Abstract
A critical event of pharyngeal swallowing is the elevation of the hyolaryngeal complex to open the upper esophageal sphincter. Current swallowing theory assigns this function to the submental and thyrohyoid muscles. However, the attachments of the long pharyngeal muscles indicate that they could contribute to this function, yet their role is uninvestigated in humans. In addition, there is evidence the posterior digastric and stylohyoid contribute to hyoid elevation. A cadaver model was used to document the structural properties of muscles. These properties were used to model muscle groups as force vectors and analyze their potential for hyolaryngeal elevation. Vector magnitude was determined using physiological cross-sectional areas (PCSAs) of muscles calculated from structural properties of muscle taken from 12 hemisected cadaver specimens. Vector direction (lines of action) was calculated from the three-dimensional coordinates of muscle attachment sites. Unit force vectors in the superior direction of submental, suprahyoid (which includes the submental muscles), long pharyngeal, and thyrohyoid muscles were derived and compared by an analysis of variance (ANOVA) to document each muscle’s potential contribution to hyolaryngeal elevation. An ANOVA with Tukey HSD post hoc analysis of unit force vectors showed no statistically significant difference between the submental (0.92 ± 0.24 cm2) and long pharyngeal (0.73 ± 0.20 cm2) muscles. Both demonstrated greater potential to elevate the hyolaryngeal complex than the thyrohyoid (0.49 ± 0.18 cm2), with P < 0.01 and P < 0.05, respectively. The suprahyoid muscles (1.52 ± 0.35 cm2) demonstrated the greatest potential to elevate the hyolaryngeal complex: greater than both the long pharyngeal muscles (P < 0.01) and the thyrohyoid (P < 0.01). The submental and thyrohyoid muscles by convention are thought to elevate the hyolaryngeal complex. This study demonstrates that structurally the long pharyngeal muscles have similar potential to contribute to this critical function, with the suprahyoid muscles having the greatest potential. If verified by functional data, these findings would amend current swallowing theory.
Keywords: Deglutition, Laryngeal elevation, Physiological cross-sectional area, Structural properties, Hyolaryngeal complex, Deglutition disorders
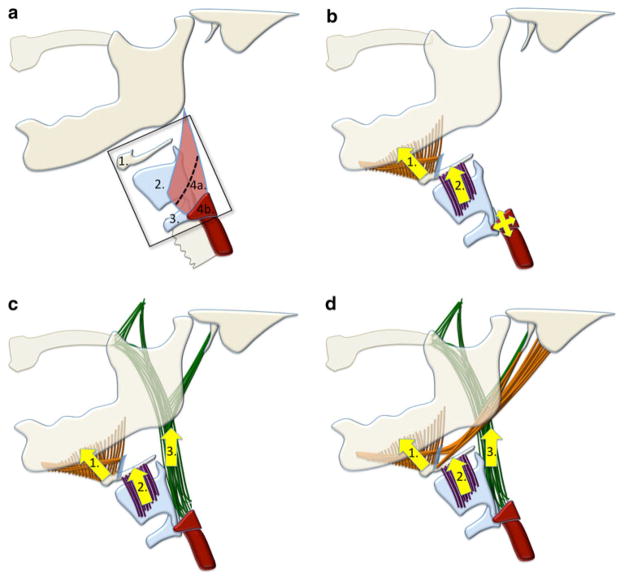
A crucial event in the pharyngeal phase of swallowing is the elevation of the hyolaryngeal complex following bolus transfer into the pharynx and preceding pharyngeal constriction (Fig. 1a). Hyolaryngeal elevation occurs concomitant with the opening of the upper esophageal sphincter. This combination of movements displaces the larynx away from the trajectory of an oncoming bolus, shortens the pharynx, and pulls open the otherwise closed upper esophageal sphincter to receive the ingested bolus [1]. Reduced elevation of the larynx has been shown to be associated with aspiration [2]. Submental muscles (mylohyoid, geniohyoid, and anterior digastric) and the thyrohyoid muscle are thought to elevate the hyolaryngeal complex in swallowing [1, 3] (Fig. 1b). The hyolaryngeal complex includes the hyoid bone, thyrohyoid membrane, and laryngeal cartilages serving as an attachment site for the cricopharyngeus that forms the upper esophageal sphincter. Other muscles attaching to the hyolaryngeal complex, including the posterior digastric, stylohyoid, and long pharyngeal muscles, have been named in the literature as potential contributors to this movement, but their roles have not been explicitly investigated [4–6]. In a prior study, a cadaver model was used to evaluate the architecture of muscles positioned to move the hyoid anteriorly and superiorly [7]. The purpose of this study was to document the structural properties of muscles attaching to the hyolaryngeal complex and evaluate the potential contribution of various muscle groups in laryngeal elevation (Fig. 1c, d).
