Abstract
Due to its non-pathogenic lifecycle, little is known about the cellular determinants of infection by adeno-associated virus (AAV). To identify these critical cellular factors, we took advantage of the gene transfer abilities of AAV in combination with a forward genetic selection to identify proteins critical for transduction by this virus. AAV serotype 5 (AAV5) vectors encoding the furin gene were used to transduce furin-deficient cells followed by selection with furin-dependent toxins. A population of cells specifically resistant to AAV5 transduction was identified and sequence analysis suggested all had a single amino acid mutation in the leader sequence of the platelet-derived growth factor receptor alpha (PDGFRα) gene. Characterization of this mutation suggested it inhibited PDGFRα trafficking resulting in limited expression on the plasma membrane. Mutagenesis and transfection experiments confirmed the effect of this mutation on PDGFRα trafficking, and the AAV5 resistant phenotype could be rescued by transfection with wild type PDGFRα.
Keywords: Forward genetics, AAV5, furin, toxin, CHO cells, anthrax
INTRODUCTION
The entry process of a virus into a cell is not strictly determined by the virus but is very much dependent upon the proteins that are expressed by the cell and the interactions that occur between the virus and these proteins. Identification of these proteins has not only resulted in development of antiviral therapies but has also advanced our understanding of cell biology. Identification of the receptor(s) or intracellular proteins associated with viral infection can be a formidable task. In addition to the use of engineered cell lines and biochemical assays, several powerful genomic techniques have been used to accomplish this task. These include a radiation hybrid approach that identified the receptor for jaagsiekte virus entry (Rai et al., 2001) and a bioinformatics-based approach termed comparative genome analysis (CGA) developed in our laboratory which identified a receptor for adeno-associated virus type 5 (AAV5) and AAV6 and several genes important to the infection of Ebola virus (Brindley et al., 2011; Di Pasquale et al., 2003; Kondratowicz et al., 2011; Quinn et al., 2009; Weller et al., 2010). While these approaches have been successful, each has limitations. Genetic selection is a powerful approach for understanding complex biological processes. While reverse genetics is a common approach for understanding the function of a known gene by deleting it, forward genetics uses selective pressure on hemizygous cells to identify genes conferring a specific phenotype. Genetic screens using gene knockout techniques or complementation with cDNA libraries followed by selection have been used to identify genes involved in apoptosis or cytokine response pathways (Kumar et al., 1997, Velazquez et al., 1992).
In this study we investigated whether forward genetic selection could help to understand critical steps in virus infection processes. As a model virus, we chose AAV5. While AAV5 is not associated with any disease, vectors based on this virus have utility for gene transfer to the eye and lung as well as for delivering a genetic vaccination against viral agents (Alexander et al., 2007; Kuck et al., 2006; Zabner et al., 2000). Efficient transduction with AAV5 is known to require both N-linked α2–3 sialic acid as an attachment receptor as well as expression of the platelet-derived growth factor receptors (PDGFRα) for internalization (Di Pasquale et al., 2003; Kaludov et al., 2001). However, other proteins are likely to be important in this process. Following selection of a spontaneously mutated cell population for variants that resisted transduction, we confirmed the importance of PDGFRα in AAV5 transduction and identified a region in the leader sequence of PDGFRα which is responsible for the proper trafficking of this protein. Transfection experiments demonstrated that the AAV5-resistant phenotype could be rescued by wild type PDGFRα.
EXPERIMENTAL PROCEDURES
Construction of AAV2F, AAV5F, AAV2B and AAV5B and vector production
A HindIII and BlpI fragment containing the furin cDNA was isolated from the pAGEFur plasmid (Hatsuzawa et al., 1990) and ligated into HindIII- and BlpI-digested AAV2rnLacZ and AAV5rnLacZ plasmids to create AAV2F and AAV5F plasmids, respectively. AAV2B and AAV5B encoding the beta galactosidase gene were previously described (Kaludov et al., 2001). Recombinant particles were produced in 293T cells as previously described (Alisky et al., 2000).
Cloning of cDNA
RNA was isolated from CHO cells by extraction of the cells with Triazol (Invitrogen, Carlsbad, CA). DNA was synthesized using the Smart Race kit (Clontech, Mountain View CA) and sequenced using a series of primers based on regions conserved across the mouse, rat, and human cDNAs of PDGFRα. Plasmids expressing either human or CHO PDGFRα were assembled and expressed in the DualGC vector (Stratagene) using the CMV promoter (phPDGFRα or pcPDGFRα respectively).
