Abstract
This open-label randomized trial compared isoniazid (9 months) to rifampin (4 months) on toxicity and completion in a jailed population with latent tuberculosis infection. Rifampin resulted in fewer elevated liver function tests (risk ratio [RR] 0.39, 95% confidence interval [CI] [0.18, 0.86]) and less toxicity requiring medication withdrawal (RR 0.51, 95% CI [0.13, 2.01]), although one participant receiving rifampin experienced an allergic reaction. Completion was achieved for 33% receiving rifampin compared to 26% receiving isoniazid (p = .10). With careful monitoring rifampin is a safe and less toxic regimen and appears to be a reasonable alternative because of its shorter duration, allowing more people to complete treatment behind bars. Therapy completion in released inmates is unacceptably low and ensuring follow-up after discharge must be part of a decision to treat.
Keywords: tuberculosis, preventive therapy, isoniazid, rifampin, correctional health care
Treatment of latent tuberculosis infection (LTBI) is a critical strategy for TB control in the United States (American Thoracic Society, 2000). Incarcerated persons have risk factors for LTBI and progression to disease (White, Tulsky, Menendez, Goldenson, & Kawamura, 2005), coming from racial/ethnic minority groups (Minton & Sabol, 2009; Sabol & Minton, 2008) and low-income, homeless, and mentally ill populations (American Thoracic Society, 2000; Barnes et al., 1996; Greenberg & Rosenheck, 2008) and having high rates of injection drug use associated with HIV infection (Maruschak, 2004) and limited health care access (Wilper et al., 2009). But treatment has not been successful in jails because inmates often are released before completion, and characteristics associated with incarceration are also associated with low rates of follow-up in the community (White et al., 2002). Success in this strategy has been an elusive goal and remains an important public health issue (Glaser & Greifinger, 1993; White et al., 2005).
Isoniazid (INH) has been the mainstay of treatment for LTBI, but the recommended 9-month regimen has been limited by poor adherence, even among nonincarcerated individuals (Horsburgh et al., 2010; Shieh et al., 2006; Sterling, 2008), and concerns about hepatotoxicity (Fountain, Tolley, Chrisman, & Self, 2005; Nolan, Goldberg, & Buskin, 1999). An alternative, rifampin (RIF) for 4 months (American Thoracic Society, 2000), has shown promising results (Fountain, Tolley, Jacobs, & Self, 2009; Lardizabal, Passannante, Kojakali, Hayden, & Reichman, 2006; Menzies et al., 2004, 2008; Page et al., 2006; Young, Wessolossky, Ellis, Kaminski, & Daly, 2009; Ziakas & Mylonakis, 2009). But to date there have been no studies in a jailed population. We report here the results of a randomized trial to test the toxicity and treatment outcomes of INH compared to RIF in a jailed population.
Method
Design, Setting, and Sample
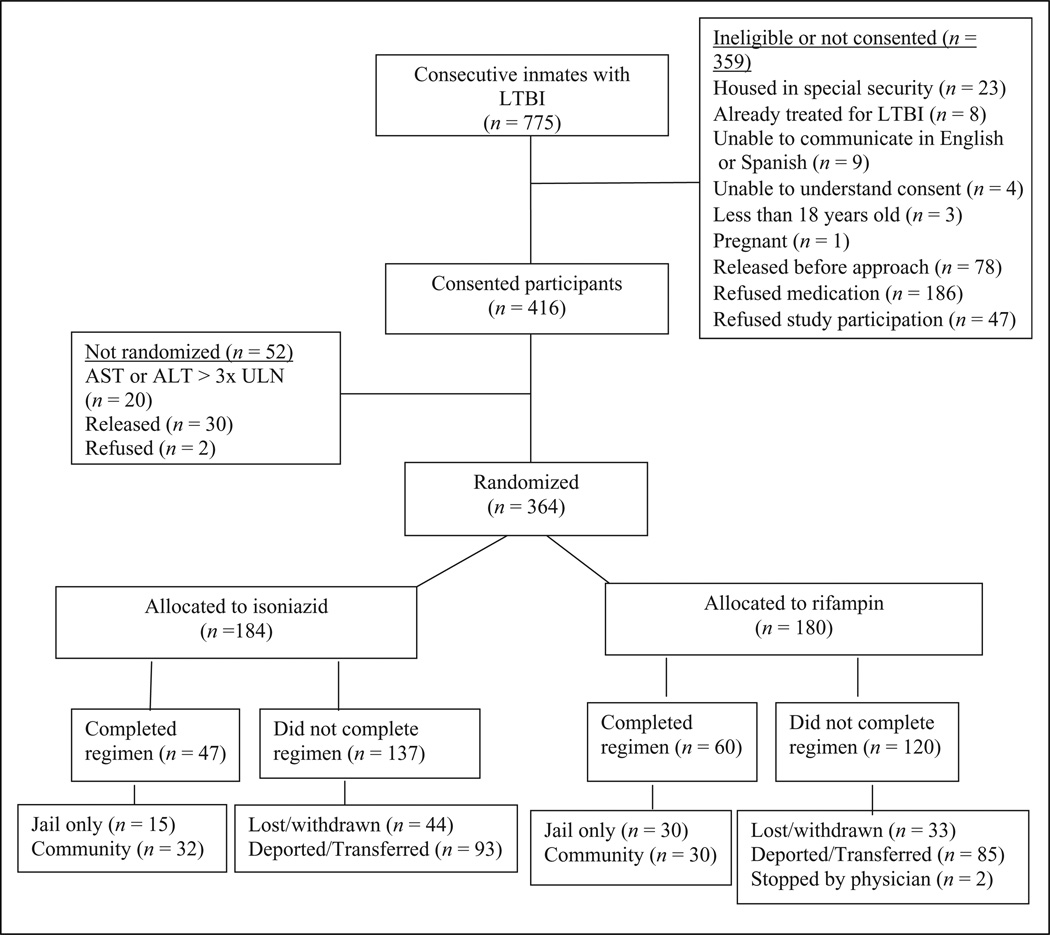
This open-label, randomized trial compared two medications for LTBI (American Thoracic Society, 2000): INH, which is widely accepted, and RIF, which has less evidence of efficacy and although acceptable, is not currently the preferred treatment (Centers for Disease Control and Prevention, 2006). The sample included inmates in the San Francisco City and County Jail diagnosed with LTBI at jail entry. Excluded were inmates with history of treatment-limiting reaction to INH or rifamycins, pregnancy or breast-feeding, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 3 times the upper limit of normal (ULN), bilirubin > 2 times ULN, platelets < 150 K/mm3, or taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. Also excluded were those not speaking English or Spanish, not in the routine level of jail security, or unable or unwilling to consent, some because of known transfer or imminent deportation. Sample size was determined to be 360 based on the toxicity rates for INH and RIF (Menzies et al., 2004) while accounting for loss from deportation or transfer to prison (White et al., 2002). Figure 1 shows participants consented and enrolled between November 30, 2004, and September 24, 2007, and followed through withdrawal, completion, or loss to follow-up.