Fig. 1.
a Illustrated hyolaryngeal complex including (1) hyoid bone, (2) thyroid cartilage, (3) cricoid cartilage, and (4) upper esophageal sphincter comprising the (a) inferior portion of the thyropharyngeus and (b) cricopharyngeus. b Illustration of the current theory of hyolaryngeal elevation by the (1) submental (mylohyoid, geniohyoid, anterior digastric) and (2) thyrohyoid muscles. This action is thought to open the upper esophageal sphincter. c Illustration of the two-sling theory with (1) the submental muscles as an anterior sling, (2) the thyrohyoid muscle, and (3) the long pharyngeal muscles (stylopharyngeus, palatopharyngeus, and salpingopharyngeus) as a posterior sling. d Illustration of the two-sling theory with (1) the suprahyoid muscles group (submental, posterior digastric, and stylohyoid) as an anterior sling, (2) the thyrohyoid muscle, and (3) the long pharyngeal muscles as a posterior sling
We propose that an anterior and posterior sling of muscles suspends the hyolaryngeal complex and elevates it in swallowing, aided by the thyrohyoid (Fig. 1c, d). The submental muscles (mylohyoid, geniohyoid, and anterior digastric) attach to the hyoid and by convention are thought to form the anterior sling (Fig. 1c). Additionally, the distal insertions of the remaining suprahyoid muscles, posterior digastric and stylohyoid, suggest that these muscles also contribute to the anterior sling [6, 8] (Fig. 1d). The long pharyngeal muscles, which include the stylopharyngeus, salpingopharyngeus, and palatopharyngeus, stabilized by the levator veli palatini, form a posterior sling. The distal attachments of these muscles blend together on the posterior edge of the thyroid cartilage and lateral pharyngeal walls proximal to the upper esophageal sphincter [9]. Finally, the thyrohyoid muscle is intrinsic to the hyolaryngeal complex and approximates the thyroid and hyoid. These muscle groups are summarized in Fig. 1d.
To test the potential contribution of the submental group, suprahyoid (submental + posterior digastric and stylohyoid) group, long pharyngeal group, and thyrohyoid muscle to hyolaryngeal elevation, we modeled each muscle as a potential force vector based on structural properties and used these data to analyze the potential contribution of the functional muscle groups to elevate the hyolaryngeal complex (Fig. 1c, d). Muscles traditionally considered to have a secondary role in hyolaryngeal elevation during the pharyngeal phase of swallowing were excluded from analysis. It should be underscored from the start that structural properties are not necessarily the same as functional realities. It is unlikely that force vectors based on morphology alone are entirely predictive of swallowing function [10]. Structural analysis of muscles in a cadaver can say nothing about the motor unit recruitment that ultimately determines the relative contribution of muscles in vivo. However, neuromuscular control of swallowing is delimited by muscular architecture. If the usual list of muscles assumed to underlie a swallowing function is incomplete, then a function may be erroneously attributed to the wrong muscles.