Cell culture
CHO-K1 (ATCC CCL-61) and CHO FD11 cells (Gordon et al, 1995), were cultured at 37°C, 5% CO2 in alpha minimal essential medium (AMEM) (Biofluids) supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 100 U/ml streptomycin. A549 cells were maintained at 37°C, 5% CO2 in HAM’s F12 and D10 medium (Dulbecco’s modified Eagle’s medium) containing 10% fetal bovine serum, 100 μg/ml penicillin, and 100 U/ml streptomycin. Madin-Darby Canine Kidney (MDCK) cells were a gift from Bob Weller. Cells were cultured at 37°C, 5% CO2 in Dulbecco’s modified essential medium (DMEM) (Biofluids) supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 100 U/ml streptomycin.
Toxin screen
Sensitivity to Pseudomonas exotoxin A (PE) toxin was determined by plating 1 × 104 CHO-K1 and CHO FD11 cells and then incubating them with increasing amounts of PE (List Biologics) for 48 h. Two days post-selection, surviving cells were counted using a microscope and a calibrated ocular. Sensitivity to anthrax toxins was determined using protective antigen (PA, List Biologics) from Bacillus anthracis in combination with fusion protein FP59 (a fusion of anthrax lethal factor with the catalytic domain of PE (Gordon et al., 1995; Klimpel et al., 1992). A modified form of PA (PA-RAAR, having amino acids 164-RKKR-167 changed to RAAR) was also used in the screen to improve the specificity for cleavage by furin. CHO-K1 and CHO FD11 cells were incubated with increasing amounts of PA for 48 h in concert with 20 ng/ml FP59. Two days post-selection surviving cells were counted using a microscope and a calibrated ocular.
Confocal immunofluorescence
Parental and mutant CHO cells were fixed with 1% paraformaldehyde for 15 min at room temperature prior to permeabilizing with 0.2% Triton X-100 in PBS and blocking. Cell surface PDGFRα expression was evaluated by incubating non-permeablized cells with primary mouse monoclonal anti-PDGFRα antibody (1:500) (Santa Cruz Biotechnology, CA) and secondary antibody (Alexa Fluor 488 anti-mouse, Molecular Probes ) (1:1000).
RESULTS
Design of AAV-based Genetic Selection
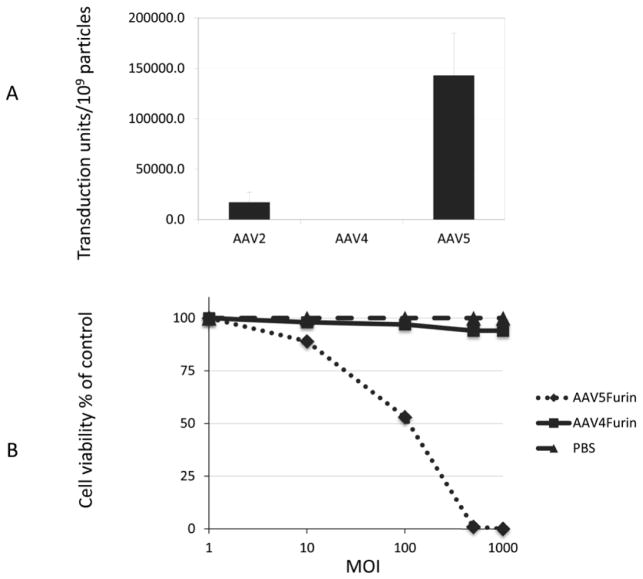
A recessive screen involves the identification of a change in phenotype because of the loss of gene expression. An example of this technique is the use of selection in a polyclonal population in which mutagenesis or loss of a gene results in a block in gene expression and alters the cell phenotype. Hemizygotic cells are an advantage in recessive screens and we chose to use the hemizygous furin-deficient Chinese Hamster Ovary cell mutant CHO FD11 (Gordon et al., 1995), which has been successfully used in recessive screens. Although the original FD11 isolate was clonal following the initial selection, subsequent passages and expansions can result in drift and spontaneous mutations to accumulate at other hemizygous loci, so that the resulting population of cells can be useful in subsequent screenings. Thus, the strategy to identify genes associated with AAV transduction involved transducing this population of CHO FD11 cells with AAV vectors encoding furin (AAVF) and then selecting resistant cells with either PE or PA toxin. To determine which serotypes could be screened using this strategy, CHO FD11 cells were transduced with three different serotypes (AAV2, AAV4, AAV5) of AAV encoding the β-gal gene and their transduction efficiencies compared (Fig. 1a). CHO FD11 cells were permissive for both AAV2 and AAV5 but resistant to AAV4 transduction. Because AAV5 exhibited a higher transduction efficiency, it was chosen for the recessive screen.
