Abstract
Background
Approximately one fourth of bone and soft-tissue sarcomas recur after prior treatment. GLV-1h68 is a recombinant, replication-competent vaccinia virus that has been shown to have oncolytic effects against many human cancer types. We sought to determine whether GLV-1h68 could selectively target and lyse a panel of human bone and soft-tissue sarcoma cell lines in vitro and in vivo.
Methods
GLV-1h68 was tested in a panel of four cell lines including: fibrosarcoma HT-1080, osteosarcoma U-2OS, fibrohistiocytoma M-805 and rhabdomyosarcoma HTB-82. Gene expression, infectivity, viral proliferation, and cytotoxicity were characterized in vitro. HT-1080 xenograft flank tumors grown in vivo were injected intratumorally with a single dose of GLV-1h68.
Results
All four cell lines supported robust viral transgene expression in vitro. At a multiplicity of infection (MOI) of 5, GLV-1h68 was cytotoxic to three cell lines, resulting in >80% cytotoxicity over 7 days. In vivo, a single injection of GLV-1h68 into HT-1080 xenografts exhibited localized intratumoral luciferase activity peaking at days 2–4, with gradual resolution over 8 days and no evidence of spread to normal tissues. Treated animals exhibited near-complete tumor regression over a 28-day period without observed toxicity.
Conclusion
GLV-1h68 has potent direct oncolytic effects against human sarcoma in vitro and in vivo. Recombinant vaccinia oncolytic virotherapy could provide a new platform for the treatment of patients with bone and soft tissue sarcomas. Future clinical trials investigating oncolytic vaccinia as a therapy for sarcomas are warranted.
Keywords: oncolysis, replication-competent, recombinant, virus
INTRODUCTION
In the United States, bone and soft-tissue sarcomas result in approximately 13,170 diagnoses with over 3,900 deaths per year, and represent 14.5% of childhood malignancies 1. Treatment often requires a multidisciplinary approach including surgery, chemotherapy and radiation therapy 2. Despite many advances in the treatment, such as immune-based therapy, neoadjuvant and adjuvant multi-agent chemotherapy, and aggressive surgery, approximately one fourth of sarcomas recur, leading to a poor prognosis 1. Therefore, novel therapeutic agents are needed in order to improve the outcome of these patients.
Preclinical studies have suggested that oncolytic viral therapy may be a promising approach for the treatment of malignant tumors. Oncolytic viral therapy relies on the natural ability of viruses to infect, replicate within and ultimately lyse a host cell 3. Viruses with oncolytic properties include: vaccinia virus, herpes simplex virus, adenovirus, Newcastle disease virus, myxoma virus, vesicular stomatitis virus, reovirus, among others 4-7. Vaccinia virus has many favorable characteristics, including the ability to infect a wide range of tumor types with efficient infection and gene expression, and potent lytic activity, which make it an excellent candidate for recombinant viral therapy 8. Further, vaccinia virus replication occurs exclusively in the cytoplasm, which eliminates the possibility of chromosomal integration. Vaccinia virus has already demonstrated an acceptable safety features in humans, since it was administered historically to millions of people as a smallpox vaccine. Importantly, vaccinia virus has previously demonstrated a natural ability to selectively target and infect cancer cells in vivo 9.
GLV-1h68 is a recombinant, replication-competent vaccinia virus expressing transgenes for Renilla luciferase, green fluorescent protein (GFP), and β-galactosidase. It has exhibited effective oncolytic ability against human thyroid cancer, head and neck squamous cell carcinoma, breast cancer, malignant pleural mesothelioma, and pancreatic cancer 3, 10-14. In the current study, we sought to determine whether GLV-1h68 could selectively target and lyse a panel of sarcoma cell lines in vitro and in vivo using a murine flank tumor model. These experiments explore the potential application of vaccinia as a novel, clinically relevant, therapeutic agent for bone and soft-tissue sarcoma.