This study investigates structural data to document the anatomical substrate underlying this critical event in pharyngeal deglutition. We propose that two muscular slings, along with the thyrohyoid, elevate the hyolaryngeal complex. Our hypothesis is that the long pharyngeal muscles have as much structural potential to elevate the hyolaryngeal complex as the submental or thyrohyoid muscles. We predict that the physiological cross-sectional area (PCSA) force vector analysis of these muscle groups will show that there is no statistically significant difference between the long pharyngeal muscles and submental muscles and that the suprahyoid muscles as a group have a greater potential for hyolaryngeal elevation than all other groups.
Methods
Subjects
The Boston University School of Medicine Anatomical Gifts program granted ethical permission for this research in compliance with the Institutional Review Board and the wishes of body donors. After specimens were used for educational purposes, seven less dissected bodies were chosen for this study. Of the possible 14 head and neck formalin-fixed hemisections, two were not fully intact and excluded.
Muscles examined in the present study include geniohyoid, anterior digastric, mylohyoid, posterior digastric, stylohyoid, thyrohyoid, stylopharyngeus, palatopharyngeus, and salpingopharyngeus. The stylopharyngeus was analyzed in its two parts: the proximal portion, inserting into the lateral pharyngeal wall, and the distal portion, inserting into the posterior edge of the thyroid cartilage. This distinction was made based on our consistent observation of these features. The salpingopharyngeus and the palatopharyngeus were measured as one muscle since their fibers are blended except for their most proximal attachment sites.
The muscles were grouped in two configurations to analyze the potential of muscle groups to elevate the hyolaryngeal complex. One configuration reflected current theory and compared the long pharyngeal muscles (stylopharyngeus, palatopharyngeus, and salpingopharyngeus) to the submental muscles (mylohyoid, geniohyoid, and anterior digastric) and the thyrohyoid. A second configuration added the posterior digastric and the stylohyoid to the submental group to form a suprahyoid group of muscles. While it is quite likely that each of these muscles can act independently and has separate neural control, our question is the potential net effect of these muscles in the superior direction.
Vectors
Methods for modeling muscles as vectors to compare their potential for force in a particular direction are thoroughly described in a previous study of the suprahyoid muscles [7]. PCSA of each muscle, a proxy for the potential maximum tetanic tension the muscle is capable of producing, is utilized as a measure of vector magnitude [11, 12]. The PCSA calculation uses muscle mass and density, muscle fiber pennation angles, and optimized fascicle lengths to calculate the functional cross-sectional area of the muscle (in cm2):
Muscle tissue samples from distal, proximal, and midpoints of muscle fascicles were wet mounted with glycerol to avoid tissue-altering processing [13]. Photomicrographs of muscle fascicles were captured under 60× magnification. The digital photomicrograph workstation included a Zeis Axioskop microscope (Zeis, Germany) equipped with an Olympus Q-Colors 5 digital camera (Olympus, Center Valley, PA) connected to a Dell Precision T5400 computer (Dell Computer Co., Round Rock, TX) equipped with QCapture version 2.98.0 software (Quantitative Imaging Corporation, Surrey, BC, Canada). Images were analyzed with Image J software (ImageJ, National Institutes of Health, Bethesda, MD). Ten sarcomeres in series were measured from the middle of the I-band to the middle of the adjacent I-band using ImageJ dynamic profiler plug-in to calculate an optically precise average sarcomere length. An important step of proper PCSA calculation is to adjust mean fascicle lengths to their optimum length using a ratio of optimal sarcomere length to mean sarcomere length in the sample [14]. In this study we tested a new method of measuring sarcomere lengths of thyrohyoid and long pharyngeal muscles using an ImageJ plug-in called dynamic profiler. We measured sarcomere lengths twice on a sample of palatopharyngeus muscles (n = 8), once using the dynamic profiler to set the measure of the length tool and once without. A t test demonstrated that there was no significant difference in its accuracy (P = 0.48). Because the dynamic profiler allowed for a more efficient identification of boundaries between sarcomeres, this method was used for all muscles in this study.