FIG. 1. AAV transduction and sensitivity to PE of CHO FD11 cells.
A) Cells were transduced with AAV2B, AAV4B, or AAV5B and the transduction activity determined by end point dilution. N=3. No transduction was observed with AAV4 compared with AAV2 or AAV5. B) CHO FD11 cells transduced with AAV5F or AAV4F at increasing MOIs were challenged with PE for 48 h and the number of surviving cells was counted. Cells treated with PBS were included as a control. CHO FD11 cells transduced with AAV5 were very sensitive to toxin compared with AAV4-treated cells.
CHO FD11 Transduction and PE selection
To determine the optimal toxin concentration, cell killing experiments were first done using the furin expressing cells (CHO-K1) and the furin deficient and toxin-resistant CHO FD11 cell line. Concentrations of 0.3 μg/ml PE and 0.03 μg/ml PA were chosen for use in the screening. All PA selections were carried out in concert with addition of 20 ng/ml FP59 (Gordon et al., 1995). An MOI of 500 AAV5F virions/cell led to a 99% kill of the cells subsequently treated with PE (Fig. 1b), while at an MOI of 1000 no cells survived. However, even at an MOI of 1000, transduction with AAV4F did not affect cell viability, confirming the previous data in Fig. 1a regarding the different cell tropisms of AAV serotypes. From this data, we concluded that AAV5 transduction (i.e., rAAV5F) could complement furin deficiency in CHO FD11 cells.
Selection of CHO FD11 cells resistant to AAV5 transduction
To conduct the selection, four rounds of transduction were carried out with increasing amounts of vector on pooled cells. As AAV vectors tend to remain episomal, they will eventually be lost in a dividing tissue culture cell population allowing for subsequent rounds of selection. To minimize the potential of false positives that might occur due to resistance to PE selection, the cells were challenged with PA in the second round of selection instead of PE and then challenged with PE in subsequent rounds. The number of resistant clones was tested at each round and was observed to increase suggesting a selection of resistant cells. In the final round at an MOI of 3500, 11 of the 20 colonies tested had a decrease in transduction with AAV5 of at least 10-fold. At an MOI of 7000, seven clones out of 13 exhibited a decrease in transduction with AAV5 vectors compared to CHO FD11 (Table 1). Overall the transduction activity of AAV5 decreased 10–1000 fold while the activity of AAV2 (another AAV serotype with distinct cell tropism and cell binding activities) was not affected. This finding suggests the mutation(s) in the selected clones are specific to AAV5 transduction.
Table 1. Selection of CHO FD11 cells resistant to AAV5 transduction.
The data summarize the number of colonies obtained after each round of selection. The colony number was calculated for MOI = 700 transductions by measuring the average number of colonies per cm2. For MOIs of 3500 and 7000, colonies were counted directly. Resistance phenotypes of isolated colonies to AAV5 transduction (two columns at right) were determined by transduction with AAV encoding β-galactosidase and the titer compared to that of the parent cells. Resistance to AAV5 is defined as a >10-fold decrease in transduction with AAV5β-gal. Two parallel selections were performed at round 4.