MATERIALS AND METHODS
Cell lines
Four human sarcoma cell lines were studied: fibrosarcoma HT-1080, osteosarcoma U-2 OS, fibrohistiocytoma M-805 and rhabdomyosarcoma HTB-82. All cells were grown in a humidified incubator at 37°C under 5% CO2. HT-1080 was grown in MEM medium. M-805 was grown in RPMI medium. U-2 OS, HTB-82 and CV-1 African green monkey kidney cells used for viral tittering were grown in DMEM medium. All medium contain 10% FCS with 1% penicillin and streptomycin.
Virus
GLV-1h68 is a replication-competent, recombinant vaccinia virus that was derived from the LIVP strain (Lister strain from the Institute of Viral Preparations, Moscow, Russia). The construction of GLV-1h68 has been previously described 10. GLV-1h68 carries the following gene cassettes inserted into the viral genome: a Renilla luciferase-green fluorescent protein (RUC-GFP) fusion cassette at the F14.5L locus, a reversely inserted human transferrin receptor (rTfr), β-galactosidase cassette at the J2R locus (encoding thymidine kinase), and a β-glucuronidase cassette at the A56R locus (encoding hemagglutinin).
GFP expression and X-gal cytochemistry in vitro
Cells were plated at 1×105 per well in 24-well plates in 1 mL of medium per well. After 6 hours, cells were infected by GLV-1h68 at a multiplicity of infection (MOI) of 1. At 12, 24, and 48 hours after infection, cells were examined with an inverted fluorescence microscope (Nikon Eclipse TE300; Nikon, Tokyo, Japan) for GFP expression, and photographs were taken at 10×. At 6, 12 and 24 hour postinfection, cells were fixed with 1% glutaraldehyde for 5 minutes and stained with X-Gal (1 mg/mL) in an iron solution of 5 mM K4Fe (CN)6, 5 mM K3Fe (CN)6, with 2 mM MgCl2 and incubated at 37°C for 4 hours. Cells were then washed with phosphate-buffered saline (PBS) and examined at 10× by light microscopy.
β-galactosidase assays
Cells (1×104) were seeded in 96-well plates with 100 uL of medium per well. After 6 hours, cells were infected with GLV-1h68 at an MOI of 5. At 6 hours after infection, cells were lysed and β-galactosidase activity was measured with an enhanced β-galactosidase assay kit (Genlantis, San Diego, CA), using a spectrophotometer (EL321e; Bio-Tek Instruments, Winooski, VT) at 570 nm. All samples were measured in triplicate.
In vitro quantification of luciferase activity
Cells were plated in 96-well plates at 1×104 cells per well and 6 hours later GLV-1h68 was added at an MOI of 5. At 12 hours after infection, 0.25 μg coelenterazine (Biotium, Hayward, CA) in 50 μL PBS was added to each well. After 10 minutes, emitted photons were measured for 30 seconds with a cooled CCD camera (Xenogen IVIS, Xenogen, Alameda, CA). Images were analyzed using Living Image software (Xenogen). Samples were analyzed in triplicate.
Viral plaque assays
Cells were plated at 2 ×104 cells per well in 12-well plates. After 6 hours, GLV-1h68 in 100 μL PBS was added to each well at an MOI of 0.1 and incubated 20 minutes at room temperature. One mL of medium was added to each well and incubated at 37°C for 72 hours. Supernatant from each well was collected and frozen. CV-1 cells were grown to confluence on 6-well plates. Supernatant samples were thawed, and serial ten-fold dilutions were added to the CV-1 cells and incubated for 4 hours. Wells were washed with medium and covered with 1% agarosein medium. After 48 hours of incubation, 2 mL of neutral red solution (2% by volume) was added and viral plaques were counted after 24 hours. Samples were measured in triplicate.