Figure 1.
Flow diagram of the study population.
Study Treatment Groups
INH (900 mg) was administered twice weekly in a 9-month regimen for a total of 76 doses that could be given over 12 months. Any participant off treatment < 1 month in the first 3 months had the regimen extended by the number of missed doses. If off treatment for ≥ 1 month in the first 3 months, the regimen was restarted. RIF (600 mg) was administered once per day in a 4-month regimen for a total of 120 doses that could be given over 6 months. If a participant missed a dose, medication was extended by the doses missed. Up to 2 weeks of missed doses could be added to the regimen; if a participant missed > 2 weeks, medication was restarted.
Treatment began in jail, by directly observed therapy (DOT). After release, field workers at the San Francisco County TB clinic gave medication by DOT using clinic protocols, including incentives (lunch, restaurant coupon, and bus token) and a case management team throughout the course. If a released participant failed to come to clinic within 1 week, field workers attempted contact by visits to places or person/persons identified at baseline. Reincarcerated participants were continued on treatment, but if lost to follow-up restarted twice, allowing a maximum of three regimen attempts, per jail protocol.
Study Procedures
All participants had symptom reviews every 2 weeks for the first 6 weeks for possible drug toxicity including hepatitis, peripheral neuropathy, arthralgia, rash, memory loss, and other clinical symptoms. Subsequent reviews were 3- and 6-month reviews (INH) and 2-week reviews (RIF), resulting in the same number for each. Liver function tests (LFTs) were drawn at 4, 8, 12, 16, and 32 weeks (INH) and 2, 4, 8, 12, and 16 weeks (RIF).
Measures
Baseline interviews included sociodemographic and health information. Previous treatment and incarcerations came from jail records. Baseline laboratory data included hepatitis BsAg and hepatitis C antibody testing.
Adverse events were recorded from LFTs and symptom reviews. In jail, many participants went to “sick call” for many reasons; all were collected and evaluated by study personnel for their relatedness to the study medication.
Data Analysis
Baseline data were compared to evaluate equivalence of study groups, using chi-square or Fisher’s exact tests for categorical data and t tests for continuous data. Analyses of toxicity and adherence were by chi-square with risk ratios (RRs) and 95% confidence intervals (CI).
Toxicity was measured by counting adverse laboratory or clinical events related to study medication and the timing of the first event in relation to doses received. Because participants could begin medication with LFTs up to 3 times ULN (Grade 1), new adverse events were a change from normal to 1.25 to 3.0 times ULN, or a change from up to 3 times ULN to > 3 times ULN (Grade 2 or higher). Although we measured any changes in LFTs, we examined and presented separately those whose LFTs represented clinically significant hepatotoxity (> 3 times ULN). Adverse events also included new clinical symptoms possibly, probably, or certainly related to the medication. Each occurrence of a new symptom and change in lab result counted independently as one event. Toxicity was also measured as the decision to withhold the medication, defined as LFT > 5.0 times ULN (Grade 3 or higher) or clinical judgment of jail or TB clinic physicians. The last regimen was used for the analysis if participants were restarted.