| Selection Round | Selection conditions | Surviving colonies | Phenotype of surviving colonies | |||
|---|---|---|---|---|---|---|
| Cells transduced | MOI | number | Percent of cells plated | Colonies tested | Colonies resistant to AAV5 | |
| 1 | 2 × 106 | 700 | 39800 | 2 | 159 | 1 |
| 2 | 1 × 105 | 700 | 23000 | 23 | - | - |
| 3 | 1 × 105 | 700 | 44250 | 44 | 30 | 1 |
| 4a | 1 × 104 | 3500 | 20 | 0.2 | 20 | 11 |
| 4b | 1 × 104 | 7000 | 13 | 0.13 | 13 | 7 |
Characterization of CHO FD11 cells resistant to AAV5 transduction
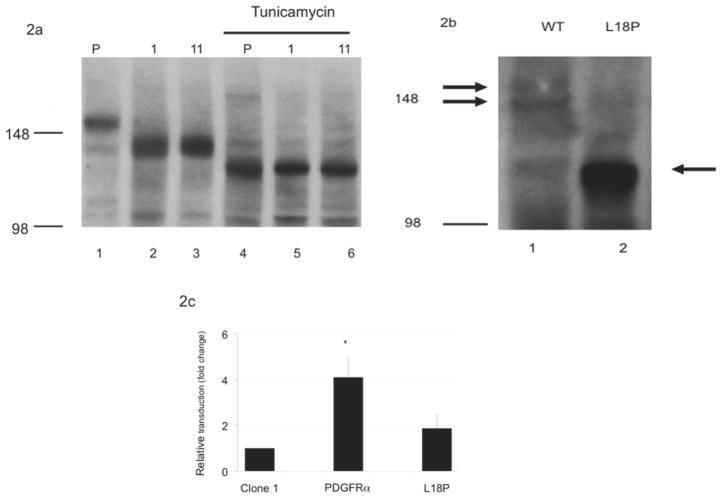
Previous data from our group demonstrated that the PDGFRα plays a key role in AAV5 transduction by serving as an internalization receptor for virus entry (Di Pasquale et al., 2003). To test if PDGFRα expression had changed in the transduction-resistant clones, cell lysates were prepared from two clones (clones 1 and 11) and a western blot was probed with anti-PDGFRα antibody (Fig. 2a). This blot suggested a similar level of expression for the two clones compared with the WT parent, but a higher mobility for the major band, which migrated at a position similar to that of a minor band in the WT PDGFRα. Five other randomly selected clones with resistance to AAV5 transduction from the MOI 3500 and 7000 selections were also tested and yielded the same banding patterns (data not shown). This suggests that these clones may be siblings and that this mutation was present early in the selection process and was expanded during subsequent selections. Human PDGFRα is glycosylated at as many as nine sites, with the added glycans accounting for up to 20% of the apparent molecular weight (Daniel et al., 1987). To distinguish whether the difference in mobility was the result of a change in glycosylation or in the protein sequence, cells were treated with tunicamycin to block the addition of N-linked carbohydrates to the protein. Tunicamycin treatment resulted in a similar mobility for the PDGFRα in the parental cells and clones 1 (MOI 3500 selection) and 11 (MOI 7000 selection), suggesting that the original difference in mobility was indeed due to decreased glycosylation of PDGFRα (Fig. 2a).
FIG. 2. PDGFRα expression and activity.
A) CHO PDGFRα glycosylation was detected by Western blot of whole cell lysates prepared from either untreated (lanes 1–3) or tunicamycin treated (lane 4–6) parental (lane 1, 4) or CHO FD11 clones 1 or 11 (lanes 2,5 and 3,6, respectively). Blots were probed for PDGFRα expression and mobility with an anti-PDGFRα antibody. Mobility of molecular weight markers of 148 and 98 kDa are indicated. B) Altered mobility of the CHO PDGFRα L18P clone compared with the CHO PDGFRα WT was determined by western blotting of whole cell lysates from MDCK cells transfected with the respective clones and probing with anti-PDGFRα antibodies. Arrows indicate the specific bands. Molecular weight markers at 148 and 98 kDa are indicated. C) Increased AAV5 transduction was observed upon transient transfection of CHO FD11 clone 1 cells with WT PDGFRα, compared with transfection of the L18P mutant with AAV5 vector expressing LacZ. N=3.
The change in glycosylation could be the result of alterations to either the primary sequence of the CHO FD11 PDGFRα, the cellular enzymes responsible for the addition of carbohydrates, or intracellular trafficking. To clarify the mechanism of this change in glycosylation, we cloned and sequenced the PDGFRα gene from the WT parental CHO FD11 cells as well as from clones 1 and 11. The CHO FD11 PDGFRα gene sequence that we determined (and deposited as Genbank HQ424162) is approximately 91% identical to the human sequence (data not shown). Sequence alignment of PDGFRα isolated from clones 1 and 11 with PDGFRα cloned from the parental CHO FD11 cells identified a single amino acid change in both clones as compared to WT, a leucine to proline substitution at position 18 (L18P). Domain analysis of the sequence of the WT CHO FD11 PDGFRα indicated that the first 23 amino acids constitute the leader sequence, suggesting this mutation could affect protein trafficking in the cell. The consensus sequence associated with a peptide leader region of protein is ill-defined but highly related to the hydrophobicity and structure of the domain (Ryan and Edwards, 1995). Thus, a substitution of a proline for leucine, which would both decrease the overall hydrophobicity as well as introduce a β-turn, could prevent proper trafficking of PDGFRα, resulting in both the altered glycosylation and lack of cell surface expression.