Cytotoxicity assays
Each cell line was plated in 12-well plates at 2 ×104 cells per well. After 6 hours, cells were infected with GLV-1h68 at an MOI of 0 (control), 0.01, 0.1, 1 and 5. Virus-induced cytotoxicity was measured daily for 7 days. Cells were washed with PBS and lysed with Triton X (1.35%, Sigma). Lactate dehydrogenase (LDH) was measured using a Cytotox96 kit (Promega, Madison, WI) and by spectrophotometry (EL321e, Bio-Tek Instruments, Winooski, VT) at 450 nm. Results were expressed as the percentage of surviving cells determined from comparing the measured LDH of each infected sample with that in non-infected control cells. Samples were measured in triplicate.
In vivo GLV-1h68 gene expression
In vivo experiments were performed under an approved animal protocol by the Memorial Sloan-Kettering Institutional Animal Care and Use Committee. Six-week old male athymic nude mice (from National Cancer Institute) were anesthetized with inhalational isoflurane. HT-1080 cells (5 × 106) in 100 L of PBS were injected into the right subcutaneous flanks. Established HT-1080 flank tumors were distributed into two experimental groups (n=3 per group) and were injected with a single dose of GLV-1h68 at 1 ×107 pfu in 50 L PBS, or 50 L PBS alone as control. For luciferase assessment, 2.5 μg of coelenterazine (Biotium) in 97.5 L PBS was injected via the retro-orbital sinus at varying times. Luciferase activity was detected with a cooled CCD camera (Xenogen IVIS). Emitted photons were measured for 5 seconds. Images were analyzed using Living Image software (Xenogen). For histology, animals were sacrificed at day 3 and flank tumors excised, frozen in Tissue Tek solution, and sectioned (10 μm). Slides were fixed with 1% glutaraldehyde and stained with X-Gal (1 mg/mL) in an iron solution of 5 mM K4Fe (CN)6, 5 mM K3Fe (CN)6 and 2 mM MgCl2 at 37°C for 4 hours. Slides were counterstained with nuclear fast red solution.
GLV-1h68 therapy of sarcoma xenografts in mice
Animals with established HT-1080 flank tumors were distributed into two experimental groups (n = 7 per group) with similar starting tumor volumes. Flank tumors were treated with intratumoral injections of a single dose of GLV-1h68 (1×107 pfu) in 50 L PBS, or 50 L PBS alone as control. Tumor dimensions were serially measured with calipers and volumes calculated by the formula: volume = length × width2 × π/6. Body weights were measured every other day. Photographs of representative animals were taken at day 28. Animals were sacrificed by CO2 inhalation.
RESULTS
In vitro β-galactosidase and GFP expression
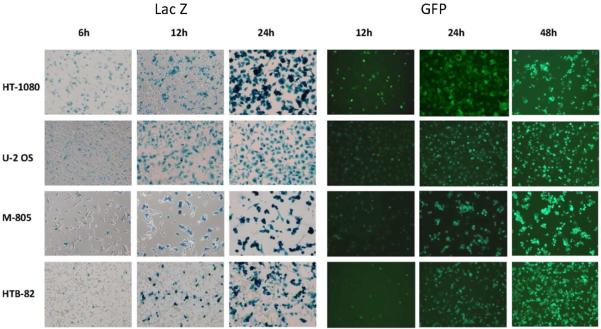
X-gal cytochemistry demonstrated β-galactosidase expression by GLV-1h68 by 6 hours after viral exposure at an MOI of 1 in all cell lines (Figure 1). There was strongly positive staining in all cell lines by 24 hours after viral exposure. HT-1080 was most sensitive to GLV-1h68, expressing β-galactosidase and GFP earlier and more intensely than the other cell lines. GFP expression peaked at 72 hours, with relative cell line sensitivity consistent with X-gal staining results.
Figure 1.
Effective early infection by GLV-1h68 of human sarcoma cell lines. Four human sarcoma cell lines were infected by GLV-1h68 at an MOI of 1. At varying times after infection, the cells were fixed in 1% glutaraldehyde for X-gal staining or visualized directly with a GFP filter. Representative photomicrographs were taken.