Three-dimensional coordinates of the muscle attachments were recorded using digital image analysis of hemisections to generate x and y coordinates of muscle attachments. A handheld Mitutoyo digital caliper (model No. CD-600 CSX, Mitutoyo Corp., Kanogawa, Japan) was used to measure z coordinates. Vector directions were derived from the muscle lines of action, which were derived from connecting the three-dimensional coordinates of muscle attachments [7]. The potential for each muscle to create movement in the superior direction was derived for each muscle by isolating the portion of each unit vector in the superior direction. By multiplying PCSA values by the ĵ (superior–inferior) unit vector of that muscle, we calculated PCSA force unit vectors that represent the potential maximum tetanic tension of each muscle in the superior direction. These vectors were summed to form the functional groups described above and compared to evaluate the potential of each group to elevate the hyolaryngeal complex.
Analysis
We compared muscle groups using one-way analyses of variance with Tukey HSD post hoc analysis performed using the VassarStats website for statistical computation (http://faculty.vassar.edu/lowry/VassarStats.html). To test the hypothesis that structurally the long pharyngeal muscles have the potential to produce the same force to elevate the hyolaryngeal complex as do the submental or thyrohyoid muscles, we compared the vertical (superior-inferior) PCSA force vectors of the long pharyngeal muscles with those of the submental muscles (geniohyoid, mylohyoid, and anterior digastric) and the thyrohyoid. A second analysis was conducted comparing vertical PCSA vectors of the long pharyngeal muscles to those of the suprahyoid muscles (geniohyoid, mylohyoid, anterior digastric, posterior digastric, and stylohyoid) as a group and to that of the thyrohyoid.
Results
The means and standard deviations of PCSA values calculated from structural properties were as follows (in cm2): posterior portion of mylohyoid (0.43 ± 0.12), anterior portion of mylohyoid (0.82 ± 0.18), posterior digastric (0.64 ± 0.16), anterior digastric (0.55 ± 0.12), stylohyoid (0.27 ± 0.09), geniohyoid (0.46 ± 0.16), thyrohyoid (0.51 ± 0.18), stylopharyngeus (pharyngeal insertion) (0.12 ± 0.06), stylopharyngeus (thyroid insertion) (0.19 ± 0.07), and palatopharyngeus and salpingopharyngeus (0.48 ± 0.17) (Table 1). The means and standard deviations of the PCSA force unit vectors in the superior direction were as follows (in cm2): posterior portion of mylohyoid (0.85 ± 0.05), anterior portion of mylohyoid (0.66 ± 0.18), posterior digastric (0.58 ± 0.06), anterior digastric (−0.15 ± 0.13), stylohyoid (0.80 ± 0.05), geniohyoid (0.24 ± 0.13), thyrohyoid (0.49 ± 0.18), stylopharyngeus (pharyngeal insertion) (0.08 ± 0.04), stylopharyngeus (thyroid insertion) (0.18 ± 0.07), and palatopharyngeus and salpingopharyngeus (0.48 ± 0.17) [7].
Table 1.