To verify that this mutation was responsible for the change in mobility and glycosylation observed with the PDGFRα in the mutant clones, site directed mutagenesis was used to correct the mutation and the corrected gene was transfected into Madin-Darby Canine Kidney MDCK cells, which have been extensively used for studying protein trafficking. Transfection of the L18P mutant or corrected PDGFRα-encoding plasmids generated distinct bands of 114 kDa and 156/148 kDa respectively (Fig. 2b), suggesting this amino acid is responsible for the change in mobility and glycosylation observed in the mutant CHO clones 1 and 11. This change in molecular weight is similar to that previously reported for deglycosylated PDGFRα (Daniel et al., 1987).
It would be expected that transfection of the WT PDGFRα (pcPDGFRα) gene should rescue the phenotype of the CHO mutants. CHO clone 1 cells were transiently transfected with either the pcPDGFRα sequence or the L18P mutation and the relative transduction activity compared to cells transfected with an empty vector (Fig. 2c). Although only a fraction of the cells were transfected, PDGFRα expression in the CHO clone 1 cell population increased the transduction activity of AAV5 by over 4-fold compared with cells transfected with an empty vector (P<0.05). In contrast, transfection of the PDGFRα gene containing the L18P mutation increased AAV5 transduction only slightly, and the change was not statistically significant. Similar results were seen if the cells were transfected with the human version, phPDGFRα (data not shown).
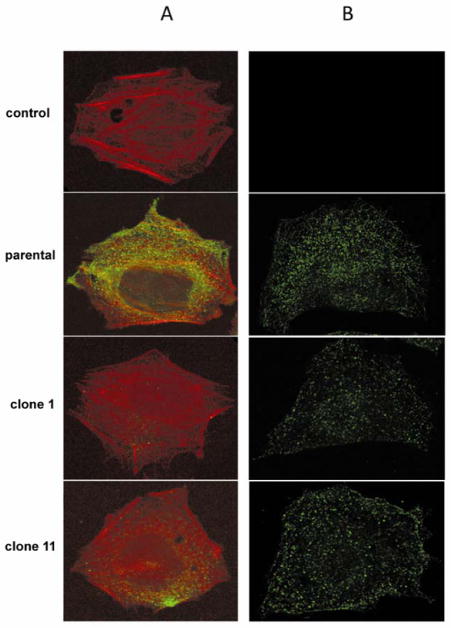
Altered glycosylation and intracellular trafficking has been associated with poor cell surface trafficking of membrane proteins (Vagin, Kraut, and Sachs, 2009). To determine the cell surface expression of PDGFRα in WT CHO FD11 and clone 1 or 11 cells, non-permeabilized cells were incubated with anti-PDGFRα antibody followed by a fluorescently labeled secondary antibody and then counter stained with rhodamine labeled phalloidin. Compared with control cells, the WT parental CHO FD11 cells express abundant levels of PDGFRα on their surface (Fig. 3). In contrast, very little PDGFRα was detected on the surface of clone 1 or clone 11. Permeabilization of the cells showed similar levels of PDGFRα expression in all three cell lines. This finding suggests that the L18P mutation affects the trafficking of the PDGFRα receptor and suggests the loss of AAV5 transduction is the result of altered trafficking of PDGFRα and lack of surface availability to function as a receptor for AAV5.
FIG. 3. Immunofluorescent staining for PDGFRα.
Parental and CHO FD11 Clones 1 and 11 were stained for PDGFRα expression. A) Cell surface PDGFRα expression was detected on unpermeabilized cells by staining with anti-PDGFRα (green) and counter staining with phalloidin (red). B) Intracellular PDGFRα was detected by permeabilizing the cells with Triton X-100 and then staining with anti-PDGFRα (green). Anti-PDGFRα was omitted in control samples in top row. Limited cell surface expression was detected in the CHO FD11 clone 1 and 11 cells compared with parental CHO K1 cells.