In vitro quantification of β-galactosidase and luciferase activity
Quantitative assays for β-galactosidase expression by GLV-1h68 at an MOI of 5 were performed. HT-1080 showed the highest β-galactosidase expression and HTB-82 the lowest expression (Figure 2A). All of the cell lines expressed some degree of susceptibility to infection by GLV-1h68. Luciferase expression demonstrated similar results as the quantitative β-galactosidase assays, with the same relative order of expression noted (Figure 2B).
Figure 2.
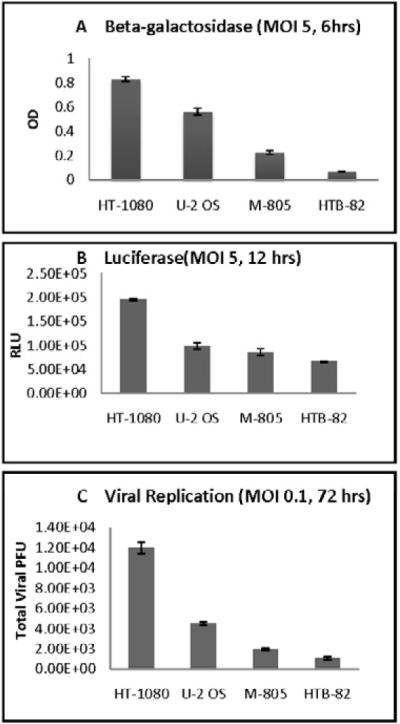
In vitro quantification of gene expression and viral replication by GLV-1h68 in human sarcoma. (A) Cell lines were infected with GLV-1h68 at an MOI of 5. Six hours later, quantitative beta-galactosidase assays were performed. (B) Cell lines were infected with GLV-1h68 at an MOI of 5, and 12 hours later coelenterazine was added. Bioluminescence was imaged with a cooled CCD camera and quantified with software analysis. (C) Cell lines were exposed to GLV-1h68 at an MOI of 0.1 and incubated for 72 hours. Supernatants were collected and viral titers quantified by plaque assays on confluent CV-1 cells.
Viral replication
Viral replication was assessed after infection with GLV-1h68 at an MOI of 0.1 (2×103 pfu). Viral tiers measured 72 hours later ranged from a 6-fold increase in HT-1080, to a doubling in U-2OS, to baseline or lower viral titers for M-805 and HTB-82 (Figure 2C).
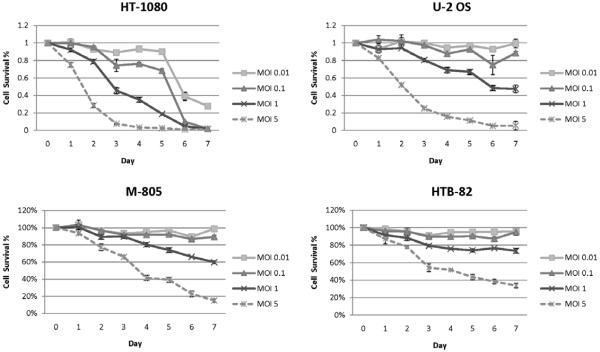
Cytotoxicity assays
All of the cell lines tested demonstrated sensitivity to cytotoxic effects by GLV-1h68 at MOI of 1 and 5 (Figure 3). At MOI 5, HT-1080, showed nearly complete cell death by day 4 after viral infection, and U-2OS showed similar susceptibility by day 6. The other two cell lines also demonstrated significant sensitivity to GLV-1h68, with <20% viable M-805 cells and <40% viable HTB-82 cells remaining by day 7 at MOI of 5. The MOI of 1 curves reflect a similar relative order of decreasing susceptibility: HT-1080, U-2OS, M-805, and HTB-82. HT-1080 was remarkably sensitive to GLV-1h68, and exhibited significant cytotoxicity at even very low MOI’s of 0.01 and 0.1 by day 6.
Figure 3.
