHC. None of them were indeterminate for QFT-IT or IP-10 assay. TST results were available for 180 HC and 166 HHC and among them TST showed a positivity of 13.3% (95%CI: 8.3%–18.3%) and 52% (95% CI: 43.6%–58.8%) respectively at 10mm cut-off point.
Table 2.
Positivity of QFT-IT, IP-10 and TST
| Parameter | TST | QFT-IT | IP-10 | |||
|---|---|---|---|---|---|---|
| N | No. of pos over total (%) | N | No. of pos over total (%) | N | No. of pos over total (%) | |
| Healthy controls | 180 | 24 (13.3) | 186 | 46 (24.7) * | 186 | 53 (26.5) * |
| Healthy contacts | 166 | 85 (51.2) | 200 | 128 (64) * | 200 | 136 (68) * |
The difference between the positivity of tests was calculated using Fisher’s exact test. The cut-off point of 10mm was used for tuberculin skin test.
represents significantly higher when compared to TST. A p value of <0.05 is considered significant.
Pos - Positive
QFT-IT - QuantiFERON TB Gold (In-tube)
TST - Tuberculin Skin test
IP-10 - Interferon gamma inducible protein-10
N - Number of subjects
% - Percentage
There was no significant difference observed between the positivity of QFT-IT and IP-10 in both HC (p=0.217) and HHC (p=0.460). However, the positivity of TST was significantly lower than QFT-IT (p=0.008 in HC and p=0.015 in HHC) and IP-10 (p<0.001 in HC and p<0.0005 in HHC) in both groups.
The positivity of tests did not differ significantly between males and females. The agreement between IP-10 and QFT-IT was moderate (k=0.556). While the agreement between TST and QFT-IT was moderate (k=0.490), it was fair (k=0.259) between IP-10 and TST (Table 3).
Table 3.
Agreement between QFT-IT, IP-10 and TST
| Groups | κ value | 95% CI | Agreement |
|---|---|---|---|
| QFT-IT vs TST | 0.490 | 0.304 to 0.675 | Moderate |
| QFT-IT vs IP-10 | 0.556 | 0.397 to 0.715 | Moderate |
| IP-10 vs TST | 0.259 | 0.047 to 0.471 | Fair |
The strength of agreement between the tests was calculated using kappa statistics. <0.2 – poor; 0.2–0.4 – Fair; 0.4–0.6 - moderate; 0.6–0.8 – good; >0.8 – excellent.
QFT-IT - QuantiFERON-Gold in tube
IFN-γ - Interferon gamma
IP-10 - Interferon gamma inducible protein-10
TST - Tuberculin skin test
CI - Confidence of Interval
Age stratification
Study subjects were stratified by age and the performance of all three tests was analyzed (Table 4). When the study subjects were stratified based on their age range as ≤17, 18–30, 31–45 and >45 years, the positivity of QFT-IT was 2.3%, 24.1%, 31.3% and 90% in HC and 50.5%, 64.7%, 68.1%, and 89.5% in HHC. IP-10 was positive in 2.3%, 33.3%, 40% and 93% of HC and 62.7%, 70.6%, 80.9% and 84.2% of HHC, where as TST was positive in 1.2%, 20.5%, 28.6% and 33.3% of HC and 37.3%, 55.8%, 66.7% and 46.2% of HHC. Only in HC, the positivity of QFT-IT, IP-10 and TST was significantly lower in children (<17 years age) compared to older (>17 years) (p<0.001).
Table 4.
Age stratification analysis
| Age | Percentage of positivity | |||||
|---|---|---|---|---|---|---|
| TST | QFT-IT | IP-10 | ||||
| HC | HHC | HC | HHC | HC | HHC | |
| ≤17 years | 1.2# | 37.3 | 2.3# | 50.5 | 2.3# | 62.7* |
| 18–30 years | 20.5 | 55.8 | 24.1 | 64.7 | 33.3 | 70.6 |
| 31–45 years | 28.6 | 66.7 | 31.3 | 68.1 | 40.0 | 80.9 |
| >45 years | 33.3 | 46.2 | 90* | 89.5* | 93.0* | 84.2* |
The difference between the positivity of tests was calculated using Fisher’s exact test. The cut-off point of 10mm was used for tuberculin skin test.
represents significantly higher when compared to TST.
represents significantly lower compared to subjects >17 years. A p value of <0.05 is considered significant.