Structural properties necessary to calculate the PCSA for the thyrohyoid and long pharyngeal muscles (n = 12; 6 males, 6 females)
| Mass (g) | Pennation angles | Fiber length (mm) | Sarcomere lengths (lm) | PCSA (cm2) | |
|---|---|---|---|---|---|
| Thyrohyoid | 1.56 ± 0.49 | 3.78 ± 1.47 | 30.68 ± 6.56 | 2.90 ± 0.24 | 0.51 ± 0.18 |
| Stylopharyngeus (pharyngeal insertion) | 0.54 ± 0.26 | 3.92 ± 1.57 | 40.52 ± 7.69 | 2.76 ± 0.40 | 0.12 ± 0.06 |
| Stylopharyngeus (thyroid insertion) | 1.35 ± 0.53 | 2.89 ± 1.20 | 63.98 ± 15.58 | 2.62 ± 0.48 | 0.19 ± 0.07 |
| Palatopharyngeus/salpingopharyngeus | 4.01 ± 0.75 | 3.53 ± 1.27 | 87.31 ± 21.39 | 2.80 ± 0.36 | 0.48 ± 0.17 |
| Posterior mylohyoida | 2.17 ± 0.56 | 2.39 ± 0.85 | 47.50 ± 3.92 | 2.95 ± 0.43 | 0.43 ± 0.12 |
| Anterior mylohyoida | 3.03 ± 0.47 | 6.99 ± 4.49 | 32.87 ± 4.26 | 2.75 ± 0.34 | 0.82 ± 0.18 |
| Posterior digastrica | 2.53 ± 0.65 | 7.10 ± 3.71 | 30.27 ± 4.28 | 2.43 ± 0.51 | 0.64 ± 0.16 |
| Anterior digastrica | 2.37 ± 0.46 | 9.29 ± 3.40 | 33.30 ± 4.50 | 2.43 ± 0.30 | 0.55 ± 0.12 |
| Stylohyoida | 1.39 ± 0.46 | 5.02 ± 1.83 | 46.93 ± 7.11 | 2.80 ± 0.35 | 0.27 ± 0.09 |
| Geniohyoida | 2.21 ± 0.59 | 7.30 ± 1.58 | 35.32 ± 3.69 | 2.31 ± 0.55 | 0.46 ± 0.16 |
Data are given as mean ± SD
Previously published data
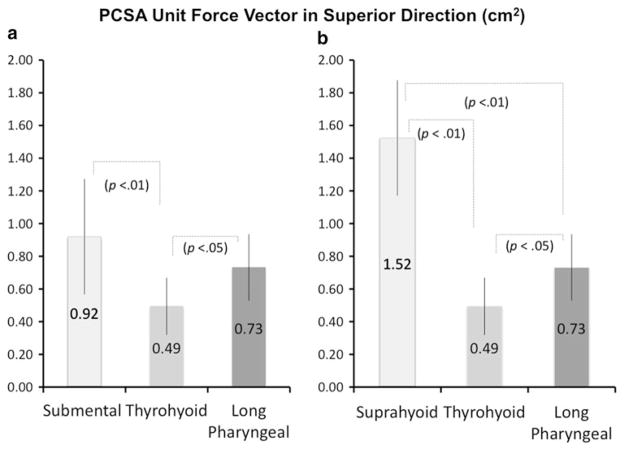
Comparison of the muscle groups (summed PCSA force unit vectors in the superior direction) showed no statistically significant difference between the submental (0.92 ± 0.24 cm2) and long pharyngeal groups (0.73 ± 0.20 cm2), though both were significantly larger than that for the thyrohyoid (0.49 ± 0.18 cm2), with P < 0.01 and P < 0.05, respectively (Fig. 2a). Comparison of the suprahyoid group, long pharyngeal, and thyrohyoid muscles showed the superior force vector of the suprahyoid group (1.52 ± 0.35 cm2) is greater than that of the long pharyngeal (P < 0.01) and thyrohyoid (P < 0.01), and that of the long pharyngeal is greater than that of the thyrohyoid (P < 0.05) (Fig. 2b).
Fig. 2.
a, b Means and standard deviations of superior PCSA force unit vectors (cm2) of a submental versus thyrohyoid versus long pharyngeal and of b suprahyoid versus thyrohyoid versus long pharyngeal. An analysis of variance with Tukey HSD of the unit force vector shows that the long pharyngeal muscles have as much potential to elevate the hyolaryngeal complex as the submental muscles, though the suprahyoid muscles as a group have the greatest potential for force in hyolaryngeal elevation
Discussion
The submental muscles and thyrohyoid are thought to underlie hyolaryngeal elevation opening the upper esophageal sphincter [1]. The results of this study suggest that the proposed anterior and posterior muscular slings have the structural potential to synergistically elevate the hyolaryngeal complex during pharyngeal swallowing. The anterior sling muscles, which attach to the anterior portion of the hyoid (Fig. 1c, d), may conceptually include the submental muscles (mylohyoid, geniohyoid, and anterior digastric) or the suprahyoid muscles (submental muscles plus stylohyoid and posterior digastric). The posterior sling, formed by the long pharyngeal muscles, attaches to the length of the posterior edge of the thyroid and the lateral pharyngeal walls in the hypopharynx. The results of this study indicate no statistically significant difference in the potential for force between the submental muscles as an anterior sling and the long pharyngeal muscles as a posterior sling. However, if the anterior sling is defined to include all of the suprahyoid muscles, these muscles have a statistically significantly greater potential for force than do the long pharyngeal muscles. The attachments of the thyrohyoid, analyzed separately from either sling in our model, indicate that it also aids in laryngeal elevation. If either of these two-sling models is verified in functional studies, this would amend current swallowing theory, which currently does not acknowledge the potential role of the long pharyngeal muscles.