Oncolytic effects of GLV-1h68 in human sarcoma. Four human sarcoma cell lines were exposed to GLV-1h68 at MOI of 0.01, 0.1, 1 and 5. Viral-induced cytotoxicity was measured at daily intervals for seven days by lactate dehydrogenase assay. Results were expressed as the percentage of surviving cells as determined by comparing the LDH of each test sample to untreated samples that were considered 100% viable. The assay was performed on 3 replicate samples for each condition, and mean values and standard error were plotted.
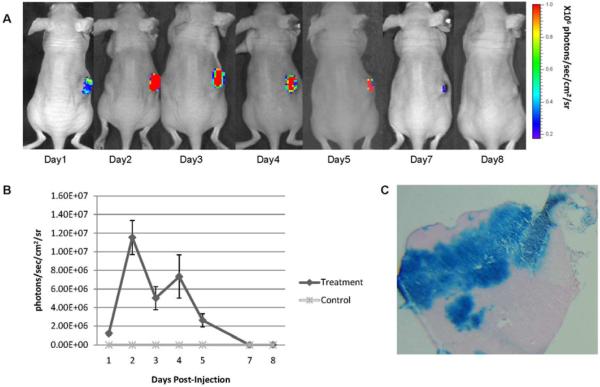
In vivo GLV-1h68 gene expression
In vivo luciferase imaging demonstrated intense activity localized specifically to the HT-1080 flank tumor after the GLV-1h68 injection, with peak levels noted at days 2–4 (Figure 4A). There was a subsequent decline of luciferase activity by day 8. No luciferase activity was detected at any other sites in the animal. Quantification of luciferase expression in vivo was measured, which was reflective of the luciferase imaging results (Figure 4B). At day 3, β-galactosidase expression of tumors sections, as measured by X-gal staining (Figure 4C), demonstrated intense and widespread staining, reflecting effective viral penetration and infection of treated tumors.
Figure 4.
GLV-1h68-mediated luciferase and β-galactosidase expression in fibrosarcoma murine xenograft flank tumors. Fibrosarcoma (HT-1080) flank tumors were treated with a single dose of GLV-1h68 (1×107 pfu) or saline as control. (A) Luciferase activity was measured after retro-orbital coelenterazine injections over a 8-day period. The reduction of luciferase activity correlated with tumor size regression. (B) Quantification of luciferase activity over a 8-day time course using software assessment of emitted protons was performed (n=3 mice). (C) HT-1080 flank tumors were excised 3 days after GLV-1h68 injection and stained for β-galactosidase expression. Representative photograph was taken by microscopy (10×). No animals demonstrated any evidence of toxicity.
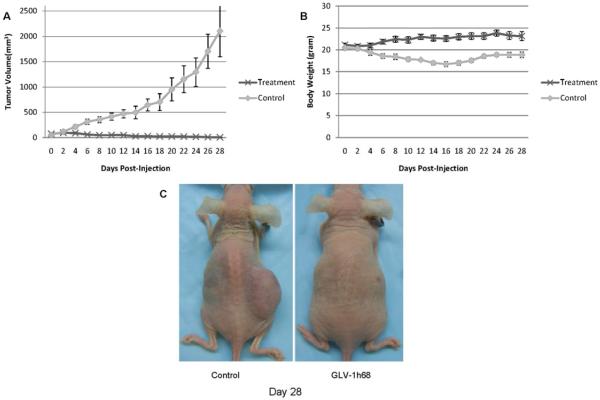
GLV-1h68 therapy of sarcoma xenografts in mice
When flank tumors reached a mean volume of 60 mm3, a single dose of GLV-1h68 (1×107 pfu) or PBS alone was injected intratumorally and followed for 28 days. Treated tumors demonstrated progressive tumor volume regression over a one week period; by day 28, mean tumor volume was just 5.65 ± 3.80 mm3 (Figure 5A). Five out of seven animals had complete regression of tumor, with the other two animals demonstrating only a 3 mm remnant of skin thickening at the regressed tumor site. In sharp contrast, all control tumors progressively increased in size to a mean volume of 2029 ± 570 mm3 over the same period (p < 0.001, t-test). In the control group, the body gross weights decreased slightly, while the mean body weight of the GLV-1h68 group increased slightly (Figure 5B). The control mice looked thin, whereas the treated mice looked healthy (Figure 5C). The experiment was concluded at day 28, since the length of the control tumors had exceeded 20 mm in dimension.