Adherence was measured by number of doses, from the last regimen, with completion as 76 doses in 12 months (INH) and 120 doses in 6 months (RIF). Therapy length was days between first and last dose in the final regimen. We believed adherence was dependent on participants remaining in jail, and because RIF therapy was shorter, dependent on study medication; remaining in jail virtually guaranteed completion. To evaluate adherence among those released and thus allowed to fail, participants were further divided into remained in jail (Jail), released (Community), and deported or sent to prison (Deported/Transferred). A survival analysis compared adherence by study group, stratified by these subgroups to allow for direct comparisons among released inmates, with time to non-adherence (withdrawal) as the outcome and observations censored after completion.
Analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina) with α = .05 for determining statistical significance. The protocol was approved by the Institutional Review Board of the University of California, San Francisco, California.
Results
Analysis of those who were consented and interviewed but then released, refused, or had elevated LFTs before randomization showed no differences in any sociodemographic or medical variables compared to those randomized to study group. Baseline characteristics (Table 1) and laboratory values did not differ by study group. Overall, 30 (8%) were positive for hepatitis C and 4 (1%) were positive for chronic hepatitis B.
Table 1.
Sample Baseline Characteristics of 364 Participants by Study Group, San Francisco Jail, 2004–2007
| INH N = 184 |
RIF N = 180 |
Total N = 364 |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | (%) | N | (%) | N | (%) | p |
| Gender | .5 | ||||||
| Male | 173 | (94) | 166 | (92) | 339 | (93) | |
| Female | 11 | (6) | 14 | (8) | 25 | (7) | |
| Age group | .08 | ||||||
| < 35 | 138 | (75) | 120 | (67) | 258 | (71) | |
| ≥35 | 46 | (25) | 60 | (33) | 106 | (29) | |
| Ethnicity | .96 | ||||||
| Latino | 114 | (62) | 107 | (59) | 221 | (61) | |
| Black | 24 | (13) | 26 | (14) | 50 | (14) | |
| White | 16 | (9) | 14 | (8) | 30 | (8) | |
| Asian | 15 | (8) | 18 | (10) | 33 | (9) | |
| Other, mixed | 15 | (8) | 15 | (8) | 30 | (8) | |
| Birth country | .90 | ||||||
| United States | 44 | (24) | 42 | (23) | 86 | (24) | |
| Foreign | 140 | (76) | 138 | (77) | 278 | (76) | |
| Language | .68 | ||||||
| English | 84 | (46) | 86 | (48) | 170 | (47) | |
| Spanish | 100 | (54) | 94 | (52) | 194 | (53) | |
| Marital status | .64 | ||||||
| Married/partnered | 76 | (41) | 70 | (39) | 146 | (40) | |
| Not partnered | 108 | (59) | 110 | (61) | 218 | (60) | |
| Housing before jail | .62 | ||||||
| Relative/friend | 104 | (57) | 99 | (55) | 203 | (56) | |
| Own apartment/house | 48 | (26) | 48 | (27) | 96 | (26) | |
| Park/street | 13 | (7) | 12 | (7) | 25 | (7) | |
| Hotel | 13 | (7) | 14 | (8) | 27 | (7) | |
| Shelter | 0 | (0) | 3 | (2) | 3 | (1) | |
| Other | 6 | (3) | 4 | (2) | 10 | (3) | |
| Stable housing before jail | .99 | ||||||
| Yes | 142 | (77) | 139 | (77) | 281 | (77) | |
| No | 42 | (23) | 41 | (23) | 83 | (23) | |
| Jailed before | .80 | ||||||
| Yes | 130 | (71) | 125 | (69) | 255 | (70) | |
| No | 54 | (29) | 55 | (31) | 109 | (30) | |
| Drug/alcohol problem | .21 | ||||||
| Yes | 100 | (54) | 86 | (48) | 186 | (51) | |
| No | 84 | (46) | 94 | (52) | 178 | (49) | |
| Employed before jail | .16 | ||||||
| Yes | 117 | (64) | 127 | (71) | 244 | (67) | |
| No | 67 | (36) | 53 | (29) | 120 | (33) | |
| Regular place for health care | .08 | ||||||
| Yes | 29 | (16) | 41 | (23) | 70 | (19) | |
| No | 155 | (84) | 139 | (77) | 294 | (81) | |
| On INH before | .40 | ||||||
| Yes | 23 | (13) | 28 | (16) | 51 | (14) | |
| No | 161 | (88) | 152 | (84) | 313 | (86) | |
| Health status | .83 | ||||||
| Poor | 15 | (8) | 17 | (9) | 32 | (9) | |
| Fair | 45 | (24) | 52 | (29) | 97 | (27) | |
| Good | 63 | (34) | 54 | (30) | 117 | (32) | |
| Very good | 38 | (21) | 37 | (21) | 75 | (21) | |
| Excellent | 23 | (13) | 20 | (11) | 43 | (12) | |
| Any medical insurance | .36 | ||||||
| Yes | 31 | (17) | 37 | (21) | 68 | (19) | |
| No | 153 | (83) | 143 | (79) | 296 | (81) | |
Notes. INH = isoniazid; RIF = rifampin.