QFT-IT - QuantiFERON TB Gold (In-tube),
TST - Tuberculin Skin test
N - Number of subjects
NS - Not significant
Positivity of IP-10 was significantly higher than TST (p=0.001) but not than QFT-IT (p=0.194) in subjects ≤17 years of age. QFT-IT and IP-10 were significantly higher than TST in subjects with >45 years of age (p=0.002 and p=0.043 respectively).
QFT-IT cut-off point
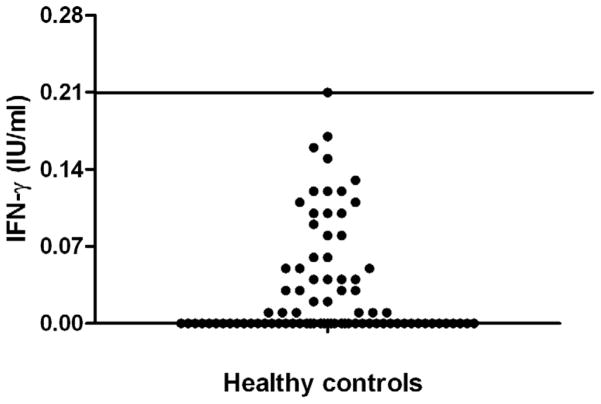
Further, we compared the QFT-IT TB antigen specific IP-10 level between children (<17 years) from HC and HHC groups. In QFT-IT negative HC, the range of IFN-γ secretion in response to QFT-IT TB antigens was 0–0.21 IU/ml. (Figure 3).
Figure 3. IFN-γ secretion in response to TB antigens in QFT-IT negative healthy control children.
The scatter plot shows the range of IFN-γ secretion in response to TB specific antigens in QFT-IT negative healthy control group children. All the QFT-IT negative healthy control group children had IFN-γ level below 0.21 IU/ml.
When ≥0.22 IU/ml was used as cut-off point, 5 more HHC children turned as positive for QFT-IT and showed a positivity of 57.6%. At this cut-off point, positivity of QFT-IT was significantly higher than TST (p=0.023) among children age ≤17 years of age.
DISCUSSION
Our study results showed that the risk for TB infection after exposure to adults with smear positive pulmonary TB were 64% and 68% as determined by QFT-IT and IP-10 respectively. However, the lower rate of infection determined by TST (51%) suggests that TST might underestimate the risk for infection for contacts of adult pulmonary TB patients.
Recent studies evidenced the higher level of IP-10 secretion in response to TB specific antigens.8–10,13–22 In our present study, while only 2.3% of children from HC group were positive for IP-10, the 62.7% of positivity of IP-10 in children from HHC indicate that IP-10 is a accurate marker for LTBI. Therefore, this study observation corroborates to the earlier findings and suggests that IP-10 may serve as a potential diagnostic marker for LTBI in children and adults.
The sub-group analysis, based on the age, carried out in this study revealed that QFT-IT and IP-10 outperformed TST in detecting LTBI particularly among children (≤17 years of age) and elder (>45 years of age) subjects. The risk of progression from LTBI to active TB is relatively higher in young children. Approximately one half of infants and one fifth of older children develop active TB up to 2 years after infection, without specific treatment.25 In particular, children <4 years of age fail to contain the spread of intracellular pathogens as a consequence of an impaired T-cell response.26–28 Therefore, this age group is considered a priority in the strategies to control TB worldwide. The higher positivity of QFT-IT and IP-10 among children indicate their usefulness over TST in detecting LTBI. The studies conducted in other endemic areas such as South Africa29 and Nigeria 30 also reported higher sensitivity of IGRA over TST in children.
Optimizing the cut-off point is one of the ways to improve the performance of a diagnostic test. In this study, we observed that the secretion of IFN-γ in response to TB antigens was below 0.22 IU/ml in QFT-IT negative children belonged to HC group. When the cut-off point 0.22 IU/ml was applied, the positivity of QFT-IT was significantly improved. However, further studies are needed to know whether the reduced cut-off points will improve the diagnostic potential QFT-IT in children.