Figure 5.
A single injection of GLV-1h68 results in complete regression of established fibrosarcoma flank tumors in vivo. Established human fibrosarcoma (HT-1080) flank tumors (n=7 mice per group) were treated with a single intratumoral injection of GLV-1h68 (1×107 pfu) or saline as control. (A) Tumor dimensions were measured and mean tumor volumes with standard errors were calculated over a 28-day course. Measured tumor volumes for the GLV-1h68 group demonstrate near complete tumor regression. (B) Mean body weights were recorded. (C) A representative mouse from each group was photographed at day 28.
DISCUSSION
Sarcomas are a very diverse group of malignant tumors with varying clinical behavior that can recur despite conventional therapy with surgery, chemotherapy and radiation therapy 15. New treatment options with completely novel mechanisms of therapeutic activity are needed for these diseases to improve patient outcome. Replication-competent oncolytic viruses are a novel, attractive approach to treating malignancy that harnesses a virus’s natural ability to infect, replicate within, and lyse a host cell as part of its natural life cycle 4, 8.
Many replication-competent oncolytic viruses have been designed with deletions of specific genes that allow for preferential viral replication and lytic activity in malignant tumor cells, but not normal cells. In addition, many oncolytic viruses carry inserted transgenes to allow for assaying for the virus, and for tracking sites of viral localization. The insertion of transgenes into the viral genome can assist in identifying viral transit to sentinel lymph node metastases 16.
Vaccinia virus is an attractive candidate as a therapeutic oncolytic vector for several reasons. First, it it has a large genome which is able to accommodate multiple transgenes without impeding the replicative ability of the virus. Second, vaccinia virus has exhibited a natural affinity for preferentially infecting malignant tumors. And third, vaccinia virus already has an established track record of safety for in humans. Most oncolytic viruses such as adenovirus and herpes simplex virus are natural pathogens; even with built in viral attenuation strategies, their safety for clinical use remains a concern 4. In contrast, however, the widespread historical use of vaccinia virus as a vaccine for smallpox has demonstrated its safety for clinical application in humans.
Here we describe a novel application of GLV-1h68, an attenuated, replication-competent, oncolytic vaccinia virus. GLV-1h68 has a mutation in the J2R gene encoding thymidine kinase that makes viral replication dependent on host cell thymidine kinase 10. This attribute may increase the selectivity for malignant tumors but attenuate its ability to replicate in normal tissues. GLV-1h68 also carries an insertional mutation in hemagglutinin gene and the F14.5L locus. Inactivation of hemagglutinin is thought to reduce the virulence of vaccinia. F14.5L has a unique NotI site, which makes this locus suitable for insertion of the luciferase-GFP cassette. GLV-1h68 injections into established anaplastic thyroid tumors in murine flanks results in specific and prolonged luciferase expression within tumor tissues, but not in normal tissue sites 12. Vaccinia virus have also been shown to have a positive therapeutic effect on head and neck squamous cell carcinoma without infection of normal cells or evidence of toxicity 3.
In addition to direct cell lysis, indirect tumoricidal mechanisms may play an important role in the antitumor activity by oncolytic vaccinia virus. Immune responses induced by vaccinia can provide beneficial anti-tumor effects [17, 18]. These immunologic effects may be further exploited by engineering vaccinia to express a variety of cytokines, which may enhance anti-tumor effects in both pre-clinical and clinical studies [19-23]. Viral infection and replication may lead to lysis of tumor cells, a release of tumor antigens into the extracellular environment, and a subsequent generation of a long-term memory immune response targeting tumor antigens [24].