Toxicity
Among INH participants, 34% experienced ≥ 1 adverse events compared to 27% taking RIF (ns). The proportion experiencing > 1 event was higher among INH participants than RIF participants (12% vs. 7%). A total of 161 adverse events were recorded for the 111 participants experiencing > 1 event.
Twenty-nine (11.4%, 21 INH participants and 4.4%, 8 RIF participants) had ≥ 1 elevated LFTs (p = .18) when considering all LFT elevations. None with elevated LFTs was positive for hepatitis B; 2 each from the 21 INH participants and the 8 RIF participants were positive for hepatitis C. Of the 21 INH participants with elevated LFTs, two had two separate events and three had three separate events; none of the 8 RIF participants had > 1 LFT elevation.
Of those with elevated LFTs, 10 participants on INH and 2 on RIF had elevations > 3 times ULN. As this population has high rates of substance abuse, we examined whether testing in jail or the community accounted for differences seen. But it appears that location of testing, and the influence of community access to alcohol, did not account for differences between groups (Table 2).
Table 2.
Percentage of Elevated LFTs (> 3 times ULN) by Timea and by Location of Testing in Each Study Group at Follow-Up
| Study Group and Test Location |
2 Weeks % (N Tested) |
4 Weeks % (N Tested) |
8 Weeks % (N Tested) |
12 Weeks % (N Tested) |
16 Weeks % (N Tested) |
32 Weeks % (N Tested) |
|---|---|---|---|---|---|---|
| INH in jail | – | 1 (107) | 1 (82) | 3 (73) | 4 (50) | 9 (22) |
| INH in community | – | 5 (20) | 5 (20) | 0 (19) | 0 (25) | 4 (27) |
| RIF in jail | 1 (131) | 0 (82) | 0 (60) | 3 (40) | 0 (33) | – |
| RIF in community | 0 (14) | 0 (22) | 0 (23) | 0 (26) | 0 (25) | – |
Notes. INH = isoniazid; RIF = rifampin; LFT = liver function test; ULN = upper limit of normal.
Testing was done for the INH group at 4, 8, 12, 16, and 32 weeks and for the RIF group at 2, 4, 8, 12, and 16 weeks (before medication was completed). Elevations were defined as a change from any baseline to > 3 times ULN.
Clinical symptoms are presented in Table 3. For INH, 2 participants (1%) experienced elevated LFTs combined with gastrointestinal (GI) symptoms, 19 (10%) experienced elevated LFTs without GI symptoms, and 163 (89%) had no elevated LFTs. By contrast, for RIF, 1 participant (0.6%) had elevated LFT with GI symptoms, 7 (4%) had elevated LFTs alone, and 172 (96%) had no elevated LFTs. RIF participants had a lower risk of elevated LFTs (RR 0.39, 95% CI [0.18, 0.86] p < .05) as compared to INH participants. Use of RIF also resulted in lower risk of elevated LFTs combined with GI symptoms (RR 0.51), but this was not statistically significant (p > .6).
Table 3.
Number of Adverse Events (Clinical Symptoms and LFTs) by Study Group
| INH | RIF | Total | ||||
|---|---|---|---|---|---|---|
| Adverse Eventa | N | (% of 184) | N | (% of 180) | N | (% of 364) |
| LFTs | 21 | (11) | 8 | (4) | 29 | (8) |
| Gastrointestinal | 19 | (10) | 16 | (9) | 35 | (10) |
| Rash/pruritis | 12 | (6) | 16 | (9) | 29 | (8) |
| Central nervous system | 20 | (7) | 6 | (3) | 26 | (7) |
| Allergic reaction | 0 | (0) | 1 | (1) | 1 | (0) |
| Otherb | 14 | (8) | 13 | (7) | 27 | (7) |
Notes. INH = isoniazid; RIF = rifampin; LFT = liver function test.
Categories are not mutually exclusive; participants could experience symptoms in more than one body system category. Therefore, the number and percentage represent the number of participants and the percentage of the study group or total that had an adverse event in the category.
Other category includes symptoms such as appetite loss, muscle/body pain, fatigue, weight loss, malaise, cold symptoms, change of urine color, fever, and eye redness.
Nine participants experienced 10 adverse events of Grade 3 or higher or clinically were found to need their medication withheld (Table 4). Six (3.3%) INH participants experienced seven such events, while three (1.7%) RIF participants each experienced one event. Of the 7 who experienced elevated LFTs, only one had AST of 92 at study start; the remainder had LFTs within normal limits at baseline. Use of RIF resulted in half the likelihood of toxicity requiring medication withdrawal (RR 0.51, 95% CI [0.13, 2.01]), but true rates of toxicity resulting in permanent medication stoppage are unknown because of loss to follow-up in four of the nine participants. One RIF participant experienced an allergic reaction, characterized by shortness of breath, oxygen saturation of 85, and pruritic diffuse rash that resolved after administration of oxygen, antihistamine, and epinephrine.