To date there are no functional data to confirm the activity and action of the long pharyngeal muscles in human swallowing. However, there is a strong indication of their involvement from animal studies. Electromyography data from a porcine model verify activity of the palatopharyngeus during pharyngeal swallowing in that animal [8, 15]. Transection of the pharyngeal branches of the vagus nerve in a rabbit model results in diminished laryngeal elevation, a functional deficit that could be attributed in part to the loss of the long pharyngeal muscles [16].
In human studies, Aydogdu et al. [17] documented an apparent relationship between diminished neurologic control of these muscles and dysphagia. These researchers investigated the effects of Wallenburg’s syndrome in 20 human subjects, 95% of whom had clinical signs of dysphagia. In this syndrome, infarction of the lateral medulla affects a number of cranial nerve nuclei. One of these nuclei is the nucleus ambiguus, which provides motor innervation to the long pharyngeal muscles, among other important swallowing muscles. The same syndrome does not affect the nuclei for cranial nerves V and VII, which innervate the mylohyoid, digastrics, and stylohyoid. In that study, subjects with Wallenburg’s syndrome demonstrate prolonged submental muscle activity during swallowing. One interpretation of this study may be that the anterior sling muscles compensate for the loss of posterior sling muscles.
Our structural observations provide some evidence that the current understanding of hyolaryngeal approximation is likely to be incomplete. If the usual list of muscles is assumed, the thyrohyoid is solely responsible for approximating the larynx to the hyoid bone [4, 18]. Population data of mean hyolaryngeal approximation distances in humans across bolus sizes is 1.09 ± 0.57 cm in females and 1.29 ± 0.47 cm in males [19]. Muscle fiber at optimal length is shown to maximally contract by as much as 28% [20]. In our cadaver sample, the mean optimal fascicle length of rehydrated thyrohyoid muscle was 2.77 ± 0.58 cm in females and 3.04 ± 0.75 cm in males [21]. Maximum thyrohyoid contraction in our sample would theoretically result in thyrohyoid approximation of only 0.78 cm in females and 0.85 cm in males. These structural analyses indicate that as much as 33% of laryngeal elevation may be attributed to the actions of other muscles that attach to the hyolaryngeal complex besides the thyrohyoid, such as the stylopharyngeus or the palatopharyngeus.