DISCUSSION
Forward genetic screens offer a powerful approach for understanding complex phenotypes such as virus entry. However, to be effective, this approach must be combined with strong selection techniques. By taking advantage of the gene transfer activities of virus in combination with our increased understanding of toxin biology, it is possible to achieve the strong selection necessary for a successful screen. Although not utilized in this study the incorporation of insertional mutagenesis strategies such as retroviral insertion and gene trap vectors to disrupt gene expression (Carette et al., 2009; Liu and Leppla, 2003) or the use of large scale siRNA libraries would further increase the discovery rate. One limitation in our study is the relatively low transduction rescue of the CHO FD11 Clone 1 cells. This is likely due to a combination of factors including low transfection efficiency of this clone (<20% at 48 h, data not shown), and competition from the endogenous mutant PDGFRα. Alternatively there could be additional mutations that affect transduction efficiency not related to PDGFRα expression.
In addition to confirming the central and likely rate limiting role of PDGFRα in the lifecycle of AAV5, the identification of an amino acid in the leader sequence necessary for optimal expression of PDGFRα maybe useful in characterizing the trafficking of this protein in the cell. Mutations in the leader sequence of PDGFRα have not been previously reported. However, mutations in the leader sequences of several other genes are associated with either disease or increased susceptibility to infection (Jarjanazi et al., 2008). Just as in our findings with clones 1 and 11, leucine to proline substitutions have been found in the calcium-sensing receptor gene, where an L13P substitution is associated with Familial hypocalciuric hypercalcemia as well as neonatal hyper-parathyroidism, and in the sonic hedgehog gene, where an L17P substitution is associated with holoprosencephaly (Kato et al., 2000; Pidasheva et al., 2005).
We demonstrated that the PDGFRα L18P mutation results in very low cell surface expression. Mutations in other regions of PDGFRα are associated with human diseases. Congenital diaphragmatic hernia (CDH) (Bleyl et al., 2007), a common birth defect with an estimated prevalence of 1 in 2500 live births, is attributed to 12 different polymorphisms in exons 3–22. Mutations in the promoter region of PDGFRα are associated with low PDGFRα expression and disease (Wu et al., 2006). In asthma, the frequency of the PDGFRα low expression allele H1 in either homozygous or heterozygous genotypes in nonallergic asthmatics was significantly higher compared with controls. In contrast, the high expression allele H2 was reduced in this population compared with controls. Single nucleotide polymorphism analysis of the leader peptide of the PDGFRα gene may identify new alleles associated with these and other diseases.
Highlights.
Forward genetic selection is a powerful tool for understanding complex host:virus interactions.
A mutation in the leader sequence of PDGFRα blocks receptor trafficking and AAV5 transduction.
This finding suggests that PDGFRα is a critical protein in the transduction of AAV5.
Virus entry is a rate-limiting step in the lifecycle of AAV5.
Acknowledgments
The authors acknowledge the assistance of Medha Bhagwat and Lynn Young with the sequence confirmation of CHO PDGFRα and review and editorial assistance from Mini Varughese.
This work was supported by a National Institutes of Health, National Institute of Dental and Craniofacial Research intramural research grant to JAC, by funds of the Molecular Physiology and Therapeutics Branch, National Institute of Dental and Craniofacial Research, and by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Agnieszka Rzadzinska, Email: agnieszka.rzadzinska@addenbrookes.nhs.uk.
Stephen H. Leppla, Email: sleppla@nih.gov.