Table 4.
Line Listing of 9 Participants Experiencing 10 Events Requiring Medication to Be Held or Stopped by Study Group
| Study Group | Event Description | Gradea | Time Since Start of Regimen |
Final Study Status |
|---|---|---|---|---|
| INH | LFT elevation | 3 | 8 weeks | Participant withdrew soon after elevation |
| INH | LFT elevation | 3 | 32 weeks | Medication resumed and completed |
| INH | LFT elevation | 3 | 4 weeks | Medication resumed and completed |
| INH | LFT elevation | 3 | 16 weeks | Medication resumed but participant transferred to another jail, not completed |
| INH | LFT elevation | 3 | 8 weeks | Medication resumed but participant transferred to another jail after second elevation, not completed |
| LFT elevation | 3 | 16 weeks | ||
| INH | Allergic reaction | 3 | 8 weeks | Medication resumed but participant withdrew from study, not completed |
| RIF | LFT elevation | 4 | 4 weeks | Medication stopped |
| RIF | LFT elevation | 3 | 12 weeks | Medication resumed and completed |
| RIF | Nausea and vomiting | 2 | 3 weeks | Medication stopped |
Note. INH = isoniazid; RIF = rifampin; LFT = liver function test; AST = aspartate aminotransferase; ALT = alanine aminotransferase.
Grade 3 for LFTs was AST or ALT >5.0–10.0 times ULN.
Adherence
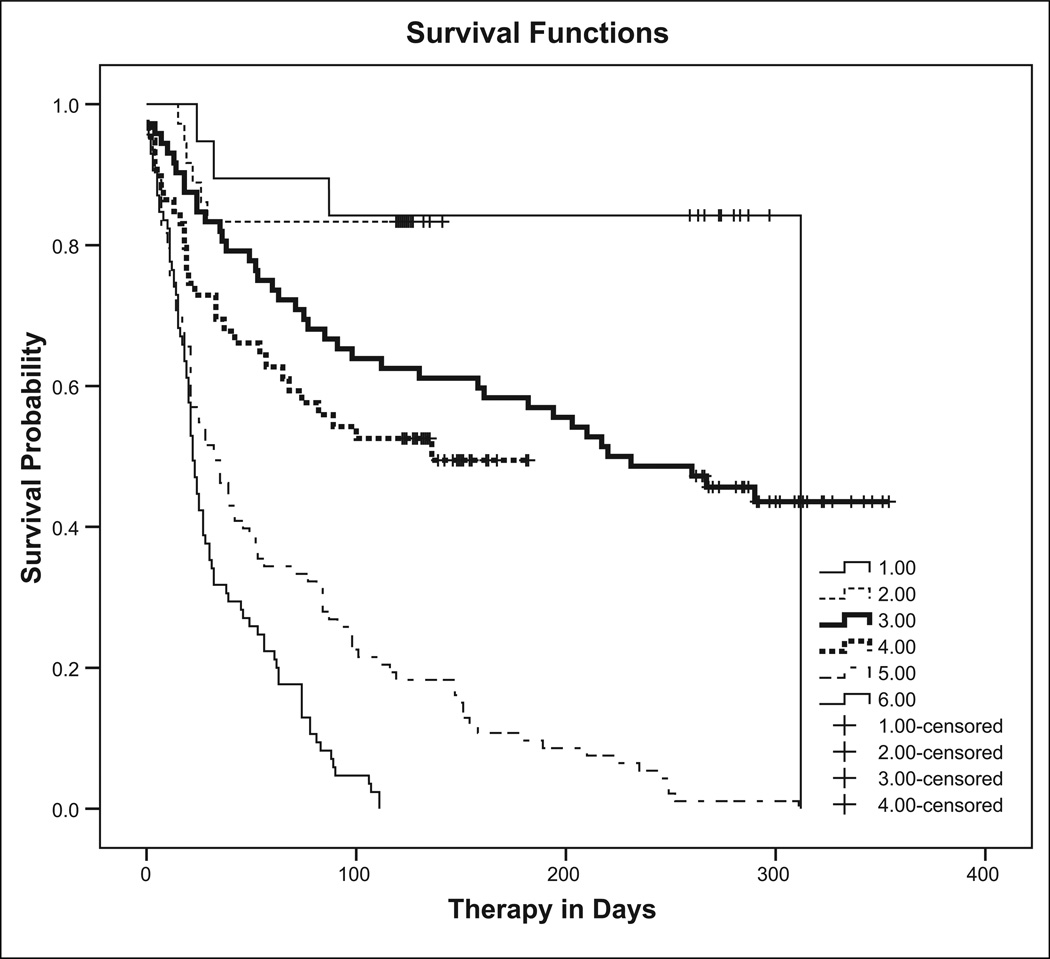
Overall, 107 of 364 completed therapy, 26% (47 of 184) of INH participants and 33% (60 of 180) of RIF participants (p = .10). Survival analysis evaluating time to nonadherence is shown in Figure 2, stratified by disposition: The top two curves represent those remaining in jail for the duration of therapy (79% completed in INH group, 83% in RIF group); the bottom two curves represent those deported or transferred to another facility, none of whom by definition completed therapy; and the middle two curves represent those released while still taking medication (44% completed in INH group, 51% in RIF group). As expected because of its shorter duration, a higher proportion of RIF participants completed treatment both inside and outside of jail compared to INH participants, although neither was statistically different (p = .72 and p = .49, respectively). Treatment completion excluding deported/transferred participants produced results in the same direction as results for the total sample, as the proportion deported/transferred was similar for both groups.