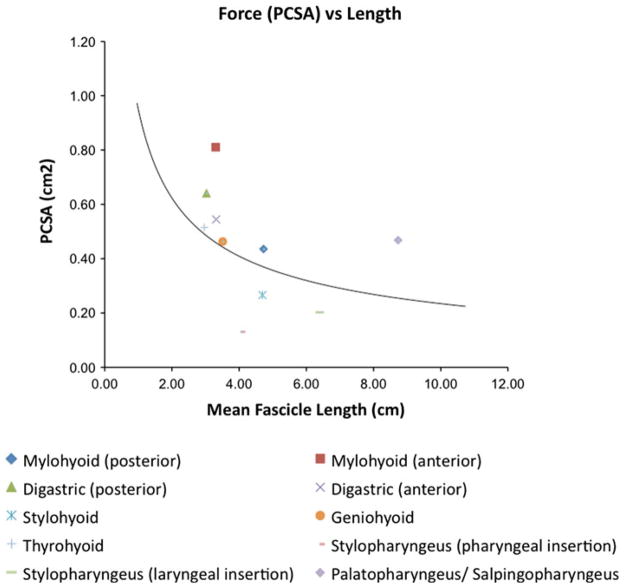
The anterior sling muscles are oriented to execute anterior and superior displacement of the hyolaryngeal complex [7]. While their structure indicates that they can contribute more force to elevation of the hyolaryngeal complex, this does not necessarily mean they are more essential to hyolaryngeal elevation. As reflected in the calculation of PCSA, there is an inverse relationship between muscle force and fiber length. While the suprahyoid muscles have greater potential for force, the long pharyngeal muscles may be more responsible for laryngeal excursion based on their length advantage (Fig. 3). Further functional studies are needed to more thoroughly elucidate the relationship between structure and function in these muscles.
Fig. 3.
Mean PCSA (cm2) versus mean fascicle length (cm) of muscles in sample (n = 12), with trend line indicating an inverse relationship between the potential for maximum tetanic tension and the potential for excursion. While in general the long pharyngeal muscles demonstrate less potential for force, they show greater potential for excursion given their length advantage
A limitation of the current study is the exclusion of the pharyngeal constrictor and hyoglossus muscles. These muscles are not accommodated by this method of force vector analysis: a line of action cannot represent the complex morphology of the pharyngeal constrictors, and the hyoglossus lacks a fixed distal attachment site. Obliquely oriented fibers of the middle and inferior pharyngeal constrictors inserting into the hyoid and thyroid may elevate the hyolaryngeal complex. However, it may also be that the mechanical advantage of obliquely oriented fibers of the pharyngeal constrictors is nullified by hyolaryngeal elevation occurring prior to pharyngeal constriction [5]. The hyoglossus could contribute to hyoid elevation when the tongue is stabilized. The attachment of the hyoglossus-palatoglossus complex to the hard palate may be functionally relevant to tongue placement or to hyolaryngeal elevation during bolus transit through the hypopharynx. Interestingly, it has been noted that following a complete glossectomy, including the hyoglossus, patients can still safely swallow a bolus if the larynx is elevated [22]. While this limitation takes nothing away from the present findings, it does leave the structural contribution of these muscles to hyolaryngeal elevation as an open question. Three-dimensional computer modeling of digitized muscle fiber bundles may provide a method with which to investigate the morphology of these muscles in the future [23].
Another limitation that should be noted is that the swallowing function of the subject population was unverified before expiration. More importantly, it should be reiterated that structural properties are not the same as functional realities. Anatomical data, while suggestive of function, must be corroborated by functional studies to establish its clinical usefulness. If verified, we propose that the most useful model of hyolaryngeal elevation during the pharyngeal phase of swallowing is that an anterior and a posterior muscular sling elevate the hyolaryngeal complex in addition to the thyrohyoid muscle.
Conclusion
The morphology of the long pharyngeal muscles suggests that they potentially have a significant role in working with the thyrohyoid and submental muscles to elevate the hyolaryngeal complex during swallowing.
Acknowledgments
The authors acknowledge Boston University School of Medicine Anatomical Gifts Program administrated by Rob Bouchie and Lee Iacopucci. Completion of this project was supported in part by grant No. F31DC011705 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Contributor Information
William G. Pearson, Jr., Email: bp1@bu.edu, Department of Anatomy and Neurobiology, Boston University, School of Medicine, 715 Albany Street, L-1004, Boston, MA 02118, USA.
Susan E. Langmore, Speech-Language Pathology, Boston University Medical Center for Speech and Hearing Sciences, Boston University, FGH Building, 4th floor, Boston, MA 02118, USA.
Louis B. Yu, Department of Anatomy and Neurobiology, Boston University, School of Medicine, 715 Albany Street, L-1004, Boston, MA 02118, USA.