References
- Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, Timmers AM, Hawes NL, Pang JJ, Barlow RB, Hauswirth WW. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13(6):685–7. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisky JM, Hughes SM, Sauter SL, Jolly D, Dubensky TW, Jr, Staber PD, Chiorini JA, Davidson BL. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport. 2000;11(12):2669–73. doi: 10.1097/00001756-200008210-00013. [DOI] [PubMed] [Google Scholar]
- Bleyl SB, Moshrefi A, Shaw GM, Saijoh Y, Schoenwolf GC, Pennacchio LA, Slavotinek AM. Candidate genes for congenital diaphragmatic hernia from animal models: sequencing of FOG2 and PDGFRalpha reveals rare variants in diaphragmatic hernia patients. Eur J Hum Genet. 2007;15(9):950–8. doi: 10.1038/sj.ejhg.5201872. [DOI] [PubMed] [Google Scholar]
- Brindley MA, Hunt CL, Kondratowicz AS, Bowman J, Sinn PL, McCray PB, Jr, Quinn K, Weller ML, Chiorini JA, Maury W. Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology. 2011;415(2):83–94. doi: 10.1016/j.virol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette JE, Guimaraes CP, Varadarajan M, Park AS, Wuethrich I, Godarova A, Kotecki M, Cochran BH, Spooner E, Ploegh HL, Brummelkamp TR. Haploid genetic screens in human cells identify host factors used by pathogens. Science. 2009;326(5957):1231–5. doi: 10.1126/science.1178955. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Milfay DF, Escobedo J, Williams LT. Biosynthetic and glycosylation studies of cell surface platelet-derived growth factor receptors. J Biol Chem. 1987;262(20):9778–84. [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, Chiorini JA. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9(10):1306–12. doi: 10.1038/nm929. Epub 2003 Sep 14. [DOI] [PubMed] [Google Scholar]
- Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun. 1995;63(1):82–7. doi: 10.1128/iai.63.1.82-87.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsuzawa K, Hosaka M, Nakagawa T, Nagase M, Shoda A, Murakami K, Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990;265(36):22075–8. [PubMed] [Google Scholar]
- Jarjanazi H, Savas S, Pabalan N, Dennis JW, Ozcelik H. Biological implications of SNPs in signal peptide domains of human proteins. Proteins. 2008;70(2):394–403. doi: 10.1002/prot.21548. [DOI] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75(15):6884–93. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Nanba E, Akaboshi S, Shiihara T, Ito A, Honma T, Tsuburaya K, Hayasaka K. Sonic hedgehog signal peptide mutation in a patient with holoprosencephaly. Ann Neurol. 2000;47(4):514–6. [PubMed] [Google Scholar]
- Kerr JR, Cotmore SF, Bloom ME, Linden RM, Parrish CR. Parvoviruses. Edward Arnold Limited; London, UK: 2005. [Google Scholar]
- Klimpel KR, Molloy SS, Thomas G, Leppla SH. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci U S A. 1992;89(21):10277–81. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB, Jr, Chiorini J, Maury W. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A. 2011;108(20):8426–31. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck D, Lau T, Leuchs B, Kern A, Muller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. J Virol. 2006;80(6):2621–30. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278(5343):1630–2. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- Liu S, Leppla SH. Retroviral insertional mutagenesis identifies a small protein required for synthesis of diphthamide, the target of bacterial ADP-ribosylating toxins. Mol Cell. 2003;12(3):603–13. doi: 10.1016/j.molcel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Canaff L, Simonds WF, Marx SJ, Hendy GN. Impaired cotranslational processing of the calcium-sensing receptor due to signal peptide missense mutations in familial hypocalciuric hypercalcemia. Hum Mol Genet. 2005;14(12):1679–90. doi: 10.1093/hmg/ddi176. [DOI] [PubMed] [Google Scholar]
- Quinn K, Brindley MA, Weller ML, Kaludov N, Kondratowicz A, Hunt CL, Sinn PL, McCray PB, Jr, Stein CS, Davidson BL, Flick R, Mandell R, Staplin W, Maury W, Chiorini JA. Rho GTPases modulate entry of Ebola virus and vesicular stomatitis virus pseudotyped vectors. J Virol. 2009;83(19):10176–86. doi: 10.1128/JVI.00422-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci U S A. 2001;98(8):4443–8. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, Edwards CO. Systematic introduction of proline in a eukaryotic signal sequence suggests asymmetry within the hydrophobic core. J Biol Chem. 1995;270(46):27876–9. doi: 10.1074/jbc.270.46.27876. [DOI] [PubMed] [Google Scholar]
- Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol. 2009;296(3):F459–69. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70(2):313–22. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Weller ML, Amornphimoltham P, Schmidt M, Wilson PA, Gutkind JS, Chiorini JA. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med. 2010;16(6):662–4. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LS, Tan CY, Wang LM, Lin CG, Wang JY. Variant in promoter region of platelet-derived growth factor receptor-alpha (PDGFRalpha) gene is associated with the severity and allergic status of childhood asthma. Int Arch Allergy Immunol. 2006;141(1):37–46. doi: 10.1159/000094180. [DOI] [PubMed] [Google Scholar]
- Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL, Chiorini JA. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74(8):3852–8. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]