Figure 2.
Completion of therapy by study group and disposition: product-limit survival function estimates of treatment completion. 1 = INH participants who never left jail (Jail); 2 = RIF participants who never left jail (Jail); 3 = INH participants released on medication (Community); 4 = RIF participants released on medication (Community); 5 = INH participants deported or delivered to another correctional facility (Deported/Transferred); 6 = RIF participants deported or delivered to another correctional facility (Deported/Transferred); + Censored observation (medication completion). INH = isoniazid; RIF = rifampin.
Discussion
This is the first study to compare INH and RIF in a jail setting. Jail medical providers are often not linked to community providers, and therapy is complicated by inmate characteristics, recidivism, and fragmented care. The high rate of refusal among this population may be an indication of another difficulty in treating these patients, mistrust of health care in the jail setting (Maruschak, 2006; Wang, Tulsky, & White, 2007; White, Tulsky, Estes, Jamison, & Long, 2008). Although the study is limited by its open label, our purpose was to examine these medications in the usual care of an urban jail. Therefore, the study demonstrates low completion rates not because of the regimens themselves but because of the difficulties in coordinating care for patients who are transferred among institutions and at risk of deportation.
Overall adverse event rates were not statistically different by study drug, but participants taking INH were 2.57 times as likely to experience elevated LFTs (95% CI [1.17, 5.65], p < .05). Elevated LFTs combined with GI symptoms occurred slightly more often but still infrequently in the INH group (1%) as compared to the RIF group (0.6%; p > .6). INH is mostly well tolerated but up to 5% report side effects, most commonly hepatitis, rash, and GI upset. Hepatotoxicity, the most serious side effect, is associated with fatal and near-fatal liver failure, although transient asymptomatic AST elevations occur in 10% to 20% and represent hepatic adaptation (Centers for Disease Control and Prevention, 1993; Saukkonen et al., 2006). AST elevations in treatment studies have ranged from 0.1% to 0.6% in large reviews to 1% to 4% in smaller studies (Saukkonen et al., 2006).
In one study of RIF for 3 months, none developed hepatitis and none had ALT elevations compared with INH (Hong Kong Chest Service, 1992). Three chart reviews found similar results comparing INH with RIF among nonincarcerated patients. One concluded that RIF was no different from INH in symptomatic adverse events, but INH patients were more likely to experience neuropathy (4.5%vs. 0%) and symptomatic ALT elevations (Young et al., 2009). In another, adverse reactions resulting in permanent medication discontinuation occurred in 4.6% of INH patients compared to 1.9% of RIF patients (Page et al., 2006). And in the third, more reactions were observed for INH (5.8%) than for RIF (3.1%), but findings were not statistically significant (Lardizabal et al., 2006). In a randomized trial, Menzies and colleagues (2008) described adverse events resulting in permanent discontinuation in 14% of INH patients, compared to 3% of RIF patients, and drug-induced hepatitis only among INH patients. Our findings for elevated LFTs combined with GI symptoms were considerably lower, which may be explained by the young age of the sample or the high loss to follow-up. A recent meta-analysis demonstrated a reduced risk of hepatotoxicity leading to drug discontinuation for RIF (range 0% to 0.7%) compared to INH (range 1.4% to 5.2%; Ziakas & Mylonakis, 2009). Our findings for all events leading to drug discontinuation are in the same direction and point to the same conclusion. The notable exception is the allergic reaction observed in one RIF participant, an event that is rare (Martinez, Collazos, & Mayo, 1999) but of concern.
Therapy completion was better for RIF than INH, but not statistically significant. Of concern is the fact that nearly half the participants started on either INH or RIF were lost to follow-up by transfer to another facility or deportation. We had no way to obtain data for these participants and assumed none in either group continued or completed, but acknowledge that their inclusion may have changed our findings. In studies of free populations, 80% versus 53% (Lardizabal et al., 2006), 91% versus 77% (Young et al., 2009), and 91% versus 76% (Menzies et al., 2008) completed RIF and INH therapy, respectively, more than twice the completion of either drug in our study. Our findings among those who remained in jail (83% for RIF and 79% for INH) were closer to the findings of these other researchers. Horsburgh’s analysis demonstrated 45% INH and 69% RIF completion including public clinics with high-risk populations (Horsburgh et al., 2010); these rates are closer to our 44% and 51% rates, respectively, among the released. DOT for high-risk groups, including released inmates, is usual care at the San Francisco TB clinic, and has improved completion compared to self-administered therapy (White, Gournis, Kawamura, Menendez, & Tulsky, 2003). But this may not be common practice elsewhere, and completion rates for either drug would be expected to be lower when self-administration is employed.