Ann C. Zumwalt, Department of Anatomy and Neurobiology, Boston University, School of Medicine, 715 Albany Street, L-1004, Boston, MA 02118, USA.
References
- 1.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingjie L, Tong Z, Xinting S, Jianmin X, Guijun J. Quantitative videofluoroscopic analysis of penetration-aspiration in post-stroke patients. Neurol India. 2010;58:42–7. doi: 10.4103/0028-3886.60395. [DOI] [PubMed] [Google Scholar]
- 3.Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2007;22:1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 1989;257:748–59. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103:128. doi: 10.1016/0016-5085(92)91105-d. [DOI] [PubMed] [Google Scholar]
- 6.Kurt T, Gürgör N, Seçil Y, Yıldız N, Ertekin C. Electrophysiologic identification and evaluation of stylohyoid and posterior digastricus muscle complex. J Electromyogr Kinesiol. 2006;16:58–65. doi: 10.1016/j.jelekin.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Pearson WG, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–51. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102:587–600. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 9.Okuda S, Abe S, Kim HJ, Agematsu H, Mitarashi S, Tamatsu Y, Ide Y. Morphologic characteristics of palatopharyngeal muscle. Dysphagia. 2008;23:258–66. doi: 10.1007/s00455-007-9133-0. [DOI] [PubMed] [Google Scholar]
- 10.German RZ, Campbell-Malone R, Crompton AW, Ding P, Holman S, Konow N, Thexton AJ. The concept of hyoid posture. Dysphagia. 2011;26:97–8. doi: 10.1007/s00455-011-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber R, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–66. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.van Eijden T, Korfage J, Brugman P. Architecture of the human jaw-closing and jaw-opening muscles. Anat Rec. 1997;248:464–74. doi: 10.1002/(sici)1097-0185(199707)248:3<464::aid-ar20>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Kamibayashi LK, Richmond FJ. Morphometry of human neck muscles. Spine. 1998;23:1314–23. doi: 10.1097/00007632-199806150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J Exp Biol. 2005;208:3275–9. doi: 10.1242/jeb.01763. [DOI] [PubMed] [Google Scholar]
- 15.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102:1017–25. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima S, Shingai T, Kitagawa J, Takahashi Y, Taguchi Y, Noda T, Yamada Y. Role of the pharyngeal branch of the vagus nerve in laryngeal elevation and UES pressure during swallowing in rabbits. Dysphagia. 2003;18:58–63. doi: 10.1007/s00455-002-0082-3. [DOI] [PubMed] [Google Scholar]
- 17.Aydogdu I, Ertekin C, Tarlaci S, Turman B, Kiylioglu N, Secil Y. Dysphagia in lateral medullary infarction (Wallenberg’s Syndrome): an acute disconnection syndrome in premotor neurons related to swallowing activity? Stroke. 2001;32:2081–7. doi: 10.1161/hs0901.094278. [DOI] [PubMed] [Google Scholar]
- 18.Mepani R, Antonik S, Massey B, Kern M, Logemann J, Pauloski B, Rademaker A, Easterling C, Shaker R. Augmentation of deglutitive thyrohyoid muscle shortening by the Shaker Exercise. Dysphagia. 2009;24:26–31. doi: 10.1007/s00455-008-9167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:146–52. doi: 10.1007/s004550010017. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths RI. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991;436:219. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J Biomech. 2005;38:2317–20. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 22.McConnel FM, O’Connor A. Dysphagia secondary to head and neck cancer surgery. Acta Otorhinolaryngol Belg. 1994;48:165–70. [PubMed] [Google Scholar]
- 23.Ravichandiran K, Ravichandiran M, Oliver ML, Singh KS, McKee NH, Agur AMR. Determining physiological cross-sectional area of extensor carpi radialis longus and brevis as a whole and by regions using 3D computer muscle models created from digitized fiber bundle data. Comput Methods Programs Biomed. 2009;95:203–12. doi: 10.1016/j.cmpb.2009.03.002. [DOI] [PubMed] [Google Scholar]