In the current manuscript, we used an athymic mouse to support the growth of human sarcoma flank tumors. This is not a suitable model for studying the immunotherapeutic potential of oncolytic vaccinia virus. Therefore, we focused our studies on investigating direct sarcoma lysis resulting from therapeutic oncolytic vaccinia virus. All four human sarcoma cell lines supported effective viral transgene expression (β-galactosidase, green fluorescent protein, luciferase) beginning as early as 6 hours after viral exposure. Viral replication was observed in two of the four cell lines by 72 hours, suggesting that there are sarcoma cell factors that are determinants of permissiveness to vaccinia. The low dose of GLV-1h68 used in the viral replication studies was intended to allow assessment of maximal viral replication, and may have been insufficient to allow for initial infection of the two less sensitive cell lines.
At MOI of 5, GLV-1h68 was highly cytotoxic to two of the cell lines (HT-1080 and U-2 OS), resulting in ≥ 90% cytotoxicity over a 7-day period. Even at an extremely low MOI of 0.01, HT-1080 cell line still demonstrated > 70% cell death over 7 days. The two other sarcoma cell lines were also susceptible to oncolysis at MOI of 5, demonstrating that the virus exhibits broad efficacy at even this modest dose. Markers of early gene expression correlated well with susceptibility to oncolysis. These correlations suggest that initial viral entry and early gene expression by GLV-1h68 may define subsequent cytotoxic susceptibility to the virus.
A single injection of intratumoral GLV-1h68 (1×107 pfu) in vivo into HT-1080 xenografts in mice exhibited localized intratumoral luciferase activity peaking at days 2-4, with gradual resolution over 8 days. Furthermore, there was no evidence of viral spread to normal organs or non-tumor sites based on luciferase imaging, demonstrating tumor-specific viral replication and activity. Animals treated with GLV-1h68 exhibited near complete tumor regression over a 28-day period without any observed toxicity, while control animals demonstrated rapid tumor progression requiring sacrifice by day 28. GLV-1h68 treated animals did not exhibit any morbidity or mortality. These results demonstrate that a single intratumoral injection GLV-1h68 exhibits significant therapeutic efficacy leading to near complete tumor regression without toxicity.
In this study we explored the efficacy of direct intratumoral injection as a therapeutic agent against locally recurrent sarcomas. Our main objective was to determine the oncolytic potential of vaccinia in treating sarcomas, and we therefore selected the most direct method of applying the virus. Direct vaccinia application may be useful in treating locally aggressive tumors [3]. Most recently, a phase I clinical trial in which oncolytic vaccinia virus was administered intratumorally to patients with refractory primary or metastatic liver cancer showed promising results [23]. Intravenous mode of delivery has also been shown to be efficacious in both preclinical and clinical phase 1 trials [9, 24-27]. Oncolytic vaccinia virus injected intravenously was able to infect, replicate within and express transgenic products in cancer cells [28].
In conclusion, this study demonstrates that GLV-1h68 may exhibit significant therapeutic effects against human sarcoma cell lines in vitro and in vivo. Recombinant vaccinia oncolytic virotherapy could provide a new platform for the treatment of patients with bone and soft tissue sarcomas. Based on these encouraging results, further preclinical and clinical studies should be planned.