Although TST is an ideal test for TB infection, there are several practical disadvantages like the need for trained personnel for administration and reading of the test; visit of the subject twice within 72 hrs; inter-observer variation in reading the results; difficulty in deciding the exact cut-off induration etc. The test cannot be repeated without the well-known booster effect.1,2 In this situation, we suggest QFT-IT or IP-10 can act as better alternative for TST in children. However, further studies are needed on the cost effectiveness and feasibility of applying QFT-IT and IP-10 in our settings.
The >80% positivity obtained with QFT-IT and IP-10 among subjects >45 years of age indicate the high TB infection load in our setting. Logically, the possibility of being TB infected in endemic countries is higher among elder subjects than the younger due to the long and repeated exposure to M. tuberculosis. This explains the reason for higher positivity of QFT-IT and IP-10 in elder subjects (age >45 years). A previous study conducted using ESAT-6 and CFP-10 based ELISPOT in the Mumbai city (another major city in India) reported that 80% of healthy adults (age range 18–70; mean 47 years) were positive for M. tuberculosis infection31. However, TST could detect only <50% of LTBI cases among subjects >45 years of age. This poor positivity of TST suggests the age specific anergy for TST 2 and questions the reliability of using TST for LTBI diagnosis in elder subjects. On the other hand, QFT-IT and IP-10 may not have any role in detecting active TB cases among elder patients, due to the high LTBI rate.
Overall, our study results suggest that QFT-IT and IP-10 may serve as better alternative to TST in children and elderly subjects for identification of LTBI. However, before firm conclusions are drawn, larger studies including a larger number of subjects are needed.
Acknowledgments
The authors wish to thank all the study subjects, who participated in this study. The work contributed by Mr. Saravanakannan, Counselor, Ms. Jennath, Health visitor and Ms. Wincy Saravanan, Laboratory technician is gratefully acknowledged.
The authors thank the project consultant Dr. Lee W Riley, Division of Infectious Diseases, School of Public Health, University of California, Berkeley, CA, USA for fruitful discussions.
Basirudeen Syed Ahamed Kabeer is the recipient of Senior Research Fellowship from Indian Council of Medical Research (ICMR), New Delhi, India.
This project is financially supported by NIH (R03) grant (AI064055).
Footnotes
All authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000 Apr;161(4 Pt 2):S221–47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 2.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993 Dec;17(6):968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000 Sep 23;356(9235):1099–104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 4.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004 Dec;4(12):761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 5.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008 Aug 5;149(3):177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalvani A, Pareek M. A 100 year update on diagnosis of tuberculosis infection. Br Med Bull. 93:69–84. doi: 10.1093/bmb/ldp039. [DOI] [PubMed] [Google Scholar]
- 7.Ruhwald M, Bodmer T, Maier C, Jepsen M, Haaland MB, Eugen-Olsen J, et al. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J. 2008 Dec;32(6):1607–15. doi: 10.1183/09031936.00055508. [DOI] [PubMed] [Google Scholar]
- 8.Dheda K, Van-Zyl Smit RN, Sechi LA, Badri M, Meldau R, Symons G, et al. Clinical diagnostic utility of IP-10 and LAM antigen levels for the diagnosis of tuberculous pleural effusions in a high burden setting. PLoS One. 2009;4(3):e4689. doi: 10.1371/journal.pone.0004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goletti D, Raja A, Ahamed Kabeer BS, Rodrigues C, Sodha A, Butera O, et al. IFN-gamma, but not IP-10, MCP-2 or IL-2 response to RD1 selected peptides associates to active tuberculosis. J Infect. Jul;61(2):133–43. doi: 10.1016/j.jinf.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Goletti D, Raja A, Syed Ahamed Kabeer B, Rodrigues C, Sodha A, Carrara S, et al. Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS One. 5(9):e12577. doi: 10.1371/journal.pone.0012577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001 Feb;2(2):123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon NK, Peng F, Ransohoff RM, Buch S. PDGF synergistically enhances IFN-gamma-induced expression of CXCL10 in blood-derived macrophages: implications for HIV dementia. J Immunol. 2007 Sep 1;179(5):2722–30. doi: 10.4049/jimmunol.179.5.2722. [DOI] [PubMed] [Google Scholar]