Conclusions
With careful monitoring, RIF is a safe and less toxic regimen, requiring fewer clinical visits because of fewer side effects. RIF’s shorter duration allows more to complete inside jail and fewer DOT visits after release. RIF has been suggested as a better alternative because of increasing levels of INH resistance among new immigrants (Reichman, Lardizabal, & Hayden, 2004), an important issue for urban jails with a high proportion of foreign-born inmates. Further work should demonstrate rates of progression to active TB, which was high in jail inmates (White et al., 2005); this study lacks efficacy data. It remains premature to recommend RIF until the relative efficacy and effectiveness compared to INH are known, but it is a suitable alternative, in particular when the patient is likely to be in the facility for the duration of therapy.
Completion with either regimen after jail release is unacceptably low, despite dedicated outreach and state-of-the-art case management. We suggest that starting LTBI treatment in a correctional setting should be discouraged unless the confinement term is known and treatment can be achieved, or there are other compelling individual or public health reasons, such as TB exposure during incarceration, severely compromised immune status, or multiple risk factors for disease progression. Ensuring follow-up after release should be part of a decision to treat, and research is crucial to identify discharge planning methods that are effective. Those about to be deported should be given information about how to obtain proper treatment in their home country. The shorter course of RIF combined with close monitoring and the 24-hour availability of care inside the correctional facility can both assure detection of side effects and improve completion rates in this high-risk population.
Acknowledgments
This study was supported by Award Number U01 AI 51315 from the National Institute of Allergy and Infectious Diseases. ClinicalTrials.gov identifier: NCT00128206. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors disclosed no conflicts of interest with respect to the authorship and/or publication of this article. For information about JCHC’s disclosure policy, please see the Self-Study Exam.
References
- American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Morbidity and Mortality Weekly Report. 2000;49:1–51. [PubMed] [Google Scholar]
- Barnes PF, el-Hajj H, Preston-Martin S, Cave MD, Jones BE, Otaya M, Eisenach KD. Transmission of tuberculosis among the urban homeless. Journal of the American Medical Association. 1996;275:305–307. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Severe isoniazid-associated hepatitis–New York, 1991–1993. Morbidity and Mortality Weekly Report. 1993;42:545–547. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Prevention and control of tuberculosis in correctional and detention facilities: Recommendations from CDC. Morbidity and Mortality Weekly Report. 2006;55(RR-9):1–44. [PubMed] [Google Scholar]
- Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: A 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128:116–123. doi: 10.1378/chest.128.1.116. [DOI] [PubMed] [Google Scholar]
- Fountain FF, Tolley EA, Jacobs AR, Self TH. Rifampin hepatotoxicity associated with treatment of latent tuberculosis infection. American Journal of Medical Science. 2009;337:317–320. doi: 10.1097/MAJ.0b013e31818c0134. [DOI] [PubMed] [Google Scholar]
- Glaser J, Greifinger R. Correctional health care: A public health opportunity. Annals of Internal Medicine. 1993;118:139–145. doi: 10.7326/0003-4819-118-2-199301150-00010. [DOI] [PubMed] [Google Scholar]
- Greenberg GA, Rosenheck RA. Jail incarceration, homelessness, and mental health: A national study. Psychiatric Services. 2008;59:170–177. doi: 10.1176/ps.2008.59.2.170. [DOI] [PubMed] [Google Scholar]
- Hong Kong Chest Service, Tuberculosis Research Centre, Madras, British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. American Review of Respiratory Diseases. 1992;145:36–41. doi: 10.1164/ajrccm/145.1.36. [DOI] [PubMed] [Google Scholar]
- Horsburgh CR, Jr, Goldberg S, Bethel J, Chen S, Colson PW, Hirsch-Moverman Y Tuberculosis Epidemiologic Studies Consortium. Latent tuberculosis infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137:401–409. doi: 10.1378/chest.09-0394. [DOI] [PubMed] [Google Scholar]
- Lardizabal A, Passannante M, Kojakali F, Hayden C, Reichman LB. Enhancement of treatment completion for latent tuberculosis infection with 4 months of rifampin. Chest. 2006;130:1712–1717. doi: 10.1378/chest.130.6.1712. [DOI] [PubMed] [Google Scholar]
- Martínez E, Collazos J, Mayo J. Hypersensitivity reactions to rifampin. Pathogenetic mechanisms, clinical manifestations, management strategies, and review of the anaphylactic-like reactions. Medicine. 1999;78:361–369. doi: 10.1097/00005792-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Maruschak LM. HIV in prisons and jails, 2002. Washington, DC: Bureau of Justice Statistics; 2004. (BJS Bulletin, NCJ 205333). [Google Scholar]