Acknowledgements
RJW is supported by NIH grants R21DE019015 and R01CA157686.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert NF, Cannon CP, Lin PP, Lewis VO. Soft-tissue sarcoma. J Am Acad Orthop Surg. 2009;17:40–47. doi: 10.5435/00124635-200901000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Yu Z, Li S, Brader P, Chen N, et al. Oncolytic vaccinia therapy of squamous cell carcinoma. Mol Cancer. 2009;8:45. doi: 10.1186/1476-4598-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu TC, Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- 5.Mullen JT, Tanabe KK. Viral oncolysis for malignant liver tumors. Ann Surg Oncol. 2003;10:596–605. doi: 10.1245/aso.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Chen CH, Li S, Givi B, et al. Therapeutic effects of a fusogenic newcastle disease virus in treating head and neck cancer. Head Neck. 2010 Nov 4; doi: 10.1002/hed.21609. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo Y, Kelly KJ, Stanford MM, Galanis C, et al. Myxoma virus is oncolytic for human pancreatic adenocarcinoma cells. Ann Surg Oncol. 2008;15:2329–2335. doi: 10.1245/s10434-008-9924-z. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, Neumunaitis J. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther. 2005;11:180–195. doi: 10.1016/j.ymthe.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Yu YA, Wang E, Chen N, et al. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 11.Lin SF, Yu Z, Riedl C, Woo Y, et al. Treatment of anaplastic thyroid carcinoma in vitro with a mutant vaccinia virus. Surgery. 2007;142:976–983. doi: 10.1016/j.surg.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Lin SF, Price DL, Chen CH, Brader P, et al. Oncolytic vaccinia virotherapy of anaplastic thyroid cancer in vivo. J Clin Endocrinol Metab. 2008;93:4403–4407. doi: 10.1210/jc.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly KJ, Woo Y, Brader P, Yu Z, et al. Novel oncolytic agent GLV-1h68 is effective against malignant pleural mesothelioma. Hum Gene Ther. 2008;19:774–82. doi: 10.1089/hum.2008.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu YA, Galanis C, Woo Y, Chen N, et al. Regression of human pancreatic tumor xenografts in mice after a single systemic injection of recombinant vaccinia virus GLV-1h68. Mol Cancer Ther. 2009;8:141–151. doi: 10.1158/1535-7163.MCT-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maki RG. Role of chemotherapy in patients with soft tissue sarcomas. Expert Rev Anticancer Ther. 2004;4:229–236. doi: 10.1586/14737140.4.2.229. [DOI] [PubMed] [Google Scholar]
- 16.Brader P, Kelly K, Gang S, Shah JP, et al. Imaging of lymph node micrometastases using an oncolytic virus herpes virus and [F]FEAU PET. PLoS One. 2009;4:e4789. doi: 10.1371/journal.pone.0004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 18.Thorne SH, Hwang TH, Kirn DH. Vaccinia virus and oncolytic virotherapy of cancer. Curr Opin Mol Ther. 2005;7:359–65. [PubMed] [Google Scholar]
- 19.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne SH, Hwang TH, O’Gorman WE, Bartlett DL, Sei S, Kanji F, Brown C, Werier J, Cho JH, Lee DE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–8. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park B-H, Hwang T-H, Kim S, Rhee SS, Ahn Y-J, Kwon H-C, Oh S-Y, Han J, Speth K, Crompton AM, et al. A Phase I-II clinical trial with JX-594, a targeted and GM-CSF-armed oncolytic poxvirus, by intratumoral injection in patients with liver tumors; AACR-NCI-EORTC international conference; San Francisco. 2007. [Google Scholar]
- 22.Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008;16:1637–42. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 23.Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, Oh SY, Han SY, Yoon JH, Hong SH, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–42. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 24.Thorne SH. Immunotherapeutic potential of oncolytic vaccinia virus. Immunol Res. 2011;50(2-3):286–93. doi: 10.1007/s12026-011-8211-4. [DOI] [PubMed] [Google Scholar]
- 25.Worschech A, Chen N, Yu YA, et al. Systemic treatment of xenografts with vaccinia virus GLV-1h68 reveals the immunologic facet of oncolytic therapy. BMC Genomics. 2009;10:301. doi: 10.1186/1471-2164-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentschev I, Donat U, Hofmann E, Weibel S, et al. Regression of human prostate tumors and metastases in nude mice following treatment with the recombinant oncolytic vaccinia virus GLV-1h68. J Biomed Biotechnol. 2010 doi: 10.1155/2010/489759. Epub 2010 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baril P, Touchefeu Y, Cany J, et al. Differential biodistribution of oncolytic poxvirus administered systemically in an autochthonous model of hepatocellular carcinoma. J Gene Med. 2011 doi: 10.1002/jgm.1624. Epub 2011 Oct. 26. [DOI] [PubMed] [Google Scholar]
- 28.Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancertargeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]