- 13.Ruhwald M, Bjerregaard-Andersen M, Rabna P, Kofoed K, Eugen-Olsen J, Ravn P. CXCL10/IP-10 release is induced by incubation of whole blood from tuberculosis patients with ESAT-6, CFP10 and TB7.7. Microbes Infect. 2007 Jun;9(7):806–12. doi: 10.1016/j.micinf.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Kabeer BS, Sikhamani R, Raja A. Comparison of interferon gamma and interferon gamma-inducible protein-10 secretion in HIV-tuberculosis patients. AIDS. Jan 16;24(2):323–5. doi: 10.1097/QAD.0b013e328334895e. [DOI] [PubMed] [Google Scholar]
- 15.Syed Ahamed Kabeer B, Raman B, Thomas A, Perumal V, Raja A. Role of QuantiFERON-TB gold, interferon gamma inducible protein-10 and tuberculin skin test in active tuberculosis diagnosis. PLoS One. 5(2):e9051. doi: 10.1371/journal.pone.0009051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabeer BS, Raja A, Raman B, Thangaraj S, Leportier M, Ippolito G, et al. IP-10 response to RD1 antigens might be a useful biomarker for monitoring tuberculosis therapy. BMC Infect Dis. 2011;11:135. doi: 10.1186/1471-2334-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whittaker E, Gordon A, Kampmann B. Is IP-10 a better biomarker for active and latent tuberculosis in children than IFNgamma? PLoS One. 2008;3(12):e3901. doi: 10.1371/journal.pone.0003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruhwald M, Petersen J, Kofoed K, Nakaoka H, Cuevas LE, Lawson L, et al. Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS One. 2008;3(8):e2858. doi: 10.1371/journal.pone.0002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrucci R, Abu Amer N, Gurgel RQ, Sherchand JB, Doria L, Lama C, et al. Interferon gamma, interferon-gamma-induced-protein 10, and tuberculin responses of children at high risk of tuberculosis infection. Pediatr Infect Dis J. 2008 Dec;27(12):1073–7. doi: 10.1097/INF.0b013e31817d05a3. [DOI] [PubMed] [Google Scholar]
- 20.Lighter J, Rigaud M, Eduardo R, Peng CH, Pollack H. Latent tuberculosis diagnosis in children by using the QuantiFERON-TB Gold In-Tube test. Pediatrics. 2009 Jan;123(1):30–7. doi: 10.1542/peds.2007-3618. [DOI] [PubMed] [Google Scholar]
- 21.Lighter-Fisher J, Peng CH, Tse DB. Cytokine responses to QuantiFERON(R) peptides, purified protein derivative and recombinant ESAT-6 in children with tuberculosis. Int J Tuberc Lung Dis. Dec;14(12):1548–55. [PubMed] [Google Scholar]
- 22.Yassin MA, Petrucci R, Garie KT, Harper G, Arbide I, Aschalew M, et al. Can interferon-gamma or interferon-gamma-induced-protein-10 differentiate tuberculosis infection and disease in children of high endemic areas? PLoS One. 6(9):e23733. doi: 10.1371/journal.pone.0023733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Global TB data base: TB County Profile – India. 2008. [Google Scholar]
- 24.Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin Infect Dis. 2004 Apr 1;38(7):966–73. doi: 10.1086/382362. [DOI] [PubMed] [Google Scholar]
- 25.Khan EA, Starke JR. Diagnosis of tuberculosis in children: increased need for better methods. Emerging infectious diseases. 1995 Oct-Dec;1(4):115–23. doi: 10.3201/eid0104.950402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int J Tuberc Lung Dis. 2004 May;8(5):658–74. [PubMed] [Google Scholar]
- 27.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. The Lancet infectious diseases. 2008 Aug;8(8):498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyawaki T, Seki H, Taga K, Sato H, Taniguchi N. Dissociated production of interleukin-2 and immune (gamma) interferon by phytohaemagglutinin stimulated lymphocytes in healthy infants. Clinical and experimental immunology. 1985 Feb;59(2):505–11. [PMC free article] [PubMed] [Google Scholar]
- 29.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004 Dec 18–31;364(9452):2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 30.Nakaoka H, Lawson L, Squire SB, Coulter B, Ravn P, Brock I, et al. Risk for tuberculosis among children. Emerg Infect Dis. 2006 Sep;12(9):1383–8. doi: 10.3201/eid1209.051606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. The Journal of infectious diseases. 2001 Feb 1;183(3):469–77. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]