- Maruschak LM. Medical problems of jail inmates. Washington, DC: Bureau of Justice Statistics; 2006. (BJS Special Report, NCJ 210696). [Google Scholar]
- Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. American Journal of Respiratory and Critical Care Medicine. 2004;170:445–449. doi: 10.1164/rccm.200404-478OC. [DOI] [PubMed] [Google Scholar]
- Menzies D, Long R, Trajman A, Dion MJ, Yang J, Al Jahdali H, Schwartzman K. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: A randomized trial. Annals of Internal Medicine. 2008;149:689–697. doi: 10.7326/0003-4819-149-10-200811180-00003. [DOI] [PubMed] [Google Scholar]
- Minton TD, Sabol WJ. Jail inmates at midyear 2008–Statistical tables. Washington, DC: Bureau of Justice Statistics; 2009. (BJS Bulletin, NCJ 225709). [Google Scholar]
- Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: A 7-year study from a public health tuberculosis clinic. Journal of the American Medical Association. 1999;281:1014–1018. doi: 10.1001/jama.281.11.1014. [DOI] [PubMed] [Google Scholar]
- Page KR, Sifakis F, Montes de Oca R, Cronin WA, Doherty MC, Federline L, Dorman SE. Improved adherence and less toxicity with rifampin vs. isoniazid for treatment of latent tuberculosis: A retrospective study. Archives of Internal Medicine. 2006;166:1863–1870. doi: 10.1001/archinte.166.17.1863. [DOI] [PubMed] [Google Scholar]
- Reichman LB, Lardizabal A, Hayden CH. Considering the role of four months of rifampin in the treatment of latent tuberculosis infection. American Journal of Respiratory and Critical Care Medicine. 2004;170:832–835. doi: 10.1164/rccm.200405-584PP. [DOI] [PubMed] [Google Scholar]
- Sabol WJ, Minton TD. Jail inmates at midyear 2007. Washington, DC: Bureau of Justice Statistics; 2008. (BJS Bulletin, NCJ 221945). [Google Scholar]
- Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: Hepatotoxicity of antituberculosis therapy. American Journal of Respiratory and Critical Care Medicine. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- Shieh FK, Snyder G, Horsburgh CR, Bernardo J, Murphy C, Saukkonen JJ. Predicting non-completion of treatment for latent tuberculous infection: A prospective survey. American Journal of Respiratory and Critical Care Medicine. 2006;174:717–721. doi: 10.1164/rccm.200510-1667OC. [DOI] [PubMed] [Google Scholar]
- Sterling TR. New approaches to the treatment of latent tuberculosis. Seminars in Respiratory and Critical Care Medicine. 2008;29:532–541. doi: 10.1055/s-0028-1085704. [DOI] [PubMed] [Google Scholar]
- Wang E, Tulsky JP, White MC. Clinical care for persons with a history of incarceration. In: King TE Jr, Wheeler MB, Bindman AB, Fernandez A, Grumbach K, Schilinger D, Villela TJ, editors. Medical management of vulnerable and underserved patients: Principles, practice and populations. New York, NY: McGraw-Hill; 2007. pp. 235–245. [Google Scholar]
- White MC, Gournis E, Kawamura M, Menendez E, Tulsky JP. Effect of directly observed preventive therapy for latent tuberculosis infection in San Francisco. International Journal of Tuberculosis and Lung Disease. 2003;7:30–35. [PubMed] [Google Scholar]
- White MC, Tulsky JP, Estes M, Jamison R, Long HL. Health and health behaviors in HIV-infected jail inmates, 1999 and 2005. AIDS Patient Care and STDs. 2008;22:221–231. doi: 10.1089/apc.2007.0043. [DOI] [PubMed] [Google Scholar]
- White MC, Tulsky JP, Goldenson J, Portillo CJ, Kawamura M, Menendez E. Randomized controlled trial of interventions to improve follow-up for latent tuberculosis infection after release from jail. Archives of Internal Medicine. 2002;162:1044–1050. doi: 10.1001/archinte.162.9.1044. [DOI] [PubMed] [Google Scholar]
- White MC, Tulsky JP, Menendez E, Goldenson J, Kawamura LM. Incidence of TB in inmates with latent TB infection: 5-year follow-up. American Journal of Preventive Medicine. 2005;29:295–301. doi: 10.1016/j.amepre.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Wilper AP, Woolhandler S, Boyd JW, Lasser KE, McCormick D, Bor DH, Himmelstein DU. The health and health care of U.S. prisoners: Results of a nationwide survey. American Journal of Public Health. 2009;99:666–672. doi: 10.2105/AJPH.2008.144279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H, Wessolossky M, Ellis J, Kaminski M, Daly JS. A retrospective evaluation of completion rates, total cost, and adverse effects for treatment of latent tuberculosis infection in a public health clinic in central Massachusetts. Clinical Infectious Diseases. 2009;49:424–427. doi: 10.1086/600394. [DOI] [PubMed] [Google Scholar]
- Ziakas PD, Mylonakis E. 4 months of rifampin compared with 9 months of isoniazid for the management of latent tuberculosis infection: A meta-analysis and cost-effectiveness study that focuses on compliance and liver toxicity. Clinical Infectious Diseases. 2009;49:1883–1889. doi: 10.1086/647944. [DOI] [PubMed] [Google Scholar]